Abstract
The generation of oxidative stress by ambient air pollution particles contributes to the development of allergic sensitization and asthma, as demonstrated by intranasal challenge with well-characterized diesel exhaust particle (DEP) suspensions in humans. This effect is due to the presence of redox active organic chemicals in DEP, and can be suppressed by antioxidants and inducers of phase II enzymes in animals. In this communication, we determined whether the administration of a standardized broccoli sprout extract (BSE), which contains a reproducible amount of the sulforaphane (SFN) precursor, glucoraphanin, could be used to suppress the nasal inflammatory response in human subjects challenged with 300 μg of an aqueous DEP suspension (equivalent to daily PM exposure levels on a Los Angeles freeway). SFN is capable of inducing an antioxidant and phase II response via activation of the nuclear transcription factor (erythroid-derived 2)-like 2 (Nrf2). Previous studies have shown that 70–90% SFN delivered by BSE is absorbed, metabolized, and excreted in humans. An initial intranasal challenge with DEP in 29 human subjects was used to characterize the magnitude of the inflammatory response. Following a 4 week washout, a BSE that delivers a reproducible and standardized dose of 100 μmol SFN in mango juice was administered daily for four days. The nasal DEP challenge was repeated and lavage fluid collected to perform white blood cell (WBC) counts. The average nasal WBC increased by 66% over the initial screening levels and by 85% over the control levels 24 hours after DEP exposure. However, total cell counts decreased by 54% when DEP challenge was preceded by daily BSE administration for 4 days (p < 0.001). Since the SFN dose in these studies is equivalent to the consumption of 100–200 g broccoli, our study demonstrates the potential preventive and therapeutic potential of broccoli or broccoli sprouts rich in glucoraphanin for reducing the impact of particulate pollution on allergic disease and asthma.
Introduction
Allergic diseases affect approximately one-third of the general population and asthma is a common heterogeneous disease, characterized by reversible airway obstruction and bronchial hyper-responsiveness that is commonly associated with atopy.1,2 Among the multiple factors that contribute to asthma are genetic predisposition, generation of allergic immune activation, and the possible involvement of a variety of pro-inflammatory and noxious environmental factors.3 Epidemiological studies, in particular, have suggested that worldwide increases in allergic and respiratory disease may be associated with environmental pollutants such as ambient air pollution.4,5 A major source of the particulates contributing to ambient air pollution in urban environments consists of diesel exhaust particles (DEP), which can enhance the allergic inflammatory response6 through the ability of these ultrafine particles to generate the production of reactive oxygen species (ROS) at sites of deposition in the upper and lower respiratory tract.7–9 The accompanying oxidative stress effects on the mucosal lining of the respiratory tract can include the generation of antioxidant defense responses as well as pro-inflammatory effects that can exacerbate the response of the nose and the lung to common environmental allergens. In fact, the antioxidant defense is triggered at a lower level of oxidative stress and suppresses the pro-inflammatory effects of air pollutant chemicals. A major component of the antioxidant defense is the induction of a large number of phase II antioxidant and anti-inflammatory genes through oxidative stress-mediated activation of the transcription factor, nuclear factor (erythroid-derived 2)-like 2 (Nrf2).10–12 Among the phase II enzymes that are induced is the expression of several glutathione S-transferases, including GSTM1. Up to 50% of normal individuals have a GSTM1 null mutation, which putatively could predispose these individuals to a higher risk of acquiring environmentally related diseases such as bladder cancer.12 It has also been reported that individuals with this null mutation have a higher induction of IgE in response to DEP plus second-hand smoke exposure.13 Moreover, individuals expressing the GSTP1 Ile/Ile105 genotype exhibit higher levels of histamine release when exposed to DEP and second-hand smoke.14 Although DEP is capable of inducing oxidative stress and inflammation in the bronchial epithelial tissue of healthy human subjects, asthmatics are even more sensitive to the effects of DEP.14,15 It is worth considering, therefore, that boosting of the Nrf2 gene response pathways could alleviate the pro-oxidative and pro-inflammatory effects of DEP. One practical way in which this can be accomplished is through the oral administration of the isothiocyanate chemical, sulforaphane (SFN), which is present in high quantities in broccoli or broccoli extracts.
The sulforaphane-rich broccoli sprout extract (BSE) utilized in this study was specifically prepared to deliver a reproducible and standardized dose of glucoraphanin, a precursor of SFN.16–18 In a prior study, the UCLA Asthma Center has shown that consumption of a broccoli sprout homogenate resulted in a dose-dependent increase in phase II enzyme RNA expression in nasal lavage cells with a maximum response at 200 grams.16 The current preparation is a significant advance over what was used in the previous studies, since it utilizes a thoroughly standardized boiling water extract in which levels of SFN are known. Thus the variability observed when delivering fresh vegetables is avoided and it has been demonstrated that 70–90% of SFN delivered in this way is absorbed, metabolized, and excreted.19 The dose utilized is also modest and is in the nutritional range obtained by consuming between 100 and 200 g of broccoli. While commercial broccolis vary in their content of glucoraphanin by a factor of about 10 and the conversion of ingested glucoraphanin to SFN varies from about 2–40% of dose,18,19 it is not unreasonable to extrapolate the findings in this study to the impact of the consumption of 200 grams of broccoli by an enthusiastic broccoli consumer.
The present study assessed the impact of a standardized BSE in individuals with both a cat allergy as well as a strong pro-inflammatory response to DEP after intranasal challenge. Our hypothesis was that the antioxidant and anti-inflammatory effects of the SFN in the BSE will interfere in the generation of nasal inflammation by the DEP. Cat allergy affects about one-third of the U.S. population and provides a convenient test platform for assessing the impact of DEP challenge on allergic inflammation because of the lack of seasonal variation as compared to individuals who are allergic to pollen allergy, e.g., grasses and ragweed.20 In subjects with asthma, a significant increase in the number of white blood cells in their nasal lavage fluid was detected both immediately and 24 h after exposure to 240 ppb ozone, as was a significant increase in epithelial cells immediately after exposure, indicating that asthmatic individuals are more sensitive to the acute inflammatory effects of ozone than nonasthmatic individuals.21
Materials and methods
Study design
Volunteers who were positive for cat allergens were consented to a pre-post experimental study design, wherein each subject served as his or her own control. The study involved three sequential phases: screening, control, and intervention (BSE treatment). During each phase the response of the nasal passages to a standard diesel exhaust particle (DEP) challenge was determined by counting the total number of cells (leukocytes) recovered from nasal lavage fluid (Fig. 1). These cell counts which were performed just prior to DEP challenge (time 0) as well as at 6 and 12 h later, and served as a biomarker for the inflammatory responsiveness of each subject to DEP exposure. The DEP challenge during the screening phase identified the number of cat allergen sensitive subjects who were responsive to DEP (29 of 35). Only subjects sensitive to DEP remained enrolled in the study and completed all of the 10 visits; others were excluded from the study after the Screening phase (visits 1–3). Two to four weeks later, the remaining subjects who entered the control phase (visits 4 and 5) were exposed to DEP, and their nasal lavage cell responses determined. After a 4-week “washout” period, subjects entered the Intervention phase (visits 6–10). Their basal white cell number in nasal lavages was determined (visit 6) and they received 4 daily oral doses of BSE containing 100 μmol sulforaphane (visits 6–9). The response to DEP challenge was then determined by comparing the nasal inflammatory response (visits 9 and 10). Safety assessments, including evaluation of symptoms, nasal exam, vital signs, hematology, blood chemistries, and pregnancy determinations, were also carried out at several visits and urine was collected on visit 10 for measurement of SFN metabolites.
Fig. 1.
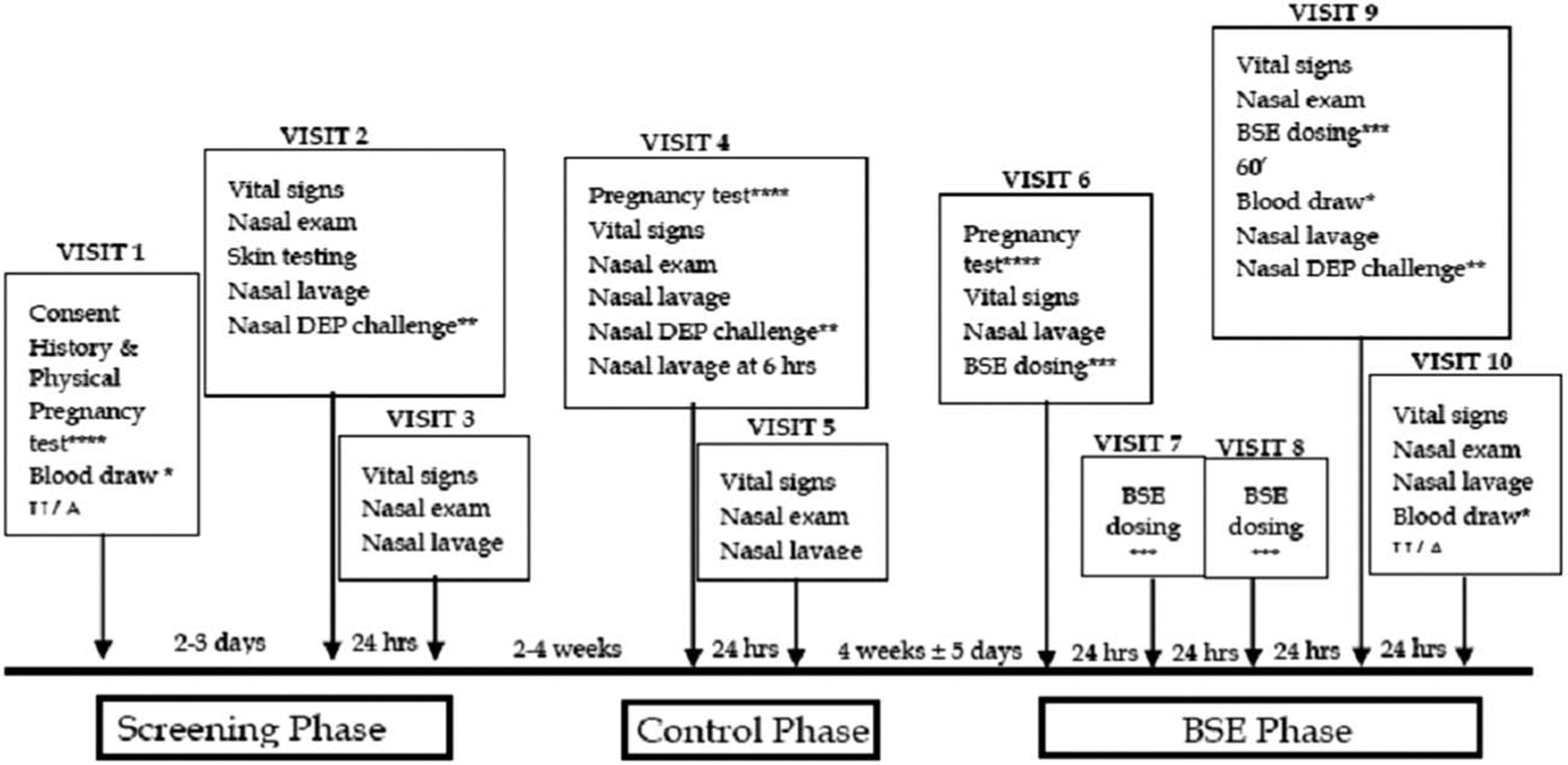
Clinic visits and their relative timing included nasal lavage, BSE administration, and/or DEP challenge. Not shown are safety assessments, which were performed on visits 1–10 as described in the text.
Subjects
Healthy subjects over age 18 who tested positive for cat allergens via skin prick test were enrolled for study, and screening phase subjects who demonstrated a robust intranasal inflammatory response of >10 000 cells per mL (in a 5 mL nasal lavage fluid) 24 hours after DEP nasal challenge were entered into the control and BSE phases. Subjects were asked to refrain from eating cruciferous vegetables and isothiocyanate-containing condiments (such as wasabi and horseradish) within 3 days of study start and for the duration of the study. Subjects were prohibited from using inhaled, topical, or oral allergy medication. Smokers were excluded from study. The UCLA Office for Protection of Research Subjects and the JHU Institutional Review Board approved this study. All subjects provided written informed consent. The study was approved by the U.S. Food and Drug Administration.
Preparation of BSE
Sulforaphane-rich BSE for this study was provided (under IND) by Paul Talalay and Jed Fahey of Johns Hopkins University through a material transfer agreement. The sulforaphane-rich BSE was prepared by the Johns Hopkins team as previously described.22–24 The reproducible and standardized dose of SFN produced was 100 μmol and this was administered once a day, for 4 consecutive days, in a single oral dose of ~1.25 grams BSE,16,22,25 suspended in juice just prior to each dosing.
Nasal challenge and lavage
Nasal challenges were performed with 300 μg DEP in 200 μL saline. This dose is equivalent to a 40 h exposure to ambient polluted air on a typical Los Angeles freeway. Diesel exhaust particles were a gift from Dr Masaru Sagai (National Institute of Environment Studies, Tsukuba, Ibaraki, Japan). These particles were collected in 2001 from the exhaust in a 4JB1-type LD, 2.741, 4-cylinder Isuzu diesel engine under a load of 10 torque onto a cyclone impactor equipped with a dilution tunnel constant volume sampler.20 DEP was collected on high capacity glass fiber filters, from which the scraped particles were stored as a powder in a glass container under nitrogen gas. The particles consist of aggregates in which individual particles are <1 μm in diameter. The chemical composition of these particles, including PAH and quinone analysis, was previously described.26 DEP aliquots were stored at −80 °C in the dark under nitrogen to prevent oxidation or loss of volatile chemicals. As a check for stability to ensure no chemical change, we repeated the chemical analysis of select organic chemical compounds and also assessed the redox-cycling ability of the stored aliquots every 3 months, using the dithiothreitol (DTT) assay.
Nasal lavage was performed as previously described by Diaz-Sanchez et al.27 Briefly, 5 mL of 0.9% saline solution was delivered to each nostril and lavage was collected after 10 seconds by having subjects lean forward, allowing the refluxed saline to be collected and pooled in a tube. Lavages were pooled from both nostrils. The lavage fluid was collected prior to nasal DEP exposure (baseline) and at 6 and 24 hours after DEP exposure. Nasal lavage fluid was centrifuged at 350 × g for 10 min at 4 °C to separate the supernatants from the cell pellets. The cell pellets were washed once with 500 μL phosphate buffer and then were resuspended in 100 μL phosphate buffer solution. 10 μL cell suspension was mixed with 10 μL trypan blue for cell counts.
Genomic DNA isolation
Peripheral blood was drawn into a BD Vacutainer K2 EDTA tube (BD Biosciences; San Jose, CA). Genomic DNA was extracted from blood using the GenElute Mammalian Genomic DNA Miniprep Kit (Sigma-Aldrich; St. Louis, MO) according to manufacturer’s instructions. Briefly, 200 μL blood was treated with Proteinase K followed by RNase for 2 min before lysing cells in 55 °C water for 10 min. DNA was precipitated with 100% ethanol and bound to a spin column. After two washes, DNA was eluted from column with TE buffer.
TaqMan polymerase chain reaction genotyping of GSTP1 IIe105VaI
Genotyping was performed using SNP Genotyping Assays for GSTP1 (rs1695) (Applied Biosystems; Foster City, CA) on a StepOnePlus Real-Time PCR Machine (Applied Biosystems). Real-time PCR was performed with 25 ng DNA in a 20 μL reaction containing TaqMan Genotyping Master Mix and SNP genotyping assay. Reactions were heated for 95 °C for 10 minutes following by 40 cycles at 92 °C for 15 s and 60 °C for 1 min. Alleles were determined using the allelic discrimination plot in the StepOne software genotyping program. Each sample was performed in quadruplicate.
GSTM1 copy number
GSTM1 copy number was determined using Pre-designed TaqMan Copy Number Assays (Applied Biosystems). GSTM1 was run in a duplex assay with RNase P as a reference gene. Reactions were heated for 95 °C for 10 minutes, followed by 40 cycles at 92 °C for 15 s and 60 °C for 1 min. Results were analyzed using Copy Caller version 1.0 software (Applied Biosytems). Each sample was performed in quadruplicate.
HPLC-MS/MS identification of SFN metabolites in plasma
R,S-Sulforaphane was purchased from LKT Laboratories, Inc. (Minnesota, USA). All solvents and other chemicals used were HPLC grade and were purchased from Fisher Scientific (USA). Water was ultrafiltered using a Milli-Q pure water system.
To the 4 °C plasma sample (200 μL) in a 1.5 mL micro centrifuge tube (Fisher Scientific, USA), pre-cooled (4 °C) trifluoroacetic acid (20 μL) (TFA) was added to precipitate plasma proteins (30 seconds) in the sample followed by brief mixing and refrigerated centrifugation at 15 900 × g for 10 min. The resulting supernatant was filtered (0.2 μm pore size) and 20 μL injected for analysis.
A LCQ Advance mass (Thermo Finnegan) and a Survey HPLC equipped with binary pump, degasser, cooled auto-sampler and a PDA detector (USA) were used for the LC-MS analysis. The HPLC column was a Zorbax SB-C18 5 μm (150 × 2.1 mm). The flow rate was 0.25 mL min−1 and gradient elution was used: solution A (1% acetic acid solution): solution B (acetonitrile). The gradient started at 2% solution B increasing over 20 min to 30% B and 50% in 30 minutes, finally re-equilibrating to 2% B for 10 min. The mobile phase solutions were filtered through 0.22 μm pore size cellulose nitrate or nylon membrane filters using a vacuum filtration apparatus. The LC eluent flow of 0.25 mL min−1 was sprayed into the mass spectrometer interface without splitting. Electrospray ionization in the positive mode (ESI+) was applied. The analytes were identified by molecular weight and their MS/MS, respectively. The identification of the MS/MS of SFN-Cys, SFN-Cys-Gly; SFN-NAC; and SFN-GSH at m/z of 178; 179; 178 and 179, respectively were used to confirm consumption of the BSE supplement by subjects in the study. Subjects had no detectable SFN metabolites prior to consuming the BSE supplement and the presence of SFN metabolites was taken as evidence of bioavailability of SFN from BSE in our subjects as shown independently in prior studies using BSE.
Statistical analysis
Quantile plots and histograms were examined to determine if the data followed the normal (Gaussian) distribution on both the original and the log-transformed values. A parametric repeated measure (mixed model) analysis of variance (ANOVA) model was used to compare means and mean differences for data following a normal distribution. This model accounted for the fact that subjects were being compared to themselves. For data that did not follow a normal distribution, medians were reported in addition to means, and non-parametric Wilcoxon signed rank methods were used to compute p values.
Within each of the three phases (screening, control and BSE) there are data at baseline, at time 0, and 6 and 24 h after DEP challenge. In the BSE phase there is also an additional “no BSE” observation before the BSE baseline observation. The main focus of interest was comparisons of the 24 h minus baseline change in the BSE phase to the corresponding 24 h minus baseline change in the control phase. Comparisons were also made to the 24 h minus baseline change in the screening phase. In addition, 6 hour minus baseline changes were compared across the three phases. The data are expressed as mean ± standard deviation (SD).
Results
Study subjects characteristics
Among 100 subjects recruited, 38 subjects tested positive for cat allergy and met the entry-level inclusion criteria. Three subjects withdrew from the study before the intervention. Of the remaining 35 subjects, 29 were DEP responders as defined by mounting an inflammatory response, characterized as >10 000 cells per mL in the nasal lavage fluid, 24 hours after DEP nasal challenge. Among the latter group, one subject did not give consent for genotyping. Genotyping was performed in 28 DEP responder subjects and the results of the GSTM1 null/GSTP1 Ile/Ile105 genotyping are shown in Table 2.
Table 2.
Descriptive statistics of DEP Responders
| N | % | |
|---|---|---|
| Total | 28 | 100 |
| Female | 14 | 50 |
| Male | 14 | 50 |
| Race | ||
| White | 17 | 61 |
| African American | 4 | 14 |
| Asian | 3 | 11 |
| Other | 4 | 14 |
| Genotype | ||
| GSTM1 | ||
| Null | 19 | 68 |
| Present | 09 | 32 |
| GSTP1 IlelO5Val | ||
| Ile/Ile | 16 | 57 |
| Ile/Val | 10 | 36 |
| Val/Val | 02 | 07 |
There was no significant difference among responders before or after BSE administration based on GSTM-1 null status. There were no serious adverse events or unexpected adverse events during the study. Mild gastrointestinal upset was the most common reported side effect reported.
LCMS identification of sulforaphane metabolites for compliance
SFN metabolites were identified by their respective mass numbers using MS/MS (SFN-Cys-Gly MS2 179 from precursor of 356, SFN-NAC MS2 178 from precursor of 341, and SFN-GSH MS2 179 from precursor of 485) as reported in the analytical literature.26 The presence of any combination of the metabolites was considered as detection of sulforaphane. SFN metabolites were detected in all 29 DEP responders after consuming BSE (Table 1).
Table 1.
Sulforaphane metabolites detection by LCMS in plasma samples 15 minutes after administering a single dose of sulforaphane-rich broccoli sprout extract
| Sulforaphane metabolites (ng mL−1) | |||||
|---|---|---|---|---|---|
| Subjects | SFN-Cys | SFN-Cys-Gly | SFN-NAC | SFN-GSH | Compliance |
| 1 | 3.1 | 16.3 | 50.4 | 0.4 | Yes |
| 2 | ND | 0.3 | 18.4 | 0.9 | Yes |
| 3 | ND | 1.5 | 20.6 | ND | Yes |
| 4 | 0.3 | 22.7 | 57.8 | 0.6 | Yes |
| 5 | 0.7 | 26.9 | 25.8 | ND | Yes |
| 6 | 1.8 | 12.7 | 19.6 | 5.1 | Yes |
| 7 | 1.4 | 25.3 | 16.7 | 1.6 | Yes |
| 8 | ND | 4.3 | 7.9 | 10.3 | Yes |
| 9 | 2.5 | 74.6 | 1.3 | 39.2 | Yes |
| 10 | 1.4 | 21.2 | 2.7 | 34.3 | Yes |
| 11 | 9 | 26.4 | 2.3 | 14.9 | Yes |
| 12 | ND | 7.7 | 4.7 | 25.2 | Yes |
| 13 | 2.6 | 27.2 | 8.7 | 30.9 | Yes |
| 14 | 2.3 | 21.3 | 6.2 | 15.1 | Yes |
| 15 | 2.1 | 59.4 | 4.6 | 87.3 | Yes |
| 16 | 0.6 | 42.8 | 3.1 | 66.5 | Yes |
| 17 | 1.2 | 37.7 | 9.8 | 32.1 | Yes |
| 18 | ND | 26.7 | 13 | 64.3 | Yes |
| 19 | 1 | 90 | 18.2 | 78.7 | Yes |
| 20 | 2.6 | 36.9 | 10.9 | 71.5 | Yes |
| 21 | 0.8 | 6 | 2.1 | 65.4 | Yes |
| 22 | 1 | 6.8 | 3.2 | 73.6 | Yes |
| 23 | 1.6 | 23.2 | 2.5 | 146.4 | Yes |
| 24 | ND | ND | ND | 46.4 | Yes |
| 25 | ND | 10.6 | ND | 15.2 | Yes |
| 26 | ND | 11.8 | ND | 38.4 | Yes |
| 27 | ND | 7.9 | 3.7 | 11.8 | Yes |
| 28 | ND | ND | ND | 16.2 | Yes |
| 29 | ND | ND | ND | 23.3 | Yes |
Broccoli sprout extract attenuates DEP-induced nasal white blood cells
Total cell counts in the nasal lavage followed a normal distribution. The log total cell counts in the nasal lavage samples are shown in Fig. 2. The baseline log cell count of the 29 DEP responders were 4.38 ± 0.54 at screening, 4.44 ± 0.51 at control phase and 4.70 ± 0.54 at BSE phase. The total log nasal white blood cell counts 6 and 24 h post-DEP exposure increased to 4.50 ± 0.56, 4.73 ± 0.52 at control phase and 4.44 ± 0.51, 4.57 ± 0.46 (Fig. 2). However, when these individuals consumed a broccoli sprout extract (BSE) for 4 days, this increase was abrogated and fell to levels similar to the baseline levels and washout levels as shown in Fig. 2. The log total cell count after BSE were significantly lower compared to screening phase and control phase cell counts (p < 0.01 at 6 hours and p < 0.001 at 24 hours).
Fig. 2.
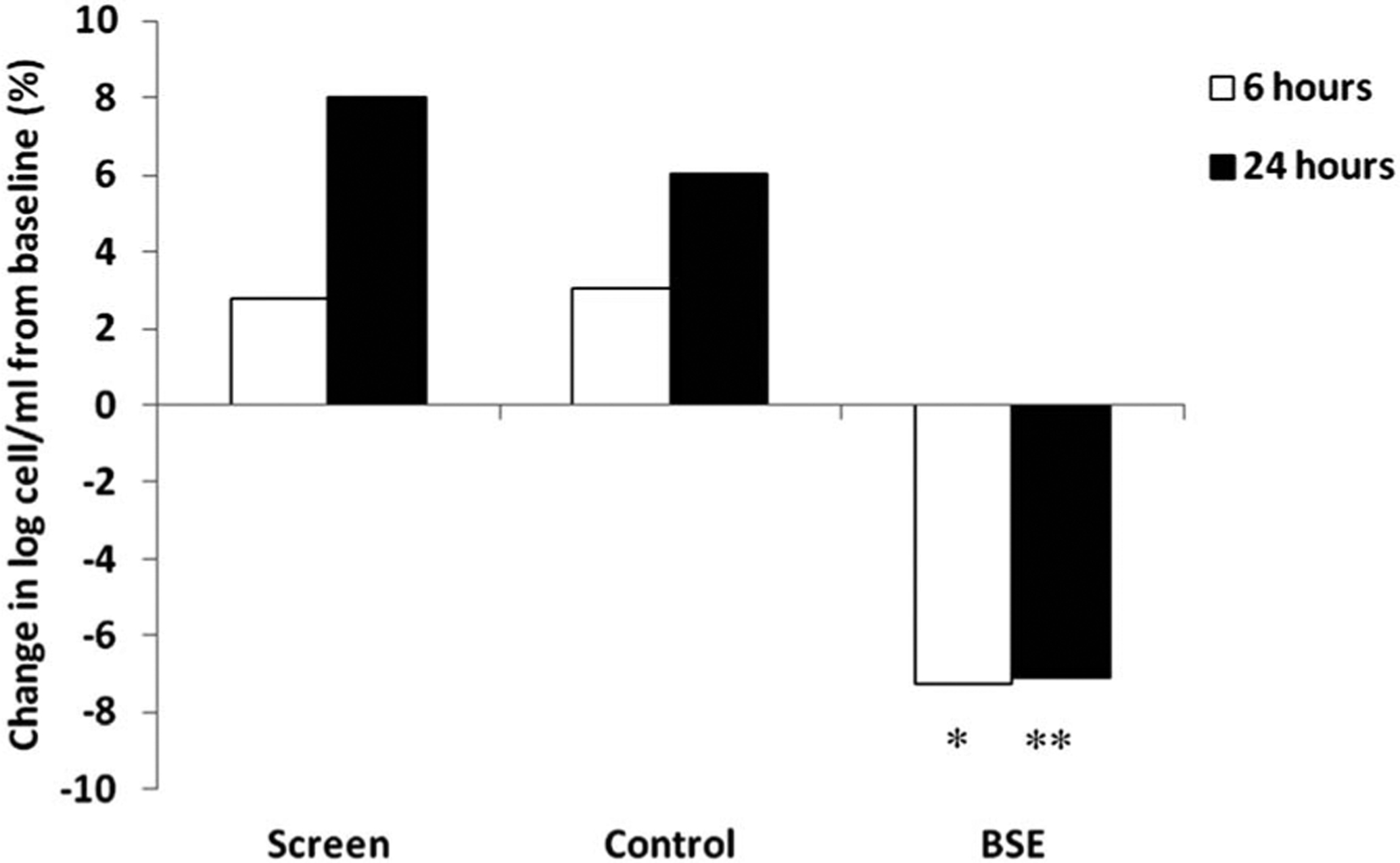
DEP responders increased total nasal cell counts at 6 and 24 h post-DEP. After 4 days of drinking broccoli sprout extract, DEP response was attenuated in responders at 6 hours and 24 hours compared to the screen and control phases. Cell count change from baseline as a percentage *(p < 0.01 comparing with screen or control phase). ** (p < 0.001 comparing with screen or control phase).
Discussion
Intranasal DEP exposure induced an increase in nasal white blood cell counts in 29 atopic cat-allergic individuals studied, providing a clinical model on which to base the study of the expected antioxidant effects of SFN-enriched BSE on the exacerbation of oxidative stress and airways inflammation by particulate air pollutants, including vehicular fossil fuel combustion emissions. Genetic polymorphisms in the antioxidant response pathway have been proposed as an explanation for variations in the allergic response to DEP. Previously, individuals with the GSTM1 null/GSTP1 Ile/Ile105 were reported to have enhanced nasal allergic responses to DEP or DEP plus ragweed.28 Individuals who were either GSTM1 null or GSTP1 Ile/Ile105 were reported to have enhanced nasal allergic responses to DEP and second-hand smoke.13 However, in the present study, the presence of GSTM1 and GSTP1 Ile/Ile105 polymorphisms did not significantly affect the DEP-induced pro-inflammatory effects in nasal lavage fluid. Due to the small sample size in this study, we cannot absolutely exclude the possibility that there could be a modulating effect of GSTM1 or GSTP1 genotype on allergic responses to DEP. The mechanism of the anti-inflammatory effects of oral SFN is related to the transcriptional activation of genes called vitagenes encoding for antioxidant enzymes as well as proteins with anti-inflammatory effects.29,30
These include phase 2 enzymes such as GST, superoxide dismutase, catalase, as well as anti-inflammatory gene products such as heme oxygenase 1. In addition to the increase in synthesis of glutathione due to the expression of phase 2 enzymes, there is also demonstrated interference in the pro-inflammatory NF-κB and AP1-mediated response pathways, which are activated by oxidative stress (including by DEP).31–37
Given that the dose of SFN administered was in the nutritional range,18,19,22,25 the present study provides encouragement that consumption of broccoli may be a useful strategy for managing the impact of particulate air pollution on atopic disease. While in the current study we did not have the opportunity to perform co-challenge with DEP plus cat allergen to assess whether BSE can also suppress the enhanced IgE and allergic inflammatory response to cat allergen in atopic individuals, we have observed that atopic individuals who generate a robust non-specific pro-inflammatory response to DEP are also more prone to demonstrate an exaggerated allergic inflammatory response due to the effects of pro-oxidative DEP chemicals to affect antigen presenting cells in the mucosal tract. This the results in skewing of the subsequent immune response to common environmental allergens, resulting in increased IgE production.38,39 Since the basis for the immune adjuvant effect involves the generation of a cascading Th2 immune response pathway that includes considerable amplification, it is possible that BSE effects could be even more potent at the level of comparing dual challenge IgE and allergic inflammatory effects. Larger clinical trials that include DEP plus allergen co-challenge will be needed to demonstrate protection against allergic inflammation in both allergic rhinitis and asthmatic subjects to determine the extent to which SFN or related compounds could ameliorate the symptoms and exacerbation of allergic disease by air pollution. Pursuit of this research is of particular importance from the perspective of inner-city asthma, including the impression from our mobile asthma clinic at UCLA that difficult-to-treat asthma cases among the pediatric population in Los Angeles are concentrated in more heavily polluted environments.
Acknowledgements
David Heber: Designed the study protocol and supervised the overall conduct of the study and the data analysis. He also participated in reporting the results and writing the manuscript. Zhaoping Li: Collaborated in the design of the study protocol and the conduct of the study. She also participated in data analysis and abstract, report, and manuscript preparation Maria Garcia-Lloret: Collaborated in the design of the study protocol and the conduct of the study. She also participated in data analysis, abstract, report and manuscript preparation Angela M. Wong: Performed the nasal lavage cell counts and the genotyping. Tsz Ying (Amy) Lee: Participated in the conduct of the study, and in manuscript preparation. Gail Thames: Collaborated in the design and approval of the study through communication with NIH and the FDA. She also prepared all documents related to the conduct of the study. She coordinated the study, oversaw data entry and data verification. Michael Krak: Participated in data entry, verification and analysis. Yanjun Zhang: Performed SFN chemical analysis by GC MS/MS. Andre Nel: UCLA Asthma Center Director, collaborated in the design the study, the conduct of the study, all data analysis and the preparation of all reports and the writing of this manuscript
Funding
This study was supported by NIH U19 AI070453-01, Xenobiotics, Oxidative Stress and Allergic Inflammation AADCRC-UCLA-01 (UCLA Asthma Center).
Footnotes
Conflict of interest
None of the authors reported any relevant conflict of interest.
References
- 1.Hwang CY, Chen YJ, Lin MW, et al. , Prevalence of atopic dermatitis, allergic rhinitis and asthma in Taiwan: a national study 2000 to 2007, Acta Derm.-Venereol, 2010, 90, 589–594. [DOI] [PubMed] [Google Scholar]
- 2.Nadif R, Siroux V, Oryszczyn MP, et al. , Heterogeneity of asthma according to blood inflammatory patterns, Thorax, 2009, 64, 374–380. [DOI] [PubMed] [Google Scholar]
- 3.Gibson PG, Inflammatory phenotypes in adult asthma: clinical applications, Clin. Respir.J, 2009, 3, 198–206. [DOI] [PubMed] [Google Scholar]
- 4.Lewtas J, Air pollution combustion emissions: characterization of causative agents and mechanisms associated with cancer, reproductive, and cardiovascular effects, Mutat. Res., Rev. Mutat. Res, 2007, 636, 95–133. [DOI] [PubMed] [Google Scholar]
- 5.Dockery DW, Schwartz J and Spengler JD, Air pollution and daily mortality: associations with particulates and acid aerosols, Environ. Res, 1992, 59, 362–373. [DOI] [PubMed] [Google Scholar]
- 6.Polosa R, Salvi S and Di Maria GU, Allergic susceptibility associated with diesel exhaust particle exposure: clear as mud, Arch. Environ. Health, 2002, 57, 188–193. [DOI] [PubMed] [Google Scholar]
- 7.Muller L, Riediker M, Wick P, et al. , Oxidative stress and inflammation response after nanoparticle exposure: differences between human lung cell monocultures and an advanced three-dimensional model of the human epithelial airways, J. R. Soc. Interface, 2010, 7(Suppl_1), S27–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auger F, Gendron MC, Chamot C, et al. , Responses of well-differentiated nasal epithelial cells exposed to particles: role of the epithelium in airway inflammation, Toxicol. Appl. Pharmacol, 2006, 215, 285–294. [DOI] [PubMed] [Google Scholar]
- 9.Nel A, Atmosphere. Air pollution-related illness: effects of particles, Science, 2005, 308, 804–806. [DOI] [PubMed] [Google Scholar]
- 10.Li N, Alam J, Venkatesan MI, et al. , Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals, J. Immunol, 2004, 173, 3467–3481. [DOI] [PubMed] [Google Scholar]
- 11.Baulig A, Sourdeval M, Meyer M, et al. , Biological effects of atmospheric particles on human bronchial epithelial cells. Comparison with diesel exhaust particles, Toxicol. in Vitro, 2003, 17, 567–573. [DOI] [PubMed] [Google Scholar]
- 12.Brockmoller J, Cascorbi I, Kerb R, et al. , Combined analysis of inherited polymorphisms in arylamine N-acetyltransferase 2, glutathione S-transferases M1 and T1, microsomal epoxide hydrolase, and cytochrome P450 enzymes as modulators of bladder cancer risk, Cancer Res, 1996, 56, 3915–3925. [PubMed] [Google Scholar]
- 13.Gilliland FD, Li YF, Gong H Jr, et al. , Glutathione s-transferases M1 and P1 prevent aggravation of allergic responses by secondhand smoke, Am. J. Respir. Crit. Care Med, 2006, 174, 1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayram H, Devalia JL, Khair OA, et al. , Comparison of ciliary activity and inflammatory mediator release from bronchial epithelial cells of nonatopic nonasthmatic subjects and atopic asthmatic patients and the effect of diesel exhaust particles in vitro, J. Allergy Clin. Immunol, 1998, 102, 771–782. [DOI] [PubMed] [Google Scholar]
- 15.Takizawa H, Diesel exhaust particles and their effect on induced cytokine expression in human bronchial epithelial cells, Curr. Opin. Allergy Clin. Immunol, 2004, 4, 355–359. [DOI] [PubMed] [Google Scholar]
- 16.Riedl MA, Saxon A and Diaz-Sanchez D, Oral sulforaphane increases Phase II antioxidant enzymes in the human upper airway, Clin. Immunol, 2009,130, 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye L, Dinkova-Kostova AT, Wade KL, et al. , Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: pharmacokinetics of broccoli sprout isothiocyanates in humans, Clin. Chim. Acta, 2002, 316, 43–53. [DOI] [PubMed] [Google Scholar]
- 18.Fahey JW, Zhang Y and Talalay P, Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens, Proc. Natl. Acad. Sci. U. S. A, 1997, 94, 10367–10372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahey JW, Wehage SL, Holtzclaw WD, et al. , Protection of humans by plant glucosinolates: efficiency of conversion of glucosinolates to isothiocyanates by the gastrointestinal microflora, Cancer Prev. Res, 2012, 5, 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diaz-Sanchez D, Dotson AR, Takenaka H, et al. , Diesel exhaust particles induce local IgE production in vivo and alter the pattern of IgE messenger RNA isoforms, J. Clin. Invest, 1994, 94, 1417–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McBride DE, Koenig JQ, Luchtel DL, et al. , Inflammatory effects of ozone in the upper airways of subjects with asthma, Am. J. Respir. Crit. Care Med, 1994, 149, 1192–1197. [DOI] [PubMed] [Google Scholar]
- 22.Fahey JW, Talalay P and Kensler TW, Notes from the field: “green” chemoprevention as frugal medicine, Cancer Prev. Res, 2012, 5, 179–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kensler TW, Ng D, Carmella SG, et al. , Modulation of the metabolism of airborne pollutants by glucoraphanin-rich and sulforaphane-rich broccoli sprout beverages in Qidong, China, Carcinogenesis, 2012, 33, 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Egner PA, Chen JG, Wang JB, et al. , Bioavailability of sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China, Cancer Prev. Res, 2011, 4, 384–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro TA, Fahey JW, Dinkova-Kostova AT, et al. , Safety, tolerance, and metabolism of broccoli sprout glucosinolates and isothiocyanates: a clinical phase I study, Nutr. Cancer, 2006, 55, 53–62. [DOI] [PubMed] [Google Scholar]
- 26.Al Janobi AA, Mithen RF, Gasper AV, et al. , Quantitative measurement of sulforaphane, iberin and their mercapturic acid pathway metabolites in human plasma and urine using liquid chromatography-tandem electrospray ionisation mass spectrometry, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci, 2006, 844, 223–234. [DOI] [PubMed] [Google Scholar]
- 27.Diaz-Sanchez D, Jyral M, Ng D, et al. , In vivo nasal challenge with diesel exhaust particles enhances expression of the CC chemokines rantes, MIP-1alpha, and MCP-3 in humans, Clin. Immunol, 2000, 97, 140–145. [DOI] [PubMed] [Google Scholar]
- 28.Gilliland FD, Li YF, Saxon A, et al. , Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study, Lancet, 2004, 363, 119–125. [DOI] [PubMed] [Google Scholar]
- 29.Calabrese V, Cornelius C, Dinkova-Kostova AT, Iavicoli I, Di Paola R, Koverech A, Cuzzocrea S, Rizzarelli E and Calabrese EJ, Cellular stress responses, hormetic phytochemicals and vitagenes in aging and longevity, Biochim. Biophys. Acta, Mol. Basis Dis, 2012, 1822, 753–783. [DOI] [PubMed] [Google Scholar]
- 30.Calabrese V, Cornelius C, Cuzzocrea S, Iavicoli I, Rizzarelli E and Calabrese EJ, Hormesis, cellular stress response and vitagenes as critical determinants in aging and longevity, Mol. Aspects Med, 2011, 32, 279–304. [DOI] [PubMed] [Google Scholar]
- 31.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M and Biswal S, Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray, Cancer Res, 2002, 62, 5196–5203. [PubMed] [Google Scholar]
- 32.Munday R and Munday CM, Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds, J. Agric. Food Chem, 2004, 52, 1867–1871. [DOI] [PubMed] [Google Scholar]
- 33.Antolino-Lobo I, Meulenbelt J, Molendijk J, Nijmeijer SM, Scherpenisse P, van den Berg M and van Duursen MB, Induction of glutathione synthesis and conjugation by 3,4-methylenedioxymethamphetamine (MDMA) and 3,4-dihydroxymethamphetamine (HHMA)in human and rat liver cells, including the protective role of some antioxidants, Toxicology, 2011, 289, 175–184. [DOI] [PubMed] [Google Scholar]
- 34.Higgins LG and Hayes JD, Mechanisms of induction of cytosolic and microsomal glutathione transferase (GST) genes by xenobiotics and pro-inflammatory agents, Drug Metab. Rev, 2011, 43, 92–137. [DOI] [PubMed] [Google Scholar]
- 35.Zhu H, Jia Z, Strobl JS, Ehrich M, Misra HP and Li Y, Potent induction of total cellular and mitochondrial antioxidants and phase 2 enzymes by cruciferous sulforaphane in rat aortic smooth muscle cells: cytoprotection against oxidative and electrophilic stress, Cardiovasc. Toxicol, 2008, 8, 115–125. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Munday R, Jobson HE, Munday CM, Lister C, Wilson P, Fahey JW and Mhawech-Fauceglia P, Induction of GST and NQO1 in cultured bladder cells and in the urinary bladders of rats by an extract of broccoli (Brassica oleracea italica) sprouts, J. Agric. Food Chem, 2006, 54, 9370–9376. [DOI] [PubMed] [Google Scholar]
- 37.McWalter GK, Higgins LG, McLellan LI, Henderson CJ, Song L, Thornalley PJ, Itoh K, Yamamoto M and Hayes JD, Transcription factor Nrf2 is essential for induction of NAD(P)H:quinone oxidoreductase 1, glutathione S-transferases, and glutamate cysteine ligase by broccoli seeds and isothiocyanates, J. Nutr, 2004, 134, 3499S–3506S. [DOI] [PubMed] [Google Scholar]
- 38.Nel AE, Diaz-Sanchez D, Ng D, Hiura T and Saxon A, Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system, J. Allergy Clin. Immunol, 1998, 102, 539–554. [DOI] [PubMed] [Google Scholar]
- 39.Kim HJ, Barajas B, Chan RC and Nel AE, Glutathione depletion inhibits dendritic cell maturation and delayed-type hypersensitivity: implications for systemic disease and immunosenescence, J. Allergy Clin. Immunol, 2007, 119, 1225–1233. [DOI] [PubMed] [Google Scholar]


