Abstract
A quantitative nucleic acid sequence-based amplification (QT-NASBA) assay for the detection of Plasmodium parasites has been developed. Primers and probes were selected on the basis of the sequence of the small-subunit rRNA gene. Quantification was achieved by coamplification of the RNA in the sample with one modified in vitro RNA as a competitor in a single-tube NASBA reaction. Parasite densities ranging from 10 to 108 Plasmodium falciparum parasites per ml could be demonstrated and quantified in whole blood. This is approximately 1,000 times more sensitive than conventional microscopy analysis of thick blood smears. Comparison of the parasite densities obtained by microscopy and QT-NASBA with 120 blood samples from Kenyan patients with clinical malaria revealed that for 112 of 120 (93%) of the samples results were within a 1-log difference. QT-NASBA may be especially useful for the detection of low parasite levels in patients with early-stage malaria and for the monitoring of the efficacy of drug treatment.
Malaria due to infection with Plasmodium falciparum is an acute parasitic disease which kills an estimated 3 million people per year, mainly children in developing countries. The fight against malaria is more and more hampered by the occurrence of widespread resistance of P. falciparum against first-line and second-line antimalarial drugs such as chloroquine and pyrimethamine-sulfadoxine (Fansidar).
In view of this increasingly widespread drug resistance, laboratory techniques are becoming more and more important: first, in initial diagnosis to prevent treatment with antimalarial drugs for patients with diseases other than malaria and, subsequently, during and after treatment to assess the efficacy of treatment.
At present, the parasite is routinely detected by microscopy of Giemsa-stained thick blood films. This technique is cheap, is easy to perform, and allows quantification. However, thick blood film examination of samples from patients with low levels of parasitemia, as may be found after treatment, is time-consuming. As the detection limit is about 20 parasites per μl (3), a substantial number of patients with low levels of parasitemia may be missed by this technique (10).
In the last decade, a number of new techniques have become available. Antigen detection methods such as Parasight-F (Becton Dickinson, Franklin Lakes, N.J.), ICT MalariaPf (ICT Diagnostics, Sydney, Australia), and OptiMAL (Flow Inc., Portland, Oreg.) (10) are fast and simple to perform; but for patients with low levels of parasitemia (<100 parasites/μl) the sensitivity decreases (10), making these tests unsuitable for the detection of low levels of parasitemia. A number of PCR assays have been developed and evaluated (1, 6, 10, 12, 14, 16). The technique was shown to be sensitive (lowest limit of detection, 1 parasite/50 μl of blood) (6) and can be applied to whole blood or blood spots stored on filter paper, but it allows only the semiquantification of parasites (9).
The availability of a fast, sensitive, reliable, and quantitative method for the detection of parasite survival during and after drug treatment will help clinicians monitor and, if necessary, adjust the treatment regimen. Here we describe the development and evaluation of a quantitative nucleic acid sequence-based amplification (QT-NASBA) assay for the detection and quantification of P. falciparum in blood samples.
MATERIALS AND METHODS
Sample collection and storage.
Blood samples were collected from malaria patients visiting two health centers, Oyugis Bay and Kendu Bay in Kenya, located in an area where malaria is holoendemic. Fifty microliters of EDTA-anticoagulated blood was mixed with 950 μl of guanidinium isothiocyanate (GuSCN) L6 lysis buffer, and the mixture was stored in liquid nitrogen until it was processed for RNA isolation. At the same time, thick blood films were prepared from these blood samples for counting of the parasites microscopically.
Lysis buffer was made by dissolving 120 g of GuSCN in 100 ml of 0.1 M Tris-HCl (pH 6.4), after which 22 ml of 0.2 M EDTA (pH 8.0) and 2.6 g of Triton X-100 were added (2).
Microscopy.
The microscopic examination of thick blood smears was used as the “gold standard” for comparison with the quantification of parasites by QT-NASBA. Thick blood films were stained with Giemsa and were examined microscopically (8). Parasites and white blood cells were counted to a total of 200 white blood cells. The number of parasites per microliter of blood was calculated by assuming that there are 8,000 white blood cells per μl of blood.
Cloning and production of in vitro RNA.
Primers Plas-1F (5′-TCAGATACCGTCGTAATCTTA-3′) and Plas-2R (5′-AACTTTCTCGCTTGCGCGAA-3′) were used to amplify by PCR a 170-bp region of the P. falciparum asexual-phase 18S rRNA gene. The amplified fragment was cloned into plasmid pCR2.1-TOPO (Invitrogen, Carlsbad, Calif.), and large quantities of in vitro RNA were produced with the transcription kit SP6/T7 (Boehringer, Mannheim, Germany).
Site-directed mutagenesis.
In order to quantify the number of parasites in the blood samples by QT-NASBA, an internal standard RNA was coamplified as a competitor by using the same amplification primers used for the wild-type (WT) RNA.
For this construct, 20 bases in the center of the WT target sequence were randomly rearranged by using a custom-made computer program designed by Organon Teknika, Boxtel, The Netherlands. The resulting sequence was checked by computer analysis for the presence of secondary structures and possible homology with the original 170-bp sequence. Next, the modified sequence was constructed by site-directed mutagenesis (7) and was cloned into plasmid pCR2.1-TOPO. In vitro RNA was transcribed as described above. This modified in vitro RNA is referred to as “quantification RNA” (Q-RNA).
For the quantification of parasites in blood, 107 molecules of in vitro Q-RNA were added to each sample.
Q-RNA.
We determined the detection limits of in vitro WT and Q-RNAs with their homologous, ruthenium-labeled detection probes. After amplification by NASBA followed by electrochemiluminescence (ECL) detection, amounts as low as 200 copies of in vitro RNA could be detected for both RNAs. No cross hybridization between the two probes and target RNAs could be observed. This made the in vitro Q-RNA ideally suited for use as competitor RNA since Q-RNA and WT RNA were amplified with equal efficiencies.
Nucleic acid isolation.
After thawing of the blood sample-L6 lysis buffer mixture, 107 molecules of in vitro Q-RNA were added as competitor RNA. Next, the nucleic acids were isolated from the blood samples by the GuSCN-silica procedure, as described by Boom et al. (2). Briefly, the blood-lysis buffer mixture was mixed with activated silica. The nucleic acids bound to the silica were washed twice with wash buffer (10 M GuSCN, 100 mM Tris-HCl [pH 6.4]), twice with 70% ethanol, and once with acetone. The nucleic acids were eluted from the silica with 100 μl of water.
For each experiment a calibration series of blood samples spiked with 108, 106, 104, 103, and 0 P. falciparum parasites per ml of blood were used as control samples and for the standardization of parasite quantification.
QT-NASBA.
Primers and probes were selected on the basis of the published sequences of the 18S rRNA genes of P. falciparum and were described earlier by Smits et al. (13). For the NASBA, primers Plas-1F (5′-TCAGATACCGTCGTAATCTTA-3′) and Plas-2R T7 (5′-AATTCTAATACGACTCACTATAGGGAGAGAACTTTCTCGCTTGCGCGAA-3′) were used. On the basis of the nucleotide sequence data, RNAs from P. falciparum, P. malariae, P. vivax, and P. ovale should all be amplified by the NASBA isolate with these primers.
Two microliters of the nucleic acid isolated was used in the QT-NASBA. The NASBA reaction was performed as described by Smits et al. (13), but instead of 41 mM KCl, a final concentration of 70 mM KCl was used in the NASBA mixture. The NASBA products were stored at −20°C until further analysis.
Detection.
The NASBA amplification products were diluted 25 times in water and were subsequently hybridized to a capture probe (5′-ACCATAAACTATGCCGACTAGG-3′) which was bound to streptavidin-coated magnetic beads. The samples were then separately hybridized to ruthenium-labeled WT (5′-CCTTATGAGAAATCAAAGTC 3′) and Q (5′-AATAACTGCACCAGTGTATA-3′) detection probes, followed by ECL detection in a NucliSens ECL reader (Organon Teknika). The light emitted is detected by a photoelectric cell, thus offering a precise measurement for quantification (15).
Calculations and statistical analysis.
In order to quantify the number of parasites in the blood samples by QT-NASBA, the WT RNA was coamplified with the internal standard RNA (Q-RNA) as a competitor by using the same amplification primers in a single-tube NASBA reaction.
After hybridization of the NASBA product to the WT and Q detection probes, the ECL provided for each sample signals for WT RNA and Q-RNA. Due to the competition for the amplification primers, a low parasite concentration in the sample generates a low signal for WT RNA and a high signal for Q-RNA, whereas a high parasite concentration shows a high signal for WT RNA and a low signal for Q-RNA. The signals for WT RNA and Q-RNA are directly correlated to the number of parasites present in the samples.
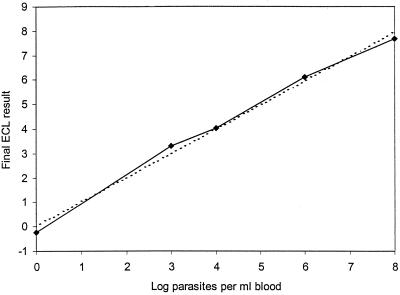
In each experiment, a standard curve was made by using blood samples containing known numbers of parasites: 108, 106, 104, 103, and 0 parasites per ml of blood mixed with 107 molecules of in vitro Q-RNA. Final ECL results were calculated as log [(WT RNA signal/Q-RNA signal) × 1,000] and were plotted on a standard curve in which the x axis represents the log parasite concentration and the y axis represents the final ECL result. Best-fit regression analysis was performed with the Excel software package. For an example, see Fig. 1.
FIG. 1.
Correlation of the final ECL results and the number of parasites in the sample. The final ECL results were calculated as indicated in the Materials and Methods section. The best-fit regression line (dotted line) was generated as indicated in the Materials and Methods section.
The parasite concentration in samples with unknown numbers of parasites was then calculated by using the mathematical equation y = ax + b, in which y is the calculated log number of parasites, a is the slope as calculated from the best-fit regression line through the standard curve, x is the final ECL result, and b is the intercept with the y axis of the best-fit regression line through the standard curve.
Detection limit.
One-milliliter blood samples were spiked with various numbers of P. falciparum parasites ranging from 0 to 440,000 parasites and were mixed with 107 molecules Q-RNA. These samples were tested blindly by the QT-NASBA.
Reproducibility.
The reproducibility of the QT-NASBA was evaluated by using the guidelines (EP10-A) of the National Committee for Clinical Laboratory Standards (11).
Nine samples were analyzed on 5 successive days by using in each run three samples each with low, middle, and high levels of parasites in a specific order, as indicated in the guidelines. For these samples, “low” comprises samples with 200 P. falciparum parasites per ml of blood, “middle” comprises samples with 105 parasites per ml of blood and “high” comprises samples with 107 parasites per ml blood. All samples were tested by the procedure described above.
The imprecision of the test method was determined by calculating the mean and standard deviation (SD) log number of parasites for all samples with low, medium, and high parasite loads. The imprecision was calculated by using the following formula: (SD/mean) · 100. The within-run variability was determined by calculating the mean ± SD log number of parasites for the samples with low, medium, and high parasite loads per day by the same formula described above. The between-run variability was determined by calculating the mean ± SD log number of parasites for the samples with low, medium, and high parasite loads over 5 days, again by the formula mentioned above.
RESULTS
Detection limit.
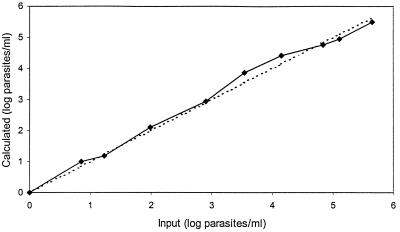
Figure 2 shows the relationship between the number of parasites present in the sample and the number calculated from the QT-NASBA results.
FIG. 2.
Results obtained by QT-NASBA with a series of blood samples spiked with different numbers of cultured P. falciparum parasites.
From Fig. 1 and 2 it follows that in spiked blood samples a range between 10 and 108 parasites per ml of blood could be quantified.
Reproducibility.
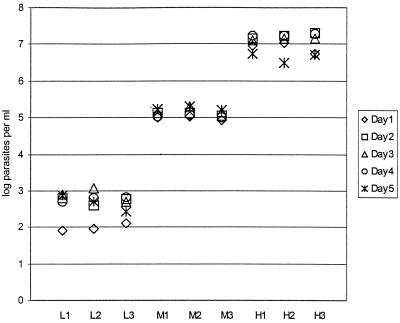
Figure 3 shows the log number of parasites per milliliter calculated by the QT-NASBA on 5 different days.
FIG. 3.
Reproducibility of QT-NASBA. Three sets of samples (with low, medium, and high parasite loads; three samples each [L1, L2, and L3, M1, M2, and M3, and H1, H2, and H3, respectively]) were tested on 5 consecutive days. The results are expressed as log number of parasites per milliliter.
The mean log number of parasites for the samples with low, medium, and high parasite loads were 2.61 (SD = 0.34), 5.11 (SD = 0.11), and 7.03 (SD = 0.24), respectively. The imprecision of the method was found to be 13.1% for the samples with low parasite loads, 2.1% for the samples with medium parasite loads, and 3.5% for the samples with high parasite loads.
The within-run variability was calculated to be 2.0 to 7.1% (samples with low parasite loads), 0.7 to 1.4% (samples with medium parasite loads), and 0.3 to 1.8% (samples with high parasite loads). The between-run variability was 10.3 to 14.1% (samples with low parasite loads), 1.5 to 2.2% (samples with medium parasite loads), and 2.5 to 4.0% (samples with high parasite loads).
Comparison with microscopy.
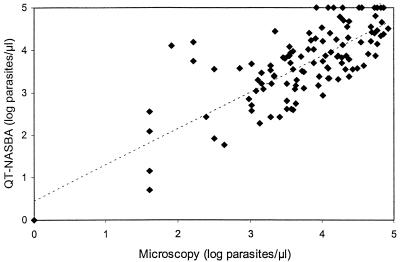
Comparison of the counts obtained by microscopic examination of thick blood smears with the results obtained by the QT-NASBA with 120 blood samples from patients with clinical malaria revealed for 112 of 120 samples (93%) a less than 1-log difference between the results obtained by both methods (Fig. 4). The differences between NASBA and microscopy were randomly distributed, as evidenced by a mean difference of 0.08 log (SD = 0.62). The remaining eight samples had log differences of 1.04 (two samples), 1.08 (three samples), 1.53 (one sample), 2 (one sample), and 2.22 (one sample). Fifteen samples had relatively low levels of parasitemia (less than 500 parasites/μl). Four of the eight samples with a greater than 1-log difference between microscopy and QT-NASBA belonged to this group of samples from patients with low levels of parasitemia.
FIG. 4.
Comparison of microscopy and QT-NASBA results with blood samples from 120 Kenyan patients with clinical malaria. The best-fit regression line (dotted line) was generated.
Four blood samples that were negative by microscopy had extremely small numbers of parasites (0.01, 0.2, 1.1, and 1.2 parasites/μl of blood) when they were tested by the QT-NASBA. These samples were not included in the group of eight samples with a greater than 1-log-unit difference mentioned above.
DISCUSSION
Microscopic examination of Giemsa-stained thick blood films is, for many good reasons, still the most widespread method for the diagnosis of malaria (7). The method is cheap, is relatively fast, and allows species identification as well as quantification of parasites. However, for pregnant women and for nonimmune individuals such as small children a detection threshold which is as low as possible is desirable. Especially in the early stages of malaria, when patients have a low parasite burden, microscopic examination does not always fulfill this requirement.
Many alternative approaches for the diagnosis of malaria have been developed and applied, such as antigen detection tests, acridine orange (a fluorescent dye for staining of parasite DNA and RNA) microscopy, PCR, and serology, all with more or less success (10). Since PCR is able to detect small numbers of parasites (one parasite/50 μl of blood) (6), a semiquantitative test has been used to predict P. falciparum treatment failure (9).
The NASBA has proved to be a sensitive and specific assay in diagnostic microbiology. In addition, there is extensive evidence, especially from the field of virology, that the NASBA can be used for the quantification of infectious agents (5). Smits et al. (13) previously described the development of a qualitative NASBA assay for the detection and semiquantification of Plasmodium. Here we describe the further development of this qualitative NASBA into a QT-NASBA with modified Q-RNA, which is added to each sample. The addition of in vitro Q-RNA, which serves as a competitor, allows the simple and reproducible quantification of parasites in a single-tube assay. The QT-NASBA is suitable for the detection of all human Plasmodium species.
In our hands, the QT-NASBA allows quantification of P. falciparum over a wide range of levels of parasitemia (10 to 108 parasites per ml of blood). The threshold of detection of 10 parasites per ml of blood makes the QT-NASBA approximately 1,000 times more sensitive than routine microscopy. Since only 50 μl of blood is subjected to nucleic acid isolation, we consider 50 parasites/ml to be a more reliable lower threshold of detection.
In addition to the low detection threshold, the QT-NASBA proved to be a reproducible test with respect to imprecision, within-run variability, and between-run variability. It was found that the highest imprecision and variability were encountered at low parasite concentrations. However, this higher imprecision at 200 parasites/ml did not result in differences with a clinical significance.
The results of routine microscopy and QT-NASBA gave an excellent correlation for 112 of 120 (93%) of the samples; for only 8 of 120 blood samples from patients with clinical malaria did we observe a discrepancy between microscopy and QT-NASBA of more than 1 log unit. Four of these eight samples were from patients with low levels of parasitemia. It is well known that the microscopy count is not very exact, especially for patients with low levels of parasitemia (4, 10). In addition, QT-NASBA was capable of detecting low levels of parasitemia in four blood samples which were originally scored as negative by routine microscopy. Since all samples were from patients with clinical signs of malaria, this shows that the QT-NASBA indeed has a lower threshold of detection than routine microscopy. Whether the detection of such low levels of parasitemia is of clinical importance is a subject for further studies.
In conclusion, the QT-NASBA for Plasmodium is a sensitive and reproducible tool for the quantification of Plasmodium parasites in blood samples from malaria patients. The QT-NASBA will not replace routine microscopy for patients with high levels of parasitemia as the test is more expensive, is relatively labor-intensive, and requires a high level of expertise and a well-equipped molecular biology laboratory. However, the QT-NASBA, in contrast to routine microscopy, is excellently suited for the detection and quantification of parasites in patients with low levels of parasitemia, making it a valuable tool both for the diagnosis of malaria in those patients who have low levels of parasitemia and for the monitoring of the efficacy of drug treatment in malaria patients in areas where malaria parasites have a high level of resistance to antimalarial drugs.
ACKNOWLEDGMENTS
This work was financially supported by EU grant ERBIC18-CT97-0227, provided within the INCO-DC program.
We thank Organon Teknika for the provision of various NASBA materials and design of the Q-probe and W. Eling, Catholic University, Nijmegen, The Netherlands, for provision of cultured P. falciparum parasites. Anja Schuitema gave technical support in the initial phase of this work, and Stella van Beers assisted with the statistical analysis of results.
REFERENCES
- 1.Barker R H, Jr, Branchongaskorn T, Courval J M, Suwonkerd W, Rimwungtragoon K, Wirth D F. Plasmodium falciparum and P. vivax: factors affecting sensitivity and specificity of PCR based diagnosis of malaria. Exp Parasitol. 1994;79:41–49. doi: 10.1006/expr.1994.1057. [DOI] [PubMed] [Google Scholar]
- 2.Boom R, Sol C J A, Salimans A A A, Jansen C L, Wertheim-van Dillen P M E, van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruce-Chwatt L J. DNA probes for malaria diagnosis. Lancet. 1984;i:795. doi: 10.1016/s0140-6736(84)91308-4. [DOI] [PubMed] [Google Scholar]
- 4.Carballo A, Ache A. The evaluation of a dipstick for Plasmodium falciparum in mining areas of Venezuela. Am J Trop Med Hyg. 1996;55:482–484. doi: 10.4269/ajtmh.1996.55.482. [DOI] [PubMed] [Google Scholar]
- 5.Chan A B, Fox J D. NASBA and other transcription based amplification methods for research and diagnostic microbiology. Rev Med Microbiol. 1999;10:185–196. [Google Scholar]
- 6.Ciceron L, Jaureguiberry G, Gay F, Danis M. Development of Plasmodium PCR for monitoring efficacy of antimalarial treatment. J Clin Microbiol. 1999;37:35–38. doi: 10.1128/jcm.37.1.35-38.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ho S N, Hunt H D, Horton R M, Pullen J K, Pease L R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- 8.Hommel M, Gilles H M. Topley and Wilson's microbiology and microbial infections. 9th ed. London, United Kingdom: Arnolds; 1998. Malaria; pp. 384–409. [Google Scholar]
- 9.Kain K C, Kyle D E, Wongsrichanalai C, Brown A E, Webster H K, Vanijanonta S, Looareesuwan S. Qualitative and semiquantitative polymerase chain reaction to predict Plasmodium falciparum treatment failure. J Infect Dis. 1994;170:1626–1630. doi: 10.1093/infdis/170.6.1626. [DOI] [PubMed] [Google Scholar]
- 10.Makler M T, Palmer C J, Ager A L. A review of practical techniques for the diagnosis of malaria. Ann Trop Med Parasitol. 1998;92:419–433. doi: 10.1080/00034989859401. [DOI] [PubMed] [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Uniformity of claims for in vitro diagnostic tests. Preliminary evaluation of quantitative clinical laboratory methods. Approved guideline EP 10-A, vol. 18, no. 6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 12.Pieroni P, Mills C D, Ohrt C, Harrington M A, Kain K C. Comparison of the Parasight®-F test with the polymerase-chain reaction for the diagnosis of Plasmodium falciparum in travelers. Trans R Soc Trop Med Hyg. 1998;92:166–169. doi: 10.1016/s0035-9203(98)90730-1. [DOI] [PubMed] [Google Scholar]
- 13.Smits H L, Gussenhoven G C, Terpstra W J, Schukkink R A F, van Gemen B, van Gool T. Detection, identification and semi-quantification of malaria parasites by NASBA amplification of small subunit ribosomal RNA sequences. J Microbiol Methods. 1997;28:65–75. [Google Scholar]
- 14.Tham J, Lee M S H, Tan T M C, Ting R C, Kara U A K. Detection and species determination of malaria parasites by PCR: comparison with microscopy and with ParaSight-F and ICT Malaria Pf tests in a clinical environment. J Clin Microbiol. 1999;37:1269–1273. doi: 10.1128/jcm.37.5.1269-1273.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Gemen B, van Beuningen R, Nabbe A, Strijp D, Jurriaans S, Lens P, Kievits T. A one tube quantitative HIV-1 RNA NASBA nucleic acid amplification assay using electrochemiluminescent (ECL) labeled probes. J Virol Methods. 1994;49:157–168. doi: 10.1016/0166-0934(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 16.Weiss J B. DNA probes and PCR for diagnosis of parasitic infections. Clin Microbiol Rev. 1995;8:113–130. doi: 10.1128/cmr.8.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]