Abstract
Introduction
The purpose of this study was to evaluate the therapeutic effectiveness of Chinese patent medicines containing red yeast rice for the treatment of hyperlipidaemia.
Methods
Relevant literature published until 13 August 2021, was retrieved from six electronic databases. Randomized clinical trials of Chinese patent medicines containing red yeast rice in patients with hyperlipidaemia were included in the review. Network meta‐analysis was performed using Stata 13.1 software. Methodological quality was assessed using the Cochrane risk of bias tool. The surface under the cumulative ranking (SUCRA) curve probability values were used to rank the treatments.
Results
This study included 47 trials involving 4824 subjects. In terms of reduced total cholesterol levels, Xuezhikang (SUCRA: 84.5%) had the highest probability of being the most effective formulation, with Simvastatin (66.4%) and Zhibitai (65.4%) ranked second and third, respectively. Xuezhikang also had the highest probability of reducing low‐density lipoprotein cholesterol levels to the greatest extent (SUCRA: 82.6%) with Simvastatin (SUCRA: 74.9%) and Zhibituo (SUCRA: 52.8%) being the second and third choices, respectively. For reduced triglyceride levels, Zhibituo (SUCRA: 80.2%) exhibited the highest probability of being the most effective, with Xuezhikang (SUCRA: 63.4%) and Simvastatin (SUCRA: 57.6%) in second and third places, respectively. Finally, in terms of improving high‐density lipoprotein cholesterol levels, Zhibituo (SUCRA: 90.1%) had the highest probability of being the most effective, with Simvastatin (SUCRA: 82.1%) and Xuezhikang (SUCRA: 51.1%) ranked second and third, respectively.
Conclusions
Xuezhikang was found to have the highest probability of being the most effective formulation for reducing total cholesterol and low‐density lipoprotein cholesterol levels, while Zhibituo had the highest probability of being the most effective for controlling triglyceride and high‐density lipoprotein cholesterol levels. The studies included in the review exhibited certain limitations and, therefore, more rigorously designed studies should be performed.
Trial registration: INPLASY registration number: INPLASY202130017.
Keywords: Chinese patent medicine, evidence‐based medicine, network meta‐analysis
The purpose of the present study was to evaluate the therapeutic effectiveness of Chinese patent medicines containing red yeast rice for the treatment of hyperlipidaemia. Xuezhikang was found to have the highest probability of being the most effective formulation for reducing total cholesterol and low‐density lipoprotein cholesterol levels, while Zhibituo had the highest probability of being most effective for controlling triglyceride levels and high‐density lipoprotein cholesterol levels. The studies included in the review exhibited a number of limitations, and therefore, more rigorously designed studies should be performed.

1. INTRODUCTION
Hyperlipidaemia is a common, global metabolic syndrome associated with lipid abnormalities, including increased levels of triglycerides (TG), total cholesterol (TC) and low‐density lipoprotein cholesterol (LDL‐C) and decreased levels of high‐density lipoprotein cholesterol (HDL‐C). 1 , 2 Serum lipid levels in the Chinese population have gradually increased, and the prevalence of dyslipidaemia among Chinese adults has reached 40.4%. 3 , 4 , 5 Hyperlipidaemia is a major contributory factor to various diseases, including cardiovascular diseases, type 2 diabetes, Alzheimer's disease and Parkinson's disease. 6 , 7 , 8 Therefore, measures to effectively and treat dyslipidaemia are crucial for preventing cardiovascular and cerebrovascular diseases.
Statins are currently the drug of choice for the treatment of hypercholesterolemia. 9 , 10 However, the side effects caused by their use often limit their application. 11 Previous studies have suggested that extracts of red yeast rice (RYR) reduce blood lipid levels. 12 , 13 There are many varieties of oral Chinese patent medicines containing RYR for the treatment of hyperlipidaemia, such as Xuezhikang, Zhibituo and Zhibitai capsules, which are widely used in China for the treatment of hyperlipidaemia. 14
However, the efficacy of these Chinese patent medicines has not been directly compared for the treatment of hyperlipidaemia; therefore, it is not possible to select an optimal formulation for patients with hyperlipidaemia. Consequently, we conducted a network meta‐analysis to compare the therapeutic effectiveness of Chinese patent medicines for treating hyperlipidaemia and identify which of them was consistently ranked as the most effective.
2. MATERIALS AND METHODS
The protocol for this meta‐analysis was registered using the INPLASY (No. INPLASY202130017), available on Inplasy.com (https://doi.org/10.37766/inplasy2021.3.0017). Ethics approval for this study was not required, as the meta‐analysis did not involve identifiable patient data.
2.1. Bibliographic search strategy
Two authors (XGQ and DXL) conducted the literature searches. Supplementary searches were performed using Google Scholar software. Searches were restricted to studies published in English or Chinese. A representative example of the search strategy using the PubMed database is as follows: #1 (hyperlipidemias[MeSH Terms]) OR (cholesterol[MeSH Terms]); #2 (random allocation[MeSH Terms]) OR (randomized)) OR (placebo) OR randomized controlled trial; #3 Simvastatin[MeSH Terms]; #4 ((Xuezhikang[Title/Abstract]) OR (Zhibituo[Title/Abstract])) OR (Zhibitai[Title/Abstract]); #5 #1 AND #2 AND #3 AND #4.
2.2. Inclusion criteria
Trials were included in the present study following the PICOS framework (population, intervention, comparisons, outcomes and study type). Population (P): participants were diagnosed with hyperlipidaemia according to recognized diagnostic criteria, with no limitations in terms of age, gender, or ethnicity. Intervention (I): The experimental group was prescribed any of the following Chinese patent medicines: Xuezhikang capsules, Zhibituo capsules or Zhibitai capsules, without the co‐administration of Western medicine. Comparisons (C): The control group received Simvastatin (Zocor) or placebo, and pairwise comparisons of the above Chinese patent medicines were performed. Outcomes (O) were serum lipid levels, including TC, TG, LDL‐C, HDL‐C levels and adverse drug reactions (ADRs). Study type (S): Only randomized controlled trials (RCTs) were included, and trials in languages other than Chinese or English were excluded.
2.3. Exclusion criteria
Studies were excluded for the following reasons: (1) duplicate publications; (2) case reports, reviews, or studies with animals as research subjects; (3) patient comorbidities (eg diabetes, cardiovascular diseases and cerebrovascular diseases), (4) incorrect or missing data or (5) trials with <50 cases.
2.4. Outcome measures
The main outcomes were serum lipid levels, including TC, TG, LDL‐C and HDL‐C levels. The secondary outcomes included ADRs.
2.5. Data extraction
Two reviewers (XGQ and DXL) independently selected the studies. Titles and abstracts were screened to identify potential articles, and then, the full texts of the screened articles were read to determine suitable studies based on the inclusion and exclusion criteria. Discrepancies in selection were resolved through team discussion. The selection procedures were based on the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow chart. 15
2.6. Assessment of quality
Two researchers (XGQ and DXL) independently assessed the risk of bias in the studies included in this review using the risk of bias tool from the Cochrane Handbook. Any disagreements were resolved using an arbiter. The following items were evaluated: selection bias (random sequence generation, allocation concealment), performance bias (blinding of participants and personnel), detection bias (blinding of assessors), attrition bias and other types of bias. The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to assess the quality of evidence. 16 According to the GRADE approach, evidence quality is classified into four levels: high, moderate, low and very low. RCTs provide high‐quality evidence; however, the evidence can be downgraded from high to low quality owing to five factors: study limitation (risk of bias), indirectness, inconsistency, imprecision and publication bias.
2.7. Statistical analysis
A statistical software (Stata 13.1; Stata Corporation) was used for the present study. The results were reported as mean differences (MDs) with 95% confidence intervals (CIs). Heterogeneity assessment was performed using chi‐squared (χ 2) tests; if I 2 was ≤50%, the heterogeneity was considered to be low, and network meta‐analysis could be performed; if I 2 > 50%, heterogeneity was deemed to be high, and the study could be conducted when the source of heterogeneity could be found. For indirect comparisons, the treatment effects of all regimens were estimated using a two‐stage network meta‐analysis as follows: Initially, an inconsistency test was performed using a node‐splitting model, and fitting consistency or inconsistency models were constructed and presented using the network command; if inconsistency was not statistically significant (p > .05), a consistency model was used; otherwise, an inconsistency model was employed. Pairwise comparisons were conducted using the ‘interval plot’ command. Ranking probabilities for each intervention were then estimated using the ‘network rank’ command. Surface under the cumulative ranking (SUCRA) curve values were calculated to rank the efficacy of each intervention. Larger SUCRA values indicate a more effective intervention. Publication bias was evaluated using comparison‐adjusted funnel plots.
3. RESULTS
3.1. Descriptions of studies
A total of 597 relevant studies were initially selected, of which 53 duplicates were excluded. Another 409 articles were excluded after reviewing their titles and abstracts based on the inclusion and exclusion criteria. Finally, the full text of the remaining 132 articles was read, from which 47 RCTs were identified as satisfying the inclusion criteria and were included in the final analysis. A flowchart describing the retrieval and screening processes is shown in Figure 1.
FIGURE 1.
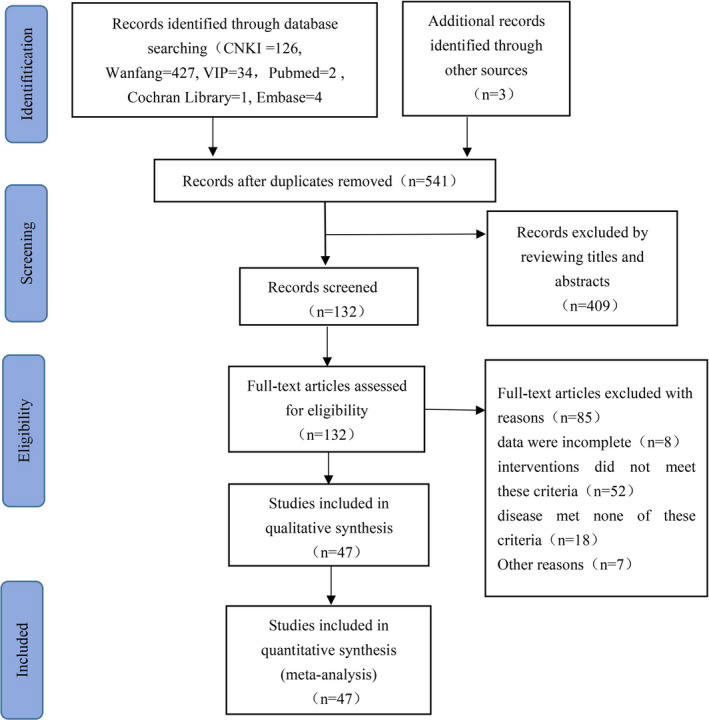
Flow chart of selection of studies
3.2. Baseline characteristics of included studies
A total of 47 RCTs involving 4824 participants diagnosed with hyperlipidaemia satisfied the study selection criteria and were included in the study. From these 47 RCTs, the effects on hyperlipidaemia resulting from the use of three Chinese patent medicines containing RYR were summarized. The characteristics of the included studies are summarized in Table 1. The included studies showed that all baseline values were comparable.
TABLE 1.
Characteristics of included studies in the network meta‐analysis
| No. | Study | Year | No. of patients | Average age (T/C) | Treatment 1 drug dose | Treatment 2 drug dose | Duration | Outcome measures | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | He Y 17 | 2013 | 88 | 48.2 ± 13.5/47.2 ± 14.4 | ZBTai | 240 mg, Bid | Simvastatin | 10 mg, QD | 8 weeks | ①②③④⑤ |
| 2 | Wu GZ 18 | 2010 | 192 | 60.5 | ZBTuo | 300 mg, Tid | Simvastatin | 10 mg, QD | 12 weeks | ②③④⑤⑥ |
| 3 | Hu XZ 19 | 2011 | 128 | 61.2 ± 3.5 | ZBTuo | 300 mg, Tid | Simvastatin | 10 mg, QD | 3 months | ②③④⑤⑥ |
| 4 | Zhao S 20 | 2008 | 99 | 51.2/51.5 | XZK | 600 mg, QD | Simvastatin | 10 mg, QD | 4 weeks | ②③④⑤⑥ |
| 5 | Li ZH 21 | 2017 | 92 | 74.47 ± 5.38/62.34 ± 2.72 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ②③④⑤ hs‐CRP |
| 6 | Xue SL 22 | 2010 | 108 | 50.34 ± 10.28/51.61 ± 10.09 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 24 weeks | ②③④⑤⑥ hs‐CRP, IMT |
| 7 | Zhang G 23 | 1998 | 80 | 57.67 ± 9.69/57.58 ± 8.74 | XZK | 600 mg, Bid | Simvastatin | 10 mg, QD | 4 weeks | ①②③④⑤⑥ |
| 8 | Xi BL 24 | 2002 | 60 | 69.57 ± 6.99/70.60 ± 5.65 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 4 weeks | ②③④⑤⑥ |
| 9 | Chen QY 25 | 2007 | 82 | 59.4 ± 3.2 /60.2 ± 4.2 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 4 weeks | ②③④⑤⑥ |
| 10 | Chen LL 26 | 2002 | 65 | 55.2 ± 3.8/56.7 ± 3.1 | XZK | 1200 mg, QN | Simvastatin | 10 mg, QD | 4 weeks | ①②③④⑤⑥ |
| 11 | Zhang XF 27 | 2010 | 76 | 55.6 ± 8.7/56.5 ± 9.1 | XZK | 1200 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ②③④⑤⑥ |
| 12 | Liu SP 28 | 2013 | 120 | 58 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 13 | Zhu QF 29 | 2003 | 159 | 62.32 ± 12.27/61.53 ± 11.69 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 3 months | ②③④⑤⑥⑦⑧ |
| 14 | Zheng W 30 | 2013 | 61 | 59.8 ± 9.7 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 12 weeks | ①②③④⑤⑥ |
| 15 | Li KL 31 | 2006 | 80 | 55.53 ± 10.56/56.05 ± 10.22 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ②③④⑥ |
| 16 | Qi MY 32 | 2004 | 224 | Not reported | XZK | 1200 mg, Bid | Simvastatin | 10 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 17 | Hua C 33 | 2011 | 100 | 45–75 | XZK | 600 mg, Bid | Simvastatin | 10 mg, QD | 8 weeks | ②④ |
| 18 | Wang SH 34 | 2003 | 120 | 69.57 ± 6.99/70.60 ± 5.65 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 4 weeks | ②③④⑤⑥ |
| 19 | Chen FJ 35 | 2003 | 286 | 60.17 ± 8.52 | XZK | 600 mg, Bid | Simvastatin | 10 mg, QD | 12 weeks | ②③④⑤⑥⑦⑧ |
| 20 | Zhou H 36 | 2012 | 139 | 59.13 ± 9.20/61.42 ± 8.52 | ZBTai | 240 mg, Bid | Simvastatin | 20 mg, QD | 12 months | ②③④⑤⑥⑨ |
| 21 | Pang J 37 | 2018 | 60 | 65/63.5 | ZBTai | 240 mg, Bid | Simvastatin | 20 mg, QD | 4 weeks | ②③④ |
| 22 | Yang WJ 38 | 2003 | 70 | 64.03 ± 5.71/63.07 ± 6.20 | ZBTuo | 240 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 23 | Liu JX 39 | 2005 | 68 | 58 ± 12/56 ± 11 | ZBTuo | 240 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 24 | Guo XM 40 | 1999 | 63 | 56 ± 12/58 ± 11 | ZBTuo | 480 mg, Bid | Simvastatin | 10 mg, QD | 6 weeks | ①②③④⑤⑥⑦⑧ |
| 25 | Zhang GR 41 | 2002 | 137 | 59.25/58.85 | ZBTuo | 1050 mg, Tid | Simvastatin | 10 mg, QD | 4 weeks | ①②③④⑤⑥ |
| 26 | Peng KL 42 | 2011 | 220 | Not reported | ZBTuo | 300 mg, Tid | Simvastatin | 10 mg, QD | 4 months | ②③④⑤⑥ |
| 27 | Xu J 43 | 2009 | 100 | 56.2/56.9 | ZBTuo | 1050 mg, Tid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 28 | Zhang QL 44 | 2004 | 60 | 56.5/58 | ZBTuo | 1050 mg, Tid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 29 | Feng ZH 45 | 2006 | 100 | 57.9/56.8 | ZBTuo | 1050 mg, Tid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 30 | Guo SH 46 | 2019 | 100 | 55.3 ± 3.2/54.2 ± 3.5 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 12 weeks | ①②③④⑤⑥ |
| 31 | Li XL 47 | 2011 | 68 | 58 ± 12/56 ± 11 | ZBTuo | 240 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ②③④⑤⑥ |
| 32 | Zhao PF 48 | 2011 | 120 | 67.32 ± 9.42/65.93 ± 8.83 | ZBTuo | 1050 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ②③④⑤⑥ |
| 33 | Zhang Q 49 | 2015 | 96 | 62.6 ± 7.8/60.8 ± 8.3 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤, ⑥, CRP, Hcy, Cost‐effect analysis |
| 34 | Duan CM 50 | 2014 | 70 | 41.9 ± 3.7/42.8 ± 3.1 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 16 weeks | ①②③④⑤⑥ |
| 35 | Yang WX 51 | 2013 | 84 | 49 ± 8.7/49 ± 9.1 | XZK | 600 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤ |
| 36 | Lu XB 52 | 2012 | 60 | 50.75 ± 3.72/43.33 ± 6.03 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 8 weeks | ①②③④⑤⑥⑩ |
| 37 | Xu NF 53 | 2011 | 60 | 51.84 ± 10.16/51.36 ± 10.65 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 8 weeks | ①②③④⑤⑥⑩ |
| 38 | Chen L 54 | 2010 | 100 | 56.5 ± 7.1 | Simvastatin | 20 mg, QD | XZK | 600 mg, Bid | 8 weeks | ②③④⑤⑥ |
| 39 | Wang M 55 | 2005 | 160 | 56.3 ± 9.1/57.4 ± 10.2 | ZBTuo | 1050 mg, Bid | Simvastatin | 20 mg, QD | 8 weeks | ①②③④⑤⑥ |
| 40 | Chen L 56 | 2003 | 117 | 56 ± 15/53 ± 18 | ZBTuo | 1050 mg, Bid | XZK | 600 mg, Bid | 30 days | ②③④⑤⑥ |
| 41 | Chen ZM 57 | 2001 | 90 | 54 ± 9/56 ± 9 | Simvastatin | 10 mg, QD | ZBTuo | 1050 mg, Tid | 8 weeks | ②③④⑤⑥ |
| 42 | Chen SM 58 | 2001 | 58 | 57.8/56.4 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 8 weeks | ②③④⑤, internal diameter of the brachial artery |
| 43 | Lu YS 59 | 1997 | 62 | 53 ± 8/53 ± 6 | XZK | 600 mg, Bid | ZBTuo | 1050 mg, Tid | 8 weeks | ②③④⑤ |
| 44 | Zhao DY 60 | 2012 | 60 | 52.6 ± 9.34/55.46 ± 11.70 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 8 weeks | ②③④⑤⑥, improvement of clinical symptoms |
| 45 | Yu JB 61 | 2014 | 60 | 18–75 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 8 weeks | ②③④⑤⑥ |
| 46 | Qi RY 62 | 2007 | 162 | 65.5 ± 4.32/67.4 ± 5.2 | XZK | 600 mg, Bid | Placebo | 600 mg, Bid | 8 weeks | ①②③④⑤⑥ |
| 47 | Peng DY 63 | 1998 | 60 | 68 ± 3/66 ± 5 | ZBTuo | 1050 mg, Tid | Placebo | 1050 mg, Tid | 6 weeks | ①②③⑤⑥ |
Abbreviations: ①, clinical efficacy; ②TC, total cholesterol; ③TG, triglycerides; ④LDL‐C, low‐density lipoprotein cholesterol; ⑤HDL‐C, high‐density lipoprotein cholesterol; ⑥ADR, adverse drug reaction; ⑦Apolipoprotein A1;⑧Apolipoprotein B;⑨Liver and kidney function, creatine kinase, carotid intima thickness;⑩Chinese medicine syndrome; C, control group; T, experimental group; XZK, Xuezhikang capsules; ZBTai, Zhibitai capsules; ZBTuo, Zhibituo capsules or Zhibituo tablets.
3.3. Risk of bias in included studies
The risk‐of‐bias graphs for the 47 studies are shown in Figure 2. All studies included randomization, five 21 , 22 , 28 , 37 , 46 described the generation of a random sequence using a random number table, three 36 , 52 , 53 were randomized, double‐blind, controlled trials, and four 50 , 58 , 61 , 63 were randomized, placebo‐controlled trials. Other studies have not described a specific method for random sequence generation. None of the unblinded studies stated the details of allocation concealment. For the blinding of participants and personnel, a high risk was identified in the unblinded studies. Because the outcomes were tested in the lab, the risk of blinding on the outcome assessment was low. All studies reported complete outcome data and were free of selective reporting. The unblinded studies were unclear in terms of other biases.
FIGURE 2.
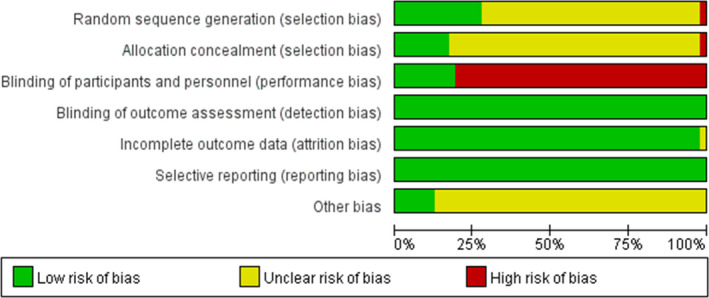
Risk of bias in the selected trials
3.4. Outcome measures
3.4.1. Test of inconsistency and network plot
The evidence network is shown in Figure 3. The network meta‐analysis included closed loops, and formal tests for inconsistency were performed. We found inconsistencies were not statistically significant (TC, p = .58; TG, p = .26; LDL‐C, p = .64; and HDL‐C, p = .32), and a network meta‐analysis was performed using a consistency model.
FIGURE 3.
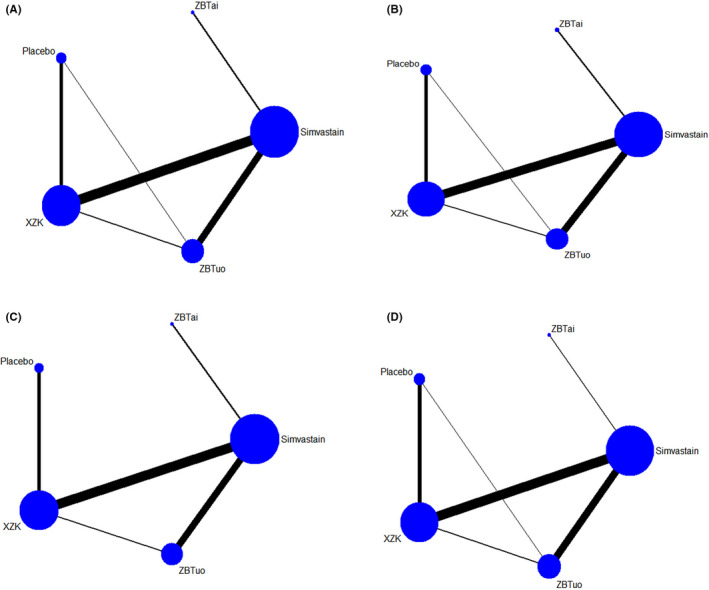
Network plot for TC (A), TG (B), LDL‐C (C) and HDL‐C (D). The nodes in the figure represent the following interventions XZK, Xuezhikang capsules; ZBTuo, Zhibituo capsules
3.4.2. TC—Total cholesterol levels
For a total of 4803 patients in 47 RCTs, 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 changes in TC levels after administration of three types of Chinese patent medicines were compared with changes due to Simvastatin or placebo. Chi‐squared tests showed no heterogeneity between studies (p = 1.0, I 2 = 0.0%). There was a statistically significant difference in all treatments compared with the placebo group. No statistically significant differences were observed between the other comparisons. The results of the network meta‐analysis are summarized in Table 2. The intervention ranking probabilities based on SUCRA were as follows: Xuezhikang (84.5%) > Simvastatin (66.4%) > Zhibitai (65.4%) > Zhibituo (33.7%) > placebo (0.0%) (Figure 4), suggesting that Xuezhikang was the most effective intervention for TC, with Simvastatin and Zhibitai ranking second and third, respectively.
TABLE 2.
Network meta‐analysis for TC (MD [95% CI])
| Zhibituo | ||||
| 0.17 (−0.35, 0.70) | Zhibitai | |||
| 0.25 (−0.01, 0.50) | 0.07 (−0.44, 0.59) | Xuezhikang | ||
| 0.17 (−0.03, 0.38) | 0.00 (−0.48, 0.48) | −0.07 (−0.25, 0.10) | Simvastatin | |
| −0.87 (−1.24, −0.49) | −1.04 (−1.63, −0.45) | −1.11 (−1.42, −0.81) | 1.04 (−1.38, −0.69) | Placebo |
Bold values indicate statistically significant values p < 0.05.
FIGURE 4.
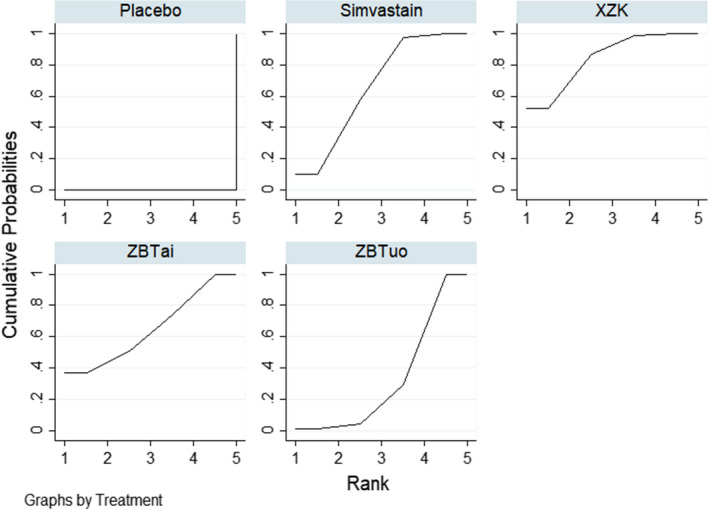
SUCRA curves for TC
3.4.3. TG—Triglyceride levels
A total of 4591 patients in 45 RCTs 17 , 18 , 19 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 were treated with three types of Chinese patent medicines for TG, which were compared with Simvastatin or placebo. Heterogeneity tests showed no heterogeneity between studies (p = 1.0, I 2 = 0.0%). One study 20 was excluded from the analysis because it contained an outlier value. There was a statistically significant difference in all treatments compared with the placebo group. No statistically significant differences were observed between the other comparisons. The results of the network meta‐analysis are summarized in Table 3. Based on the SUCRA values (Figure 5), Zhibituo (80.2%) > Xuezhikang (63.4%) > Simvastatin (57.6%) > Zhibitai (48.7%) > placebo (0.1%), suggesting that Zhibituo was the most effective intervention for TG, with Xuezhikang and Simvastatin ranking second and third, respectively.
TABLE 3.
Network meta‐analysis for TG (MD [95% CI])
| Zhibituo | ||||
| 0.07 (−0.12, 0.26) | Zhibitai | |||
| −0.08 (−0.50, 0.34) | −0.15 (−0.61, 0.31) | Xuezhikang | ||
| 0.01 (−0.15, 0.18) | −0.06 (−0.29, 0.18) | 0.09 (−0.36, 0.55) | Simvastatin | |
| −0.79 (−1.12, −0.46) | −0.86 (−1.22, −0.50) | −0.71 (−1.25, −0.17) | −0.80 (−1.10, −0.51) | Placebo |
Bold values indicate statistically significant values p < 0.05.
FIGURE 5.
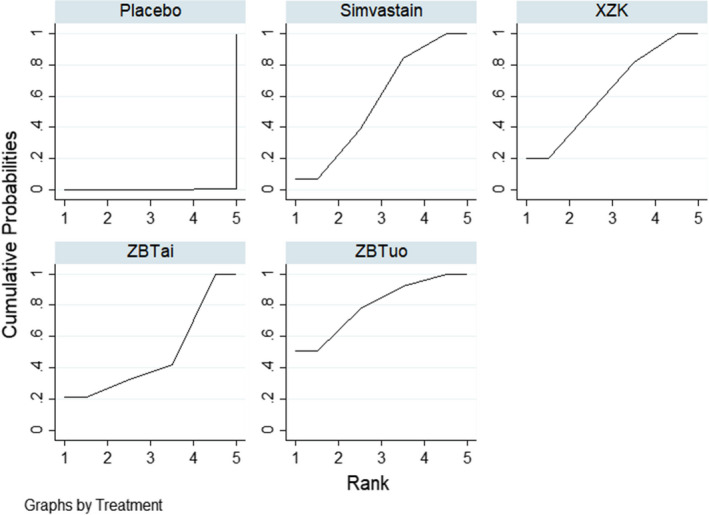
SUCRA curves for TG
3.4.4. LDL‐C—Low‐density lipoprotein cholesterol levels
In a total of 4724 patients in 46 RCTs, 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 three types of Chinese patent medicines were analysed for changes in LDL‐C levels compared to Simvastatin or placebo. Chi‐squared tests showed no heterogeneity between studies (p = 1.0, I 2 = 0.0%). There was a statistically significant difference in all treatments compared with the placebo group. No statistically significant differences were observed between the other comparisons. The results of the network meta‐analysis are summarized in Table 4. The ranking probabilities based on SUCRA values (Figure 6) were Xuezhikang (82.6%) > Simvastatin (74.9%) > Zhibituo (52.8%) > Zhibitai (39.5%) > placebo (0.2%), indicating that Xuezhikang was the most effective intervention for LDL‐C, followed by Simvastatin and Zhibituo, respectively.
TABLE 4.
Network meta‐analysis for LDL‐C (MD [95% CI])
| Zhibituo | ||||
| −0.16 (−0.68, 0.36) | Zhibitai | |||
| 0.12 (−0.14, 0.38) | 0.28 (−0.23, 0.79) | Xuezhikang | ||
| 0.09 (−0.12, 0.30) | 0.25 (−0.23, 0.72) | −0.03 (−0.21, 0.15) | Simvastatin | |
| −0.94 (−1.35, −0.52) | −0.78 (−1.38, −0.18) | −1.06 (−1.38, −0.73) | −1.02 (−1.39, −0.65) | Placebo |
Bold values indicate statistically significant values p < 0.05.
FIGURE 6.
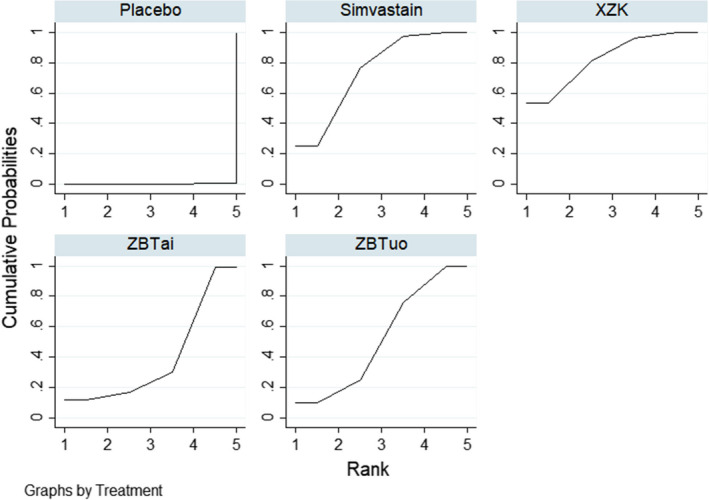
SUCRA curves for LDL‐C
3.4.5. HDL‐C—High‐density lipoprotein cholesterol levels
A total of 4473 patients in 43 RCTs 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 32 , 34 , 35 , 36 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 were used to analyse three Chinese patent medicines for changes in HDL‐C levels compared to Simvastatin or placebo. Heterogeneity tests showed no heterogeneity between studies (p = .24, I 2 = 0.0%). One study 30 was excluded from the analysis because it identified an outlier value. The results suggested that in five comparisons (Zhibituo vs. Zhibitai; Zhibituo vs. placebo; Simvastatin vs. Zhibitai; Xuezhikang vs. placebo; Simvastatin vs. p‐placebo), the differences were statistically significant (MD = 0.16, 95% CI [0.02, 0.30]; MD = 0.16, 95% CI [0.07, 0.25]; MD = −0.15, 95% CI [−0.28, −0.02]; MD = 0.11, 95% CI [0.03, 0.18]; MD = 0.1, 95% CI [0.06, 0.23], respectively). No statistically significant differences were observed between the other comparisons. The results of the network meta‐analysis are summarized in Table 5. The ranking probabilities based on SUCRA values (Figure 7) were as follows: Zhibituo (90.1%) > Simvastatin (82.1%) > Xuezhikang (51.1%)> Zhibitai (14.0%) > placebo (12.7%), indicating that Zhibituo was the most effective intervention for HDL‐C, followed by Simvastatin and Xuezhikang.
TABLE 5.
Network Meta‐analysis of HDL‐C (MD [95% CI])
| Zhibituo | ||||
| 0.16 (0.02, 0.30) | Zhibitai | |||
| 0.05 (−0.01, 0.11) | −0.11 (−0.24, 0.03) | Xuezhikang | ||
| 0.01 (−0.04, 0.06) | −0.15 (−0.28, −0.02) | −0.04 (−0.09, 0.00) | Simvastatin | |
| 0.16 (0.07, 0.25) | −0.00 (−0.15, 0.15) | 0.11 (0.03, 0.18) | 0.15 (0.06, 0.23) | Placebo |
Bold values indicate statistically significant values p < 0.05.
FIGURE 7.
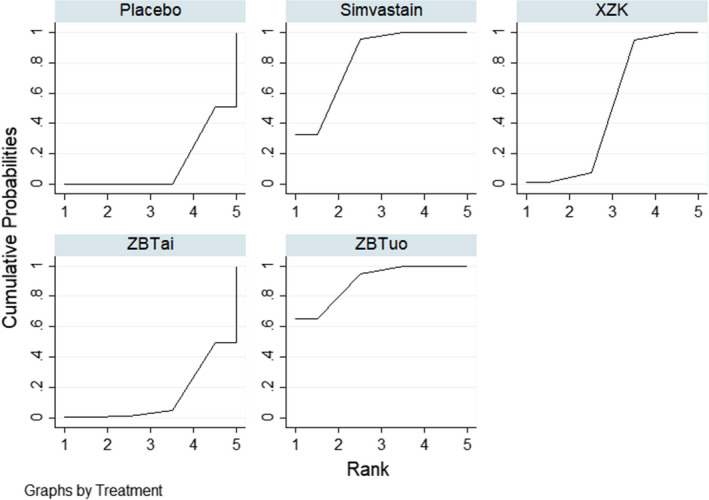
SUCRA curves for HDL‐C
3.5. Adverse drug reactions
No serious adverse events were reported in the 47 RCTs included in this study. Of the 40 trials that described adverse reactions during treatment, 13 reported no adverse reactions, while 27 RCTs reported adverse events in detail.
Of the interventions that involved treatment with Simvastatin, 26 RCTs (1546 patients in total) reported adverse events, including gastrointestinal symptoms (80 cases, such as abdominal pain, bloating and nausea), slightly increased aspartate aminotransferase (AST) and alanine aminotransferase (ALT) levels (41 cases), headache (1 case), fatigue (4 cases) and muscle spasms (3 cases).
For treatment with Zhibituo, 15 RCTs (a total of 837 patients) reported adverse events, including gastrointestinal symptoms (35 cases, such as abdominal pain, bloating and nausea).
Thirteen RCTs (a total of 811 patients) reported adverse events after treatment with Xuezhikang, including gastrointestinal symptoms (39 cases, such as abdominal pain, bloating and nausea) and slightly increased levels of AST and ALT (6 cases).
3.6. Publication bias
A comparison‐adjusted funnel plot of all outcomes demonstrated, by its asymmetry, that some publication bias existed, for which that of TC is displayed in Figure 8.
FIGURE 8.
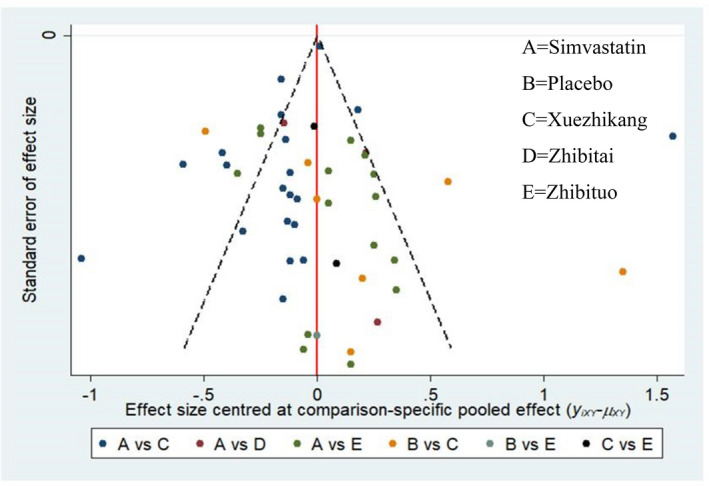
Funnel plot for TC. A Simvastatin; B Placebo; C Xuezhikang; D Zhibitai, E Zhibituo
3.7. Quality of evidence
The GRADE approach was used to assess the quality of evidence. The quality of evidence for the outcomes was low, and the results are presented in Table 6, and the reasons for downgrading included study limitations (risk of bias) and imprecision. Most of the included studies were classified as high risk; there was imprecision because the ranking probabilities based on SUCRA values were very close, and publication bias was observed.
TABLE 6.
Results of GRADE evidence evaluation
| Outcome | No. of participants (studies) | Certainty of evidence | Downgrading due to |
|---|---|---|---|
| TC | 4803 (47) | Low | Study limitations; publication bias |
| TG | 4591 (45) | Low | Study limitations; publication bias |
| LDL‐C | 4724 (46) | Very low | Study limitations; imprecision; publication bias |
| HDL‐C | 4473 (43) | Very low | Study limitations; imprecision; publication bias |
4. DISCUSSION
The incidence of hyperlipidaemia has increased owing to heredity, nutrition, diet, medication and other factors. 64 Hyperlipidaemia is a major risk factor for cardiovascular diseases and atherosclerosis. 65 There is increasing evidence that traditional Chinese medicines that eliminate phlegm and blood stasis can successfully reverse the symptoms of hyperlipidemia. 66 , 67 Previous meta‐analyses have compared the efficacy and safety of RYR for hyperlipidaemia. In 2006, Liu et al. 68 compared the effectiveness of RYR with placebo, no treatment, statins or other active lipid‐lowering agents in the treatment of hyperlipidaemia, whereas the control group in our study received Simvastatin (Zocor); the inclusion criteria in our study were more specific. In 2014, only 13 RCTs were included by Li et al., 69 and in 2015, 20 studies were included by Gerards et al. 70 However, 47 RCTs were included in our study. In 2019, Fogacci et al. 71 performed a meta‐analysis on the safety data surrounding RYR, whereas the purpose of our study was to evaluate the therapeutic effectiveness. In 2020, Sungthong et al. 72 performed a meta‐analysis to analyse the efficacy of RYR on cardiovascular outcomes in patients with myocardial infarction, while the participants of our study were diagnosed with hyperlipidaemia. RYR showed overall tolerability and safety for hyperlipidaemia, based on a previous meta‐analysis. 71 The results of our meta‐analysis provide evidence that Chinese patent medicines containing RYR are highly efficient for the treatment of hyperlipidaemia.
The present network meta‐analysis is the first study to assess and rank the effectiveness of Chinese patent medicines that eliminate phlegm and remove blood stasis in treating hyperlipidaemia. By adopting rigorous inclusion criteria, 47 RCTs with 4824 participants were included in the analyses. The results indicated that different Chinese patent medicines have different benefits for the treatment of hyperlipidaemia. In terms of reducing the levels of TC, Xuezhikang (SUCRA: 84.5%) displayed the highest probability of being the most effective option, followed by Simvastatin (SUCRA: 66.4%) and Zhibitai (SUCRA: 65.4%). For reducing TG levels, Zhibituo (SUCRA: 80.2%) exhibited the highest probability of being the most effective, with Xuezhikang (SUCRA: 63.4%) and Simvastatin (SUCRA: 48.7%) in second and third places, respectively. In terms of reducing LDL‐C levels, Xuezhikang (SUCRA: 82.6%) had the highest probability of being the most effective, followed by Simvastatin (SUCRA: 74.9%) and Zhibituo (SUCRA: 52.8%). Finally, in terms of improving HDL‐C levels, Zhibituo (SUCRA: 90.1%) had the highest probability of being the most effective, with Simvastatin (SUCRA: 82.1%) and Xuezhikang (SUCRA: 51.1%) ranked second and third, respectively. Furthermore, no additional severe toxicity was identified in any experimental group compared to the control group. However, there was no significant difference between Zhibituo and Xuezhikang.
The RYR has been widely used in China for many years. 73 Previous studies have shown that it can reverse the symptoms of hyperlipidaemia, the mechanism of action of which is similar to that of statins. 74 Statins are the key lipid‐lowering medications and are the current recommended initial therapy for blood lipid disorders. 75 , 76 The mechanism is that its efficacy component, monacolin K, acts like the synthetic drug Lovastatin but without the severe side effects of statins. 77 In addition, experimental studies have indicated that the main chemical component of RYR, responsible for its lipid‐reducing properties, is ergosterol. 78 Clinical studies have suggested that other compounds in RYR may also decrease serum lipid levels. 79 Our network meta‐analysis yielded results similar to those of previous studies.
Both Xuezhikang and Zhibituo are made from RYR, which can alleviate drug properties, enhance or change drug effects, reduce toxicity and expand the range of clinical applications of fermented medicines. 80 However, there are different active ingredients in Xuezhikang and Zhibituo. 81 Xuezhikang is made by high‐tech biotechnology, containing Lovastatin, a statin homolog, a variety of essential amino acids, unsaturated fatty acids, sterols and small amounts of flavonoids. 82 , 83 The main bioactive components of Zhibituo are Lovastatin and Lovastatin acid. 84 The content of Lovastatin in Zhibituo and Xuezhikang was shown to be 2.7 and 11.1 g/kg, respectively. 81 Xuezhikang provides hypotriglyceridemic performance superior to Simvastatin in terms of reduction in levels, and the underlying mechanism has been attributed to more significant apoA5 upregulation via the PPARα signalling pathway. 85 Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a crucial regulator of plasma cholesterol homeostasis. 86 A previous study demonstrated that Xuezhikang increases PCSK9 levels through the SREBP‐2 pathway, 87 and isoflavones and phytosterols in Xuezhikang play a role in lowering cholesterol levels through a mechanism different from that of Lovastatin, which elevates the excretion of lipids and bile acids in faeces. 88 In summary, Xuezhikang and Zhibituo contain natural statins, which are safer than synthetic statins and can lower blood lipids. 89
However, the present study has several limitations: (1) all studies included in the review were from China, which might be a potential source of publication bias. (2) Publication bias was also observed. (3) The quality of the studies included in the review was not considered high, with five studies 21 , 22 , 28 , 37 , 46 reporting the specific methods of random sequence generation, three randomized, double‐blind, placebo‐controlled trials 36 , 52 , 53 and four randomized, placebo‐controlled trials. 50 , 58 , 61 , 63 In addition, the quality of evidence for the outcomes was low. (4) The number of trials that compared some of the medicines was relatively small, causing the comparative results to not be incredibly persuasive, and those cases should be considered with caution. Thus, additional, well‐designed, double‐blinded, multicentre RCTs are required to establish the efficacy of Chinese patent medicines for the treatment of hyperlipidaemia.
5. CONCLUSIONS
To reduce the levels of TC and LDL‐C, Xuezhikang displayed the highest probability of being the most effective option. To reduce TG levels, Zhibituo exhibited the highest probability of being the most effective, and Zhibituo may have the highest probability of ameliorating levels of HDL‐C, whereas there was no significant difference between Zhibituo and Xuezhikang. Owing to the limitations of this study, the validity of our results requires confirmation using large‐sample, high‐quality, multicentre, prospective RCTs.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest regarding this study.
AUTHOR CONTRIBUTIONS
Guiqin Xu: Conceptualization (equal); Data curation (equal); Software (equal); Writing‐original draft (lead); Writing‐review & editing (equal). Mingxin Lin: Conceptualization (equal); Data curation (equal); Project administration (lead); Software (lead); Writing‐original draft (lead); Writing‐review & editing (lead). Xueli Dai: Data curation (equal); Software (equal). Jingqing Hu: Conceptualization (lead); Methodology (lead); Writing‐original draft (equal); Writing‐review & editing (equal).
ACKNOWLEDGEMENTS
This study is funded by the National Key Research and Development Program of China (grant number 2019YFC1708501) and the Fundamental Research Funds for the Central public welfare research institutes (grant number YZ‐202022).
Xu G, Lin M, Dai X, Hu J. Comparing the effectiveness of Chinese patent medicines containing red yeast rice on hyperlipidaemia: A network meta‐analysis of randomized controlled trials. Endocrinol Diab Metab.2022;5:e314. 10.1002/edm2.314
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Chen H, Miao H, Feng YL, Zhao YY, Lin RC. Metabolomics in dyslipidemia. Adv Clin Chem. 2014;66:101‐119. [DOI] [PubMed] [Google Scholar]
- 2. Zhou PP, Yang XL, Yang ZL, Huang W, Kou J, Li F. Akebia saponin D regulates the metabolome and intestinal microbiota in high fat diet‐induced hyperlipidemic rats. Molecules. 2019;24:1‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pan L, Yang Z, Wu Y, et al. The prevalence, awareness, treatment and control of dyslipidemia among adults in China. Atherosclerosis. 2016;248:2‐9. [DOI] [PubMed] [Google Scholar]
- 4. Joint Committee for Guideline Revision . 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15:1‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ding WQ, Dong HB, Mi J. Prevalence of dyslipidemia in Chinese children and adolescents: a meta‐analysis. Chin J Epidemiol. 2015;36:71‐77. [PubMed] [Google Scholar]
- 6. Karr S. Epidemiology and management of hyperlipidemia. Am J Manag Care. 2017;23(Suppl):S139‐S148. [PubMed] [Google Scholar]
- 7. Andreadou I, Schulz R, Badimon L, et al. Hyperlipidaemia and cardioprotection: animal models for translational studies. Br J Pharmacol. 2020;177:5287‐5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen K, Ma Z, Yan X, et al. Investigation of the lipid‐lowering mechanisms and active ingredients of danhe granule on hyperlipidemia based on systems pharmacology. Front Pharmacol. 2020;11:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alonso R, Cuevas A, Cafferata A. Diagnosis and management of statin intolerance. J Atheroscler Thromb. 2019;26:207‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mollazadeh H, Tavana E, Fanni G, et al. Effects of statins on mitochondrial pathways. J Cachexia Sarcopenia Muscle. 2021;12:237‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ahmed ST, Akeroyd JM, Mahtta D, et al. Shared decisions: a qualitative study on clinician and patient perspectives on statin therapy and statin‐associated side effects. J Am Heart Assoc. 2020;9:e017915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cicero AFG, Fogacci F, Zambon A. Red yeast rice for hypercholesterolemia: JACC focus seminar. J Am Coll Cardiol. 2021;77:620‐628. [DOI] [PubMed] [Google Scholar]
- 13. Ong YC, Aziz Z. Systematic review of red yeast rice compared with simvastatin in dyslipidaemia. J Clin Pharm Ther. 2016;41:170‐179. [DOI] [PubMed] [Google Scholar]
- 14. Meng TT, Xie XL, Li TT, et al. Oral Chinese Traditional and Herbal Drugs Chinese Patent medicine combined with statins in treatment of dyslipidemia: a network Meta‐analysis. Chin Tradit Herb Drugs. 2021;53:1092‐1104. [Google Scholar]
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PLoS One. 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. He Y. Effect comparison of simvastatin and Zhibitai capsule in the treatment of simple hyperlipidemia. You Jiang Med J. 2013;41:854‐856. [Google Scholar]
- 18. Wu GZ, Xiaomei M, Jie L. Therapeutic effect of simvastatin on hyperlipidemia. Med J West China. 2010;22:1292‐1293. [Google Scholar]
- 19. Hu XZ. The efficacy of simvastatin in patients with hypercholesterolemia: Jian Kang Bi Du. 2011;2:20‐21. https://d.wanfangdata.com.cn/periodical/ChlQZXJpb2RpY2FsQ0hJTmV3UzIwMjEwNzIzEg9qa2JkLXoyMDExMDIwMTcaCGRrOXZ3dGc2 [Google Scholar]
- 20. Zhao S. Clinical study of simvastatin in treatment of hyperlipidemia. Guide Chin Med. 2008;6:86. [Google Scholar]
- 21. Li ZH, Qin SL, Le J, et al. Effect of Xuezhikang capsule on serum high sensitive C reactive protein in patients with hyperlipidemia. Pract Clin Med. 2017;18:28‐29. [Google Scholar]
- 22. Xue SL, Liu FJ. Effect of Xuezhikang capsule on blood lipid, carotid intima‐media thickness and Hs‐CRP. Chin Commun Doctors. 2010;12:198. [Google Scholar]
- 23. Zhang G, Zhang KX, Xu Z, Guang XF. Comparison of lipid—lowering effects of Xuezhikang and Shujiangzhi on hyperlipidemia. Capital Med. 1998;5:35‐36. [Google Scholar]
- 24. Xi BL, Ren JY. Effects of Xuezhikang vs simvastatin in treating hyperlipidemia. Foreign Med Sci. 2002;7:233‐234. [Google Scholar]
- 25. Chen QY. Clinical observation of Xuezhikang and simvastatin on hyperlipidemia. China Healthc Innov. 2007;2:72. [Google Scholar]
- 26. Chen LL, Liu J. The effects of Xuezhikang on hypercholesterolemia. Herald Med. 2002;21:31‐32. [Google Scholar]
- 27. Zhang XF. Comparison of the efficacy of Xuezhikang and simvastatin in the treatment of hypercholesterolemia. World Health Dig. 2010;7:96‐97. [Google Scholar]
- 28. Liu SP. Clinical observation of Xuezhikang on 60 cases with HLP. Tianjin J Trad Chin Med. 2013;30:203‐204. [Google Scholar]
- 29. Zhu QF, Jiang L, Wang Y. Effect of Xuezhikang vs simvastatin on apolipoprotien B and A1 of hyperlipidemia. Guang Ming J Trad Chin Med. 2003;18:24‐25. [Google Scholar]
- 30. Zheng W. Clinical efficacy comparison of Xuezhikang and simvastatin in the treatment of senile hyperlipidemia. Pract J Card Cereb Pneumal Vasc Dis. 2013;21:129‐130. [Google Scholar]
- 31. Li KL. Comparison study on the effect of Xuezhikang and simvastatin for treatment primary hypercholesterolemia. Med Ind Inf. 2006;17:22‐23. [Google Scholar]
- 32. Qi MY, Zhang J, Xiao JG. Clinical observation on 112 cases of hypercholesterolemia treated with Xuezhikang. Pract Clin Med. 2004;6:20‐22. [Google Scholar]
- 33. Hua C, Wang YK. Therapeutic effect of Xuezhikang on hypercholesterolemia. Chin Commun Doctors. 2011;13:23. [Google Scholar]
- 34. Wang SH, Sun JL, Liu HQ. Clinical observation of Xuezhikang in the treatment of senile hyperlipidemia in 60 patients. Clin J Anhui Trad Chin Med. 2003;6:474‐475. [Google Scholar]
- 35. Chen FJ, Yuan Q, Qi HW, et al. Clinical observation of Xuezhikang in treating middle and old age hyperlipidaemias. Shanghai J Prev Med. 2003;15:222‐223. [Google Scholar]
- 36. Zhou H, Yang HY, Zhang JH. Comparison of Zhibitai and simvastatin in hypolipemic effect and atheromatous plaque resolution of carotid artery in patients with hyperlipemia. China J Mod Med. 2012;22:64‐68. [Google Scholar]
- 37. Pang J. Clinical Observation on Zhibitai capsule in treating dyslipidemia with phlegm and blood stasis syndrome. Mingyi. 2018;4:47. [Google Scholar]
- 38. Yang WJ, Fu XJ. Comparison of therapeutic effects of Zhibituo and simvastatin on hyperlipidemia. Chin Mod Med Sci Technol. 2003;3:2‐4. [Google Scholar]
- 39. Liu JX, Rui L. Comparison of effects between Zhibituo and simvastatin in treating hyperlipemia. Chin J Cardiovasc Rehabil Med. 2005;14:353‐355. [Google Scholar]
- 40. Guo XM, Tu L, Mi SZ. Comparison of therapeutic effects of Zhibituo and simvastatin on regulating blood lipid metabolism disorder. Pharmacol Clin Chin Mater Med. 1999;15:46‐48. [Google Scholar]
- 41. Zhang GR. Comparison of therapeutic effects of Zhibituo and simvastatin on hyperlipidemia. Guangxi Med J. 2002;24:713‐714. [Google Scholar]
- 42. Peng KL, He JL. Comparison of the clinical effect of simvastatin and lipoprotection on hyperlipidemia. Clin Mediterr. 2011;31:73‐74. [Google Scholar]
- 43. Xu J. The efficacy of simvastatin and Zhibituo in lipid regulation. Chin J Mod Drug Appl. 2009;3:134‐135. [Google Scholar]
- 44. Zhang QL. The comparative interventional therapies of dyslipidemia by simvastatin and Zhibituo with 60 cases. Int Med Health Guid News. 2004;10:29‐30. [Google Scholar]
- 45. Feng ZH, Zhang CX, Hu FM, et al. The comparative interventional therapies of dyslipidemia by simvastatin and Zibituo with 100 cases. Med J Chin Peoples Health. 2006;18:3‐4. [Google Scholar]
- 46. Guo SH. Comparison of the efficacy and safety of Xuezhikang and simvastatin in the treatment of dyslipidemia. Contemp Med Forum. 2019;17:128‐130. [Google Scholar]
- 47. Li XL. The clinic observation on effect of capsule Zhibituo. Public Med Forum Mag. 2011;15:901‐902. [Google Scholar]
- 48. Zhao PF. Effect observation on 60 cases of Zhibituo tablets in the treatment of hyperlipidemia. Zhejiang J Trad Chin Med. 2011;46:310‐311. [Google Scholar]
- 49. Zhang Q, Xue SD, Ma XX. Effects of Xuezhikang and simvastatin on blood lipid, CRP and plasma homocysteine. Med Inf. 2015;28:40. [Google Scholar]
- 50. Duan CM. Randomized parallel controlled study on Xuezhikang capsule in the treatment of dyslipidemia. J Pract Trad Chin Intern Med. 2014;28:26‐28. [Google Scholar]
- 51. Yang WX. Clinical analysis of xuezhikang in the treatment of hypercholesterolemia. Contemp Book Company Med. 2013;19:143‐144. [Google Scholar]
- 52. Lu XB Xuezhikang capsule in treating hyperlipidemia re‐evaluation of the Clinical observation. Hubei University of Chinese Medicine; 2012. http://cdmd.cnki.com.cn/Article/CDMD‐10507‐1012488142.htm. Accessed August 10 2021.
- 53. Xu NF Xuezhikang capsule for the treatment of hyperlipidemia (tan zhuo zu zhi) randomized, double‐blind controlled clinical research. Chengdu University of Traditional Chinese Medicine; 2011. http://cdmd.cnki.com.cn/Article/CDMD‐10633‐1011156079.htm. Accessed August 10 2021.
- 54. Chen L. Effect of simvastatin on hyperlipoidemia. J Hainan Med Coll. 2010;16:1170‐1171. [Google Scholar]
- 55. Wang M, Yang J, Liu C, Xiao YD. Clinical application of Zhibituo in the treatment of hyperlipidemia. Aerosp Med Hum Perform. 2005;16:26. [Google Scholar]
- 56. Chen L, Qin YW, Zheng X, Guo RB. Lipid regulating effect of Di’ao Zhibituo capsule. Chin J Integr Trad West Med. 2003;23:389. [Google Scholar]
- 57. Chen ZM. Comparison of simvastatin and Zhibituo in the treatment of hyperlipidemia. J Guangxi Med Univ. 2001;18:543. [Google Scholar]
- 58. Chen SM, Yu ZM, Luo HD, Qiu YH, Chen MX. Effect of Xuezhikang on endothelial function in patients with hyperlipoidemia. Chin J Arterioscler. 2001;9:235‐237. [Google Scholar]
- 59. Lu YS, Gu JS, Zhou WG, Xu WY, Jin LF. Comparison of Xuezhikang and Zhibituo in the treatment of middle‐aged and elderly hyperlipidemia. Suzhou Univ J Med Sci. 1997;17:1126‐1127. [Google Scholar]
- 60. Zhao DY Clinical study on Xuezhikang Capsule in the treatment of dyslipidemia of different TCM Syndrome Types. Beijing University of Chinese Medicine; 2012. http://cdmd.cnki.com.cn/Article/CDMD‐10026‐1012363243.htm. Accessed August 10 2021.
- 61. Yu JB. Clinical observation of Xuezhikang in the treatment of hyperlipidemia. J Sichuan Trad Chin Med. 2014;32:170‐171. [Google Scholar]
- 62. Qi RY, Xiao HP. Therapeutic effect of Xuezhikang on the old with dyslipidemia. J Navy Med. 2007;28:23‐24. [Google Scholar]
- 63. Peng DY. Observation on the effect of Zhibituo in treating hyperlipidemia. Chin Med J Metall Ind. 1998;15:201‐203. [Google Scholar]
- 64. He NN, Ye HH. Exercise and hyperlipidemia. Adv Exp Med Biol. 2020;1228:79‐90. [DOI] [PubMed] [Google Scholar]
- 65. Li QI, Gu W, Ma X, et al. Amino acid and biogenic amine profile deviations in an oral glucose tolerance test: a comparison between healthy and hyperlipidaemia individuals based on targeted metabolomics. Nutrients. 2016;8:379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Qi XJ, Zhou RJ, Liu HP, et al. Analysis on regularity of traditional Chinese patent medicine in treating hyperlipidemia. Zhongguo Zhong Yao Za Zhi. 2019;44:4277‐4284. [DOI] [PubMed] [Google Scholar]
- 67. Dai L, Lu AP, Zhong LLD, Zheng G, Bian Z. Chinese herbal medicine for hyperlipidaemia: a review based on data mining from 1990 to 2016. Curr Vasc Pharmacol. 2017;15:520‐531. [DOI] [PubMed] [Google Scholar]
- 68. Liu JP, Zhang J, Shi Y, Grimsgaard S, Alraek T, Fønnebø V. Chinese red yeast rice (Monascus purpureus) for primary hyperlipidemia: a meta‐analysis of randomized controlled trials. Chin Med. 2006;1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li Y, Jiang L, Jia Z, et al. A meta‐analysis of red yeast rice: an effective and relatively safe alternative approach for dyslipidemia. PLoS One. 2014;9:e98611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gerards MC, Terlou RJ, Yu H, Koks CH, Gerdes VE. Traditional Chinese lipid‐lowering agent red yeast rice results in significant LDL reduction but safety is uncertain—a systematic review and meta‐analysis. Atherosclerosis. 2015;240:415‐423. [DOI] [PubMed] [Google Scholar]
- 71. Fogacci F, Banach M, Mikhailidis DP, et al. Safety of red yeast rice supplementation: a systematic review and meta‐analysis of randomized controlled trials. Pharmacol Res. 2019;143:1‐16. [DOI] [PubMed] [Google Scholar]
- 72. Sungthong B, Yoothaekool C, Promphamorn S, Phimarn W. Efficacy of red yeast rice extract on myocardial infarction patients with borderline hypercholesterolemia: a meta‐analysis of randomized controlled trials. Sci Rep. 2020;10(1):2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fukami H, Higa Y, Hisano T, Asano K, Hirata T, Nishibe S. A review of red yeast rice, a traditional fermented food in japan and east Asia: its characteristic ingredients and application in the maintenance and improvement of health in lipid metabolism and the circulatory system. Molecules. 2021;26:1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cicero AFG, Fogacci F, Banach M. Red yeast rice for hypercholesterolemia. Methodist Debakey Cardiovasc J. 2019;15:192‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Arnold SV, Gosch K, Wong ND, et al. Use of non‐LDL‐C lipid‐lowering medications in patients with type 2 diabetes. Endocrinol Diabetes Metab. 2020;3:e00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Su X, Cheng Y, Chang D. Lipid‐lowering therapy: guidelines to precision medicine. Clin Chim Acta. 2021;514:66‐73. [DOI] [PubMed] [Google Scholar]
- 77. Patel S. Functional food red yeast rice (RYR) for metabolic syndrome amelioration: a review on pros and cons. World J Microbiol Biotechnol. 2016;32:87. [DOI] [PubMed] [Google Scholar]
- 78. Liang J‐X, Zhang Q‐Q, Huang Y‐F, et al. Comprehensive chemical profiling of monascus‐fermented rice product and screening of lipid‐lowering compounds other than monacolins. J Ethnopharmacol. 2019;238:111879. [DOI] [PubMed] [Google Scholar]
- 79. Wang TJ, Lien ASY, Chen JL, Lin CH, Yang YS, Yang SH. A randomized clinical efficacy trial of red yeast rice (Monascus pilosus) against hyperlipidemia. Am J Chin Med. 2019;47:323‐335. [DOI] [PubMed] [Google Scholar]
- 80. Zhang H, Gao SM, Wang YF, Yang J, Chai X. Research progress on material and functional changes and fermentation mechanism of “fermented preparation” of traditional Chinese medicine. Chin Trad Herb Drugs. 2021;52:2473‐2479. [Google Scholar]
- 81. Klingelhöfer I, Morlock GE. Lovastatin in lactone and hydroxy acid forms and citrinin in red yeast rice powders analyzed by HPTLC‐UV/FLD. Anal Bioanal Chem. 2019;411:6655‐6665. [DOI] [PubMed] [Google Scholar]
- 82. Heber D, Yip I, Ashley JM, Elashoff DA, Elashoff RM, Go VL. Cholesterol‐lowering effects of a proprietary Chinese red‐yeast‐rice dietary supplement. Am J Clin Nutr. 1999;69:231‐236. [DOI] [PubMed] [Google Scholar]
- 83. Liang L, Shao W, Shu T, et al. Xuezhikang improves the outcomes of cardiopulmonary resuscitation in rats by suppressing the inflammation response through TLR4/NF‐κB pathway. Biomed Pharmacother. 2019;114:108817. [DOI] [PubMed] [Google Scholar]
- 84. Hao SY, Wang L, Li H, Zhang D, Xu H. Simultaneous determination of lovastatin and lovastatin acid in fermentum Rubrum and Zhibituo tablets by QAMS. Chin J Exp Trad Med Formulae. 2017;23:74‐78. [Google Scholar]
- 85. Zhao SP, Li R, Dai W, Yu BL, Chen LZ, Huang XS. Xuezhikang contributes to greater triglyceride reduction than simvastatin in hypertriglyceridemia rats by up‐regulating apolipoprotein A5 via the PPARalpha signaling pathway. PLoS One. 2017;12:e0184949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Poirier S, Mayer G, Benjannet S, et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363‐2372. [DOI] [PubMed] [Google Scholar]
- 87. Jia YJ, Zhang Y, Liu J, Guo YL, Xu RX, Li JJ. Short‐ and long‐term effects of xuezhikang, an extract of cholestin, on serum proprotein convertase subtilisin/kexin type 9 levels. Chin J Integr Med. 2016;22:96‐100. [DOI] [PubMed] [Google Scholar]
- 88. Feng D, Sun J‐G, Sun R‐B, et al. Isoflavones and phytosterols contained in Xuezhikang capsules modulate cholesterol homeostasis in high‐fat diet mice. Acta Pharmacol Sin. 2015;36:1462‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Yin MM, Ye H, Zhang XZ. Research progress of Monascus purpureus single drug and compound preparation in the treatment of Hyperlipi‐demia. Med Recapitulate. 2017;23:344‐347. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


