Abstract
Context
No meta-analyses appeared to have been conducted to examine overall correlations between resonance Raman spectroscopy (RRS)–assessed skin carotenoids and plasma/serum carotenoids.
Objective
To review the available literature and quantify the association between RRS-assessed skin carotenoids and plasma/serum carotenoids via a meta-analysis of observational studies.
Data Sources
To identify relevant publications, we searched the PubMed, Embase, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ProQuest, and Scopus databases in April 2020 for items combining 3 concepts: Raman spectroscopy, skin, and plasma or serum.
Data Extraction
Criteria for inclusion were publication in a peer-reviewed journal between 1990 and 2020, available in English language, and results reported as a baseline Pearson correlation coefficient. In teams of 2, the researchers independently reviewed titles and abstracts of 2212 nonduplicate papers with initial screening yielding 62 papers for full-text review, of which 15 were deemed eligible for inclusion.
Data Analysis
A random-effects model in R (version 4.0.0) “meta” package was used to analyze the correlation between RRS-assessed skin and plasma/serum carotenoids. A subgroup analysis was conducted for studies involving adults and children, respectively.
Conclusions
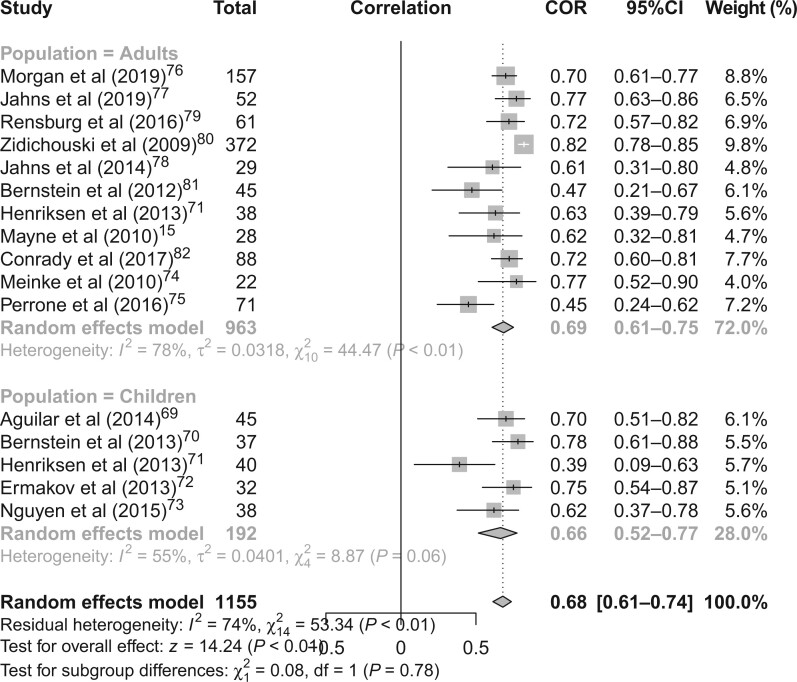
The 15 studies included 1155 individuals: 963 adults and 192 children. One study included children and adults. The random-effects model yielded an overall correlation of 0.68 (95%CI, 0.61–0.74; I2 = 74%; P < 0.01). The results were similar when grouped by adults and children. Among 963 adults, the correlation in the random-effects model was 0.69 (95%CI, 0.61–0.75; I2 = 78%; P < 0.01). Among 192 children, the correlation in the random-effects model was 0.66 (95%CI, 0.52– 0.77; I2 = 55%; P = 0.06). Overall, there was a positive, statistically significant correlation between RRS-assessed skin carotenoids and plasma/serum carotenoids in a pooled meta-analysis of 15 studies.
Systematic Review Registration
PROSPERO (record number 178835)
Keywords: fruit and vegetable intake, plasma carotenoids, resonance Raman spectroscopy, serum carotenoids, skin carotenoids, validation
INTRODUCTION
Adequate fruit and vegetable consumption is associated with reduced risk of cardiovascular disease,1,2 type 2 diabetes mellitus,3 various cancers,2,4 and obesity.5 The Dietary Guidelines for Americans recommends inclusion of at least 2.5 cups of vegetables and 2 cups of fruits per day.6 However, 9 in 10 Americans do not consume these recommended amounts.7,8 Inadequate intake of fruits and vegetables may be partially responsible for the increase in the prevalence of obesity in the United States from 30.5% to 42.4% during the years 1999 to 2018.9
To more effectively promote population-level fruit and vegetable intake, improved measures of fruit and vegetable intake are needed.10 Although self-reported measures of fruit and vegetable intake have predominated in studies, these measures are fraught with error, including recall bias and intervention-related bias.10,11 Carotenoids are pigmented phytonutrients that occur naturally in many fruits and vegetables.12 The most common dietary carotenoids found in human blood are α- and β-carotene, β-cryptoxanthin, lycopene, lutein, and zeaxanthin.13 Carotenoids contribute to vital functions such as inhibiting tumor cell growth, serving as antioxidants, and protecting against eye disease.14 Because of their predominance in fruits and vegetables, the current gold standard of measuring fruit and vegetable intake is measuring carotenoid levels in a blood sample.13 However, collecting blood samples in field-based nutrition studies is challenging because of the need for a sterile environment, a trained phlebotomist, and blood processing in standard laboratory-based conditions. Because of these drawbacks, noninvasive yet valid methods are needed to assess fruit and vegetable intake. Skin carotenoid status, as assessed using resonance Raman spectroscopy (RRS), has emerged as a valid and reliable method to assess fruit and vegetable intake,15,16 which overcomes many of the challenges related to obtaining plasma carotenoid measurements. RRS uses a small laser at a blue-light wavelength to assess carotenoid levels in the skin.16,17 Researchers use RRS-assessed skin carotenoids as an indirect biomarker of total plasma/serum carotenoid levels, and as an as an objective measure of fruit and vegetable intake (eg, see Shaping Healthy Choices Program18 and Farm Fresh Foods for Healthy Kids19).
Although there have been several studies examining associations between RRS-assessed skin carotenoids and plasma or serum carotenoids,20 to date, to our knowledge, no meta-analyses have been conducted that examined overall correlations between RRS-assessed skin carotenoids and plasma/serum carotenoids. Thus, the aim of this study was to review the available literature and quantify the association between RRS-assessed skin carotenoids and plasma/serum carotenoids via a meta-analysis of observational studies. We hypothesized that RRS-assessed skin carotenoids would be positively associated with plasma/serum carotenoids across a variety of populations.
METHODS
In accordance with established PRISMA (Preferred Reporting of Systematic Reviews and Meta-analyses) guidelines, the prospective protocol for this systematic review was registered in PROSPERO (registration no. 178835). The PICOS (participants, interventions, comparisons, outcomes, and study design) criteria used to define the study question are listed in Table 1.
Table 1.
Description of the PICOS criteria used to define the research question
| Parameter | Description |
|---|---|
| Population |
Included: adults and children Excluded: none |
| Intervention/correlation | Correlation with plasma or serum carotenoid levels |
| Comparison | Not applicable, because observational studies and correlations were reviewed, rather than interventions |
| Outcome | Resonance Raman spectroscopy–assessed skin carotenoids |
| Study design | Cross-sectional studies describing the correlation between skin and plasma or serum carotenoids |
Literature search and review
For this systematic review, our goal was to identify and synthesize findings from published studies that included correlations between RRS-assessed skin carotenoid measurement (a proxy for fruit and vegetable intake) and serum or plasma carotenoid concentrations (the accepted standard measure of fruit and vegetable intake). To identify relevant publications, we searched the PubMed, Embase, CINAHL, Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, ProQuest, and Scopus databases in April 2020 for sources combining 3 concepts: Raman spectroscopy, skin, and plasma or serum. Subject headings, which vary slightly by database, and keywords were used to create concept searches, and searches were limited to title and abstract when possible. Search terms for RRS included spectrum analysis, carotenoid sensor, Raman spectrum measurement, Raman microscopy, and Raman spectroscopy. The terms skin, epidermis, epidermal, or dermal were used as to identify studies assessing skin. The terms blood, plasma, and serum were used to identify studies assessing plasma or serum levels. Searches for each concept were then combined using the Boolean operator AND. The search strategy was adjusted as needed between databases. The MEDLINE/PubMed search query used was: (((((((((“spectrum analysis”[MeSH] or “spectrum analysis, Raman”[MeSH])) or carotenoid sensor[title/abstract]) or Raman spectrum measurement[title/abstract]) or Raman microscopy[title/abstract]) or spectrum analysis[title/abstract]) or Raman spectroscopy[title/abstract] or Raman spectrometry[title/abstract])) and ((((((“skin”[MeSH]) or “epidermis”[MeSH]) or skin[title/abstract]) or epidermis[title/abstract]) or epidermal[title/abstract]) or dermal[title/abstract])) and ((((((“blood”[MeSH]) or “plasma”[MeSH]) or “serum”[MeSH]) or blood[title/abstract]) or plasma[title/abstract]) or serum[title/abstract]).
The structure of this search was guided by our experience with a previous systematic review,20 during which including carotenoids as a search concept limited identification of studies. Criteria for inclusion were as follows: publication in a peer-reviewed journal between 1990 and 2020, available in English, and results reported as a baseline Pearson correlation coefficient between skin carotenoids as assessed by RRS and plasma or serum carotenoids.
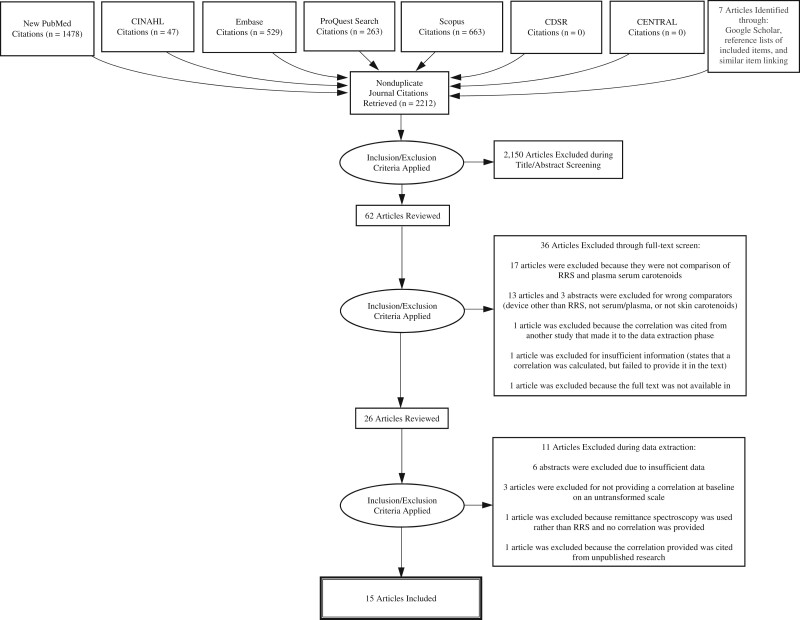
Our combined database searches yielded 2980 items. Identified publications were loaded into Endnote 9.1 (Clarivate Analytics, Philadelphia, PA), where non–peer reviewed publications and duplicates were removed, leaving 2222 items. These remaining items were loaded into Covidence (Melbourne, Victoria, Australia), where 17 more duplicates were removed. Seven additional items were identified through reference lists of included items,21 a Google Scholar search of author names from included items,22 or from a document compiled by Pharmanex (Provo, UT)23 listing papers associated with their Biophotonic Scanner.24–28 A total of 2212 nonduplicate records were identified.
Initial review criteria included that the publication be an original work published in a peer-reviewed journal, reporting a validation study of Raman spectrum analysis including plasma/serum/blood carotenoid concentration as a comparison measure. Reports were excluded if they were not original works published in peer-reviewed journals and not available in English. Our review team consisted of 2 faculty members (S.B.J.P. and G.C.F.) and 3 research assistants (A.P.K., J.O., N.S.J.). At least one faculty member (either S.B.J.P. or G.C.F.) was involved in reviewing each item. After title and abstract screening, 62 reports remained. A total of 36 reports were excluded during full-text screening. Reasons for exclusion during full-text screening include the following: 1729–45 did not meet inclusion criteria (eg, a review article, skin or plasma/serum carotenoids not assessed), 13 articles46–58 and 3 abstracts from conference proceedings59–61 used the wrong comparators (ie, devices other than RRS were used to assess skin carotenoids as compared with plasma/serum, or RRS-assessed skin carotenoids were compared with something other than plasma/serum), 121 provided a correlation that was cited from another included study,24 ,162 had insufficient information (the authors stated a correlation was calculated but did not provide it in the text), and 1 was not available in English.25
Of the 26 remaining reports, 11 were excluded during data extraction. Reports excluded and reasons for exclusion during data extraction include the following: 6 abstracts24,26–28,63,64 were from conference proceedings, which had insufficient data for the meta-analysis, 322,65,66 did not provide a correlation at baseline on an untransformed scale, 167 used remittance spectroscopy rather than RRS, and 168 provided a correlation that cited unpublished research. In total, 15 reports15,69–82 were deemed as meeting criteria for inclusion in this review and meta-analysis (See Figure 1, PRISMA diagram).15,21–82
Figure 1.
PRISMA diagram for the meta-analysis of studies examining associations between resonance Raman spectroscopy-assessed skin carotenoids and plasma carotenoids among 963 adults and 192 children. Abbreviations: CDSR, Cochrane Database of Systematic Reviews; RRS, resonance Raman spectroscopy
Two data extractors independently extracted the study reference, population characteristics (namely, age, sex, race, and body mass index, as available), sample size, statistical tests used to quantify the association between RRS-assessed skin carotenoids and plasma/serum carotenoids, and correlation or association outcomes. The data extractors came together to reach consensus on the data extracted from each article. The data were entered into an Excel spreadsheet (Microsoft, Redmond, WA) and analyzed.
Quality assessment
To measure the rigor of the studies, the Quality Appraisal Tool in Studies With Diverse Designs (QATSDD) was used as described by the developers.83 The QATSDD includes 16 criteria. However, 4 criteria were considered not applicable for the purposes of this review. Two excluded criteria only apply to qualitative study designs, and 2 other criteria (theoretical basis and evidence of user involvement in design) were excluded because they were deemed not applicable to study designs examining validity of a research tool.
The following 12 criteria were evaluated for each study: statements of aims/objectives in the main body of the report; clear description of research setting; evidence of sample size considered in terms of analysis; representative sample of target group of a reasonable size; description of procedure for data collection; rationale for choice of data collection tool; detailed recruitment data; statistical assessment of reliability and validity of measurement tool(s); fit between stated research question and method of data collection; fit between research question and method of analysis; good justification for analytical method selected; and strengths and limitations critically discussed. For the purposes of this assessment, a “data collection tool” was defined as a method of data capture (eg, REDCap), and a “measurement tool” was defined as a validated scale or measurement device (eg, a food frequency questionnaire or Schorr Height Board). Each criterion was rated using a 4-point scale, ranging from 0 to 3, with 3 representing the highest quality. Two reviewers (A.P.K. and N.S.J.) independently analyzed and assigned a score to each of the included articles. The study team then discussed the independent findings and reconciled any scoring discrepancies. Each researcher justified their rating and shared the final assessment with the faculty researcher. Final quality scores for each article were calculated as a percentage by summing the points, dividing the sum by 36 (the maximum number of points), and multiplying that result by 100.
Statistical analysis
We used aggregate participant data and created a narrative (ie, descriptive) synthesis of all studies included. We used a random-effects model with the Sidik-Jonkman84 estimator for the between-study heterogeneity variance to pool the overall correlation between RRS-assessed skin carotenoids and plasma/serum carotenoids. In the analysis, Fisher z-transformation to the correlation was applied. The result was summarized in forest plots, and the Higgin and Thompson85 heterogeneity index and Cochran Q test results were reported. Although some studies focused on adults, other focused on children. Thus, we conducted subgroup analysis to address the heterogeneity of the study effects due to age. To test the presence of publication bias, funnel plots were visually inspected and Egger tests86 were provided. Statistical analyses were performed using R (4.0.0) software packages “meta” and “dmeta.”
RESULTS
A synthesis of all studies is listed in Table 2.15,69–82 There were 15 studies overall, with 5 including children69–73 and 11 including adults.15,71,74–82 One study71 included both children and adults. Of the 11 studies among adults, 5 reported at least some participants from a racial/ethnic group other than non-Hispanic White,15,71,76,79,82 and 2 reported including at least 10% of the sample from a racial/ethnic groups other than non-Hispanic White.15,71 All the studies (100%) included information of participants’ sex (4 studies included only females).71,75–77 The mean ages ranged from 32 to 77 years. In 6 studies (54.5%), total plasma or serum carotenoid concentrations were measured71,74,77,78,80,81; 2 (18.2%) stated “total carotenoids” were used but did not specifically define total plasma/serum carotenoids76,82; and 3 (27.3%) included a subset of plasma/serum carotenoids, but not the total.15,75,79
Table 2.
Descriptive characteristics of studies included in the meta-analysis of validity of resonance Raman spectroscopy
| Reference | No. of participants | Pearson correlation coefficient | Mean age ± SD (years)a | Race/ethnicity, no. (%) | Biological sex, no. (%) | Mean BMI ± S (kg/m2) | Plasma/serum, carotenoids assessed |
|---|---|---|---|---|---|---|---|
| Studies among adults | |||||||
| Morgan et al 201976 | 157 |
r = 0.698 P < 0.0001 |
58.56 ± 9.49 |
Non-Hispanic white: 150 (95.5) Other: 6 (3.8) Not reported: 1 (0.6) |
Female: 157 (100) | Mean BMI: 35.72 ± 6.48 | Serum: total carotenoids, specific carotenoids were not named. |
| Jahns et al 201977 | 52 |
r = 0.77 P < 0.001 |
49.4 ± 0.8 (SE) | Non-Hispanic white: 50 (96) | Female: 52 (100) |
Mean BMI: 26.5 ± 0.6 (SE) Overweight: 17 (33%) Obese: 12 (23%) |
Plasma: α-, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin. |
| Rensburg et al 201679 | 61 |
r = 0.72 P < 0.001 |
Overall populationa: Males: 40.6 ± 12.2 Females: 42.8 ± 12.0 |
Overall populationa: Non-Hispanic White: 78 (96.3) Indian: 1 (1.2) African: 2 (2.5) |
Overall populationa: Male : 19 (23) Female: 62 (77) |
Mean BMI, males: 25.0 ± 2.2; females: 23.7 ± 2.7 | Serum; lutein/zeaxanthin, β-carotene, lycopene. |
| Zidichouski et al 200980 | 372 |
r = 0.82 P = 0.001 |
33.4 ± 10.0 | Not provided |
Male: 199 (53) Female: 173 (47) |
Not provided. | Serum: α-, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin. |
|
Jahns et al 201478 |
29 |
r = 0.61 P < 0.001 |
32.1 ± 2.5 (SE) | Non-Hispanic White: 28 (96) |
Male: 9 (31) Female: 20 (69) |
Mean BMI: 23.6 ± 0.6 (SE) | Plasma: α-, β-carotene, β-cryptoxanthin, lycopene and lutein/zeaxanthin. |
| Bernstein et al 201281 | 45 |
r = 0.4727 P = 0.0010 |
Overall populationa: 77.4 ± 7.7 (range, 50–85) | Not provided. |
Overall populationa: Male: 24 (45) Female: 29 (55) |
Not provided. | Serum: oxolutein, lutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene. |
| Henriksen et al 201371 | 38b |
r = 0.63 P < 0.001 |
Not provided |
Mothers of normal-weight infants: Non-Hispanic White: 53 Latino: 43 Asian: 3 Mothers of infants with IUGR: Non-Hispanic White: 30 Latino: 60 Asian: 10 |
Female: 38 (100) | Not provided. | Serum: lutein, oxolutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene. |
| Mayne et al 201015 | 28 |
r = 0.62 P = 0.006 |
Overall populationa: 37.0 (no SD reported; median: 33 y) |
Overall populationa: Non-Hispanic White: 62 (83.8) Non-White: 12 (16.2) |
Overall populationa: Male: 28 (38) Female: 46 (62) |
Overall populationa: Underweight: 4 (5.4%) Healthy weight: 45 (60.8%) Overweight: 20 (27.0%) Obese: 5 (6.8%) |
Plasma: lutein, zeaxanthin, β-cryptoxanthin, β-carotene, and lycopene |
| Conrady et al 201782 | 88 |
r = 0.722 P < 0.0001 |
59 ± 17 (range, 13–90) |
Non-Hispanic White: 74 (84) African: 1 (1) Asian: 1 (1) Hispanic: 1 (1) Multinational: 1 (1) Not recorded: 10 (11) |
Male: 39 (44) Female: 49 (56) |
Not provided | Serum: lutein, zeaxanthin, lutein + zeaxanthin, and total carotenoids, specific carotenoids were not named |
| Meinke et al 201074 | 22 | r = 0.77 |
Experimental group: 40.5 (range, 22–59; median, 42 y) Placebo group: 34.7 (range, 25–49; median, 29 y) |
Not provided. |
Experimental group: Male: 6 (54) Female: 5 (46) Placebo group: Male: 8 (73) Female: 3 (27) |
Experimental group: 27.0 (range, 18–35; median, 29.1) Placebo group: 25.0 (range, 19–32; median, 26.1) |
Serum: lutein, zeaxanthin, α-carotene, β-carotene, β-cryptoxanthin, and lycopene |
| Perrone et al 201675 | 71 |
r = 0.450 P< 0.0001 |
Age range, 36–74 | Not provided | Female: 71 (100) | 27.06 ± 2.84 | Serum: lycopene |
| Studies among children | |||||||
| Aguilar et al 201469 | 45 | r = 0.70 | 10.5 (range, 5–17) |
Non-Hispanic White: 34 (76) Hispanic: 7 (16) Asian: 3 (6) Pacific Islander: 1 (2) |
Female: 25 (55) Male: 20 (45) |
Underweight (<5th percentile): 4 (8%) Normal weight (5th to <85th percentile): 34 (76%) Overweight (>85th percentile): 7 (16%) |
Serum: β-carotene, lycopene, and lutein |
| Bernstein et al 201370 | n = 37c |
r = 0.78 P< 0.0001 |
Infants and children < 7 y |
Non-Hispanic White: 30 (81.1) Hispanic: 4 (10.8) Multiracial: 3 (8.1) |
Male: 16 (43) Female: 21 (57) |
Not provided | Serum: total carotenoids, specific carotenoids were not named other than lutein/zeaxanthin |
| Henriksen et al 201371 (Infants: normal weight and IUGR) | N = 40* |
r = 0.39 P = 0.021 |
Normal weight: gestational age: 37–38 wk: 43% 39–40+ wk: 57% IUGR: gestational age: 37–38 wk: 75% 39–40+ wk: 25% |
Normal weight: Non-Hispanic White: 53 Latino: 43 Asian: 3 IUGRc: Non-Hispanic White: 33 Latino: 58 Asian: 8 |
Normal weight: Male: 53 Female: 47 IUGR: Male: 42 Female: 58 |
Birth weight <10th percentile for age: 10 (33.3%) | Serum: lutein, oxolutein, zeaxanthin, β-cryptoxanthin, α-carotene, β-carotene, and lycopene |
| Ermakov et al 201372 | 32 | r = 0.75 | Infants aged 1 d to 6 y | Non-Hispanic White: 32 (100) | Not provided | Not provided | Serum: total carotenoids, specific carotenoids were not named |
| Nguyen et al 201573 | 38 |
r = 0.62 P < 0.001 |
11.2 ± 0.5 (range, 10–12) | Not provided |
Male: 11 (29) Female: 27 (71) |
BMI percentile ( + SD) 73.3 ± 26.3 Normal weight (5th to <85th percentile): 19 (50%) Overweight (85th to <95th percentile): 9 (23.7%) Obese (>95th percentile): 10 (26.3%) |
Plasma: α-carotene, β-carotene, lutein, zeaxanthin, and lycopene |
Numbers given for the overall population but not for the subset of individuals for which both resonance Raman spectroscopy–assessed skin carotenoids and plasma/serum carotenoids were available.
Control (healthy weight infants): 37 wk gestation or older: n = 30; IUGR: birth weight <10th percentile for age: n = 10. In this study, there were 2 sets of twins, so there are 38 mothers and 40 infants.
All demographic data were obtained from supplementary materials found at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3680006/bin/supp_13-11891_IOVS-13-11891-s01.pdf or https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3680006/bin/supp_54_6_4034__index.html.70
Abbreviations: BMI, body mass index; IUGR, intrauterine growth restriction.
Of the 5 studies among children,69–73 3 (60%) reported including at least 10% of the sample from a racial/ethnic groups other than non-Hispanic White,69–71 and 4 (80%) included information on the participants’ sex.69–71,73 The mean ages ranged from gestation of 28 weeks to 12 years. One study (20%) used total serum carotenoid levels,71 2 (40%) stated “total carotenoids” were used but did not specifically define total plasma/serum carotenoids70,72; and 2 (40%) included a subset of plasma/serum carotenoids.69,73
The quality assessment is found in Table 3.15,69–82 The scores ranged from 47.7% to 91.7%. The study by Jahns et al78 received a score of 91.7% (highest); the studies by Ermakov et al72 and Perrone et al75 each received a score of 47.7%, the lowest of all of the studies. The average score for the studies was 68.3%. The 2 criteria that most studies scored highly on were (1) fit between stated research question and method of data collection criteria and (2) the fit between research question and method of analysis.
Table 3.
Summary of methodological quality scores for 15 studies included in the meta-analysis of resonance Raman spectroscopy validity studies
| Score (range: 0–3) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Criterion | Morgan et al, 201976 | Jahns et al, 201977 | Aguilar et al, 201469 | Rensburg et al, 201679 | Zidichouski et al, 200980 | Jahns et al; 201478 | Bernstein et al; 201370 | Bernstein et al; 201281 | Henriksen et al; 201371 | Ermakov et al; 201372 | Nguyen et al; 201573 | Mayne et al; 201015 | Conrady et al; 201782 | Meinke et al; 201074 | Perrone et al; 201675 |
| Statement of aims/objectives in main body of report | 2 | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 3 | 1 | 3 | 3 | 2 | 3 | 2 |
| Clear description of research setting | 3 | 3 | 3 | 3 | 1 | 3 | 1 | 3 | 3 | 1 | 3 | 3 | 1 | 1 | 3 |
| Evidence of sample size considered in terms of analysis | 1 | 1 | 3 | 1 | 1 | 3 | 0 | 1 | 0 | 0 | 1 | 2 | 1 | 1 | 0 |
| Representative sample of target group of a reasonable size | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 |
| Description of procedure for data collection | 3 | 3 | 3 | 2 | 2 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 2 | 2 | 2 |
| Rationale for choice of data collection tool(s) | 2 | 3 | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 0 | 2 | 3 | 0 | 0 | 0 |
| Detailed recruitment data | 1 | 1 | 2 | 2 | 0 | 3 | 0 | 1 | 0 | 0 | 2 | 2 | 2 | 0 | 1 |
| Statistical assessment of reliability and validity of measurement tool(s) | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 |
| Fit between stated research question and method of data collection | 3 | 2 | 3 | 2 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| Fit between research question and method of analysis | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 |
| Good justification for analytic method selected | 3 | 3 | 3 | 2 | 3 | 3 | 1 | 2 | 2 | 2 | 3 | 2 | 1 | 2 | 2 |
| Strengths and limitations critically discussed | 2 | 3 | 2 | 2 | 1 | 3 | 1 | 2 | 2 | 0 | 2 | 2 | 2 | 0 | 0 |
| Score total/maximum score possible; % | 28/36; 77.8 | 30/36; 83.3 | 32/36; 88.9 | 25/36; 69.4 | 22/36; 61.1 | 33/36; 91.7 | 18/36; 50.0 | 23/36; 63.9 | 25/36; 69.4 | 17/36; 47.2 | 30/36; 83.3 | 31/36; 86.1 | 21/36; 58.3 | 18/36; 50.0 | 17/36; 47.2 |
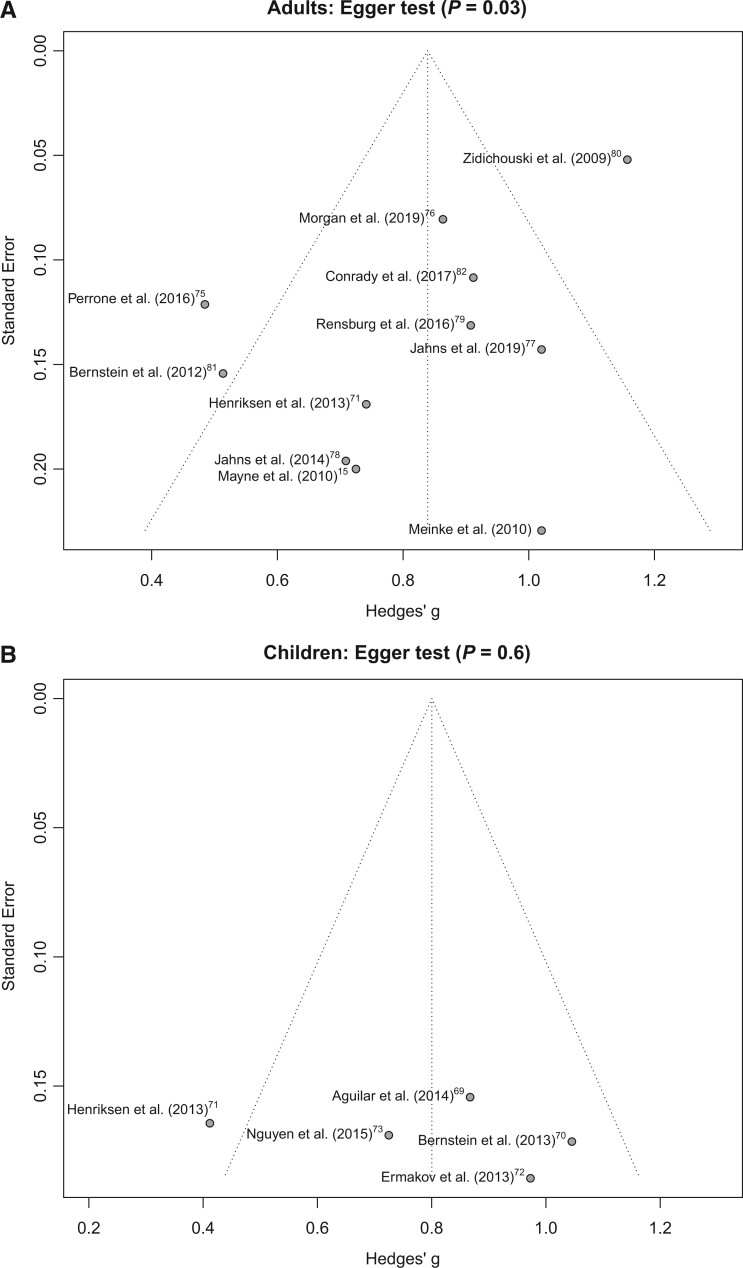
Figure 2 15 , 69–82 shows random-effects models for all studies. The random-effects model yielded an overall Pearson correlation of 0.68 (95%CI, 0.61–0.74; I2 = 74%; P < 0.01). The overall z-test of the pooled correlation was statistically significant (P < 0.01). The results were similar when grouped by adults and children. Among 963 adults, the correlation in the random-effects model was 0.69 (95%CI, 0.61–0.75; I2 = 78%; P < 0.01). Among 192 children, the correlation in the random-effects model was 0.66 (95%CI, 0.52–0.77; I2 = 55%; P = 0.06). The test for subgroup differences using Cochran Q was not statistically significant (P = 0.78). Funnel plots asymmetry and Egger test results showed the potential for publication bias among studies involving adults (P = 0.03) but no evidence of publication bias of studies involving children (P = 0.60) (Figure 3a,b).15,69–82
Figure 2.
Meta-analysis results for studies examining associations between resonance Raman spectroscopy-assessed skin carotenoids and plasma/serum carotenoids among 963 adults and 192 children. Abbreviations: COR, correlation; df, degrees of freedom
Figure 3.
Risk of bias assessment for studies examining the association between resonance Raman spectroscopy-assessed skin carotenoids and plasma/serum carotenoids among (a) adults and (b) children.
DISCUSSION
This meta-analysis of studies examining the association between RRS-assessed skin carotenoids and plasma/serum carotenoids indicated a positive correlation between RRS-assessed skin carotenoids and plasma/serum carotenoids (r = 0.68; P < 0.01), and this was similar when comparing adults and children. RRS and other techniques to assess skin carotenoids are increasingly being used to evaluate public health nutrition interventions18,19; thus, the validity of such methods needs to be clearly established. In addition, the correlation between RRS-assessed skin carotenoids and serum carotenoids among infants in the Henriksen et al71 study was relatively low (r = 0.39) and illuminates the complexity of perinatal maternal-infant physiology. More research is needed to assess the relationships between skin and plasma/serum carotenoids in mothers and infants. The results of our meta-analysis support the use of the RRS method for nutrition monitoring and evaluation of nutrition interventions. However, because only 5 of the included studies were among children,69–73 and 4 studies included > 10% of the population from a non-White racial/ethnic group,15,69–71 future studies should be conducted in populations of varying ages and racial/ethnic backgrounds, given the ultimate goal of evaluating interventions and policies to increase healthy eating among all populations.
Many factors affect carotenoid levels in the body, and each included study did not control for each possible factor. Extrinsic factors such as co-consumed lipids, food processing, molecular structure, medications, smoking, and alcohol have all been found to affect detection of plasma/serum carotenoids.87 Intrinsic factors such as age, body composition, hormones variability, and genetics also play a strong role in carotenoid detection in serum and plasma.87 Because these factors can also likely affect skin carotenoids, such factors should be considered in future validation studies of various skin carotenoid methods as an approximation of fruit and vegetable intake.
One of the strengths of this meta-analysis that we is examined overall correlations between RRS-assessed skin carotenoids and plasma/serum carotenoids; to our knowledge, this is the first meta-analyses to do so. It is noteworthy that all studies showed a positive correlation between RRS-assessed skin carotenoids and plasma/serum carotenoids, yet only 1 study examined the correlation between RRS-assessed skin carotenoids and skin biopsy specimens to confirm the validity of the skin RRS technique.15 As reported earlier, the funnel plots and Egger test findings showed evidence of publication bias of studies in adults but no publication bias of studies among children.
A limitation of this study was that some of the studies solely focused on 1 or 2 carotenoids. Another limitation is that some studies used a longitudinal design, measuring skin and plasma/serum carotenoids at multiple time points and then examining correlations between these multiple time points. We were not able to include those data in this review, given the different statistical methods used in those analyses. An additional limitation is the medium to high heterogeneity in the studies, which was 1 reason we used random-effects models. This heterogeneity indicates the correlation may depend on each specific study population and could indicate publication bias or other forms of bias.88
CONCLUSION
The findings of this meta-analysis of 15 studies suggest RRS-assessed skin carotenoids can be used to approximate intake of carotenoid-rich fruits and vegetables. More research on the validity of measurement of skin carotenoids is warranted, particularly among individuals of different ages, as well as racially and ethnically diverse populations.
Supplementary Material
Acknowledgments
Author contributions. S.B.J.P., G.C.F., and Q.W. conceptualized the study. G.C.F. conducted the systematic review and created the Covidence database. S.B.J.P., N.S.J., A.P.K., and J.O. conducted title, abstract, and full-text reviews; extracted study data, and created tables used for statistical analyses. Q.W. conducted all statistical analyses and created forest and funnel plots. G.C.F. and N.S.J. created the PRISMA diagram. N.S.J. and A.P.K. conducted the quality assessment. All authors contributed to the initial drafts of the paper. All authors reviewed and commented on subsequent drafts of the manuscript and approved the final version as submitted.
Funding . The authors gratefully acknowledge funding from the National Heart, Lung, and Blood Institute (grant R01HL142544).
Declaration of interests. The authors have no relevant interests to declare.
References
- 1. Aune D, Giovannucci E, Boffetta P, et al. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang X, Ouyang Y, Liu J, et al. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. Bmj. 2014;349:G4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Muraki I, Imamura F, Manson JE, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies [published correction appears in BMJ. 2013;347:f6935]. BMJ Br Med J. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Farvid MS, Chen WY, Rosner BA, et al. Fruit and vegetable consumption and breast cancer incidence: repeated measures over 30 years of follow-up. Int J Cancer. 2019;144:1496–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bertoia ML, Mukamal KJ, Cahill LE, et al. Changes in intake of fruits and vegetables and weight change in united states men and women followed for up to 24 years: analysis from three prospective cohort studies [published correction appears in PLoS Med. 2016;13(1):e1001956]. PLoS Med. 2016;13:e1001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. 2015–2020 Dietary Guidelines for Americans. 8th ed. Washington, DC: U.S. Department of Health and Human Services and U.S. Department of Agriculture; 2015. https://health.gov/our-work/food-and-nutrition/2015-2020-dietary-guidelines. Accessed June 8, 2020.
- 7. Lee-Kwan SH, Moore LV, Blanck HM, Harris DM, et al. Disparities in state-specific adult fruit and vegetable consumption — United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66:1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Krebs-Smith SM, Guenther PM, Subar AF, et al. Americans do not meet federal dietary recommendations. J Nutr. 2010;140:1832–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. Hyattsville, MD: National Center for Health Statistics; NCHS Data Brief, no 360; 2020. [PubMed] [Google Scholar]
- 10. Kirkpatrick SI, Collins CE, Keogh RH, et al. Assessing dietary outcomes in intervention studies: pitfalls, strategies, and research needs. Nutrients. 2018;10:1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Thompson FE, Kirkpatrick SI, Subar AF, et al. The National Cancer Institute’s dietary assessment primer: a resource for diet research. J Acad Nutr Diet. 2015;115:1986–1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26. [DOI] [PubMed] [Google Scholar]
- 13.Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. β-carotene and other carotenoids. In: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids. Washington, DC: National Academies Press; 2000:325–382. [PubMed] [Google Scholar]
- 14. Krinsky NI, Johnson EJ. Carotenoid actions and their relation to health and disease. Mol Aspects Med. 2005;26:459–516. [DOI] [PubMed] [Google Scholar]
- 15. Mayne ST, Cartmel B, Scarmo S, et al. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Scarmo S, Henebery K, Peracchio H, et al. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur J Clin Nutr. 2012;66:555–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ermakov IV, Sharifzadeh M, Ermakova M, et al. Resonance Raman detection of carotenoid antioxidants in living human tissue. J Biomed Opt. 2005;10:064028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Beccarelli LM, Scherr RE, Dharmar M, et al. Using skin carotenoids to assess dietary changes in students after 1 academic year of participating in the Shaping Healthy Choices Program. J Nutr Educ Behav. 2017;49:73–78.e1. [DOI] [PubMed] [Google Scholar]
- 19. Seguin RA, Morgan EH, Hanson KL, et al. Farm Fresh Foods for Healthy Kids (F3HK): an innovative community supported agriculture intervention to prevent childhood obesity in low-income families and strengthen local agricultural economies. BMC Public Health. 2017;17:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Radtke MD, Pitts SJ, Jahns L, et al. Criterion-related validity of spectroscopy-based skin carotenoid measurements as a proxy for fruit and vegetable intake: a systematic review [published online ahead of print, 2020 May 14]. Adv Nutr. 2020;11:1282–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gellermann W, Zidichouski JA, Smidt CR, et al. Raman detection of carotenoids in human tissue. In: Packer L, Obermueller-Jevic U, Kraemer K, eds. Carotenoids and Retinoids: Molecular Aspects and Health Issues. Urbana, IL: AOCS Press; 2005:86–114. [Google Scholar]
- 22. Spees CK, Braun AC, Hill EB, et al. Impact of a tailored nutrition and lifestyle intervention for overweight cancer survivors on dietary patterns, physical activity, quality of life, and cardiometabolic profiles. J Oncol. 2019;2019:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raman measurement of carotenoids in living tissues: a validated method for determining meaningful aspects of human health. Pharmanex. Available at: https://www.nuskin.com/content/dam/global/library/feb2017/raman-measurement-carotenoids-living-tissues.pdf. 2018. Accessed October 23, 2020.
- 24. Smidt CR, Gellermann W, Zidichouski JA. Non-invasive Raman spectroscopy measurement of human carotenoid status. FASEB J. 2004;18:A480. [Google Scholar]
- 25. Li C, Bi S, Poole S, et al. Human skin carotenoids in 88,611 subjects measured by Biophotonic Scanner. Chin J Clin Pharm. 2006;15:124–125. [Google Scholar]
- 26. Carlson JJ, Stavens S, Holubkav R, et al. Associations of antioxidant status, oxidative stress, with skin carotenoids assessed by Raman spectroscopy (RS). FASEB J. 2006;20:A1318-c-. [Google Scholar]
- 27. Zukley LM, Nguyen V, Lowndes J, et al. Effects of antioxidant supplementation on skin and serum carotenoids. FASEB J. 2006;20:A145. [Google Scholar]
- 28. Hill E, Clinton S, Grainger E, et al. Biobehavioral intervention improves dietary patterns and biomarkers of carotenoid and fatty acid intakes in overweight cancer survivors. J Acad Nutr Diet. 2017;117:A19. [Google Scholar]
- 29. Caspary L, Thum J, Creutzig A, et al. Quantitative reflection spectrophotometry: spatial and temporal variation of Hb oxygenation in human skin. Int J Microcirc. 1995;15:131–136. [DOI] [PubMed] [Google Scholar]
- 30. Eberhardt K, Stiebing C, Matthaüs C, et al. Advantages and limitations of Raman spectroscopy for molecular diagnostics: an update. Expert Rev Mol Diagn. 2015;15:773–787. [DOI] [PubMed] [Google Scholar]
- 31. Olsztyńska-Janus S, Gasior-Głogowska M, Szymborska-Małek K, et al. Spectroscopic techniques in the study of human tissues and their components. part II: Raman spectroscopy. Acta Bioeng Biomech. 2012;14:121–133. [PubMed] [Google Scholar]
- 32. Takiwaki H, Miyaoka Y, Kohno H, et al. Graphic analysis of the relationship between skin colour change and variations in the amounts of melanin and haemoglobin. Ski Res Technol. 2002;8:78–83. [DOI] [PubMed] [Google Scholar]
- 33. Pandey R, Paidi SK, Valdez TA, et al. Noninvasive monitoring of blood glucose with Raman spectroscopy. Acc Chem Res. 2017;50:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pezdirc K, Hutchesson MJ, Williams RL, et al. Consuming high-carotenoid fruit and vegetables influences skin yellowness and plasma carotenoids in young women: a single-blind randomized crossover trial. J Acad Nutr Diet. 2016;116:1257–1265. [DOI] [PubMed] [Google Scholar]
- 35. Pilotto S, Pacheco MT, Silveira L Jr, et al. Analysis of near-infrared Raman spectroscopy as a new technique for a transcutaneous non-invasive diagnosis of blood components. Lasers Med Sci. 2001;16:2–9. [DOI] [PubMed] [Google Scholar]
- 36. Taylor S, Westerhof W, Im S, et al. Noninvasive techniques for the evaluation of skin color. J Am Acad Dermatol. 2006;54:S282–S290. [DOI] [PubMed] [Google Scholar]
- 37. Wyss R. Chromatographic and electrophoretic analysis of biomedically important retinoids. J Chromatogr B Biomed Sci Appl. 1995;671:381–425. [DOI] [PubMed] [Google Scholar]
- 38. Ermakov IV, Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J Biophotonics. 2012;5:559–570. [DOI] [PubMed] [Google Scholar]
- 39. Ferguson-Pell M, Hagisawa S. An empirical technique to compensate for melanin when monitoring skin microcirculation using reflectance spectrophotometry. Med Eng Phys. 1995;17:104–110. [DOI] [PubMed] [Google Scholar]
- 40. Hajizadeh-Saffar M, Feather JW, Dawson JB. An investigation of factors affecting the accuracy of in vivo measurements of skin pigments by reflectance spectrophotometry. Phys Med Biol. 1990;35:1301–1315. [DOI] [PubMed] [Google Scholar]
- 41. Liasi FT, Samatham R, Jacques SL. Noninvasive in vivo optical characterization of blood flow and oxygen consumption in the superficial plexus of skin. J Biomed Opt. 2017;22:1. [DOI] [PubMed] [Google Scholar]
- 42. Glennie DL, Hayward JE, Farrell TJ. Modeling changes in the hemoglobin concentration of skin with total diffuse reflectance spectroscopy. J Biomed Opt. 2015;20:035002. [DOI] [PubMed] [Google Scholar]
- 43. Liu Q, Yuen C, Chen K, et al. Surface enhanced Raman spectroscopy for malaria diagnosis and intradermal measurements. In: Vo-Dinh T, Lakowicz JR, eds. Prog Biomed Opt Imaging - Proc SPIE. 2018;10509 (Plasmonics in Biology and Medicine XV). doi:10.1117/12.2294539
- 44. Mayne ST, Cartmel B, Scarmo S, et al. Resonance Raman spectroscopic evaluation of skin carotenoids as a biomarker of carotenoid status for human studies. Arch Biochem Biophys. 2013;539:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meléndez-Martínez AJ, Stinco CM, Mapelli-Brahm P. Skin carotenoids in public health and nutricosmetics: the emerging roles and applications of the UV radiation-absorbing colourless carotenoids phytoene and phytofluene. Nutrients. 2019;11:1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chauncey K, Decanio B, Larumbe E, et al. The effect of an intervention to increase fruit and vegetable intake on skin carotenoid scores: a controlled trial. Top Clin Nutr. 2019;34:233–243. [Google Scholar]
- 47. Darvin ME, Magnussen B, Lademann J, et al. Multiple spatially resolved reflection spectroscopy for in vivo determination of carotenoids in human skin and blood. Laser Phys Lett. 2016;13:095601. [Google Scholar]
- 48. Zuñiga YLMC, Plata-Que MT. Skin carotenoid levels and diabetes profiles of type 2 diabetes patients seen at the East Avenue Medical Center Outpatient Diabetes Clinic. Philipp J Intern Med. 2012;50. [Google Scholar]
- 49. Aguilar SS, Wengreen HJ, Dew J. Skin carotenoid response to a high-carotenoid juice in children: a randomized clinical trial. J Acad Nutr Diet. 2015;115:1771–1778. [DOI] [PubMed] [Google Scholar]
- 50. Choi M-H, Jo H-G, Kim M-J, et al. Fruit juice supplementation alters human skin antioxidant levels in vivo: case study of Korean adults by resonance Raman spectroscopy. Biotechnol Bioproc Eng. 2018;23:116–121. [Google Scholar]
- 51. Binder L, SheikhRezaei S, Baierl A, et al. Confocal Raman spectroscopy: in vivo measurement of physiological skin parameters – A pilot study. J Dermatol Sci. 2017;88:280–288. [DOI] [PubMed] [Google Scholar]
- 52. Ermakov IV, Ermakova M, Sharifzadeh M, et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch Biochem Biophys. 2018;646:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Perrone A, Tesoriere L, Pintaudi AM, et al. Raman spectroscopy technology to monitor the carotenoids in skin of thalassemia patients: a novel non-invasive tool relating oxidative stress with iron burden. Thalass Rep. 2014;4:38–42. [Google Scholar]
- 54. Pitts SBJ, Jahns L, Wu Q, et al. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr. 2018;21:1664–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rerksuppaphol S, Rerksuppaphol L. Effect of fruit and vegetable intake on skin carotenoid detected by non-invasive Raman spectroscopy. J Med Assoc Thail. 2006;89:1206–1212. [PubMed] [Google Scholar]
- 56. Rush E, Amoah I, Diep T, et al. Determinants and suitability of carotenoid reflection score as a measure of carotenoid status. Nutrients. 2020;12:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Scarmo S, Cartmel B, Lin H, et al. Significant correlations of dermal total carotenoids and dermal lycopene with their respective plasma levels in healthy adults. Arch Biochem Biophys. 2010;504:34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stahl W, Heinrich U, Jungmann H, et al. Increased dermal carotenoid levels assessed by noninvasive reflection spectrophotometry correlate with serum levels in women ingesting betatene. J Nutr. 1998;128:903–907. [DOI] [PubMed] [Google Scholar]
- 59. Graham ML, Morgan EH, Seguin RA. Is self‐reported fruit and vegetable consumption associated with BioPhotonic skin scan score in women 40 years and older? FASEB J. 2016;30:1153. [Google Scholar]
- 60. Sun J, Ward M, Rau C, et al. Maternal and infant carotenoid status: birth to 4 months of age. J Investig Med. 2012;60:208–209. [Google Scholar]
- 61. Ermakov IV, Whigham LD, Redelfs AH, et al. Skin carotenoids as biomarker for vegetable and fruit intake: validation of the reflection-spectroscopy based “Veggie Meter”. FASEB J. 2016;30:403–409. [Google Scholar]
- 62. Blume-Peytavi U, Rolland A, Darvin ME, et al. Cutaneous lycopene and β-carotene levels measured by resonance Raman spectroscopy: high reliability and sensitivity to oral lactolycopene deprivation and supplementation. Eur J Pharm Biopharm. 2009;73:187–194. [DOI] [PubMed] [Google Scholar]
- 63. Nguyen L, Scherr RE, Linnell J, et al. Evaluating the relationship between fruit and vegetable intake using plasma and dermal biomarkers and reported dietary intake in 4th grade children. FASEB J. 2013;27:623.4. [Google Scholar]
- 64. Conrady CD, Bell JE, Besch BM, et al. Interrelationships between macular, skin, and serum carotenoids. Invest Ophthalmol Vis Sci. 2016;57:3628. https://iovs.arvojournals.org/article.aspx?articleid=2561832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Meinke MC, Schanzer S, Lohan SB, et al. Comparison of different cutaneous carotenoid sensors and influence of age, skin type, and kinetic changes subsequent to intake of a vegetable extract. J Biomed Opt. 2016;21:107002. [DOI] [PubMed] [Google Scholar]
- 66. Chan GM, Chan MM, Gellermann W, et al. Resonance Raman spectroscopy and the preterm infant carotenoid status. J Pediatr Gastroenterol Nutr. 2013;56:556–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Prince MR, Frisoli JK. Beta-carotene accumulation in serum and skin. Am J Clin Nutr. 1993;57:175–181. [DOI] [PubMed] [Google Scholar]
- 68. Smidt CR, Burke DS. Nutritional significance and measurement of carotenoids. Curr Top Nutraceutical Res. 2004;2:79–91. [Google Scholar]
- 69. Aguilar SS, Wengreen HJ, Lefevre M, et al. Skin carotenoids: a biomarker of fruit and vegetable intake in children. J Acad Nutr Diet. 2014;114:1174–1180. [DOI] [PubMed] [Google Scholar]
- 70. Bernstein PS, Sharifzadeh M, Liu A, et al. Blue-light reflectance imaging of macular pigment in infants and children. Invest Ophthalmol Vis Sci. 2013;54:4034–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Henriksen BS, Chan G, Hoffman RO, et al. Interrelationships between maternal carotenoid status and newborn infant macular pigment optical density and carotenoid status. Invest Ophthalmol Vis Sci. 2013;54:5568–5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Ermakov IV, Ermakova MR, Bernstein PS, et al. Resonance Raman based skin carotenoid measurements in newborns and infants. J Biophotonics. 2013;6:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Nguyen LM, Scherr RE, Ermakov IV, et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch Biochem Biophys. 2015;572:73–80. [DOI] [PubMed] [Google Scholar]
- 74. Meinke MC, Darvin ME, Vollert H, et al. Bioavailability of natural carotenoids in human skin compared to blood. Eur J Pharm Biopharm. 2010;76:269–274. [DOI] [PubMed] [Google Scholar]
- 75. Perrone A, Pintaudi AM, Traina A, et al. Raman spectroscopic measurements of dermal carotenoids in breast cancer operated patients provide evidence for the positive impact of a dietary regimen rich in fruit and vegetables on body oxidative stress and bc prognostic anthropometric parameters: a five year study. Tyagi A, ed. Oxid Med Cell Longev. 2016;2016:2727403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Morgan EH, Graham ML, Marshall GA, et al. Serum carotenoids are strongly associated with dermal carotenoids but not self-reported fruit and vegetable intake among overweight and obese women. Int J Behav Nutr Phys Act. 2019;16:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jahns L, Johnson LAK, Conrad Z, et al. Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-h recalls and plasma carotenoid concentrations across one year: a cohort study. Nutr J. 2019;18:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jahns L, Johnson LK, Mayne ST, et al. Skin and plasma carotenoid response to a provided intervention diet high in vegetables and fruit: uptake and depletion kinetics. Am J Clin Nutr. 2014;100:930–937. [DOI] [PubMed] [Google Scholar]
- 79. Janse van Rensburg A, Wenhold F. Validity and reliability of field resonance Raman spectroscopy for assessing carotenoid status. J Nutr Sci Vitaminol. 2016;62:317–321. [DOI] [PubMed] [Google Scholar]
- 80. Zidichouski JA, Mastaloudis A, Poole SJ, et al. Clinical validation of a noninvasive, Raman spectroscopic method to assess carotenoid nutritional status in humans. J Am Coll Nutr. 2009;28:687–693. [DOI] [PubMed] [Google Scholar]
- 81. Bernstein PS, Ahmed F, Liu A, et al. Macular pigment imaging in AREDS2 participants: an ancillary study of AREDS2 subjects enrolled at the Moran Eye Center. Invest Ophthalmol Vis Sci. 2012;53:6178–6186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Conrady CD, Bell JP, Besch BM, et al. Correlations between macular, skin, and serum carotenoids. Invest Ophthalmol Vis Sci. 2017;58:3616–3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Sirriyeh R, Lawton R, Gardner P, et al. Reviewing studies with diverse designs: the development and evaluation of a new tool. J Eval Clin Pract. 2012;18:746–752. [DOI] [PubMed] [Google Scholar]
- 84. Sidik K, Jonkman JN. A comparison of heterogeneity variance estimators in combining results of studies. Stat Med. 2007;26:1964–1981. [DOI] [PubMed] [Google Scholar]
- 85. Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21:1539–1558. [DOI] [PubMed] [Google Scholar]
- 86. Egger M, Smith GD, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. Br Med J. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Moran NE, Mohn ES, Hason N, Erdman JW, et al. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv Nutr. 2018;9:465–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Blanchard T, Lyson T. Food availability and food deserts in the nonmetropolitan south. Vol Number 12. Southern Rural Development Center; 2006. Available at: http://srdc.msstate.edu/publications/other/foodassist/2006_04_blanchard.pdf. Accessed June 16, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.