Background: Kidney transplant recipients receiving immunosuppressive drugs have impaired immune responses to messenger RNA (mRNA) COVID-19 vaccines (1). Consequently, despite standard vaccination with mRNA vaccines, many of these patients remain at high risk for severe disease during the ongoing pandemic. The U.S. Food and Drug Administration has authorized immunocompromised people to receive a third dose of mRNA vaccine after the standard 2-dose regimen to further boost protection, and French health authorities approved a third dose on 11 April 2021. Subsequent studies found that approximately 50% of patients who did not respond after a second dose seroconverted after a third dose, which produced an overall seroconversion rate of about 65% (2, 3). Antispike IgG titers above 143 binding antibody units (BAU) per milliliter correlate with the presence of neutralizing antibodies (the most widely accepted marker of disease protection) against the wild-type virus and the Alpha, Beta, and Gamma variants, but neutralization of the Delta variant requires higher antispike IgG titers (4). Therefore, patients with low titers of antispike IgG may remain insufficiently protected. In June 2021, French health authorities approved offering a fourth vaccine dose to recipients of solid organ transplants who had a weak response after a third dose.
Objective: To investigate whether a fourth dose of an mRNA-based anti–SARS-CoV-2 vaccine would increase antispike IgG titers in kidney transplant recipients who showed a weak serologic response after 3 doses.
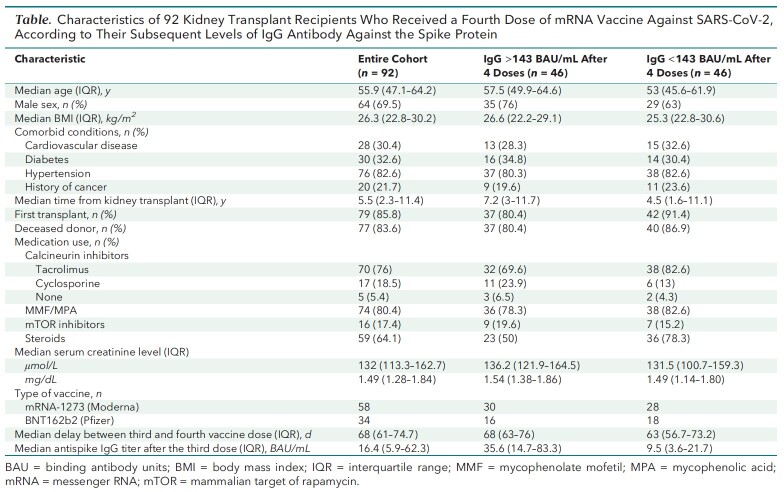
Case Report: A fourth dose of mRNA vaccine (BNT162b2 [Pfizer], n = 34; mRNA-1273 [Moderna], n = 58) was given to 92 kidney transplant recipients from 3 independent French university hospitals (Strasbourg, Lyon, and Nantes) who had antispike IgG titers less than 143 BAU/mL 1 month after a third dose. All had measurements of antispike IgG titers 2 to 6 weeks later (median, 29 days [interquartile range, 26 to 34 days]). The Table shows the characteristics of these patients.
Table.
Characteristics of 92 Kidney Transplant Recipients Who Received a Fourth Dose of mRNA Vaccine Against SARS-CoV-2, According to Their Subsequent Levels of IgG Antibody Against the Spike Protein
There were no safety concerns with the fourth vaccine dose. After a median of 29 days, median antispike IgG levels increased from 16.4 BAU/mL (interquartile range, 5.9 to 62.3 BAU/mL) to 145 BAU/mL (interquartile range, 27.6 to 243 BAU/mL) (Figure) and 50% of patients reached the threshold of 143 BAU/mL. Patients who reached this threshold had a longer interval between their transplant and fourth vaccine dose and were less frequently treated with steroids (Table). The percentage of patients who had antispike IgG titers above 143 BAU/mL after the fourth dose was 48% for the BNT162b2 vaccine and 52% for the mRNA-1273 vaccine, and patients who received the mRNA-1273 vaccine had higher IgG titers (median, 150 vs. 122 BAU/mL). Only 1 patient was subsequently diagnosed with mild COVID-19, and he had an antispike IgG level of 28 BAU/mL 1 month after his fourth vaccine dose.
Figure. Antispike IgG titers measured 2–6 wk after the third and fourth vaccine doses in 92 kidney transplant recipients.
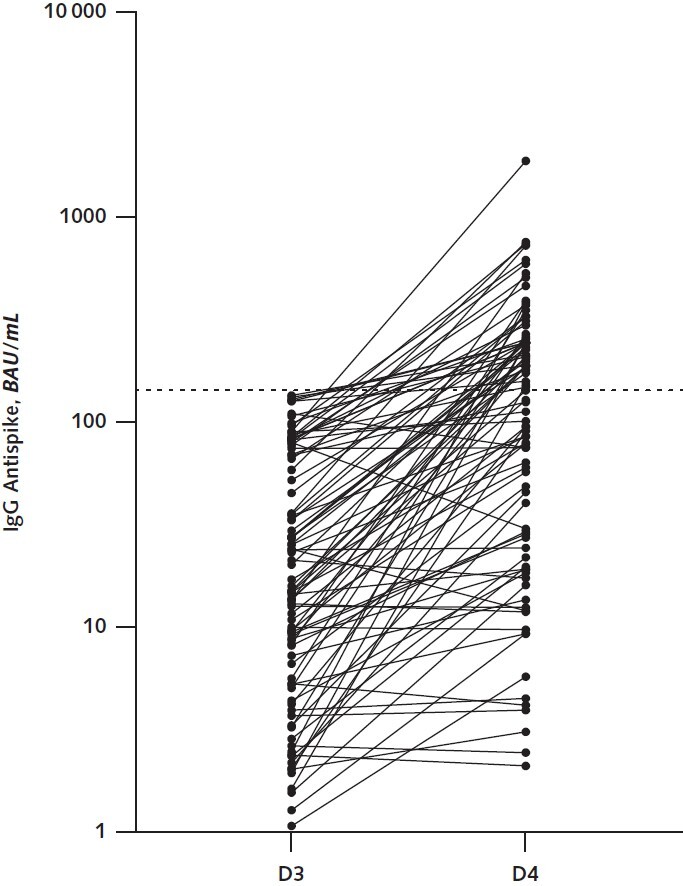
Titers are expressed in BAU after calibration to the World Health Organization standard. The dotted line indicates the threshold of 143 BAU/mL. BAU = binding antibody units; D3 = third dose; D4 = fourth dose.
Discussion: Our study indicates that a fourth dose of an mRNA-based vaccine produces a satisfactory antibody response in some kidney transplant recipients who did not respond adequately after 3 previous doses, and it supports the use of a fourth vaccine dose for these patients. We have shown in an unpublished study that kidney transplant recipients with previous COVID-19 had higher antispike IgG titers than uninfected recipients who were vaccinated. Assuming that the difference can be attributed to a higher antigen dose with infection than with vaccination, those results also support the use of additional, repeated doses of vaccine for kidney transplant recipients who do not respond adequately to standard vaccination. Prophylactic infusion of monoclonal anti–SARS-CoV-2 antibodies can be offered to patients who do not respond adequately to additional vaccine doses (5). For example, in our experience, only 10% of patients who did not respond (<1 BAU/mL) after the third dose were able to reach antispike IgG titers above 143 BAU/mL after a fourth dose. It would have been interesting to examine T-cell immunity after repeated vaccine doses in these patients, but the assays are time-consuming, which makes it challenging to implement them during routine practice. Finally, we recognize that an increase in antispike IgG titers does not invariably provide protection from infection and disease, which is why we encourage longitudinal studies with a sufficient duration of follow-up to evaluate the risk for COVID-19 in patients like these after additional vaccine doses.
Footnotes
This article was published at Annals.org on 11 January 2022.
References
- 1. Boyarsky BJ , Werbel WA , Avery RK , et al. Antibody response to 2-dose SARS-CoV-2 mRNA vaccine series in solid organ transplant recipients. JAMA. 2021;325:2204-2206. [PMID: ] doi: 10.1001/jama.2021.7489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benotmane I , Gautier G , Perrin P , et al. Antibody response after a third dose of the mRNA-1273 SARS-CoV-2 vaccine in kidney transplant recipients with minimal serologic response to 2 doses. JAMA. 2021. [PMID: ] doi: 10.1001/jama.2021.12339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamar N , Abravanel F , Marion O , et al. Three doses of an mRNA Covid-19 vaccine in solid-organ transplant recipients [Letter]. N Engl J Med. 2021;385:661-662. [PMID: ] doi: 10.1056/NEJMc2108861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Planas D , Veyer D , Baidaliuk A , et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276-280. [PMID: ] doi: 10.1038/s41586-021-03777-9 [DOI] [PubMed] [Google Scholar]
- 5. O'Brien MP , Forleo-Neto E , Musser BJ , et al; Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184-1195. [PMID: ] doi: 10.1056/NEJMoa2109682 [DOI] [PMC free article] [PubMed] [Google Scholar]