Abstract
Legend for Graphical Abstract. A time line of the study is shown. Surgery is performed on Day 0 of the experiment and the CPB circuit is illustrated (adapted from Grocott et al.2). At 5½ months post-CPB, food restriction is initiated. Starting 6 months post-CPB, behavioral testing is performed using an 8-arm maze. Animals are euthanized at the end of behavioral testing via perfusion fixation, and cellular analyses on tissue sections are performed using histological and immunohistochemical techniques. Cognitive impairment and increased neuroinflammation were key findings in the CPB group.
Advances in surgical techniques and medical management have substantially improved mortality and morbidity in patients undergoing cardiopulmonary bypass (CPB). Although post-operative cognitive dysfunction is common after CPB, the prevalence and mechanism(s) of intermediate and long-term cognitive deficits in the absence of neuronal loss remain a matter of discussion.1 Utilizing an established rat model of CPB2 that does not display neuronal loss3,4, we examined behavioral and structural effects of CPB at 6 months post-surgery. Persistent deficits in performance on a complex behavioral task and sustained activation of macrophages/microglia were observed with no evidence of neuronal loss.
Materials and Methods
A flow chart for the study is presented in Supplementary Material. Male Sprague-Dawley rats (375-400g) were randomized to two groups: 1) CPB and 2) Sham Surgery (n=6/group). The CPB procedure, involving a 60-minute period of bypass, was performed as described previously2 (see also Supplementary Material). The Sham Surgery group received an identical surgical procedure and duration of anesthesia as did the CPB group, except that the CPB circuit was not activated. At 6 months post-CPB, behavioral testing was performed using a win-shift task on an 8-arm radial maze.5 Win-shift is a challenging cognitive task, involving a multi-stage foraging strategy that requires animals to retain and compare information about the location of food over a period of 0.25 to 12 hours. The task utilizes two sequential trials separated by a variable inter-trial interval. The first trial presents four food-baited arms in the maze with the other four arms blocked by a clear Plexiglass door. During the initial stage of task acquisition, the second (retention) trial occurs 5 minutes after the first trial. In the second trial, all maze arms are open, but only those arms that were not baited in the first trial are baited. A correct choice in the second trial is when an animal visits a baited (previously unbaited) arm of the maze. An incorrect choice is when an animal visits an unbaited (previously baited) arm. Once animals have acquired the task, the interval between the first and second trials is progressively increased in duration. After behavioral testing was completed, animals were euthanized under deep anesthesia by intracardial perfusion of fixative. Brains were sectioned and processed for microscopic assessment of Nissl staining (Cresyl Violet), interneuron subtypes (anti-calretinin and anti-parvalbumin), immature neurons (anti-doublecortin: DCX), and macrophages/microglia (anti-CD68). Staining for degenerating neurons (Fluro-Jade) was performed in additional animals at 2, 4, and 7 days post-CPB (n=2 animals/time point).
Results
Animals in the CPB group exhibited deficits in performance on the win-shift task as compared with those in the Sham Surgery group (Figure 1). At shorter inter-trial intervals, performance was similar between groups, indicating that animals in both groups were able to learn and perform the task. However, at longer inter-trial intervals (3 and 6 hours) the CPB group exhibited significantly poorer performance.
Figure 1.
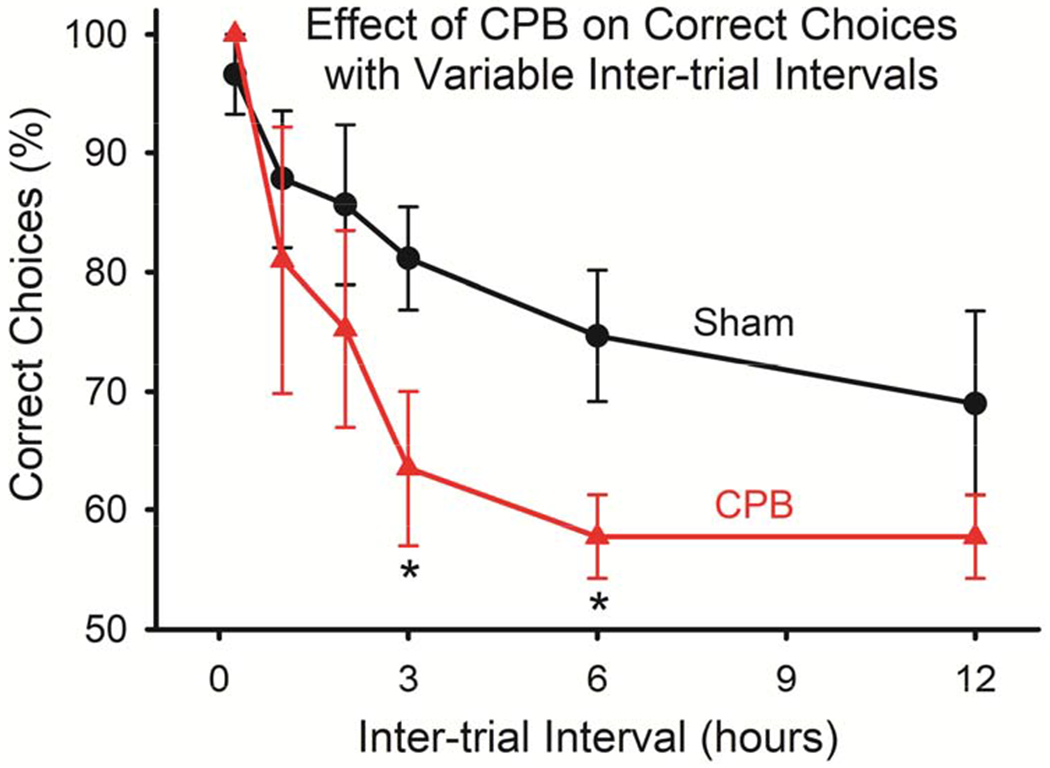
Impaired performance on the win-shift task by animals subjected to CPB. Animals in both groups acquired the task, and performed similarly at short (0.25, 1, and 2 hours) inter-trial intervals. However, at longer intervals (3 and 6 hours), the CPB group performed significantly more poorly (*P<0.05, One-way ANOVA).
Qualitative assessments of Nissl-stained and immunohistochemically-stained sections did not indicate a loss of primary neurons or interneurons in the hippocampus at 6 months post-CPB (Figure 2). In addition, Fluro-Jade staining at 2, 4, and 7 days post-CPB did not reveal degenerating neurons (data not shown). In contrast, a significant increase in the number of activated microglia, and a trend toward reduced numbers of immature neurons, were observed at 6 months post-CPB (Figure 2). The timeline and fundamental outcomes of this study are summarized in Figure 3.
Figure 2.
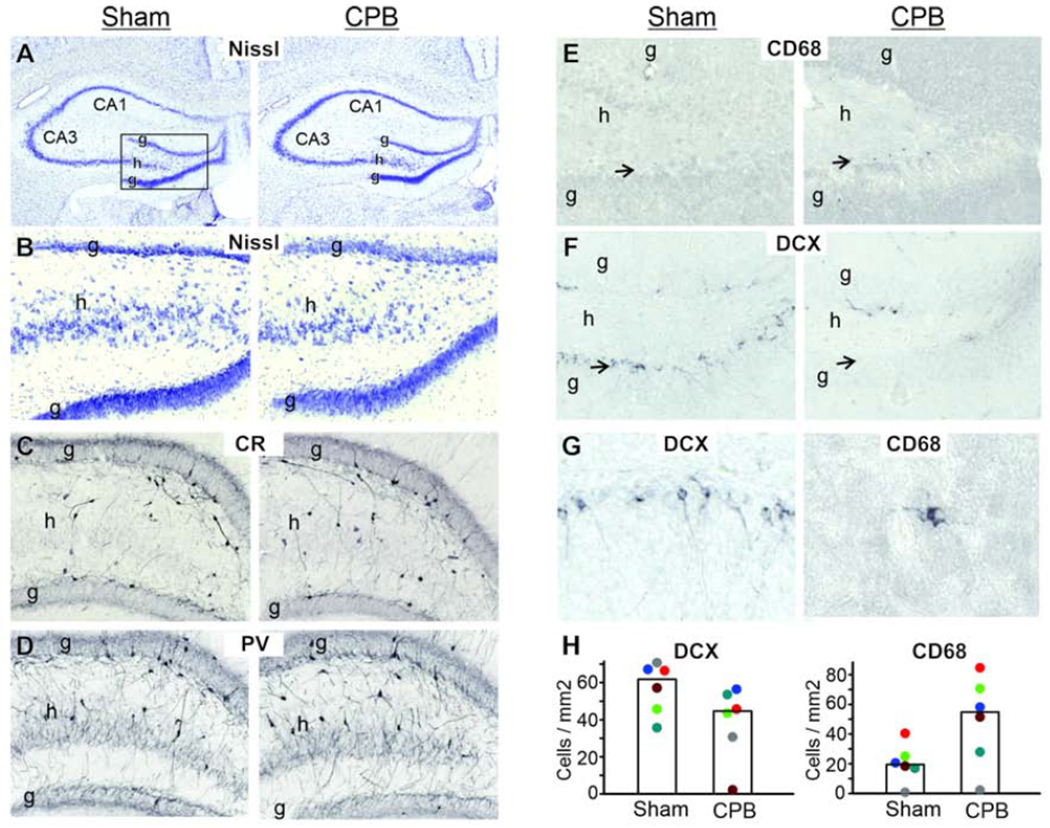
A-G:Tissue sections stained for cell bodies (Nissl), calretinin interneurons (CR), parvalbumin interneurons (PV), macrophages/activated microglia (CD68), and immature neurons (DCX) are shown. The rectangle in the left panel of A, which frames the dentate gyrus of the hippocampus, depicts the areas shown in B-F (g=granule cell layer; h=hilus). No apparent loss of primary neurons or interneurons was observed. In contrast, the population of activated microglia/macrophages appeared increased (E) while the population of immature neurons appeared decreased (F) in the CPB group (arrows indicate infragranular zone). In G, higher magnification images of DCX+ and CD68+ cells illustrate the typical morphology of immature neurons and activated microglia, respectively. H: Quantitative cell counting demonstrates a significant (p=0.04, t-test) increase of CD-68+ cells, and non-significant trend (p=0.09, t-test) for a decrease in DCX+ cells in the CPB group. Graph bars are medians and dots are individual animals.
Figure 3.
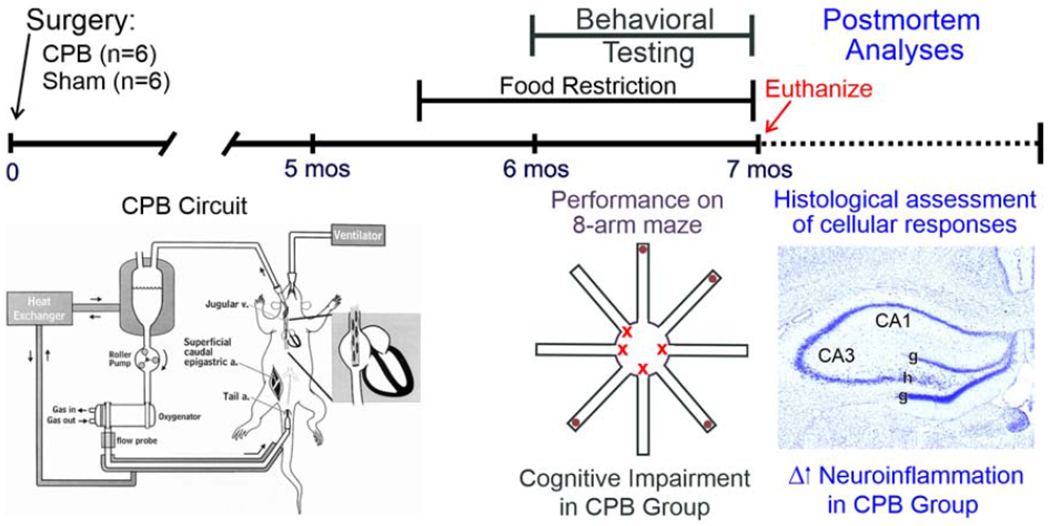
A time line of the study is shown. Surgery is performed on Day 0 of the experiment and the CPB circuit is illustrated (adapted from Grocott et al.2). At 5½ months post-CPB, food restriction is initiated. Starting 6 months post-CPB, behavioral testing is performed using an 8-arm maze. Animals are euthanized at the end of behavioral testing via perfusion fixation, and cellular analyses on tissue sections are performed using histological and immunohistochemical techniques. Cognitive impairment and increased neuroinflammation were key findings in the CPB group.
Discussion
Our findings demonstrate that persistent cognitive dysfunction exists in an established rat model of CPB. It is notable that this CPB model has been shown not to produce neuronal loss3,4, a finding corroborated by our histological outcomes. Consequently, it is reasonable to consider alternative, non-neurodegenerative mechanisms as potentially underlying the cognitive deficit. Two mechanisms that could possibly contribute to cognitive dysfunction are increased neuroinflammation and reduced adult neurogenesis. Our finding of a long-term increase in the number of activated macrophages/microglia after CPB is consistent with a sustained neuroinflammatory response. This response occurs in the infragranular zone of the dentate gyrus, an area that exhibits neurogenesis in adulthood. There was a trend toward fewer immature neurons after CPB. This is notable because alterations in adult neurogenesis can affect learning and memory. Clearly other mechanisms contributing to cognitive compromise, such as disturbances in white matter, are possible and are not ruled-out by the current study. Nonetheless, our findings are parsimonious with the hypothesis that sustained neuroinflammation and perhaps reduced neurogenesis contribute to persistent cognitive dysfunction after CPB.
Supplementary Material
Central Picture Legend:
Performance on a cognitive task was impaired 6 months after cardiopulmonary bypass.
Central Message:
Long-term cognitive dysfunction and neuroinflammation were demonstrated in an establshed rat model of cardiopulmonary bypass.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None reported
References
- 1.Fink HA, Hemmy LS, MacDonald R, Carlyle MH, Olson CM, Dysken MW, McCarten JR, Kane RL, Garcia SA, Rutkus IR, Ouelette J, Wilt TJ. Intermediate- and long-term cognitive outcomes after cardiovascular procedures in older adults: a systematic review. Annals of Internal Medicine. 2015; 163:107–117. [DOI] [PubMed] [Google Scholar]
- 2.Grocott HP, Mackensen GB, Newman MF & Warner DS Neurological injury during cardiopulmonary bypass in the rat. Perfusion. 2001; 16:75–81. [DOI] [PubMed] [Google Scholar]
- 3.Mackensen GB, et al. Cardiopulmonary bypass induces neurologic and neurocognitive dysfunction in the rat. Anesthesiology 95, 1485–1491 (2001). [DOI] [PubMed] [Google Scholar]
- 4.de Lange F, Jones WL, Mackensen GB, Grocott HP. The effect of limited rewarming and postoperative hypothermia on cognitive function in a rat cardiopulmonary bypass model. Anesthesia and Analgesia. (2008);106:739–745. [DOI] [PubMed] [Google Scholar]
- 5.Williams CL, Packard MG, McGaugh JL. Amphetamine facilitation of win-shift radial-arm maze retention: The involvement of peripheral adrenergic and central dopmaninergic systems. Psychobiology. (1994);22:141–148. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


