Abstract
Innate-like T (iT) cells comprise a population of immunoregulatory T cells whose effector function is imposed during their development in the thymus to provide protective immunity prior to antigen encounter. The molecular mechanism that drives the generation of iT cells remains unclear. Here, we report that the cytokine receptor γc plays a previously unappreciated role for thymic iT cells by controlling their cellular abundance, lineage commitment, and subset differentiation. As such, γc overexpression on thymocytes dramatically altered iT cell generation in the thymus, as it skewed the subset composition of invariant NKT (iNKT) cells and promoted the generation of IFNγ-producing innate CD8 T cells. Mechanistically, we found that the γc-STAT6 axis drives the differentiation of IL-4-producing iNKT cells, which in turn induced the generation of innate CD8 T cells. Collectively, these results reveal a cytokine-driven circuity of thymic iT cell differentiation that is controlled by the abundance of γc proteins.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00018-021-04067-3.
Keywords: CD1d, IL-4, iNKT cells, PLZF, STAT6
Introduction
T cells are generated in the thymus as antigen-inexperienced naïve T cells that require further maturation in peripheral tissues to acquire functional competence [1, 2]. However, a small population of thymocytes is generated as phenotypically and functionally mature T cells with the innate ability to produce pro-inflammatory cytokines, including IFNγ, IL-4, and IL-17 [3, 4]. At least four major populations of such thymic innate-like T (iT) cells have been identified: invariant NKT (iNKT) cells, mucosal-associated invariant T (MAIT) cells, a subset of γδ T cells, and innate CD8 T cells [5–7]. Multiple mechanisms have been proposed to give rise to thymic iT cells. These mechanisms include strong agonistic TCR signals, increased intrathymic availability of the cytokine IL-4, and the upregulation of the transcription factor promyelocytic leukemia zinc finger (PLZF), among others [3, 6, 8]. However, it remains unclear if and how all these events are interconnected and whether there is a common mechanism that governs the generation of thymic iT cells.
In addition to TCR signaling, cytokine receptor signaling is another nonredundant requirement for T cell development in the thymus [9–11]. In particular, cytokines of the common γc-chain (γc) family play critical roles in the lineage choices and maturation of conventional T cells as demonstrated by the severely impaired thymopoiesis of γc-deficient mice [12, 13]. Despite its importance in T cell development, however, the expression of the γc cytokine receptor is actively suppressed in preselection CD4+CD8+ double-positive (DP) thymocytes [14, 15]. We recently demonstrated that such a DP thymocyte-specific decrease in γc abundance is mediated by the transcription factor RORγt [16], which is selectively expressed in DP cells among thymocytes [17, 18]. However, why the abundance of γc on preselection thymocytes must be limited and what the in vivo consequence would be if γc expression failed to be suppressed in preselection thymocytes have not been explained.
Here, we report the surprising finding that γc expression is directly linked to the thymic generation of iT cells. Specifically, we show that maintaining the amounts of γc proteins at low abundance in preselection thymocytes is necessary to limit the generation of thymic iT cells. We further demonstrate that γc availability constrains the differentiation of the IL-4-producing iNKT subset and IFNγ-expressing innate CD8 T cells. Altogether, these findings propose a new regulatory mechanism for the generation of thymic iT cells that is controlled by the surface protein abundance of the γc cytokine receptor.
Results
γc availability limits γc signaling in immature thymocytes
T cell development in the thymus can be compartmentalized into distinct stages based on expression of the Heat-Stable Antigen (HSA) and the TCR (Fig. 1A) [19]. The most immature cells are HSAhiTCRβlo (stage I), while the most mature thymocytes are HSAloTCRβhi (stage III). However, the majority of thymocytes are positioned between these two stages, and they display an HSAintTCRβint phenotype (stage II) (Fig. 1A). The transition from stage I to II, and then from stage II to III, is mediated by a combination of TCR and cytokine signals, known as β-selection and positive selection, respectively [20]. While the TCR levels progressively increase with maturation, the expression of cytokine receptors is distinct and stage-specific [14]. The IL-7Rα, for example, is highly abundant on stage I and stage III thymocytes but conspicuously absent on immature stage II cells, which correspond to preselection DP thymocytes (Fig. 1A) [14, 21]. The IL-4Rα, on the other hand, is highly expressed on stage II preselection DP thymocytes, as previously reported (Fig. 1A and Supplemental Fig. 1A) [21]. Curiously, the expression of the γc cytokine receptor, which pairs with the IL-4Rα for IL-4 signaling, is expressed at uniquely low levels on DP thymocytes (Fig. 1A and Supplemental Fig. 1A) [14, 15]. Thus, IL-4 receptor signaling is possibly constrained on preselection DP cells because of the limited availability of γc.
Fig. 1.
Limited availability of γc constrains cytokine signaling in DP thymocytes. A Stage-specific expression of IL-7Rα, IL-4Rα, and γc in thymocyte subsets. The discrete developmental stages were defined by surface HSA and TCRβ expression (contour plots). The most immature cells are HSAhiTCRβlo (gate I), which differentiate into HSAintTCRβint (gate II) and then become HSAloTCRβhi (gate III) mature thymocytes (left). Contour plots show the CD4/CD8 profiles of the gated thymocyte populations (middle). Cytokine receptor expression (red lines) was assessed on gated thymocytes and overlaid with control antibody staining (gray lines) (right). Data are representative of 6 independent experiments. B Cell surface γc and IL-4Rα expression on WT and γcTg thymocytes (red lines) overlaid with control antibody staining (gray). Data are representative of 4 independent experiments. C IL-4 signaling in WT and γcTg DP thymocytes was assessed by evaluating pSTAT5 levels. IL-4-induced STAT5 phosphorylation in the DP cells (left) or CD8SP (right) thymocytes was determined as fold induction over medium treatment. Data are the summary of 4 independent experiments. *P < 0.05 and **P < 0.01 (Student’s two-tailed t test)
To examine whether the low abundance of surface γc on DP cells would suppress cytokine signaling, we analyzed γc transgenic mice (γcTg) that overexpress γc in T lineage cells [22]. We found that the protein abundance of γc was specifically and substantially increased on γcTg DP thymocytes, whereas IL-4Rα expression remained unaffected on these cells (Fig. 1B). Notably, the increased abundance of surface γc proteins dramatically improved IL-4 signaling in γcTg DP thymocytes, as demonstrated by the increased phosphorylation of the downstream signaling protein STAT5 upon IL-4 stimulation (Fig. 1C, left and Supplemental Fig. 1B). IL-4 signaling in mature CD8 single-positive (CD8SP) thymocytes, on the other hand, did not significantly change upon increased γc sexpression, indicating that γc availability is not a limiting factor for postselection thymocytes (Fig. 1C, right and Supplemental Fig. 1C). These results document that γc availability is limited on DP thymocytes and further suggest that suppression of γc expression is a mechanism to limit the signaling of IL-4, and presumably also other γc cytokines, in developing thymocytes.
γc overexpression promotes innate CD8 T cell generation
To understand the in vivo consequence of increased γc availability in the thymus, we assessed the selection and lineage differentiation of γcTg thymocytes in comparison to those from wild-type (WT) littermate controls (LMCs). While the overall thymocyte numbers were decreased in γcTg mice, we did not find noticeable changes in positive selection or T cell maturation based on CD69 versus TCRβ expression (Fig. 2A). The frequency of CD69+TCRβhi cells, which correspond to thymocytes undergoing positive selection, was unchanged, and the amount of CD69, which is an indicator of TCR signaling strength, also remained unaltered (Fig. 2A). However, we observed a marked increase in CD8SP cell frequencies in γcTg mice (Fig. 2B, C), whereby CD4SP thymocyte frequencies remained unaffected (Fig. 2C). Thus, the increased abundance of γc selectively advanced the generation of CD8 lineage T cells in the thymus.
Fig. 2.
γc overexpression induces innate CD8 T cell development. A Total thymocyte numbers and CD69 versus TCRβ profiles of WT littermate control (LMC) and γcTg mice (top). Bar graphs (bottom) show the frequency and the protein abundance of CD69 expression (Mean Fluorescence Intensity; MFI) of thymocytes undergoing positive selection (CD69+TCRβhi). Data are representative of 2 independent experiments with a total of 5 WT littermates and 7 γcTg mice. B Contour plots show the CD4/CD8 profiles of the total (top) and TCRβhi-gated (bottom) WT and γcTg thymocytes. C Bar graphs show the frequencies of CD8SP and CD4SP cells among TCRβhi-gated WT and γcTg thymocytes. Data are the summary of 7 independent experiments with a total of 16 γcTg mice and 9 WT littermate controls. D Contour plots show the CXCR3 versus CD44 and the CD122 versus CD44 profiles of mature TCRβhiCD8SP cells among WT and γcTg thymocytes. Innate CD8 T cells are gated in red. Data are representative of 7 independent experiments with a total of 9 WT and 16 γcTg mice. E IFNγ expression by WT littermate control and γcTg CD8 T cells. Freshly isolated thymocytes were stimulated for 4 h with PMA and ionomycin in the presence of brefeldin A and assessed for IFNγ expression by intracellular staining. Dot plots show representative IFNγ versus CD8 profiles from 3 independent experiments. F Intranuclear staining for eomes and T-bet expression in memory-phenotype CD44hi and naive CD44lo TCRβhiCD8SP thymocytes of γcTg mice. Histograms are representative of 2 independent experiments. G Cell surface IL-4Rα and HSA expression in CD44hi and naive CD44lo TCRβhiCD8SP thymocytes of γcTg mice. Histograms are representative of 2 independent experiments
CD8 lineage specification of post selection thymocytes is primarily driven by γc cytokines [15, 23]. In particular, IL-7 is the major intrathymic cytokine that promotes the generation of CD8 lineage cells in postselection thymocytes [23]. Therefore, we postulated that the increased γc abundance on preselection DP cells could have facilitated IL-7 signaling and driven the differentiation of CD8 T cells. However, in-depth analysis of γcTg CD8SP thymocytes revealed that these cells did not correspond to conventional CD8SP thymocytes. In fact, γcTg CD8SP thymocytes contained large fractions of CD44hiCXCR3hi and CD44hiCD122hi memory phenotype cells (Fig. 2D). In addition, γcTg CD8SP cells produced copious amounts of IFNγ upon PMA and ionomycin stimulation, while WT CD8 T cells did not (Fig. 2E). In this regard, the phenotype of γcTg CD8SP cells was reminiscent of innate CD8 T cells, which arise upon increased expression of intrathymic IL-4 and depend on IL-4R signaling [8, 24]. To examine if the γcTg memory-phenotype CD8SP cells indeed correspond to innate CD8 T cells, next, we examined these cells for markers that are associated with thymic innate CD8 T cells [4]. We found that γcTg CD8SP T cells expressed large amounts of eomesodermin (eomes) but not T-bet (Fig. 2F), which are characteristics associated with innate CD8 T cells [25, 26]. In addition, γcTg CD8SP thymocytes showed increased amounts of surface IL-4Rα but dramatically reduced amounts of HSA (Fig. 2G), further affirming that these cells correspond to innate CD8 T cells. Collectively, these results documented that the increase in γc abundance promotes the generation of thymic innate CD8 T cells.
Increased abundance of γc promotes the generation of PLZFhi thymocytes
Innate CD8 T cells depend on IL-4 signaling to acquire their memory-phenotype and effector function [8, 27]. The primary source of intrathymic IL-4 has been identified as innate-like thymocytes that express large amounts of the transcription factor PLZF, encoded by the gene Zbtb16 [8]. PLZF+ thymocytes mostly correspond to iNKT cells, among which the NKT2 subset, which is highly abundant for PLZF, is the major producer of IL-4 [24, 28]. Consequently, we asked whether the increased γc availability in γcTg mice would promote the generation or differentiation of PLZFhi iNKT cells. To this end, we utilized PBS57-loaded CD1d tetratmers to identify iNKT cells (CD1dTet+) in thymocytes of WT LMC and γcTg mice and examined them in further detail. We first noted that the frequency of thymic iNKT cells did not substantially differ between γcTg and LMC mice (Fig. 3A, left). The amount of PLZF proteins, however, was significantly increased in γcTg iNKT cells (Fig. 3A, right), suggesting that the forced expression of γc alters the differentiation of thymic iNKT cells. Indeed, γcTg iNKT cells were highly enriched for PLZFhi iNKT cells, whereas WT iNKT cells contained a significantly greater fraction of PLZFlo cells (Fig. 3B). Consistent with previous reports that IL-4 is primarily produced by PLZFhi cells [24], the PLZFhi iNKT cell population of γcTg thymocytes produced large amounts of IL-4 when stimulated with PMA and ionomycin (Fig. 3C). These results are in further support of our notion that the increased generation of innate CD8 T cells in γcTg mice is driven by the increased numbers of IL-4-producing PLZFhi thymocytes.
Fig. 3.
Increase in γc expression drives the differentiation of PLZFhi iNKT cells. A PLZF expression among thymic iNKT cells of WT LMC and γcTg mice. Dot plots show PBS57-loaded CD1d tetramer staining (CD1dTet) versus intracellular PLZF expression of whole thymocytes. Bar graph displays the amount of PLZF protein expression (MFI) in PLZF+ iNKT cells of the indicated mice. Results are the summary of 5 independent experiments with a total of 12 LMC and 12 γcTg mice. B PLZF distribution in thymic iNKT cells of WT LMC and γcTg mice. PLZF+ iNKT cells were divided into two subpopulations, i.e., PLZFhi and PLZFlo, based on the abundance of PLZF proteins. Histogram is representative and the bar graph is the summary of 5 independent experiments with a total of 12 LMC and 12 γcTg mice. C IL-4 production in γcTg thymocytes. PMA and ionomycin-stimulated γcTg thymocytes were stained for IL-4 production and then counterstained with anti-PLZF antibodies to identify IL-4-producing cells among PLZF+ cells. The results are representative of 3 independent experiments
A PLZF requirement for γcTg innate CD8 T cells
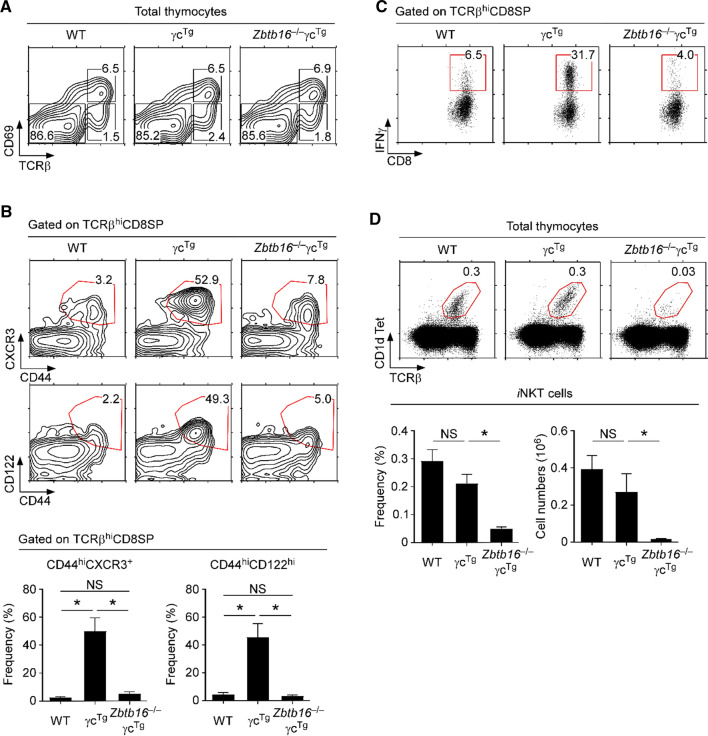
To directly demonstrate that γcTg-driven innate CD8 T cell generation depends on PLZF, we next generated γcTg mice that are deficient in Zbtb16 and thus cannot produce PLZF proteins. The thymocytes of these Zbtb16–/–γcTg mice did not show alterations in T cell development or positive selection based on their CD69 and TCRβ expression (Fig. 4A). However, we found that the genetic ablation of Zbtb16 in γcTg mice effectively reverted the frequencies of mature CD8SP thymocytes to wildtype levels (Supplemental Fig. 2). Moreover, CD8SP thymocytes of Zbtb16–/–γcTg mice were distinct from those of Zbtb16+/+ (WT) γcTg mice, because they retained a naïve phenotype (Fig. 4B, top). In fact, CD44hiCXCR3hi and CD44hiCD122hi innate phenotype CD8 T cells were virtually absent in WT and in Zbtb16–/–γcTg mice (Fig. 4B, bottom). Consistent with these findings, Zbtb16–/–γcTg CD8SP thymocytes did not produce IFNγ upon PMA and ionomycin stimulation, unlike γcTg CD8SP thymocytes (Fig. 4C).
Fig. 4.
PLZF is required to generate innate CD8 T cells in γcTg thymocytes. A CD69 versus TCRβ profiles of WT, γcTg and Zbtb16–/–γcTg thymocytes. Data are representative of 2 independent experiments with a total of 3 WT, 4 γcTg and 2 Zbtb16–/–γcTg mice. B Contour plots show the CXCR3 versus CD44 and CD122 versus CD44 profiles of mature TCRβhiCD8SP cells in WT, γcTg, and Zbtb16–/–γcTg thymocytes. Innate CD8 T cells are gated in red. Contour plots are representative (top) and bar graphs (bottom) show summary of 3 independent experiments with a total of 3 WT, 3 γcTg and 3 Zbtb16–/–γcTg mice. C IFNγ expression by WT, γcTg and Zbtb16–/–γcTg CD8 T cells. Freshly isolated thymocytes were stimulated for 4 h with PMA and ionomycin in the presence of brefeldin A, and assessed for IFNγ expression by intracellular staining. Dot plots show representative IFNγ versus CD8 profiles from 3 independent experiments. D iNKT cell frequencies in WT, γcTg and Zbtb16–/–γcTg thymocytes. Dot plots (top) are representative and bar graphs are the summary of 3 independent experiments with a total of 4 WT, 5 γcTg and 3 Zbtb16–/–γcTg mice
PLZF is a nonredundant requirement for iNKT cell generation, and all thymic iNKT cells express PLZF [29, 30]. iNKT cells have been previously identified as the main drivers of innate CD8 T cell differentiation, and PLZF is necessary to generate iNKT cells [24, 29, 30]. Consequently, we aimed to examine if iNKT cells are associated with the γcTg-mediated generation of innate CD8 T cells. To this end, we assessed iNKT cells in the thymus of γcTg and Zbtb16–/–γcTg mice by staining with PBS57-loaded CD1d tetramers and anti-TCRβ antibodies (Fig. 4D). As expected, thymic iNKT cells failed to develop in the absence of PLZF [29, 30], so that we did not detect any meaningful numbers of CD1dTet+ mature thymocytes in Zbtb16–/–γcTg mice (Fig. 4D). In fact, both the frequency and cell number of thymic iNKT cells were dramatically decreased in Zbtb16–/–γcTg mice, whereas iNKT cell frequency and numbers in Zbtb16-sufficient γcTg mice were unaltered compared to WT mice (Fig. 4D). Thus, these results suggest that γcTg promotes the generation of innate CD8 T cells through PLZF-expressing iNKT cells. Consequently, PLZF-deficiency hampers innate CD8 T cell differentiation because of the failure to produce iNKT cells in the thymus.
iNKT cells drive the differentiation of innate CD8 T cells in γcTg mice
To directly demonstrate that the γcTg induces innate CD8 T cells over an iNKT cell-dependent mechanism, we generated γcTg mice that are deficient for CD1d (Cd1d–/–γcTg). iNKT cell generation requires agonistic TCR engagement of glycolipid-presenting CD1d molecules, and CD1d-deficiency results in the lack of mature iNKT cells [31, 32]. We confirmed the lack of thymic iNKT cells in Cd1d–/–γcTg mice (Fig. 5A), and we also noted that CD8SP thymocytes in such mice had reverted to a normal phenotype (Supplemental Fig. 3). Specifically, Cd1d–/–γcTg mice showed dramatic reductions in CD44hiCXCR3hi and CD44hiCD122hi innate-like CD8SP thymocytes compared to wildtype γcTg mice (Fig. 5B and Supplemental Fig. 3). Unlike CD8SP cells from WT γcTg mice, Cd1d–/–γcTg CD8SP thymocytes also maintained high levels of HSA and did not upregulate IL-4Rα, which are consistent with a naïve CD8 T cell phenotype (Fig. 5C). Finally, we assessed IFNγ expression in both WT γcTg and Cd1d–/–γcTg CD8SP thymocytes, and we found that PMA and ionomycin stimulation induced IFNγ production in WT γcTg but not in Cd1d–/–γcTg cells (Fig. 5D). Collectively, these results directly demonstrate a requirement for iNKT cells in the γcTg-induced generation of innate CD8 T cells.
Fig. 5.
iNKT cells drive innate CD8 T cell differentiation in γcTg thymocytes. A iNKT cell frequencies in γcTg and Cd1d–/–γcTg thymocytes. Data are representative of 6 independent experiments with a total of 10 γcTg and 17 Cd1d –/–γcTg mice. B Contour plots show the CXCR3 versus CD44 and CD122 versus CD44 profiles of mature TCRβhiCD8SP cells in γcTg and Cd1d –/–γcTg thymocytes. Innate CD8 T cells are identified with red gates. Bar graphs show the summary of 6 independent experiments with a total of 16 γcTg and 17 Cd1d –/–γcTg mice. C Cell surface IL-4Rα and HSA expression in CD44hi and CD44lo TCRβhiCD8SP thymocytes of γcTg and Cd1d –/–γcTg mice. Histograms are representative of 2 independent experiments. D IFNγ expression by γcTg and Cd1d –/–γcTg CD8 T cells. Freshly isolated thymocytes were stimulated for 4 h with PMA and ionomycin in the presence of brefeldin A and assessed for IFNγ expression by intracellular staining. IFNγ versus CD8 dot plot profiles are representative (top), and the bar graph (bottom) shows the summary of 3 independent experiments with a total of 5 γcTg and 8 Cd1d –/–γcTg mice
The increased abundance of γc proteins alters thymic iNKT subset composition
To better understand how γcTg affects thymic iNKT cells to induce the generation of innate CD8 T cells, we analyzed the development and differentiation of γcTg iNKT cells in further detail. While the γcTg did not alter the frequencies of HSAlo mature iNKT cells between LMC and γcTg thymocytes (Fig. 6A), we found that the γcTg heavily interfered with the development of mature thymic iNKT cells (Fig. 6B). The maturation of thymic iNKT cells proceeds along a well-defined pathway that can be divided into three developmental stages based on the distinct expression of CD44 and NK1.1 [33, 34]. The least mature stage 1 (ST1) iNKT cells are defined as CD44–NK1.1–, which is followed by stage 2 (ST2) iNKT cells that are CD44+NK1.1–, finally culminating in the most differentiated stage 3 (ST3) iNKT cells that are CD44+NK1.1+. In WT C57BL/6 mice, the majority of mature iNKT cells correspond to ST3 cells, and previous studies had identified this population as prominent producers of IFNγ [24, 33, 34]. ST2 iNKT cells, on the other hand, are mostly IL-4-producers, and they are noted as the primary source of intrathymic IL-4 [24]. Strikingly, γcTg iNKT cells were highly enriched for ST2 cells concomitant to a substantial decrease in the ST3 population, which significantly differed from LMC iNKT cells, where ST3 iNKT cells dominate and ST2 cells are in the minority (Fig. 6B). These results indicate that the increased expression and availability of γc proteins interferes with the maturation and differentiation of thymic iNKT cells.
Fig. 6.
Increased γc abundance alters the subset composition of thymic iNKT cells. A Frequencies of mature (HSAlo) thymic iNKT cells of γcTg and WT LMC mice. Dot plots are representative (left), and bar graph (right) shows the summary of 9 independent experiments with a total of 22 γcTg and 14 WT LMC mice. B Developmental stages of thymic iNKT cells were determined in LMC and γcTg thymocytes. Mature (HSAlo) iNKT cells were identified by CD1d-tetramer and anti-HSA staining (contour plots) and assessed for stage 1 (ST1) to stage 3 (ST3) based on surface CD44 and NK1.1 expression. Contour plots are representative, and bar graph shows summary of 6 independent experiments with a total of 12 γcTg and 6 WT LMC mice. C Subset distribution among thymic iNKT cells of LMC and γcTg mice. NKT1 cells were identified by intracellular PLZF and T-bet staining, while NKT2 and NKT17 cells were identified by PLZF and RORγt staining. Dot plots are representative and the bar graphs showing the frequency of each subset are the summary of 4 independent experiments. D CD5 expression on thymic iNKT cells of LMC and γcTg mice. The abundance of surface CD5 was assessed on preselection (CD69–TCRβlo) thymocytes and mature (HSAlo) iNKT cells of LMC and γcTg mice (top). CD5 expression was also determined on individual thymic iNKT subsets of the same mice (bottom). Results are representative of 3 independent experiments with 4 γcTg and 3 WT LMC mice. E Intracellular pCD247 content in mature (HSAlo) iNKT cells of LMC and γcTg mice. Mature iNKT cells were fixed, permeabilized, and assessed for pCD247 expression either immediately after isolation (top) or PMA plus ionomycin-stimulation (bottom). Histograms are representative and bar graphs are the summary of 3 independent experiments with 4 γcTg and 3 WT LMC mice
Complementary and alternatively to the developmental classification of iNKT cells, iNKT cells can be also classified into three functionally distinct subsets, based on their cytokine and transcription factor expression profiles [24]. Accordingly, mature iNKT cells are composed of NKT1, NKT2, and NKT17 cells, analogous to their counterparts in peripheral CD4 helper T cell subsets. NKT1 cells are IFNγ producers that specifically express the transcription factor T-bet, and they correspond to ST3 iNKT cells. NKT2 cells, on the other hand, are IL-4-producers and they largely overlap with ST2 iNKT cells [24, 28]. In WT thymocytes, most iNKT cells correspond to NKT1 cells [24, 35, 36], which we confirmed by intracellular staining for T-bet (Fig. 6C). Compared to the iNKT subset composition of LMC thymocytes, however, we found that NKT1 cells were significantly underrepresented in γcTg iNKT cells. Instead, the majority of γcTg iNKT cells corresponded to NKT2 cells (Fig. 6C), which we identified by their high level of PLZF expression and the absence of RORγt [28, 37]. Because NKT2 cells are primarily IL-4 producers, these results further explain why the cytokine landscape in the γcTg thymus is skewed to favor the generation of iT cells. Altogether, these data propose that the increased protein abundance of γc would alter the subset competition of thymic iNKT cells from a mostly IFNγ-producing population into a mostly IL-4-producing population. As a result, the γcTg increases the availability of intrathymic IL-4 which promotes the differentiation of IL-4-induced innate CD8 T cells.
Multiple genetic factors have been identified to control the generation of innate-like memory CD8 T cells [4]. These findings include the seminal observation of bystander CD8 iT cells in Klf2-deficient mice [8] as well as the increased number of innate CD8 T cells in BALB/c mice that is driven by increased frequencies of NKT2 cells [24, 38] (Supplemental Fig. 4A). A unifying mechanism that would connect these different factors in driving innate CD8 T cell generation, however, has not been identified. Thus, we next aimed to assess whether the increased abundance of γc proteins would potentially play such a role. To this end, we examined γc expression on thymocytes and iNKT cells of C57BL/6 mice and BALB/c mice with the expectation that the protein abundance of γc would be increased in BALB/c mice. However, this was not the case. We found that γc expression did not differ during thymocyte differentiation (Supplemental Fig. 4B) and also not in iNKT cell development (Supplemental Fig. 4C) of C57BL/6 and BALB/c mice. Collectively, the γcTg revealed a previously unappreciated pathway of iT cell generation that is distinct by its utilization of the γc cytokine receptor.
Assessing the molecular mechanisms of increased NKT2 cell differentiation among γcTgiNKT cells
The data thus far led us to a model, where the increased protein abundance of γc would promote the generation of IL-4-producing NKT2 cells, which in turn would increase the abundance of intrathymic IL-4 to drive the differentiation of innate CD8 T cells. The cornerstone of this model lies in its ability to explain how the increased abundance of γc would increase the generation of NKT2 cells. Currently, the mechanism of iNKT subset differentiation remains incompletely understood. However, there is a consensus that TCR signaling would play a critical role in this process, and that difference in TCR signaling strength would influence iNKT subset fate [39, 40]. In this regard, a genetic model of diminished TCR signaling strength recently demonstrated that the development of NKT1 cells increased upon weakened TCR signaling, whereas NKT2 and NKT17 generation was impaired under the same circumstances [41]. Moreover, NKT2 cells express large amounts of CD5, a stringent indicator of TCR signaling strength [42], while NKT1 cells show the lowest level of CD5, documenting a hierarchy in TCR signaling strengths that is associated with iNKT subset identity [28, 40]. Thus, we considered it necessary to examine whether the forced expression of γc would alter iNKT subset differentiation by amplifying TCR signaling strength. To this end, we assessed surface CD5 expression on pre-selection thymocytes and mature iNKT cells of γcTg and LMC mice (Fig. 6D). We found that CD5 levels did not increase on thymocytes and on mature iNKT cells of γcTg mice (Fig. 6D, top), and also did not differ on individual iNKT subsets (Fig. 6D, bottom). Phosphorylation of the CD3 zeta-chain (CD247) is another highly sensitive marker of in vivo TCR engagement [43]. Notably, assessing the intracellular phospho-CD247 (p247) contents, either in freshly isolated or in in vitro stimulated iNKT cells of γcTg and LMC thymocytes, also did not reveal any difference (Fig. 6E). Altogether, these results suggested that it is rather unlikely that forced γc expression alters iNKT subset differentiation by interfering with TCR signaling.
A STAT6-requirement for NKT2 cell generation in γcTgiNKT cells
Because the γc protein is a cytokine receptor, we next aimed to assess whether it is indeed cytokine signaling that promotes NKT2 cell differentiation in γcTg mice. There are six cytokines that require γc for ligand binding and signaling [44], and all of them trigger the downstream activation of STAT5. Generation and analysis of γcTg mice that are conditionally deficient for STAT5 in T cells (Stat5cKO) [23] revealed a complete lack of thymic iNKT cells in Stat5cKOγcTg thymocytes (Fig. 7A), indicating that STAT5 is essential for iNKT cell generation in γcTg mice. The generation of conventional CD4SP thymocytes, however, was refractory to STAT5-deficiency, as previously reported (Supplemental Fig. 5) [23]. As a corollary, these results document that the generation of NKT2 cells in general, and the increased NKT2 cell generation in γcTg thymocytes in particular, depend on STAT5. Altogether, the γcTg-driven NKT2 cell differentiation depends on cytokine signaling downstream of γc, as demonstrated by its requirement for STAT5.
Fig. 7.
STAT6 promotes the generation of NKT2 cells in γcTg thymocytes. A iNKT cell frequencies in γcTg and Stat5cKOγcTg thymocytes. Dot plots are representative of 2 independent experiments with a total of 2 γcTg and 3 Stat5cKOγcTg mice. B iNKT cell frequencies in γcTg and Stat6–/–γcTg thymocytes. Dot plots are representative and bar graph is the summary of 2 independent experiments with a total of 4 γcTg and 3 Stat6 –/–γcTg mice. C Subset distribution of thymic iNKT cells in γcTg and Stat6–/–γcTg mice. NKT1 cells were identified by intracellular PLZF and T-bet staining, while NKT2 and NKT17 cells were identified by PLZF and RORγt staining. Dot plots are representative and bar graph is the summary of 3 independent experiments with a total of 6 γcTg and 5 Stat6–/–γcTg mice
To further determine which cytokine(s) are utilized by γc to impose NKT2 lineage fate, we next considered that γc signaling also activates other STAT molecules than STAT5 [44]. As such, STAT6, which was originally identified as IL-4-STAT, is uniquely induced by IL-4 among γc cytokines [44, 45]. Preselection DP cells are the immediate precursors of iNKT cells, and we demonstrated that increased protein abundance of γc dramatically increased IL-4 responsiveness in these cells (Fig. 1C). Therefore, we asked whether the γcTg would promote NKT2 cell generation in a STAT6-dependent manner. To this end, we generated STAT6-deficient γcTg mice (Stat6–/–γcTg) and assessed the generation and subset composition of thymic iNKT cells in these mice. The overall frequencies and numbers of iNKT cells in Stat6–/–γcTg thymocytes were not significantly different from those of WT thymocytes (Fig. 7B). However, the frequency of NKT2 cells was substantially reduced, whereas the frequency of NKT1 cells was conversely increased (Fig. 7C). In agreement with a lack of IL-4 signaling, the generation of innate CD8 T cells was also profoundly diminished in Stat6–/–γcTg mice (Supplemental Fig. 6). These results reveal a previously unappreciated role for γc and STAT6 in determining the iNKT lineage fate, and they suggest that the limited availability of γc in developing thymocytes constrains the generation of NKT2 cells and the intrathymic production of IL-4. Because IL-4 is also the main driver of innate CD8 T cell differentiation, the abundance of γc, albeit indirectly, controls the production of innate CD8 cells in the thymus.
Discussion
Cytokine receptor signaling plays a critical role in the development and lineage differentiation of T cells. Here, we report that the abundance of the γc cytokine receptor is a limiting factor for cytokine signaling in thymocytes, and that the increased availability of γc proteins increased the generation of IL-4-producing NKT2 cells, which, in turn, promoted the differentiation of IFNγ-producing innate CD8 T cells. Thus, the surface abundance of γc controls the generation of multiple iT cell populations in the thymus, providing a new perspective on the biological significance of γc regulation during thymocyte development.
The γc receptor was originally identified as the third subunit of the high-affinity IL-2 receptor but was later found to be shared as a nonredundant subunit by a series of other cytokines that are collectively referred to as γc family cytokines [12, 13]. γc is required for the high-affinity binding and signaling of γc family cytokines, and its abundance limits γc cytokine signaling independently of the proprietary cytokine receptors. While the cellular abundance of γc proteins has been considered to be developmentally set [46], recent reports demonstrated that the amounts of γc proteins substantially vary, depending on the maturation and activation of T cells [14, 15, 22]. As such, γc is highly expressed on the immature CD4, CD8 double-negative (DN) thymocytes but is substantially downregulated on preselection DP thymocytes. Postselection thymocytes, on the other hand, re-express high levels of γc proteins and become responsive to γc cytokine signaling [15]. The biological significance of diminished γc level selectively in DP thymocytes is intriguing but not yet fully understood. Premature γc cytokine signaling in preselection thymocytes could result in the survival of unproductive or autoreactive TCR specificities. Thus, the suppression of γc expression could serve as a mechanism to ensure that only the thymocytes that successfully underwent the process of positive and negative selection become mature T cells [21]. Distinct from but not mutually exclusive with this supposition, our current study demonstrated that dysregulated γc expression can induce increased IL-4 signaling in DP thymocytes, thus altering the subset composition of thymic iNKT cells and promoting the generation of CD8 iT cells. It is currently unclear to us how the permeation of IL-4 signaling in preselection thymocytes would skew iNKT subset differentiation. Because we identified STAT6 as a key molecule downstream of γcTg that drives NKT2 cell differentiation, we consider it likely that increased pSTAT6 abundance in preselection DP thymocytes could predispose immature iNKT cells to become NKT2 lineage cells. Further in-depth analyses of the chromatin landscape and differential gene transcription of wildtype and γcTg DP thymocytes are necessary to obtain evidence regarding the mechanisms. We are also uncertain about the identity of IL-4 producers in the thymus that would trigger γc-mediated STAT6 phosphorylation in developing iNKT cells, and we are currently in the process of addressing these points.
A role for the IL-4/STAT6 axis in iNKT subset specification, and specifically in the lineage differentiation of NKT2 cells, was surprising to us. This finding was unexpected, because the current view posits that TCR signaling—not cytokine receptor signaling—is the primary driver of iNKT subset differentiation [40, 47]. iNKT subsets are primarily defined based on their effector phenotype and distinct transcription factor expression, but the molecular mechanisms that direct their specification remain unclear [48]. The zinc finger protein PLZF is considered a nonredundant requirement and a master transcription factor for iNKT cell development [29, 30]. Notably, the amount of PLZF proteins differs depending on the iNKT subset, such that NKT2 cells are highly abundant in PLZF, followed by NKT17 cells that express intermediate amounts and NKT1 cells that express the smallest amounts of PLZF [28]. Because PLZF is induced by positive selecting TCR signaling [30], it has been proposed that iNKT subset specification would be directed by the difference in TCR signaling strength during thymic iNKT cell development [39–41, 47, 49]. Consequently, strong TCR signals would induce large amounts of PLZF and impose NKT2 lineage fate, whereas intermediate and weak TCR signaling would induce lesser amounts of PLZF and impose NKT17 and NKT1 subset differentiation, respectively [39–41]. How the difference in TCR signaling strength induces different amounts of PLZF to determine iNKT subset fate remains an intriguing issue that is not fully understood [47]. As a potential resolution, the transcription factor Egr2 can be considered as a mediator that would link TCR strength to iNKT subset differentiation. Egr2 is induced downstream of TCR signaling, and the sustained expression of Egr2 was found to be necessary for the maturation and differentiation of iNKT cells [50, 51]. Moreover, the amount of Egr2 highly correlates with the cellular abundance of PLZF in iNKT cells. Thymocytes that were engineered to become hyporesponsive to TCR stimulation failed to sustain Egr2 expression, and they also expressed diminished amounts of PLZF proteins [39]. As a result, weakening the TCR signaling strength altered the subset composition of thymic iNKT cells so that PLZFhi NKT2 cell frequencies were decreased, whereas PLZFlo NKT1 cell differentiation was conversely increased [39]. Collectively, these and other data came to suggest that the strength of TCR signaling is a major regulator of iNKT subset differentiation and that molecules in the TCR signaling cascade, such as ZAP70, NFAT, and Egr2 [39, 41], are active players in this process.
In this regard, it was striking that the increased abundance of the γc cytokine receptor had such a profound effect on thymic iNKT subset specification. In fact, a role for the γc deviates from the prevailing TCR-centric model of iNKT subset differentiation, where the strength of TCR signals drives distinct iNKT effector fates. Signaling of γc cytokines utilizes the JAK/STAT pathway which does not intersect with the TCR-triggered calcium/NFAT/Egr2 signaling pathway [52]. Therefore, it is unlikely that γc signaling co-opts the TCR signaling pathway to alter iNKT lineage fate. In agreement, we did not find any differences in the surface abundance of CD5 or the phosphorylation of CD247 between WT and γcTg thymocytes and iNKT cells. Instead, our current findings reveal a γc cytokine-driven mechanism of iNKT subset specification that might operate alternatively or in parallel to TCR-mediated iNKT lineage differentiation [53]. Evidence for γc cytokines in establishing iNKT subset identity has been previously supplied by studies, where IL-15 was found to be required to upregulate T-bet and, thus, would be necessary to specify NKT1 cell fate [36]. However, non-γc cytokines, such as TGFβ and IL-25, were also found to affect iNKT cell differentiation, indicating that γc cytokines would be not unique in their ability to shape the iNKT subset composition [53, 54]. Altogether, cytokines are evidently associated with establishing iNKT subset identity, but a definitive role for cytokines in driving iNKT subset specification had not been formulated. The current study now formally introduces a γc cytokine-driven mechanism as a regulator of iNKT subset specification that promotes NKT2 but suppresses NKT1 cell differentiation.
Notably, the increase in γc abundance also promoted the thymic generation of memory-phenotype innate CD8 T cells, albeit indirectly through the increased production of intrathymic IL-4 by PLZFhi NKT2 cells. While the role of thymus-derived innate CD8 T cells in T cell immunity is only partly understood, innate CD8 T cells are akin to virtual memory (VM) CD8 T cells, which are generated in peripheral tissues. Both CD8 T cell subsets acquire the function and phenotype of effector memory T cells prior to antigen encounter [55]. On the other hand, innate CD8 T cells differ from VM CD8 T cells in that they require IL-4, whereas VM CD8 T cells depend on IL-15 for their generation and survival [56]. In addition, VM CD8 T cells are poor producers of IFNγ, but innate CD8 T cells express copious amounts of IFNγ, indicating that the roles of VM and innate CD8 T cells are presumably distinct and that these subsets would govern different aspects of the innate immune response.
Collectively, the current study provides a new perspective on the molecular mechanism of iT cell generation that involves a necessary a role of the cytokine receptor γc and its abundance during thymocyte development. Importantly, our current data do not dismiss or contradict the requirement for TCR signaling in iT cell development, and do not oppose its role in iNKT cell differentiation. TCR expression and signaling remained unaltered in γcTg mice, but the increased abundance of γc was sufficient to skew the subset composition of iNKT cells. Thus, γc signaling is a new regulator of thymic iT cell differentiation, and this signaling pathway is controlled by the abundance of γc proteins.
Materials and methods
Animals
C57BL/6 (B6) mice and BALB/c mice were obtained from the Charles River Laboratories. Cd1d–/– mice and Stat6–/– mice were purchased from the Jackson Laboratory. Stat5cKO mice were previously described [23], and they were generated by breeding Stat5a/b-floxed mice with E8iii-Cre transgenic mice to delete STAT5a/b expression in thymocytes. Zbtb16–/– mice were previously described and kindly provided by Dr. Pier Pandolfi (Harvard University) [57]. The γcTg mice were generated by ligating a murine γc cDNA into a human CD2 (hCD2) enhancer–promoter-based vector and injecting the construct into fertilized B6 oocytes; these mice were previously described [22]. Mice of both sexes and between the age of 5–12 weeks were used for experiments. Whenever possible, littermate control (LMC) mice were used as wild-type control mice, unless indicated otherwise. The animal experiments were approved by the National Cancer Institute Animal Care and Use Committee, and all mice were cared for in accordance with US National Institutes of Health guidelines.
Cell culture and cell isolation
Single-cell suspensions were prepared by gentle tweezing of thymuses with forceps and filtering through 70 μm nylon meshes. Thymocytes were analyzed either immediately or after in vitro culture (5 × 106 cells per mL) in a 7.5% CO2 atmosphere in RPMI-1640-based cell culture medium supplemented with 10% (vol/vol) FCS. Where indicated, the cells were stimulated with mouse recombinant IL-4 (Peprotech).
Flow cytometry
Organs were processed to generate single-cell suspensions, stained, and analyzed by FACS LSRII, LSR Fortessa or FACSCalibur flow cytometers (Becton Dickinson). Dead cells were excluded by forward light scatter gating and propidium iodide staining. The data were analyzed using software designed by the Division of Computer Research and Technology, NIH. Antibodies with the following specificities were used for staining: CD3 (145-2C11), CD4 (GK1.5 and RM4.5), CD5 (53–7.3), CD8α (53–6-7), TCRβ (H57-597), CD69 (H1.2F3), γc (4G3), CD44 (IM7), IL-4Rα (M1), CD122 (TM-β1), T-bet (O4-46), RORγt (Q31-378), and phosphorylated STAT5 (pSTAT5)(47; all from BD Biosciences); HSA (M1/69), CXCR3 (CXCR3-173), PLZF (9E12), and IFNγ (XMG1.2; all from Biolegend); Eomes (Dan11mag), NK1.1 (PK136), IL-4 (11B11), phosphorylated CD247 (3ZBR4S) and IL-7Rα (A7R34; all from eBioscience). Fluorochrome-conjugated CD1d tetramers loaded with PBS-57 and unloaded controls were obtained from the NIH tetramer facility (Emory University, Atlanta, GA).
iNKT subset staining
In brief, iNKT cells were stained with PBS-57-loaded mouse CD1d tetramers followed by staining for other surface markers, as previously described [28, 58]. Specifically, for each analysis, thymocytes were stained with fluorochrome-conjugated CD1d tetramers in FACS buffer (0.5% BSA, 0.1% sodium azide in Ca2+ and Mg2+-free HBSS) for 20 min at 4 °C. Without removing the tetramer reagents, antibodies against surface proteins were added, and cells were incubated for an additional 30 min at 4 °C. After washing out excess reagents by cell centrifugation, pelleted cells were resuspended in 150 μL of 1:3 mixed concentrate/diluent working solution of the Foxp3 Transcription Factor Staining Buffer kit (eBioscience) and 100 μL of FACS buffer and incubated at room temperature for 20 min. Cells were then washed twice with 1 × permeabilization buffer (eBioscience), before adding antibodies for transcription factors, such as PLZF, RORγt, and T-bet. Cells were incubated at room temperature for 1 h, before washing out excess reagents with FACS buffer and flow cytometric analysis.
Cytokine-induced pSTAT5 analysis
Cytokine-induced STAT5 phosphorylation was assessed as previously described [23, 59]. In brief, freshly isolated thymocytes were stimulated with recombinant IL-4 for 30 min, after which the cytokine stimulation was terminated by the addition of an equal volume of 4% paraformaldehyde in PBS. After quenching the reaction with 10% FBS, the cells were washed twice in FACS buffer (0.05% Na-azide, 0.05% BSA in HBSS) and permeabilized with ice-cold MeOH. After washing the fixed and permeabilized cells two times with FACS buffer, intracellular pSTAT5 was detected with anti-pSTAT5 antibodies (clone 47, BD Bioscience).
Intracellular cytokine expression analysis
Single cell suspension of thymocytes were stimulated for 4 h with PMA (25 ng/ml) and ionomycin (1 mM) (both from Sigma) in the presence of 3.0 μg/mL brefeldin A (eBioscience) for intracellular cytokine staining. Stimulation was terminated by washing cells in ice-cold FACS buffer (0.5% BSA 0.1% sodium azide in HBSS). Dead cells were counterstained with 1 μL of Aqua Live/Dead solution per staining (eBioscience). Excess reagents were washed out by cell centrifugation with ice-cold FACS buffer after 30 min incubation at 4 °C. Surface proteins were stained with the indicated antibodies for 20 min at 4 °C, and excess reagents were washed out with FACS buffer by centrifugation for 7 min at 1500 rpm. Pelleted cells were then resuspended in 100 μL of FACS buffer, to which 150 μL of a 1:3 mixture of concentrate/diluent working solution of the Foxp3 Transcription Factor Staining Buffer kit (eBioscience) was added. Cells were then incubated at room temperature for 20 min after which cells were washed twice with 1 × permeabilization buffer (eBioscience) before adding antibodies for transcription factors and cytokines. Excess reagents were washed out with FACS buffer before flow cytometric analysis.
Statistical analysis
Statistical differences were analyzed using Student’s two-tailed t test. P values of less than 0.05 were considered significant. *P < 0.05, **P < 0.01, ***P < 0.001, and NS (not significant) (Student’s two-tailed t test).
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank Dr. Damian Kovalovsky (NCI) for ciritical comments on this manuscript. We also thank the EIB flow cytometry core for their expertise and help with FACS data acquisition and analysis.
Author contribution
JYP and HW designed and performed the experiments, analyzed the data, and contributed to the writing of the manuscript. DTD and CH performed experiments and analyzed the data. JHP conceived the project, supervised the study, and wrote the manuscript.
Funding
This study was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH, and by a National Research Foundation of Korea grant (NRF-2018R1A5A2024418) funded by the Korean government MSIT (Ministry of Science and ICT).
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Approval of animal experiments was granted by the NCI Animal Care and Use Committee under the animal protocol number EIB-076 and ASP-421. All mice were cared for in accordance with the Public Health Service policy on human care and use of laboratory animals and NIH guidelines.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joo-Young Park and Hee Yeun Won have contributed equally to this work.
References
- 1.Fink PJ, Hendricks DW. Post-thymic maturation: young T cells assert their individuality. Nat Rev Immunol. 2011;11(8):544–549. doi: 10.1038/nri3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seddon B, Yates AJ. The natural history of naive T cells from birth to maturity. Immunol Rev. 2018;285(1):218–232. doi: 10.1111/imr.12694. [DOI] [PubMed] [Google Scholar]
- 3.Alonzo ES, Sant'Angelo DB. Development of PLZF-expressing innate T cells. Curr Opin Immunol. 2011;23(2):220–227. doi: 10.1016/j.coi.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jameson SC, Lee YJ, Hogquist KA. Innate memory T cells. Adv Immunol. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banach M, Robert J. Evolutionary underpinnings of innate-like T cell interactions with cancer. Immunol Invest. 2019;48(7):737–758. doi: 10.1080/08820139.2019.1631341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermijlen D, Prinz I. Ontogeny of Innate T Lymphocytes - Some Innate Lymphocytes are More Innate than Others. Front Immunol. 2014;5:486. doi: 10.3389/fimmu.2014.00486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salou M, Legoux F, Lantz O. MAIT cell development in mice and humans. Mol Immunol. 2021;130:31–36. doi: 10.1016/j.molimm.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Weinreich MA, Odumade OA, Jameson SC, Hogquist KA. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat Immunol. 2010;11(8):709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gascoigne NR, Rybakin V, Acuto O, Brzostek J. TCR Signal Strength and T Cell Development. Annu Rev Cell Dev Biol. 2016;32:327–348. doi: 10.1146/annurev-cellbio-111315-125324. [DOI] [PubMed] [Google Scholar]
- 10.Singer A, Adoro S, Park JH. Lineage fate and intense debate: myths, models and mechanisms of CD4- versus CD8-lineage choice. Nat Rev Immunol. 2008;8(10):788–801. doi: 10.1038/nri2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zlotnik A, Moore TA. Cytokine production and requirements during T-cell development. Curr Opin Immunol. 1995;7(2):206–213. doi: 10.1016/0952-7915(95)80005-0. [DOI] [PubMed] [Google Scholar]
- 12.Waickman AT, Park JY, Park JH. The common gamma-chain cytokine receptor: tricks-and-treats for T cells. Cell Mol Life Sci. 2016;73(2):253–269. doi: 10.1007/s00018-015-2062-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiSanto JP, Muller W, Guy-Grand D, Fischer A, Rajewsky K. Lymphoid development in mice with a targeted deletion of the interleukin 2 receptor gamma chain. Proc Natl Acad Sci USA. 1995;92(2):377–381. doi: 10.1073/pnas.92.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park JY, Jo Y, Ko E, Luckey MA, Park YK, Park SH, et al. Soluble gammac cytokine receptor suppresses IL-15 signaling and impairs iNKT cell development in the thymus. Sci Rep. 2016;6:36962. doi: 10.1038/srep36962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaughtry TM, Etzensperger R, Alag A, Tai X, Kurtulus S, Park JH, et al. Conditional deletion of cytokine receptor chains reveals that IL-7 and IL-15 specify CD8 cytotoxic lineage fate in the thymus. J Exp Med. 2012;209(12):2263–2276. doi: 10.1084/jem.20121505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ligons DL, Hwang S, Waickman AT, Park JY, Luckey MA, Park JH. RORgammat limits the amount of the cytokine receptor gammac through the prosurvival factor Bcl-xL in developing thymocytes. Sci Signal. 2018 doi: 10.1126/scisignal.aam8939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun Z, Unutmaz D, Zou YR, Sunshine MJ, Pierani A, Brenner-Morton S, et al. Requirement for RORgamma in thymocyte survival and lymphoid organ development. Science. 2000;288(5475):2369–2373. doi: 10.1126/science.288.5475.2369. [DOI] [PubMed] [Google Scholar]
- 18.Kurebayashi S, Ueda E, Sakaue M, Patel DD, Medvedev A, Zhang F, et al. Retinoid-related orphan receptor gamma (RORgamma) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc Natl Acad Sci USA. 2000;97(18):10132–10137. doi: 10.1073/pnas.97.18.10132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crispe IN, Bevan MJ. Expression and functional significance of the J11d marker on mouse thymocytes. J Immunol. 1987;138(7):2013–2018. [PubMed] [Google Scholar]
- 20.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–176. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 21.Yu Q, Park JH, Doan LL, Erman B, Feigenbaum L, Singer A. Cytokine signal transduction is suppressed in preselection double-positive thymocytes and restored by positive selection. J Exp Med. 2006;203(1):165–175. doi: 10.1084/jem.20051836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong C, Luckey MA, Ligons DL, Waickman AT, Park JY, Kim GY, et al. Activated T cells secrete an alternatively spliced form of common gamma-chain that inhibits cytokine signaling and exacerbates inflammation. Immunity. 2014;40(6):910–923. doi: 10.1016/j.immuni.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, et al. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11(3):257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist KA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14(11):1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gordon SM, Carty SA, Kim JS, Zou T, Smith-Garvin J, Alonzo ES, et al. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J Immunol. 2011;186(8):4573–4578. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, Hogquist KA. KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity. 2009;31(1):122–130. doi: 10.1016/j.immuni.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang W, August A. The signaling symphony: T cell receptor tunes cytokine-mediated T cell differentiation. J Leukoc Biol. 2015;97(3):477–485. doi: 10.1189/jlb.1RI0614-293R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park JY, DiPalma DT, Kwon J, Fink J, Park JH. Quantitative difference in PLZF protein expression determines iNKT lineage fate and controls innate CD8 T cell generation. Cell Rep. 2019;27(9):2548–2574. doi: 10.1016/j.celrep.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kovalovsky D, Uche OU, Eladad S, Hobbs RM, Yi W, Alonzo E, et al. The BTB-zinc finger transcriptional regulator PLZF controls the development of invariant natural killer T cell effector functions. Nat Immunol. 2008;9(9):1055–1064. doi: 10.1038/ni.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Savage AK, Constantinides MG, Han J, Picard D, Martin E, Li B, et al. The transcription factor PLZF directs the effector program of the NKT cell lineage. Immunity. 2008;29(3):391–403. doi: 10.1016/j.immuni.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei DG, Lee H, Park SH, Beaudoin L, Teyton L, Lehuen A, et al. Expansion and long-range differentiation of the NKT cell lineage in mice expressing CD1d exclusively on cortical thymocytes. J Exp Med. 2005;202(2):239–248. doi: 10.1084/jem.20050413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNab FW, Berzins SP, Pellicci DG, Kyparissoudis K, Field K, Smyth MJ, et al. The influence of CD1d in postselection NKT cell maturation and homeostasis. J Immunol. 2005;175(6):3762–3768. doi: 10.4049/jimmunol.175.6.3762. [DOI] [PubMed] [Google Scholar]
- 33.Benlagha K, Wei DG, Veiga J, Teyton L, Bendelac A. Characterization of the early stages of thymic NKT cell development. J Exp Med. 2005;202(4):485–492. doi: 10.1084/jem.20050456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pellicci DG, Hammond KJ, Uldrich AP, Baxter AG, Smyth MJ, Godfrey DI. A natural killer T (NKT) cell developmental pathway iInvolving a thymus-dependent NK1.1(-)CD4(+) CD1d-dependent precursor stage. J Exp Med. 2002;195(7):835–844. doi: 10.1084/jem.20011544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Townsend MJ, Weinmann AS, Matsuda JL, Salomon R, Farnham PJ, Biron CA, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20(4):477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 36.Gordy LE, Bezbradica JS, Flyak AI, Spencer CT, Dunkle A, Sun J, et al. IL-15 regulates homeostasis and terminal maturation of NKT cells. J Immunol. 2011;187(12):6335–6345. doi: 10.4049/jimmunol.1003965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crosby CM, Kronenberg M. Tissue-specific functions of invariant natural killer T cells. Nat Rev Immunol. 2018;18(9):559–574. doi: 10.1038/s41577-018-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lai D, Zhu J, Wang T, Hu-Li J, Terabe M, Berzofsky JA, et al. KLF13 sustains thymic memory-like CD8(+) T cells in BALB/c mice by regulating IL-4-generating invariant natural killer T cells. J Exp Med. 2011;208(5):1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuttle KD, Krovi SH, Zhang J, Bedel R, Harmacek L, Peterson LK, et al. TCR signal strength controls thymic differentiation of iNKT cell subsets. Nat Commun. 2018;9(1):2650. doi: 10.1038/s41467-018-05026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dashtsoodol N, Bortoluzzi S, Schmidt-Supprian M. T Cell Receptor expression timing and signal strength in the functional differentiation of invariant natural killer T cells. Front Immunol. 2019;10:841. doi: 10.3389/fimmu.2019.00841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M, Svensson MND, Venken K, Chawla A, Liang S, Engel I, et al. Altered thymic differentiation and modulation of arthritis by invariant NKT cells expressing mutant ZAP70. Nat Commun. 2018;9(1):2627. doi: 10.1038/s41467-018-05095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998;188(12):2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitcher LA, Young JA, Mathis MA, Wrage PC, Bartok B, van Oers NS. The formation and functions of the 21- and 23-kDa tyrosine-phosphorylated TCR zeta subunits. Immunol Rev. 2003;191:47–61. doi: 10.1034/j.1600-065x.2003.00003.x. [DOI] [PubMed] [Google Scholar]
- 44.Lin JX, Leonard WJ. The common cytokine receptor gamma Chain Family of Cytokines. Cold Spring Harb Perspect Biol. 2018 doi: 10.1101/cshperspect.a028449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hou J, Schindler U, Henzel WJ, Ho TC, Brasseur M, McKnight SL. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- 46.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9(7):480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hogquist K, Georgiev H. Recent advances in iNKT cell development. F1000Res. 2020 doi: 10.12688/f1000research.21378.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kwon DI, Lee YJ. Lineage differentiation program of invariant natural killer T Cells. Immune Netw. 2017;17(6):365–377. doi: 10.4110/in.2017.17.6.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Joseph C, Klibi J, Amable L, Comba L, Cascioferro A, Delord M, et al. TCR density in early iNKT cell precursors regulates agonist selection and subset differentiation in mice. Eur J Immunol. 2019;49(6):894–910. doi: 10.1002/eji.201848010. [DOI] [PubMed] [Google Scholar]
- 50.Lazarevic V, Zullo AJ, Schweitzer MN, Staton TL, Gallo EM, Crabtree GR, et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat Immunol. 2009;10(3):306–313. doi: 10.1038/ni.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seiler MP, Mathew R, Liszewski MK, Spooner CJ, Barr K, Meng F, et al. Elevated and sustained expression of the transcription factors Egr1 and Egr2 controls NKT lineage differentiation in response to TCR signaling. Nat Immunol. 2012;13(3):264–271. doi: 10.1038/ni.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leonard WJ, Lin JX, O'Shea JJ. The gammac family of cytokines: basic biology to therapeutic ramifications. Immunity. 2019;50(4):832–850. doi: 10.1016/j.immuni.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 53.Buechel HM, Stradner MH, D'Cruz LM. Stages versus subsets: invariant natural killer T cell lineage differentiation. Cytokine. 2015;72(2):204–209. doi: 10.1016/j.cyto.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havenar-Daughton C, Li SM, Benlagha K, Marie JC. Development and function of murine ROR gamma t(+) iNKT cells are under TGF-beta signaling control. Blood. 2012;119(15):3486–3494. doi: 10.1182/blood-2012-01-401604. [DOI] [PubMed] [Google Scholar]
- 55.Lee JY, Hamilton SE, Akue AD, Hogquist KA, Jameson SC. Virtual memory CD8 T cells display unique functional properties. Proc Natl Acad Sci USA. 2013;110(33):13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White JT, Cross EW, Kedl RM. Antigen-inexperienced memory CD8(+) T cells: where they come from and why we need them. Nat Rev Immunol. 2017;17(6):391–400. doi: 10.1038/nri.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36(6):653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- 58.Park JY, Kwon J, Kim EY, Fink J, Kim HK, Park JH. CD24(+) Cell depletion permits effective enrichment of thymic iNKT cells while preserving their subset composition. Immune Netw. 2019;19(2):e14. doi: 10.4110/in.2019.19.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waickman AT, Ligons DL, Hwang S, Park JY, Lazarevic V, Sato N, et al. CD4 effector T cell differentiation is controlled by IL-15 that is expressed and presented in trans. Cytokine. 2017;99:266–274. doi: 10.1016/j.cyto.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Not applicable.