ABSTRACT
Carbapenem resistance in Acinetobacter baumannii is primarily due to the global spread of two main clones that carry oxa23, oxa24, and oxa58. However, new carbapenem-resistant clones are emerging that are also resistant to a wide range of antibiotics. Strains belonging to ST85IP (Institut Pasteur) carry the blaNDM metallo-β-lactamase carbapenem resistance gene. Here, we completed the genome sequence of an ST85IP strain, Cl300, recovered in 2015 in Lebanon, using a combination of Illumina MiSeq and Oxford Nanopore sequencing and a hybrid assembly approach. Cl300 is highly resistant to meropenem and amikacin, and consistent with this, a copy of the blaNDM carbapenem and 14 copies of the aphA6 amikacin resistance genes were found in the genome. Cl300 also contains the sul2 sulfonamide and the msr(E) macrolide resistance genes. All aphA6 copies and blaNDM are in a novel 76-kb Tn7 family transposon designated Tn6924. Like Tn7, Tn6924 is bounded by 29-bp inverted repeats with additional TnsB binding sites at each end. Several variants of Tn6924 were found in a set of diverse strains, including ST85IP strains as well as members of global clones 1 and 2. sul2 and msr(E) are in a 13.0-kb pseudocompound transposon (PCT) bounded by IS1008. ST85s represent a diverse group of strains, particularly in their antibiotic resistance gene content and the K and OC surface polysaccharide loci. Acquisition of Tn6924 by members of global clones indicates the significance of this transposon in spreading two clinically significant resistance genes, blaNDM and aphA6.
IMPORTANCE To date, efforts to study the resistance mechanisms of carbapenem-resistant Acinetobacter baumannii have been largely focused on the two major globally distributed clones (GC1 and GC2). ST85 is an emerging sequence type, and unlike other clones, it is associated with the carriage of the blaNDM gene. Here, we completed the genome sequence of an ST85 strain and showed that blaNDM and 14 copies of the aphA6 amikacin resistance genes are in Tn6924, a novel Tn7 family transposon. Analysis of all publicly available ST85s predicted that all strains in the main lineage carry a variant of Tn6924. Variants of Tn6924 were also found in other clones, including GC1 and GC2. Tn6924 is an important mobile element given that it carries two clinically important resistance genes (blaNDM and aphA6) and has spread to other clones. Therefore, outbreaks caused by ST85s should be studied and tracked.
KEYWORDS: Acinetobacter baumannii, aminoglycoside resistance, carbapenem resistance, aphA6, bla NDM , antibiotic resistance, Tn6924
INTRODUCTION
Acinetobacter baumannii is a Gram-negative opportunistic pathogen that causes a range of nosocomial infections. Infections caused by this microorganism have become a challenge to treat due to high levels of antibiotic resistance (1). In particular, the global spread of carbapenem-resistant A. baumannii (CRAb) strains that are also extensively drug resistant has become a major concern (2, 3).
In A. baumannii, carbapenem resistance is predominantly caused by genes encoding the class D OXA-23, OXA-24 (OXA-40), and OXA-58 β-lactamases (oxacillinases), while class B enzymes (metallo-β-lactamases) are uncommon. The majority of CRAb strains belong to two major global clones, global clone 1 (GC1) and 2 (GC2), also known as international clones (ICs) (2, 4), with GC2s being the most prevalent clone in most geographical areas (2). In the last few years, several studies have reported the emergence of CRAb strains that do not belong to the major global clones, such as those belonging to ST25IP (5–10) and, more recently, CRAb strains belonging to ST85IP (3, 11–19). Although low in number compared to the widespread GC1 and GC2 isolates, ST85 strains have been reported in Algeria, France, Tunisia, Spain, Turkey, Lebanon, and Libya (3, 11–19). Notably, in most cases, carbapenem resistance in ST85IP strains is associated with the presence of the blaNDM metallo-β-lactamase gene, which encodes a class B carbapenemase enzyme (13, 15, 16, 18, 20), as well as, in some cases, genes conferring high levels of aminoglycoside resistance, in particular, to kanamycin and tobramycin (11, 19–21). However, despite their significance, the genetic context of antibiotic resistance genes, especially the blaNDM metallo-β-lactamase gene, has not been studied in detail.
Here, we completed the genome sequence and examined the genomic context of antibiotic resistance genes in an ST85IP CRAb strain recovered in Lebanon. We show that the blaNDM and the aphA6 (amikacin, kanamycin, and neomycin resistance) genes are in a novel Tn7 family transposon designated Tn6924. Furthermore, we studied the evolution of Tn6924 using a comparative analysis of several related transposons in publicly available complete ST85IP genomes.
RESULTS
Antibiotic resistance profile.
Cl300 was found to be resistant to carbapenems (imipenem and meropenem), ampicillin, third-generation cephalosporins (cefotaxime and ceftazidime), streptomycin, amikacin, netilmicin, spectinomycin, neomycin, kanamycin, sulfamethoxazole, nalidixic acid, ciprofloxacin, trimethoprim, florfenicol, and chloramphenicol (Table S1 in the supplemental material). Cl300 is susceptible to colistin, with an MIC of <0.25 mg/L, and showed reduced susceptibility to ampicillin-sulbactam (Table S1).
Complete genome sequence of Cl300 and antibiotic resistance genes.
The genome of Cl300 was completed using a combination of Illumina MiSeq and Oxford Nanopore (MinION) data using a hybrid assembly approach. The genome assembly was repeated three times and always resulted in an identical genome assembly, which consists of a 4,007,379-bp chromosome and a 9,205-bp plasmid named pCl300.
The Cl300 genome was found to contain 14 copies of the aphA6 (amikacin resistance) gene (locus ids K9C16_00500, K9C16_00510, K9C16_00520, K9C16_00530, K9C16_00540, K9C16_00550, K9C16_00560, K9C16_00570, K9C16_00655, K9C16_00665, K9C16_00675, K9C16_00685, K9C16_00695, and K9C16_00705 in GenBank accession number CP082952) and a single copy of blaNDM (carbapenem resistance; locus id K9C16_00580), sul2 (sulfonamide resistance; locus id K9C16_12420), msr(E) (macrolide-triamilide resistance; locus id K9C16_12495) and bleMBL (bleomycin resistance; locus id K9C16_00585), accounting for its resistance phenotype observed. The blaNDM gene refers to the blaNDM-1 throughout the manuscript.
Cl300 is resistant to fluoroquinolones due to the mutations found in the gyrA DNA gyrase and parC topoisomerase IV genes, leading to GyrA S81L and ParC S84L substitutions. These specific mutations are well-known to cause resistance to fluoroquinolones (e.g., nalidixic acid) in Gram-negative bacteria, including A. baumannii (22, 23).
pCl300 is cryptic and encodes a novel putative replication initiation protein that belongs to the Rep_3 family (PFAM01051). Its closest known Rep is RepAci3 encoded by p203 (GenBank accession number GU978997). An identical plasmid was found in A. baumannii strain ACN21 (GenBank accession number CP038648). ACN21 is recovered in India but also belongs to ST85IP, the same as Cl300.
Cl300 is resistant to high levels of meropenem and amikacin.
MIC testing was used to examine whether the presence of blaNDM and the multiplication of aphA6 in Cl300 have led to higher levels of carbapenem and amikacin resistance. The meropenem MIC was found to be 512 mg/L, versus 16, 32. and 128 mg/L for D36 (with a single copy of oxa23), ACICU (carrying oxa58), and ABS201 (carrying oxa24), respectively. This signifies the contribution of a single copy of blaNDM in Cl300 toward its extensive carbapenem resistance, given that the CLSI recommends the >8-mg/L (for both imipenem and meropenem) threshold to consider a strain as resistant.
The amikacin MIC for Cl300 was found to be >512 mg/L (versus 8 mg/L for A388 with a single aphA6 copy), which is significant given the CLSI recommendation of ≥64-mg/L threshold for considering a strain as resistant. We also analyzed the Illumina read depth of the aphA6 sequence relative to the gyrA gene, which is a housekeeping gene located in the chromosome. The sequence of the gyrA gene had a 30.28 coverage, while the aphA6 sequence read depth was 399.26 (SRA accession number SRR15725578). resulting in a 13.18-fold increase in sequence depth/coverage of aphA6 relative to the chromosome, which correlates well with the presence of 14 aphA6 copies observed in the final assembly (see below) and, hence, the high amikacin resistance level observed.
Cl300 contains Tn6924, a novel Tn7 family transposon with a blaNDM and 14 copies of aphA6.
Analysis of the Cl300 genome showed that all 14 aphA6 copies, as well as the blaNDM gene, were clustered in a chromosomal region at the 3′ end of the glmS gene (locus id K9C16_00775 in GenBank accession number CP082952). We determined the boundaries of this element and found that it is bounded by 29-bp imperfect inverted repeats (IRs) and flanked by a 5-bp duplication of the target site (TGCCT at bases 92721 to 92725 in GenBank accession number CP082952). Examination of this region also indicated the presence of a set of genes encoding transposition proteins related to the TnsABCDE transposition proteins (29 to 50% amino acid identity; locus IDs K9C16_00750, K9C16_00755, K9C16_00760, K9C16_00765, and K9C16_00770 in GenBank accession number CP082952) encoded by the well-studied Tn7 transposon (24). Recently, we described Tn6171 in several A. baumannii strains, which is a Tn7 family transposon that carries the fimsbactin siderophore systems (25). Analysis of the TnsABCDE proteins found in Cl300 showed that they share 86 to 94% amino acid identity with those encoded by Tn6171. In addition, several overlapping copies of the inner part of the IRs, equivalent to the binding sites for the transposase TnsB (26), were found within 200 bp of the transposon boundaries. All these properties are reminiscent of Tn7 and its related transposons (often called Tn7 family transposons), and the transposon in Cl300 was called Tn6924. Tn6924 is a 76,190-bp novel Tn7 family transposon (bases 92726 to 168915 in GenBank accession number CP082952) with a highly complex evolutionary history and a mosaic structure composed of multiple class I transposons and insertion sequences. The 14 copies of aphA6 are in two separate locations in Tn6924, and each copy of aphA6 is interspersed with copies of ISAba125 in a structure known as TnaphA6 (27). Six tandem duplications of TnaphA6 (region A in Fig. 1) are found inside an existing transposon comprised of ISAba125-jycS-ISAba125 and have generated a 3-bp target site duplication (CTT), suggesting this structure has arisen via traditional transposition followed by tandem duplication of the individual TnaphA6 unit. The second set of eight tandem duplications of TnaphA6 (region B in Fig. 1), adjacent to blaNDM-1 in their expected location in Tn125, appear to have duplicated in place, as there are no target site duplications detectable.
FIG 1.
Tn6924. (A) Structure of Tn6924 in Cl300 (GenBank accession no. CP082952); (B) structure in Acinetobacter baumannii strains AbBAS1 (GenBank accession no. CP065392), TP1 (GenBank accession no. CP056784), TP2 (GenBank accession no. CP060011), TP3 (GenBank accession no. CP060013), ACN21 (GenBank accession no. CP038644), and 11W359501 (GenBank accession no. CP041035). Copies of ISAba125 are shown as dark green boxes, and other insertion sequences are shown as light green boxes. Inverted repeats are shown as vertical bars, and the nucleotide sequences of target site duplications are shown above the line. The orientation and extent of genes are indicated by horizontal arrows. Transposition genes are colored purple, and antibiotic resistance genes are colored orange and red. Structures of known origin are labeled.
Variants of Tn6924 found in unrelated sequence types, including major global clones 1 and 2.
A search of the GenBank nonredundant database with the Tn6924 sequence revealed that variants of Tn6924 are present in seven other A. baumannii strains, including AbBAS1, 11W359501, TP1, TP2, TP3, ACN21, and AF401 (Table 1). There appear to be issues with the assembly of the AF401 sequence, and it will not be examined further. The remaining six strains belong to multiple sequence types (STs), including two (AbBAS1 and ACN21) belonging to ST85IP (Table 1), as well as members of the major globally distributed clones GC1 (11W359501) (Table 1) and GC2 (TP1, TP2, and TP3) (Table 1). The variation in the structures (Fig. 1) is characterized by deletions, duplications, and acquisition of additional insertion sequences (IS). The structures in TP2, TP3, and ACN21 are identical to one another, and these likely represent an early evolutionary step of the structure seen in Cl300 prior to insertion of the aphA6 tandem duplications in ISAba125-jycS-ISAba125 and prior to the second set of duplications arising. The structure in TP1 is identical to that in TP2/TP3/ACN21 except for a recombination between the two directly oriented copies of ISAba14, which has removed a 6,673-bp fragment containing blaNDM-1 and aphA6.
TABLE 1.
General properties of strains containing Tn6924 or its variants
| Strain | Yr | Country | Source | STIP | STOX | GenBank acc. no. |
|---|---|---|---|---|---|---|
| Cl300 | 2015 | Lebanon | TAa | 85 | 1089 | CP082952 |
| ACN21 | 2018 | India | Blood | 85 | 1089 | CP038644 |
| AbBAS1 | 2019 | Spain | Clinical | 85 | 957 | CP065392 |
| 11W359501 | 2015 | UK | NKb | 1 | 231 | CP041035 |
| TP1 | 2016 | USA | Clinical | 570c | 1578 | CP056784 |
| TP2 | 2016 | USA | Clinical | 570 | 1578 | CP060011 |
| TP3 | 2016 | USA | Clinical | 570 | 1578 | CP060013 |
| AF401 | 2009 | Mexico | Small colon | 79 | 1974 | CP018254 |
TA, tracheal aspirate.
NK, not known.
ST570 is a single-locus variant (differs in rpoB) of ST2, represents global clone 2.
The structure in AbBAS1 is more complicated. An adjacent deletion originating from the first copy of ISAba125 in the ISAba125-jycS-ISAba125 transposon has extended into an open reading frame (ORF) downstream of mtnN and has deleted 17,032 bp relative to the structure in Cl300. There has also been a duplication of a 10,462-bp segment between the two copies of ISAba125, which has introduced a second copy of blaNDM-1. The structure in AbBAS1 has also acquired two additional copies of ISAba125 in a transposon-like structure, which has generated a 3-bp target site duplication.
The structure in 11W359501 is the simplest, and likely represents the original, or close to the original version of Tn6924. It does not contain any resistance genes and just a single IS, ISAba10.
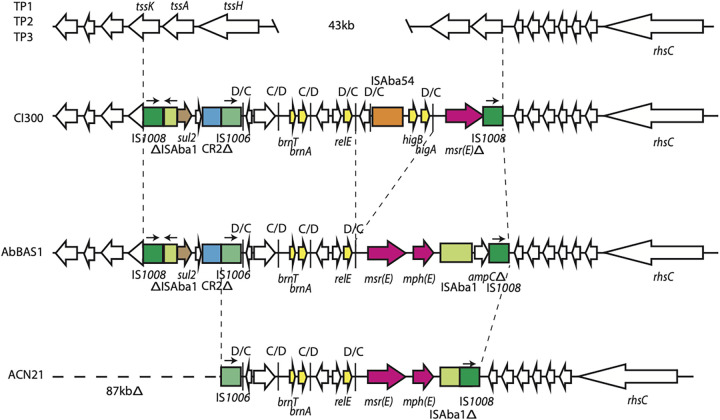
sul2 and msr(E) are located in an IS1008-bounded pseudocompound transposon.
The sul2 and msr(E) genes were not accounted for in Tn6924. An analysis of the Cl300 genome revealed that they were in a 13.0-kb pseudocompound transposon (PCT) bounded by IS1008 (Fig. 2), which has replaced 47.4 kb of the chromosome. This PCT includes multiple known pdif modules (28) containing toxin/antitoxin genes (yellow in Fig. 2) and the msr(E) resistance gene (pink in Fig. 2), the ISAba1-sul2-CR2 region associated with GIsul2, and additional IS. One of the IS, colored orange in Fig. 2, is novel and has been submitted to the ISFinder database under ISAba54. ISAba54 is related to ISAlw22 in the insertion sequence not classified yet family (ISNCY), sharing 89% nucleotide identity, and has generated a 5-bp target site duplication (TSD). The msr(E)-mph(E) dif module has been truncated by IS1008, removing mph(E) and truncating the 3′ end of msr(E). The remaining dif modules, containing the brnTA, relE, and higAB toxin/antitoxin genes, are either identical or related (∼5% nucleotide difference) to known dif modules (28).
FIG 2.
Structure of the IS1008 pseudocompound transposon (PCT). Copies of IS1008 are shown as dark green boxes, and other insertion sequences are shown as light green or orange boxes. Vertical black bars represent pdif sites, and the orientations of the pdif sites are shown above. The orientation and extent of genes are indicated by horizontal arrows. Known or predicted toxin-antitoxin genes are colored yellow, and the msr(E) and mph(E) antibiotic resistance genes are colored pink. Drawn to scale from GenBank accession numbers CP082952 (Cl300), CP056784 (TP1), CP060011 (TP2), CP060013 (TP3), CP065392 (AbBAS1), and CP038644 (ACN21).
The GenBank nonredundant database was searched with the left-hand and right-hand boundaries of this PCT. The only other genomes containing this PCT at this chromosomal location are AbBAS1 and ACN21, which also contained Tn6924. The PCT is not found in the other Tn6924-containing strains (TP1/TP2/TP3 or 11W359501). The PCT in AbBAS1 (Fig. 2) is 14.4 kb and is very similar to the structure in Cl300. A recombination between two D and C sites has removed two dif modules, including the higAB module. The msr(E)-mph(E) module is more complete than in Cl300, with both genes intact, but an ISAba1 has removed the last 38 bp of the module. The ISAba1 is associated with a truncated copy of the ampC gene, with IS1008 removing 273 bp from the 3′ end of ampC. It is not clear how this structure has arisen, but it is likely that it may be the progenitor of the structure seen in Cl300 given that more of the msr(E)-mph(E) module is intact. The PCT in ACN21 (Fig. 2) is 11.5 kb and differs from the structure seen in AbBAS1 by two IS-mediated deletions. The IS1008 at the right-hand end has been deleted into the ISAba1, removing the remainder of ampC. A deletion arising from the internal copy of IS1006 has removed the entire left-hand side of the island, including the fragment originating from GIsul2, and has deleted 87 kb of the adjacent backbone.
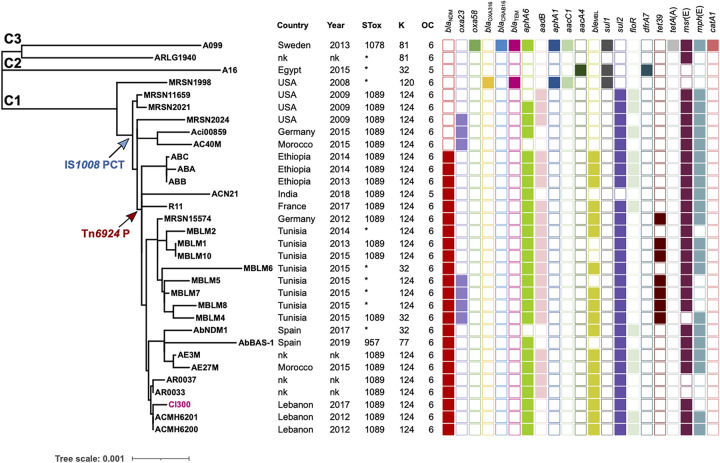
Phylogenetic analysis of ST85IP genomes reveals multiple lineages.
To examine the phylogenetic relationship of Cl300 to other ST85IP strains, all publicly available ST85IP genomes (31 genomes as of July 2021, including Cl300) were downloaded from GenBank and used to construct a whole-genome sequence phylogenetic tree (Fig. 3). All 31 genomes were screened for their important features such as antibiotic resistance genes, as well as K and OC surface polysaccharide loci (Table 2). ST85IP genomes are from a diverse geographical region including several Middle Eastern (Lebanon), African (Egypt and Tunisia), and European countries. A single strain (ACN21) was recovered in India. The four Multidrug-Resistant Organism Repository and Surveillance Network (MRSN) strains recovered in the United States are military strains and are likely to have been imported from the Middle East region. All but one, ARLG1940, contain several antibiotic resistance genes (Table 2), such as those conferring resistance to carbapenems, aminoglycosides, tetracycline, and macrolides, indicating resistance to a wide range of clinically important antibiotics (Table 2). Most ST85IP strains belong to ST1089OX. However, one belongs to ST1087OX and one to ST957OX, and eight strains belong to novel sequence types. Analysis of the K and OC surface polysaccharides indicated that all but two ST85IP genomes encode the OCL6 outer core locus. ACN21 and A15 contain OCL5. Although the majority of strains contained the KL124 capsular locus, a few strains encode KL32, KL81, or KL120.
FIG 3.
Phylogenetic tree of ST85IP genomes constructed using the whole-genome alignment of ST85IP strains. C1-3 indicate clades 1, 2, and 3. Blue and red arrows marked IS1008 PCT and Tn6926P indicate the entry of the pseudocompound transposon (PCT) and the precursor of Tn6926 in ST85IP, respectively. Color-coded filled boxes (on the right) indicate the presence/absence of antibiotic resistance genes in ST85IP genomes, and the middle panel includes genomes’ metadata. The K and OC columns indicate the capsular and outer core surface polysaccharides. STs indicated with an asterisk are novel and could not be determined. Nk indicates not known.
TABLE 2.
Properties of ST85IP strains
| Strain | Yr | Country | Source | STOX | KL | OC | Antibiotic resistance genes | GenBank acc. no.d |
|---|---|---|---|---|---|---|---|---|
| Cl300 | 2015 | Lebanon | TAa | 1089 | 124 | 6 | aphA6 (14x), blaNDM, bleMBL, sul2, msr(E) | CP082952 |
| A16 | 2015 | Egypt | NKb | c | 32 | 5 | sul1, dfrA7, aacA4 | JACSTQ |
| ACMH-6200 | 2012 | Lebanon | Wound | 1089 | 124 | 6 | msr-mph(E), floR, sul2, bleMBL, blaNDM, aphA6 | LKMA |
| ACMH-6201 | 2012 | Lebanon | Wound | 1089 | 124 | 6 | msr-mph(E), floR, bleMBL, blaNDM, sul2, aphA6 | LKMB |
| A099 | 2013 | Sweden | LLLe | 1078 | 81 | 6 | catA1 (2x), tetA (3x), sul1 (2x), msr-mph(E) (2x), oxa58 (2x), aacC1 (3x), blaTEM (2x), aphA1, blaCARB16 (2x), aphA6 | DADARN |
| AR_0037 | NK | NK | NK | 1089 | 124 | 6 | aphA6, blaNDM, bleMBL, sul2, aadB (2x) | MPBX |
| AR_0033 | NK | NK | NK | 1089 | 124 | 6 | aphA6, blaNDM, bleMBL, sul2, aadB (2x) | MPCA |
| AB-A | 2014 | Ethiopia | Wound | 1089 | 124 | 6 | bleMBL, blaNDM, sul2, floR, msr-mph(E), aadB, aphA6 | LWSM |
| AB-C | 2014 | Ethiopia | Wound | 1089 | 124 | 6 | floR, sul2, aadB, msr-mph(E), blaNDM, bleMBL, aphA6 | LWSO |
| AB-B | 2013 | Ethiopia | Wound | 1089 | 124 | 6 | aadB, msr-mph(E), floR, bleMBL, blaNDM, sul2, aphA6 | LWSN |
| ARLG1940 | NK | NK | NK | 1078 | 81 | 6 | msr(E) | NGIM |
| AE27M | 2015 | Morocco | Hospital ventilator | 1089 | 124 | 6 | msr-mph(E), aadB, blaNDM, bleMBL, floR, sul2, aphA6 | FWWO |
| AC40M | 2015 | Morocco | Anal margin | 1089 | 124 | 6 | msr-mph(E), sul2, oxa23 | FWYN |
| AE3M | NK | NK | NK | 1089 | 124 | 6 | msr-mph(E), sul2, aadB, bleMBL, blaNDM, aphA6, floR | FWFB |
| MBL_M1 | 2013 | Tunisia | Urine | 1089 | 124 | 6 | aadB, tet39, msr-mph(E), aphA6, sul2, blaNDM1 | MWTR |
| MBL_M4 | 2015 | Tunisia | Blood | 1089 | 32 | 6 | aadB, tet39, oxa23, blaNDM, bleMBL, mph(E), sul2, aphA6 | MWTU |
| MBL_M5 | 2015 | Tunisia | Urine | c | 124 | 6 | aadB, tet39, aphA6 (2x), blaNDM1, msr(E), oxa23, sul2 | MWTV |
| MBL_M6 | 2015 | Tunisia | Urine | c | 32 | 6 | bleMBL, blaNDM1, aadB, aphA6, sul2, msr-mph(E) | MWTW |
| MBL_M2 | 2014 | Tunisia | Urine | c | 124 | 6 | aadB, blaNDM1, bleMBL, aphA6, sul2, msr(E) | MWTS |
| MBL_M8 | 2015 | Tunisia | Urine | c | 124 | 6 | tet39, aadB, aphA6, aphA6, oxa23, bleMBL, blaNDM1, msr(E), sul2 | MWTY |
| MBL_M7 | 2015 | Tunisia | Blood | c | 124 | 6 | aadB, tet39, oxa23, bleMBL, blaNDM, sul2, aphA6(2x), msr(E) | MWTX |
| MBL_M10 | 2015 | Tunisia | Blood | 1089 | 124 | 6 | aadB, tet39, msr-mph(E), aphA6, sul2, blaNDM | MWUA |
| R11 | 2017 | Tahiti | NK | 1089 | 124 | 6 | aadB, floR, blaNDM, bleMBL, sul2, msr(E), mph(E), aphA6 | QKWE |
| MRSN11659 | 2009 | USA | NK | 1089 | 124 | 6 | msr-mph(E), aadB, sul2, floR | AAYNPT |
| MRSN1998 | 2008 | USA | Wound | c | 120 | 6 | blaOXA316, sul1, aacC1, blaTEM, aphA1 | AAYNTJ |
| MRSN2021 | 2009 | USA | NK | 1089 | 124 | 6 | sul2, msr-mph(E), aadB, floR, aphA6 | AAYNRB |
| MRSN2024 | 2009 | USA | Wound | 1089 | 124 | 6 | oxa23, sul2, msr-mph(E), aadB, aphA6 | AAYNNH |
| Ab-NDM-1 | 2017 | Spain | Rectal swab | c | 32 | 6 | sul2, bleMBL, blaNDM, floR, msr-mph(E) | QBBY |
| ACN21 | 2018 | India | Blood | 1089 | 124 | 5 | msr-mph(E), blaNDM, aphA6 | CP038644 |
| Aci00859 | 2015 | Germany | Wound | 1089 | 124 | 6 | msr-mph(E), sul2, floR, oxa23, aphA6 | VAGE |
| MRSN15574 | 2012 | Germany | Respiratory | 1089 | 124 | 6 | msr-mph(E), sul2, tet39, aadB, blaNDM, bleMBL, aphA6 | VHGP |
TA, tracheal aspirate.
NK, not known.
Could not be determined (novel, single-locus variants of either 1089 or 1078).
Draft genomes’ accession numbers include a set of letters followed by 00000000. Here, short forms (letters only) have been used.
Lung’s Lower Lobe sample.
The whole-genome phylogenetic tree showed that the ST85IP genomes belong to three different clades with the major clade (C1) containing 28 strains, the second clade (C2) only has 1 strain, and the last clade (C3) has 2 strains (Fig. 3). Each clade consisted of multiple sublineages. Strains from the same geographical regions appear to cluster together, suggesting an in situ evolution of lineages in each niche. Notably, 22 isolates contain the blaNDM metallo-β-lactamase gene, with all but one also containing the aphA6 amikacin resistance gene. Analysis of these draft genomes predicted that both blaNDM and aphA6 are in Tn6924 or its variants, indicating the significance of the transposon in bringing in and the spread of these important resistance genes. Analysis of the phylogenetic tree combined with the distribution of antibiotic resistance genes revealed the insertion point of both Tn6924 and the IS1008 flanked sul2-msr(E)-mph(E) PCT (blue and gray arrows in Fig. 3). The acquisition of the IS1008 flanked sul2-msr(E)-mph(E) PCT appears to be an earlier event relative to the acquisition of Tn6924.
DISCUSSION
In A. baumannii, carbapenem resistance is mainly due to the production of oxacillin carbapenemases (e.g., OXA-23, OXA-24, and OXA-58), while other acquired carbapenem resistance genes are uncommon (2). The emergence of CRAb strains that carry the blaNDM gene, a gene frequently found in Escherichia coli and Klebsiella pneumoniae, is therefore a major public health concern given that blaNDM confers resistance not only to carbapenems but also to all β-lactams (excluding aztreonam) (29). While the majority of the global spread of CRAb is due to two main global clones, GC1 and GC2, several emerging sequence types (STs) have also been reported (2), such as those belonging to ST10, ST25, and ST85IP (2, 16, 17, 20). ST85 strains, now reported in Lebanon, Tunisia, Egypt, Spain, Turkey, and Germany, have been known to carry the blaNDM carbapenem resistance gene (11–17, 19–21). Here, we completed and analyzed the genome of Cl300, a carbapenem-resistant ST85IP strain recovered in Lebanon, and determined the context of its resistance genes, including blaNDM and 14 copies of the amikacin resistance gene aphA6. We showed that in Cl300, the blaNDM and 14 aphA6 genes are present in a novel Tn7 family transposon called Tn6924. Tn6924 has a compound structure and a complex in situ evolutionary history that includes many IS/transposon insertion/deletion and multiplication events. It is likely that high amikacin selective pressure has facilitated the amplification of TnaphA6, leading to the current structure and, therefore, high levels of amikacin resistance (MIC > 512 mg/L). Analyzing the genomes of all publicly available ST85IP genomes predicted the presence of Tn6924 and/or its variants in a subclade of the main phylogenetic clade (22 strains) (blue arrow marked “Tn” in Fig. 3). In addition, several variants of Tn6924 were found in a small set of diverse strains, some of which belong to the major global clones GC1 and GC2 (Table 1), the most encountered and, indeed, the most successful clones of A. baumannii. The presence of Tn6924/variants in these strains will alarmingly increase the possibility of further spreading blaNDM and aphA6.
Moreover, we determined the structure of an IS1008 PCT carrying the sulfonamide sul2 and macrolide msr(E) resistance genes in Cl300 and in the other two complete ST85IP genomes (ACN21 and AbBAS-1) (Fig. 2). Analysis performed here predicted a complex in situ evolution of the IS1008 PCT with its central fragment originating from small A. baumannii plasmids previously described (28). Interestingly, all but one strain (MRSN1998) in the main ST85IP clade are also predicted to include a variant of the IS1008 PCT. Phylogenetic analysis, combined with the presence of resistance genes, indicates an earlier acquisition of IS1008 PCT than the entry of the Tn6924 precursor, which is in line with the history of antibiotic usage where the introduction of older antibiotics precedes the last-line carbapenem antibiotics.
In summary, Tn7 and its variants represent a diverse family of transposons that carry a wide variety of genes, including antibiotic resistance genes that contribute to the success of diverse organisms (30). Tn6924 represents a Tn7 family transposon that contributes to the success of a diverse set of ST85IP strains. Although it appears that ST85s at this stage are still limited to the Middle East region, North Africa, and a few European countries (with only a single genome from India and all MRSN U.S. strains likely imported from the Middle East), outbreaks and sporadic cases caused by these strains should be fully characterized and tracked to identify sources and potential transmission routes.
MATERIALS AND METHODS
Bacterial strain and publicly available genome sequence data used in this study.
A. baumannii Cl300 was recovered in 2015 from a tracheal aspirate of a male patient in Tripoli Governmental Hospital, Tripoli, North Lebanon. Cl300 was previously reported as part of a study investigating the epidemiology of A. baumannii strains isolated from different hospitals in Lebanon (11).
To examine the relationship of Cl300 to other ST85IP genomes, >4,000 A. baumannii genomes publicly available in GenBank (https://www.ncbi.nlm.nih.gov/genome/403) were screened for ST85IP strains using the mlst program publicly available at https://github.com/tseemann/mlst. Thirty-one ST85IP genomes (2 complete and 29 draft genomes) were found and included in phylogenetic analysis of this study.
Antibiotic susceptibility and resistance testing.
The antibiotic resistance profile was determined using the calibrated dichotomous sensitivity (CDS) disk diffusion method as described previously (31). Resistance profiles were interpreted according to the Clinical and Laboratory Standards Institute (CLSI) guidelines for Acinetobacter spp. (32) and CDS disk diffusion assay (http://cdstest.net) (31) when a CLSI breakpoint for Acinetobacter spp. was not available (e.g., for netilmicin, streptomycin, spectinomycin, sulfamethoxazole, nalidixic acid, and rifampicin). MICs of amikacin, meropenem, and colistin were determined using the standard broth microdilution method as described elsewhere (33). A. baumannii A388 (carrying a single copy of aphA6) was used as a control in amikacin MIC assay. A. baumannii strains D36 (carrying oxa23) and ACICU (containing oxa58) were used as controls in meropenem MIC assays.
Whole-genome sequencing, assembly, and annotation.
Cl300 cells were grown overnight at 37°C in LB inoculated from a single colony. Genomic DNA was isolated using the DNeasy UltraClean microbial kit (Qiagen, Germantown, MD, USA), and sequencing was performed using Illumina MiSeq and Oxford Nanopore (MinION). Library preparation and barcoding for Illumina MiSeq and MinION (Oxford Nanopore Technologies, Oxford, UK) sequencing was performed as described previously (34). Illumina sequencing generated 5,865,134 paired-end short reads with 150-fold coverage and an average length of 250 bp, and MinION generated a total of 37,640 reads with an N50 of 15.5 kbp and ∼50-fold coverage.
The FastQC (v.0.11.9) program (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was used (default setting) to check the quality of Illumina reads (e.g., per-base sequence quality of >38, per-base sequence content of <10%, etc.). Filtlong (v.0.2.0) (https://github.com/rrwick/Filtlong) was used to filter the MinION reads with low quality by discarding any read shorter than 1 kbp (--min_length 1000). In addition, PHRED read quality scores assigned in the FASTQ file during basecalling were used by Filtlong to discard the worst 10% of reads measured by bp rather than read counts. The latter was done using the parameter —keep_percent 90. The high-quality Illumina and MinION reads were assembled de novo using a hybrid assembly approach with the Unicycler program (v0.4.7) using default settings (35).
Protein coding, rRNA, and tRNA gene sequences were annotated using Prokka (36), followed by manual annotations of resistance genes, insertion sequences, and surface polysaccharide loci using ResFinder (https://cge.cbs.dtu.dk/services/ResFinder/), ISFinder (https://www-is.biotoul.fr/), BLASTp (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins), Pfam (http://pfam.xfam.org/), and Kaptive (https://kaptive-web.erc.monash.edu/) (37) searches. Multilocus sequence types (MLSTs) in the Institut Pasteur and Oxford schemes (http://pubmlst.org/abaumannii/) were determined from the genome sequence data. Figures were drawn to scale using SnapGene (v.5.2.4) and InkScape (v.1.0).
To examine the relationship of Cl300 to other ST85IP genomes, all publicly available ST85IP genomes (31 genomes as of July 2021) were downloaded from GenBank and used to construct a recombination-free whole-genome phylogenetic tree as described previously (38). The phylogenetic tree was visualized and annotated using the iTOL online phylogenetic tree visualization tool (https://itol.embl.de/).
Data availability.
The complete genome and plasmid sequences of Cl300 have been deposited in the GenBank/EMBL/DDBJ database and are publicly available under the accession numbers CP082952 (chromosome) and CP082953 (pCl300). Illumina short reads and MinION long reads are also deposited under the Sequence Read Archive (SRA) accession numbers SRR15725578 and SRR15725577, respectively.
ACKNOWLEDGMENTS
R.M. and C.G. were supported by the Australian Research Council (ARC) Discovery Project (DP180100474). M.H. was supported by an Australian Research Council (ARC) DECRA fellowship (fellowship DE200100111).
Footnotes
Supplemental material is available online only.
Contributor Information
Christopher J. Harmer, Email: christopher.harmer@sydney.edu.au.
Mohammad Hamidian, Email: mohammad.hamidian@uts.edu.au.
Ayush Kumar, University of Manitoba.
REFERENCES
- 1.Harding CM, Hennon SW, Feldman MF. 2018. Uncovering the mechanisms of Acinetobacter baumannii virulence. Nat Rev Microbiol 16:91–102. doi: 10.1038/nrmicro.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamidian M, Nigro SJ. 2019. Emergence, molecular mechanisms and global spread of carbapenem-resistant Acinetobacter baumannii. Microb Genom 5:e000306. doi: 10.1099/mgen.0.000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins PG, Hagen RM, Kreikemeyer B, Warnke P, Podbielski A, Frickmann H, Loderstädt U. 2021. Molecular epidemiology of carbapenem-resistant Acinetobacter baumannii isolates from Northern Africa and the Middle East. Antibiotics 10:291. doi: 10.3390/antibiotics10030291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zarrilli R, Pournaras S, Giannouli M, Tsakris A. 2013. Global evolution of multidrug-resistant Acinetobacter baumannii clonal lineages. Int J Antimicrob Agents 41:11–19. doi: 10.1016/j.ijantimicag.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 5.da Silva KE, Maciel WG, Croda J, Cayô R, Ramos AC, de Sales RO, Kurihara MNL, Vasconcelos NG, Gales AC, Simionatto S. 2018. A high mortality rate associated with multidrug-resistant Acinetobacter baumannii ST79 and ST25 carrying OXA-23 in a Brazilian intensive care unit. PLoS One 13:e0209367. doi: 10.1371/journal.pone.0209367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamidian M, Hall RM. 2016. The resistance gene complement of D4, a multiply antibiotic-resistant ST25 Acinetobacter baumannii isolate, resides in two genomic islands and a plasmid. J Antimicrob Chemother 71:1730–1732. doi: 10.1093/jac/dkw041. [DOI] [PubMed] [Google Scholar]
- 7.Hamidian M, Holt KE, Hall RM. 2015. Genomic resistance island AGI1 carrying a complex class 1 integron in a multiply antibiotic-resistant ST25 Acinetobacter baumannii isolate. J Antimicrob Chemother 70:2519–2523. doi: 10.1093/jac/dkv137. [DOI] [PubMed] [Google Scholar]
- 8.Montaña S, Vilacoba E, Fernandez JS, Traglia GM, Sucari A, Pennini M, Iriarte A, Centron D, Melano RG, Ramírez MS. 2020. Genomic analysis of two Acinetobacter baumannii strains belonging to two different sequence types (ST172 and ST25). J Glob Antimicrob Resist 23:154–161. doi: 10.1016/j.jgar.2020.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Rodríguez CH, Nastro M, Famiglietti A. 2018. Carbapenemases in Acinetobacter baumannii. Review of their dissemination in Latin America. Rev Argent Microbiol 50:327–333. doi: 10.1016/j.ram.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Sahl JW, Del Franco M, Pournaras S, Colman RE, Karah N, Dijkshoorn L, Zarrilli R. 2015. Phylogenetic and genomic diversity in isolates from the globally distributed Acinetobacter baumannii ST25 lineage. Sci Rep 5:15188. doi: 10.1038/srep15188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al Atrouni A, Hamze M, Jisr T, Lemarié C, Eveillard M, Joly-Guillou ML, Kempf M. 2016. Wide spread of OXA-23-producing carbapenem-resistant Acinetobacter baumannii belonging to clonal complex II in different hospitals in Lebanon. Int J Infect Dis 52:29–36. doi: 10.1016/j.ijid.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 12.Bakour S, Olaitan AO, Ammari H, Touati A, Saoudi S, Saoudi K, Rolain JM. 2015. Emergence of colistin- and carbapenem-resistant Acinetobacter baumannii ST2 clinical isolate in Algeria: first Case report. Microb Drug Resist 21:279–285. doi: 10.1089/mdr.2014.0214. [DOI] [PubMed] [Google Scholar]
- 13.Bonnin RA, Cuzon G, Poirel L, Nordmann P. 2013. Multidrug-resistant Acinetobacter baumannii clone, France. Emerg Infect Dis 19:822–823. doi: 10.3201/eid1905.121618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheikh HB, Domingues S, Silveira E, Kadri Y, Rosário N, Mastouri M, Da Silva GJ. 2018. Molecular characterization of carbapenemases of clinical Acinetobacter baumannii-calcoaceticus complex isolates from a university hospital in Tunisia. Biotech 8:297. doi: 10.1007/s13205-018-1310-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Decousser JW, Jansen C, Nordmann P, Emirian A, Bonnin RA, Anais L, Merle JC, Poirel L. 2013. Outbreak of NDM-1-producing Acinetobacter baumannii in France, January to May 2013. Euro Surveill 18:20547. doi: 10.2807/1560-7917.es2013.18.31.20547. [DOI] [PubMed] [Google Scholar]
- 16.Fernández-Cuenca F, Pérez-Palacios P, Galán-Sánchez F, López-Cerero L, López-Hernández I, López Rojas R, Arca-Suárez J, Díaz-de Alba P, Rodríguez Iglesias M, Pascual A. 2020. First identification of blaNDM-1 carbapenemase in blaOXA-94-producing Acinetobacter baumannii ST85 in Spain. Enferm Infecc Microbiol Clin (Engl Ed) 38:11–15. doi: 10.1016/j.eimc.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Heydari F, Mammina C, Koksal F. 2015. NDM-1-producing Acinetobacter baumannii ST85 now in Turkey, including one isolate from a Syrian refugee. J Med Microbiol 64:1027–1029. doi: 10.1099/jmm.0.000132. [DOI] [PubMed] [Google Scholar]
- 18.Jeannot K, Diancourt L, Vaux S, Thouverez M, Ribeiro A, Coignard B, Courvalin P, Brisse S. 2014. Molecular epidemiology of carbapenem non-susceptible Acinetobacter baumannii in France. PLoS One 9:e115452. doi: 10.1371/journal.pone.0115452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafei R, Pailhoriès H, Hamze M, Eveillard M, Mallat H, Dabboussi F, Joly-Guillou ML, Kempf M. 2015. Molecular epidemiology of Acinetobacter baumannii in different hospitals in Tripoli, Lebanon using blaOXA-51-like sequence based typing. BMC Microbiol 15:103. doi: 10.1186/s12866-015-0441-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaidane N, Naas T, Oueslati S, Bernabeu S, Boujaafar N, Bouallegue O, Bonnin RA. 2018. Whole-genome sequencing of NDM-1-producing ST85 Acinetobacter baumannii isolates from Tunisia. Int J Antimicrob Agents 52:916–921. doi: 10.1016/j.ijantimicag.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Mosqueda N, Espinal P, Cosgaya C, Viota S, Plasensia V, Alvarez-Lerma F, Montero M, Gómez J, Horcajada JP, Vila J, Roca I. 2013. Globally expanding carbapenemase finally appears in Spain: nosocomial outbreak of Acinetobacter baumannii producing plasmid-encoded OXA-23 in Barcelona, Spain. Antimicrob Agents Chemother 57:5155–5157. doi: 10.1128/AAC.01486-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vila J, Ruiz J, Goñi P, Marcos A, Jimenez de Anta T. 1995. Mutation in the gyrA gene of quinolone-resistant clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 39:1201–1203. doi: 10.1128/AAC.39.5.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vila J, Ruiz J, Goñi P, Jimenez de Anta T. 1997. Quinolone-resistance mutations in the topoisomerase IV parC gene of Acinetobacter baumannii. J Antimicrob Chemother 39:757–762. doi: 10.1093/jac/39.6.757. [DOI] [PubMed] [Google Scholar]
- 24.Craig NL. 1996. Transposon Tn7. Curr Top Microbiol Immunol 204:27–48. doi: 10.1007/978-3-642-79795-8_2. [DOI] [PubMed] [Google Scholar]
- 25.Hamidian M, Hall RM. 2021. Dissemination of novel Tn7 family transposons carrying genes for synthesis and uptake of fimsbactin siderophores among Acinetobacter baumannii isolates. Microb Genom 7:mgen000548. doi: 10.1099/mgen.0.000548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arciszewska LK, Craig NL. 1991. Interaction of the Tn7-encoded transposition protein TnsB with the ends of the transposon. Nucleic Acids Res 19:5021–5029. doi: 10.1093/nar/19.18.5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nigro SJ, Post V, Hall RM. 2011. Aminoglycoside resistance in multiply antibiotic-resistant Acinetobacter baumannii belonging to global clone 2 from Australian hospitals. J Antimicrob Chemother 66:1504–1509. doi: 10.1093/jac/dkr163. [DOI] [PubMed] [Google Scholar]
- 28.Blackwell GA, Hall RM. 2017. The tet39 determinant and the msrE-mphE genes in Acinetobacter plasmids are each part of discrete modules flanked by inversely oriented pdif (XerC-XerD) sites. Antimicrob Agents Chemother 61:e00780-17. doi: 10.1128/AAC.00780-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poirel L, Bonnin RA, Boulanger A, Schrenzel J, Kaase M, Nordmann P. 2012. Tn125-related acquisition of blaNDM-like genes in Acinetobacter baumannii. Antimicrob Agents Chemother 56:1087–1089. doi: 10.1128/AAC.05620-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parks AR, Peters JE. 2009. Tn7 elements: engendering diversity from chromosomes to episomes. Plasmid 61:1–14. doi: 10.1016/j.plasmid.2008.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell SM, Smith DD. 1975. The CDS disc method of antibiotic sensitivity testing (calibrated dichotomous sensitivity test). Pathology 7:1–48. doi: 10.3109/00313027509082602. [DOI] [PubMed] [Google Scholar]
- 32.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing; 29th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 33.Wiegand I, Hilpert K, Hancock RE. 2008. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 34.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Completing bacterial genome assemblies with multiplex MinION sequencing. Microb Genom 3:e000132. doi: 10.1099/mgen.0.000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wick RR, Judd LM, Gorrie CL, Holt KE. 2017. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput Biol 13:e1005595. doi: 10.1371/journal.pcbi.1005595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 37.Wyres KL, Cahill SM, Holt KE, Hall RM, Kenyon JJ. 2020. Identification of Acinetobacter baumannii loci for capsular polysaccharide (KL) and lipooligosaccharide outer core (OCL) synthesis in genome assemblies using curated reference databases compatible with Kaptive Microb Genom 6:e000339. doi: 10.1093/bioinformatics/btu153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holt K, Kenyon JJ, Hamidian M, Schultz MB, Pickard DJ, Dougan G, Hall R. 2016. Five decades of genome evolution in the globally distributed, extensively antibiotic-resistant Acinetobacter baumannii global clone 1. Microb Genom 2:e000052. doi: 10.1099/mgen.0.000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download SPECTRUM01745-21_Supp_1_seq9.pdf, PDF file, 0.01 MB (16KB, pdf)
Data Availability Statement
The complete genome and plasmid sequences of Cl300 have been deposited in the GenBank/EMBL/DDBJ database and are publicly available under the accession numbers CP082952 (chromosome) and CP082953 (pCl300). Illumina short reads and MinION long reads are also deposited under the Sequence Read Archive (SRA) accession numbers SRR15725578 and SRR15725577, respectively.