Abstract
Large series of patients with acute myeloid leukemia (AML) after ex vivo T cell-depleted hematopoietic stem cell transplantation (TCD allo-HSCT) have not been previously reported. We retrospectively analyzed the outcomes of 266 patients (median age 54 years) with AML who received CD34 selected TCD allo-HSCTs while in first (75%) or second (25%) complete remission (CR) at a single institution. The conditioning regimens were all myeloablative and no additional graft-versus-host disease (GVHD) prophylaxis was given. Cumulative incidence of grade II-IV and III-IV acute GVHD at 180 days were 14% (95% confidence interval [CI] CI: 10 −18) and 3% (95% CI: 1–5), respectively. Cumulative incidence of chronic GVHD at 3 years was 3% (95% CI: 1–6). The 3-year CI of non-relapse mortality (NRM) and relapse was 21% (95% CI: 16–26) and 21% (95% CI: 17–27), respectively. Overall survival (OS) and disease-free survival (DFS) at 1, 3 and 5 years were 75%, 61%, 56% and 68%, 57%, 53%, respectively. There were no significant differences in OS, DFS and relapse rates for patients transplanted in CR1 vs CR2. However, patients with high risk cytogenetics at diagnosis had significantly poorer outcomes. OS and DFS rates compare favorably to unmodified allo-HSCT but with considerably lower rates of GVHD.
Keywords: T cell depletion, Stem cell transplantation, AML, GVHD, Transplant outcomes
INTRODUCTION
Post-remission therapy with allogeneic hematopoietic stem cell transplantation (allo-HSCT) is generally recommended for adult patients with intermediate or high-risk AML in first complete remission (CR1) or more advanced disease stage (1–12). However, NRM and GVHD remain the main causes of post-transplant morbidity and mortality, particularly in older patients, and have limited its widespread use. Though use of reduced intensity regimens has expanded applicability of allogeneic HSCT to older patient populations, the lower risk of NRM has been countered with a higher incidence of relapse (13).
Ex-vivo T cell-depleted (TCD) allo-HSCT has demonstrated favorable OS and DFS in patients with AML, acute lymphoblastic leukemia, myelodysplastic syndrome and non-Hodgkin lymphoma and has been shown to significantly reduce the incidence of acute and chronic GVHD without higher relapse rates (14–23). Large case series using this modality have not been published in AML. The aim of the present study was to analyze the outcomes of a large cohort of adult patients with AML in CR1 or CR2 who underwent TCD allo-HSCT from 2001 to 2014 at Memorial Sloan Kettering Cancer Center (MSKCC).
PATIENTS and METHODS
Patients and Donors
A total of 266 consecutive adult (age ≥18 years) patients with AML in CR1 and CR2 who underwent a first TCD allo-HSCT at MSKCC from January 2001 through December 2014 were included in the study. Patients received grafts from HLA-identical or mismatched related or unrelated donors. Written informed consent for treatment was obtained from all patients. This retrospective review was conducted with approval of the Institutional Review and Privacy Board. Patient demographics, disease characteristics, treatment, GVHD, and survival data were retrieved from departmental databases. Clinical charts were additionally reviewed for missing data.
Donor-recipient human leukocyte antigen (HLA) matching was established by high resolution DNA sequence-specific oligonucleotide typing for HLA-A, -B, C, DRB1 and DQB1. A matched donor was defined as matching at 10 HLA-alleles.
Conditioning Regimen and Supportive Care
All patients received myeloablative conditioning with one of the following preparative regimens previously reported in detail (14–16): 1) hyperfractionated total-body irradiation (HFTBI) 1375 cGy over 4 days followed by thiotepa 5 mg/kg/day i.v. for 2 days and fludarabine 25 mg/m2/day i.v. for 5 days (n=52); 2) HFTBI 1375 cGy over 4 days followed by thiotepa 5 mg/kg/day and cyclophosphamide 60 mg/kg/day i.v. for 2 days (n= 69); or 3) busulfan followed by melphalan 70 mg/m2/day i.v. for 2 days, and fludarabine 25 mg/m2/day i.v. for 5 days (total n=145). Busulfan dosing was either 0.8 mg/kg i.v. every 6 hours for 9 (n=1), 10 (n=56), or 12 doses (n=67), or single daily doses of 3.2 mg/kg for 2 (n=1), 3 (n=19), or 4 doses (n=1). Busulfan doses were adjusted according to first dose pharmacokinetics. Six patients in the HFTBI + thiotepa + cyclophosphamide group were also enrolled on BMT CTN 0303 (18). Patients >60 years of age and those patients with therapy related or secondary AML received the regimen without total body irradiation (TBI). The decision to utilize one regimen over the other was based on: age, co-morbidities, diagnosis of secondary or treatment related AML, all of which led to use of the all chemotherapy regimen. In later years, based upon protocol priorities, the availability of busulfan pharmacokinetic dose targeting, and a retrospective study of transplant-related renal toxicity (24) published in 2010, the all-chemotherapy regimens became the preferred conditioning with more limited use of TBI (25) for patients with no prior history of renal impairment or for those enrolled onto specific protocols that included TBI.
T cell depletion of granulocyte colony-stimulating factor (G-CSF) mobilized peripheral blood stem cells (PBSC) was performed as previously described (26–28). Positive selection of CD34+ cells was performed using an Isolex 300i Magnetic Cell Separator (Baxter, Deerfield, IL) with subsequent sheep RBC rosette depletion to remove residual T-cells (Isolex E-) (n=129) (15) or by CD34+ cell selection alone, using the CliniMACS CD34 Reagent System (Miltenyi Biotech, Gladbach, Germany) (n=126) (21,29). The first CliniMACS system at our institution was used in 2006 for patients who were enrolled onto BMT CTN protocol 0303. For patients not enrolled onto that protocol, we continued to use the Isolex system until mid 2010 after which they were no longer available. Bone marrow (BM) grafts were used upon donor preference and were TCD by sequential soybean lectin agglutination (SBA) and sheep RBC rosetting (n= 11). Equine (total 60 mg/kg) or rabbit (2.5 mg/kg to 5 mg/kg) antithymocyte globulin was administered prior to transplant (d −3, −2) to promote engraftment in most cases except for those patients receiving a transplant from an HLA-matched related donor who were conditioned with HFTBI + thiotepa + fludarabine, where no ATG was given (15). Patients did not receive any other post-transplant immunosuppressive prophylaxis.
All patients received supportive care which included regular prospective monitoring of reactivation or infection with EBV, CMV, or toxoplasmosis, based upon patient and donor serostatus. CMV was monitored at least weekly to day +100, every 2 weeks until day +180, and then at physician discretion thereafter based upon patient specific factors. The same monitoring strategy was used for EBV. During this time period, no specific prophylaxis was used for CMV or EBV. Patients who were toxo seropositive or whose donors were seropositive received either atovaquone or TMP/SMX soon after engraftment for both pneumocystis carinii pneumonia and toxoplasmosis prophylaxis. Prophylaxis against opportunistic infections were according to standard guidelines and G-CSF was administered beginning at day +7. IVGG replacement was done per CDC recommendations at the time.
During the time period of this study, it was our institutional standard to offer a TCD transplant to patients undergoing transplant for AML in 1st or 2nd CR. Unmodified transplants were performed in 46 patients, (41 CR1, 5 CR2) over the same time period. Reasons for proceeding with an unmodified transplant included patient preference, insurance denial for the TCD transplant on protocol, or physician preference to use a non-myeloablative regimen with an unmodified graft because of concerns of age or frailty.
Definitions
Neutrophil engraftment was defined as the first of 3 consecutive days with an absolute neutrophil count (ANC) ≥ 500/μL. Platelet engraftment was defined as a platelet count of ≥20000/μL without transfusion support for seven consecutive days. Engraftment was confirmed by documentation of chimerism in the bone marrow cells using fluorescent in situ hybridization of the X and Y chromosomes in sex-mismatched donor-recipient pairs and by measurement of DNA restriction fragment length polymorphisms or short tandem repeats in sex-matched pairs. Primary graft failure was defined as the absence of neutrophil recovery (≥500/μL) by day + 28 and a bone marrow biopsy with ≤ 5% cellularity. Secondary graft failure was defined as loss of ANC >500/μL after primary engraftment, with bone marrow biopsy showing less than 5% cellularity, but with persistence of donor-type lymphoid cells.
Complete remission was defined as ≤5% blasts in the bone marrow, absence of blasts in peripheral blood platelet count ≥100K, and ANC ≥1000/μL. Cytogenetic risk was assigned according to standard criteria (30). Minimal residual disease (MRD) was assessed prior to transplant by cytogenetic analyses as well as by means of a quantitative real-time PCR assay to detect molecular abnormalities that had been detected at diagnosis.
Assessment of comorbidities and calculation of the HCT-CI was assigned for all patients (31). Acute GVHD was diagnosed clinically, confirmed pathologically whenever possible, and classified according to standard criteria (32). Chronic GVHD was defined according to National Institutes of Health consensus criteria (33). Overall survival (OS) and disease-free survival (DFS) were defined following standard criteria and causes of death were determined using a standard algorithm (34). Chronic GVH and relapse free survival (CRFS) was graded according to National Institutes of Health consensus criteria global score (33).
Statistical analysis
Data was updated as of May 2017 which allowed for at least 2 years of follow up for the last patient transplanted. Patient and treatment characteristics were compared across groups using Pearson’s chi-squared or Fisher’s exact test for categorical variables, as appropriate. Differences in continuous variables were assessed via the Wilcoxon Rank-Sum test. OS and DFS were estimated using the Kaplan-Meier method with differences in survival rates across groups accessed via a logrank test. The cumulative incidence of relapse, NRM, GVHD, and engraftment were estimated using the cumulative incidence method for competing risks. Death in the absence of relapse and disease relapse were considered competing risks for relapse and NRM, respectively, while death and relapse were both considered competing risks for GVHD. Death prior to engraftment was treated as a competing risk for engraftment. Differences in cumulative incidence between groups were evaluated using Gray’s test. A cause-specific Cox Proportional Hazards regression model was used to determine the joint effect of patient characteristics on relapse and NRM. For the purposes of estimating GVHD, all patients were considered at risk for GVHD at the time of transplant and are included in the analysis.
RESULTS
Patient, disease, and graft characteristics
A total of 266 patients fulfilled the inclusion criteria and constituted the study population. The baseline patient characteristics and disease status at transplant are summarized in Table 1. Briefly, 200 patients with AML were in CR1 (75%), whereas the remaining 66 (25%) were in CR2. Median age for the entire group was 54 years (range, 19–73), with 165 patients (62%) ≥ 50 years. One hundred and fifty-six patients (59%) were seropositive for CMV. One hundred and seventy-two patients (65%) had de novo AML, 69 (26%) had secondary AML and 25 (9%) therapy-related AML. Patients in CR1 were significantly less likely to have de novo AML and more likely to have an adverse cytogenetic risk profile. Pretransplantation monitoring of MRD by cytogenetics or molecular testing identified 16 pts who had detectable MRD at time of transplant either by cytogenetics and/or molecular testing. Seventy-six patients had normal cytogenetics at diagnosis with either an abnormal molecular finding that was not retested at time of transplant or the testing failed at time of transplant, or had normal cytogenetics at diagnosis and no evaluable molecular testing at diagnosis. Therefore of the 266 patients, only 190 could be evaluated for MRD pre-transplant and of these, 16 were found to have MRD as noted above. Of the 266, 215 (81%) received a transplant from an HLA matched donor. Patients undergoing transplant in CR2 were more likely to have an HLA-mismatched donor (32%) than patients undergoing transplant in CR1 (15%). A majority of patients in CR1 (58%) received chemotherapy without radiation as myeloablative conditioning pre-transplant while more CR2 patients (58%) received TBI. This was in part due to patient age since most patients above 60 of age were not offered the TBI containing regimen and in part by physician discretion. HCT-CI was intermediate or high risk for 76% of patients.
Table 1.
Patient, donor and graft characteristics according to disease status
| Variable | Disease Status at TCD-HSCT | P-value | ||
|---|---|---|---|---|
|
| ||||
| CR1 N=200 n (%) | CR2 N=66 n (%) | |||
| Age, years | ||||
| Median (range) | 54 (19–73) | 52 (23–71) | 0.15 | |
| <50 | 75 (38) | 26 (39) | 0.02 | |
| 50–59 | 56 (28) | 28 (42) | ||
| >60 | 69 (34) | 12 (18) | ||
|
| ||||
| Female | 91 (46) | 32 (48) | 0.78 | |
|
| ||||
| Patient CMV serostatus | 0.82 | |||
| Seropositive | 116 (58) | 40 (61) | ||
|
| ||||
| AML | 0.007 | |||
| De novo | 119 (60) | 53 (80) | ||
| Secondary | 58 (29) | 11 (17) | ||
| Therapy-related | 23 (12) | 2 (3) | ||
|
| ||||
| Cytogenetic risk profile at diagnosis | <0.001 | |||
| Favorable | 6 (3) | 22 (33) | ||
| Intermediate (I & II) | 134 (67) | 35 (53) | ||
| Adverse | 60 (30) | 9 (14) | ||
|
| ||||
| Donor/recipient gender | ||||
| Match | 104 (52) | 35 (53) | > 0.99 | |
|
| ||||
| Donor type of 10 | 0.009 | |||
| Related HLA identical | 82 (41) | 19 (29) | ||
| Matched unrelated | 88 (44) | 26 (39) | ||
| Mismatched unrelated/related | 29/1(14/1) | 19/2 (29/3) | ||
|
| ||||
| Conditioning regimen * | 0.03 | |||
| TBI-based | 83 (42) | 38 (58) | ||
| Chemotherapy-based | 117 (58) | 28 (42) | ||
|
| ||||
| Stem cell source | >0.99 | |||
| Bone marrow | 8 (4) | 3 (5) | ||
| Peripheral blood | 192 (96) | 63 (95) | ||
|
| ||||
| CD34+ selection method | <0.001 | |||
| Lectin | 8 (4) | 3 (5) | ||
| CliniMACS | 111 (56) | 15 (23) | ||
| Isolex | 81 (40) | 48 (73) | ||
|
| ||||
| HCT-CI 1 | 0.04 | |||
| Low | 45 (22) | 18 (27) | ||
| Intermediate | 77 (38) | 14 (21) | ||
| High | 78 (39) | 34 (52) | ||
Abbreviations: N, number of patients; CMV, cytomegalovirus; MRD, minimal residual disease; HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index
For detailed information on conditioning regimen, see Methods.
Low=0; Intermediate=1–2; High ≥ 3
Peripheral blood was the graft source used for most of the patients with only 11 patients (4%) receiving bone marrow. Ex vivo TCD of PBSC was achieved by the Isolex E- technique in 48% of cases, and more recently by selection of CD34+ cells with CliniMACS device alone in 47%. The median infused CD34+ cell dose and CD3+ cell dose was 7.3 × 106/kg (range, 0.37–31.2) and 2 × 103/kg (range, 0–619), respectively.
Engraftment
All but one patient who died on day +1 engrafted with a median time to neutrophil recovery of 11 days (range, 9–21). Two hundred and fifty-seven patients (97%) engrafted platelets with a median time to platelet recovery of 17 days (range, 10–54). Eight patients had secondary graft failure, 4 having received transplants from matched donors (1 related and 3 unrelated) and the other 4 transplanted from mismatched unrelated donors (3 were 9/10 and 1 was 8/10). All of these patients had received PBSC transplants. Four patients with secondary graft failure are alive with a median follow-up of 83 months (range, 63–139), 3 after a second allo-HSCT (2 from a different donor) and one after a CD34+ selected stem cell boost administered without conditioning. Of the remaining 4 patients who died, two received stem cell boosts – one died of relapse almost a year later and one died of graft failure. Two patients did not get stem cell boosts, one died of chronic GVHD over a year later and one died of graft failure.
GVHD
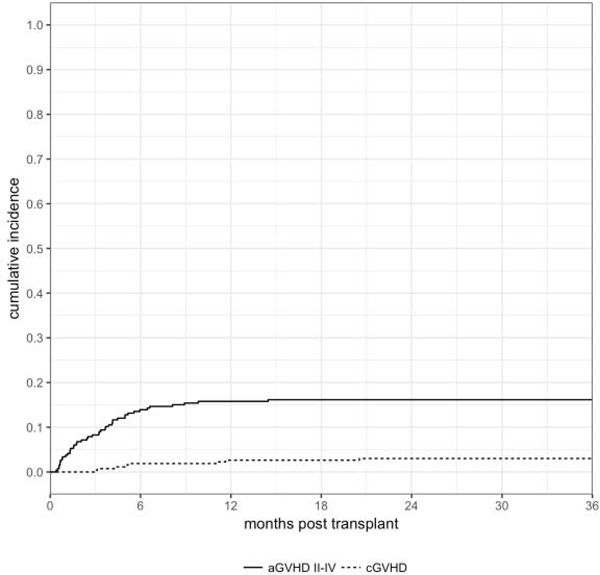
Forty-three patients developed acute grade II-IV GVHD at a median onset of 85 days (range, 12–440). The cumulative incidence of grade II-IV and III-IV acute GVHD at day + 180 was 14% (95% CI: 10–18) and 3% (95% CI: 1–5), respectively (Figure 1). In univariate analysis, none of the patient, disease or transplant characteristics, including the dose of T cells infused with the graft, were associated with acute GVHD. Eight patients had chronic GVHD at a median time of 154 days (range, 89–624) for a cumulative incidence at 3 years of 3% (95% CI: 1–6) (Figure 1). In this series, only 2 of the 43 patients developing acute GvHD did so following a donor leukocyte infusion. None of the patients with chronic GvHD had received DLI prior to developing GvHD.
Figure 1.
Cumulative incidence of grade II-IV acute GVHD and chronic GVHD
Overall Survival, Disease-Free Survival, Chronic GvH and Relapse Free Survival, and Relapse
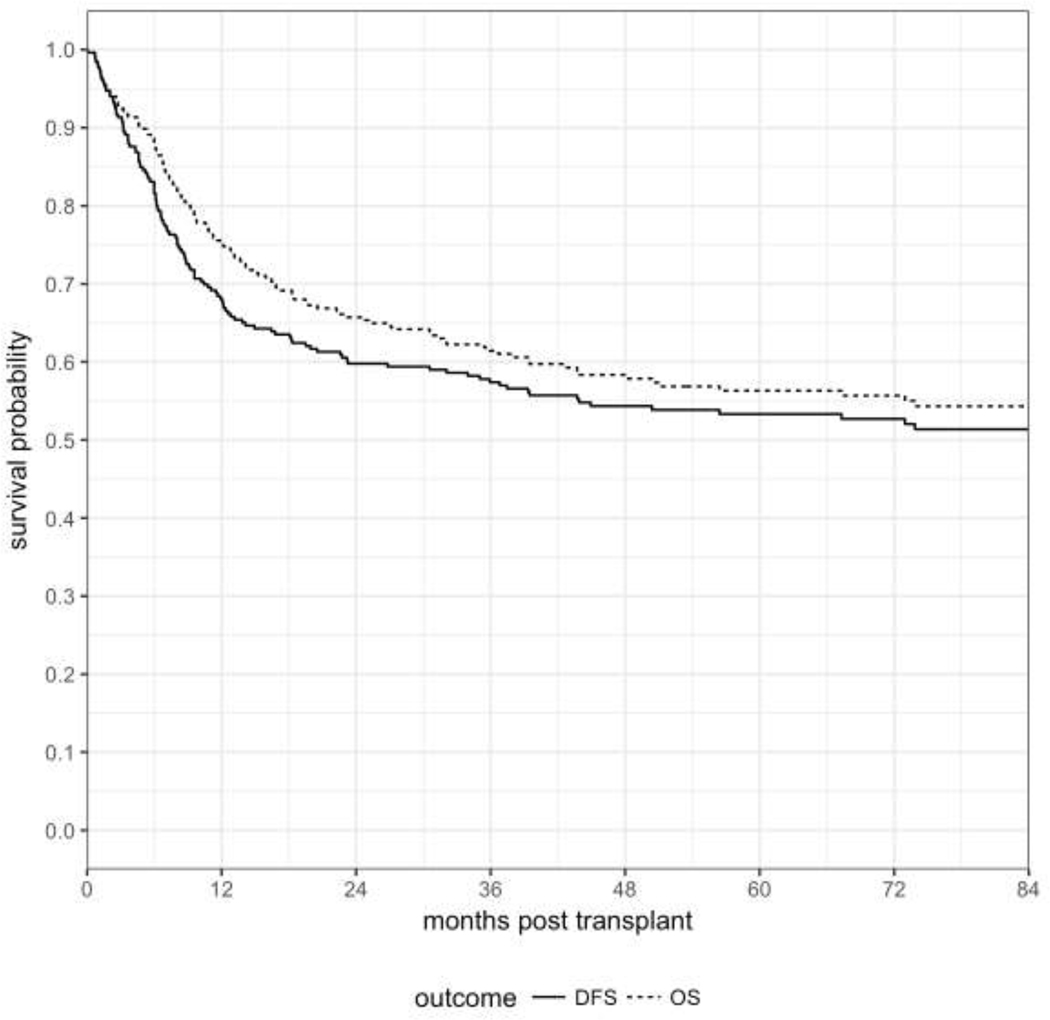
A total of 146 patients were alive at last follow-up with a median follow-up of 76 months (range, 12 to 184). Probability of OS and DFS at 1-year post-transplant were 75% (95% CI: 70–80) and 68% (95% CI: 62–73), respectively, at 3 years 61% (95% CI: 55–67) and 57% (95% CI: 51–63), and at 5-years: 56% (95% CI: 50–62) and 53% (95% CI: 47–59) (Figure 2). Univariate analysis of patient, donor, disease and transplant characteristics for OS and DFS are shown in Table 2. Intermediate and adverse cytogenetic risk and high HCT-CI were significantly associated with worse OS (p<0.001) and DFS (p<0.001). High HCT-CI was also correlated with a lower OS (p=0.014) and DFS (p=0.014). There was no statistical difference in DFS and OS for older patients ≥ 60 yrs (p=0.065 and 0.126, respectively), those receiving TCD grafts from ≤7/8 (p=0.06) or from ≤9/10 (p=0.089) compared to fully matched donors (Table 2).
Figure 2.
OS and DFS for the entire cohort
Table 2.
Correlation of OS and DFS with patient, disease and transplant characteristics
| OS % (95% CI) | DFS % (95% CI) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 year | 3 year | 5 year | p-value | 1 year | 3 year | 5 year | p-value | ||
| Overall | 75 (70, 80) | 61 (55, 67) | 56 (50, 62) | 68 (62, 73) | 57 (51, 63) | 53 (47, 59) | |||
|
| |||||||||
| CR status | 0.508 | 0.592 | |||||||
| CR1 | 77 (71, 83) | 63 (56, 69) | 57 (49, 64) | 69 (62, 75) | 58 (51, 65) | 54 (47, 61) | |||
| CR2 | 68 (55, 78) | 57 (45, 68) | 54 (41, 65) | 65 (52, 75) | 54 (42, 66) | 51 (38, 62) | |||
|
| |||||||||
| Age | 0.065 | 0.126 | |||||||
| <50 | 79 (70, 86) | 66 (56, 74) | 62 (52, 71) | 71 (61, 79) | 61 (51, 70) | 59 (48, 68) | |||
| 50–59 | 75 (64, 83) | 62 (51, 71) | 60 (49, 70) | 69 (58, 78) | 58 (47, 68) | 57 (46, 67) | |||
| ≥60 | 70 (59, 79) | 55 (44, 65) | 45 (33, 56) | 63 (51, 72) | 52 (40, 62) | 43 (32, 54) | |||
|
| |||||||||
| Patient gender | 0.31 | 0.59 | |||||||
| Female | 73 (64, 80) | 58 (49, 66) | 52 (42, 60) | 69 (60, 76) | 55 (46, 64) | 50 (40, 59) | |||
| Male | 77 (69, 83) | 64 (56, 71) | 60 (51, 68) | 67 (59, 74) | 59 (51, 67) | 56 (48, 64) | |||
|
| |||||||||
| Match of 8 | 0.06 | 0.134 | |||||||
| ≤7 | 69 (54, 79) | 55 (40, 67) | 47 (33, 60) | 65 (50, 76) | 51 (37, 64) | 45 (31, 58) | |||
| 8 | 77 (71, 82) | 63 (56, 69) | 59 (52, 65) | 69 (62, 75) | 59 (52, 65) | 55 (48, 62) | |||
|
| |||||||||
| Match of 10 | 0.089 | 0.179 | |||||||
| <=9 | 71 (58, 81) | 56 (43, 68) | 48 (35, 60) | 65 (51, 75) | 52 (39, 63) | 47 (34, 58) | |||
| 10 | 76 (70, 82) | 63 (56, 69) | 59 (52, 66) | 69 (62, 75) | 59 (52, 66) | 55 (48, 62) | |||
|
| |||||||||
| Conditioning Regimen | 0.151 | 0.187 | |||||||
| Chemotherapy | 76 (68, 82) | 59 (51, 67) | 51 (42, 59) | 68 (60, 75) | 56 (47, 63) | 48 (39, 56) | |||
| TBI-based | 74 (66, 81) | 64 (55, 72) | 62 (53, 70) | 68 (59, 75) | 59 (50, 68) | 59 (50, 68) | |||
|
| |||||||||
| HCT-CI | 0.014 | 0.014 | |||||||
| Low | 81 (69, 89) | 68 (55, 78) | 64 (51, 75) | 73 (60, 82) | 62 (49, 73) | 60 (47, 71) | |||
| Intermediate | 80 (70, 87) | 66 (55, 75) | 64 (53, 73) | 71 (61, 80) | 63 (53, 72) | 62 (51, 71) | |||
| High | 68 (58, 76) | 54 (45, 63) | 46 (36, 55) | 63 (53, 71) | 50 (40, 59) | 43 (34, 52) | |||
|
| |||||||||
| Etiology | 0.472 | 0.487 | |||||||
| De novo | 74 (67, 80) | 62 (55, 69) | 58 (50, 65) | 66 (59, 73) | 59 (51, 66) | 56 (48, 63) | |||
| Secondary | 75 (63, 84) | 61 (48, 71) | 57 (45, 68) | 70 (57, 79) | 55 (42, 66) | 52 (39, 63) | |||
| Therapy - related | 80 (58, 91) | 56 (35, 73) | 42 (23, 61) | 76 (54, 88) | 52 (31, 69) | 43 (23, 61) | |||
|
| |||||||||
| MRD | 0.926 | 0.734 | |||||||
| Negative | 75 (69, 80) | 61 (55, 67) | 56 (49, 62) | 68 (62, 74) | 57 (51, 63) | 53 (47, 59) | |||
| Positive | 75 (46, 90) | 61 (33, 80) | 61 (33, 80) | 62 (35, 81) | 56 (30, 76) | 56 (30, 76) | |||
|
| |||||||||
| Cytogenetic risk profile | < 0.001 | < 0.001 | |||||||
| Favourable | 93 (74, 98) | 85 (66, 94) | 85 (66, 94) | 93 (74, 98) | 82 (62, 92) | 82 (62, 92) | |||
| Intermediate | 75 (68, 81) | 64 (57, 71) | 59 (51, 66) | 72 (64, 78) | 61 (54, 68) | 57 (49, 64) | |||
| Adverse | 68 (56, 78) | 45 (33, 56) | 38 (26, 49) | 49 (37, 60) | 38 (26, 49) | 32 (22, 44) | |||
|
| |||||||||
| Donor/patient gender | 0.342 | 0.375 | |||||||
| match | 72 (64, 79) | 59 (51, 67) | 54 (45, 62) | 65 (57, 73) | 56 (47, 64) | 52 (43, 60) | |||
| mismatch | 79 (71, 85) | 64 (54, 71) | 59 (49, 67) | 71 (62, 78) | 59 (50, 67) | 55 (46, 63) | |||
Abbreviations: HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index; MRD, minimal residual disease
The 1, 3 and 5-year probabilities of CRFS were 67% (95% CI: 61–72), 56% (95% CI: 50–62) and 53% (95% CI: 46–59), respectively. In the univariate analyses, the factors associated with a significantly lower CRFS were high HCT-CI (p=0.025) and intermediate and adverse cytogenetics at diagnosis (p<0.001)
The cumulative incidence of relapse was 17% (95% CI: 12–21) at 1 year and 21% (95% CI: 17–27) at 3 years. Median time to relapse was 8 months (range, 2–119 months) for the CR1 group and 6 months (range, 2–45 months) for the CR2 group. T cell dose of the graft did not correlate with relapse. No statistically significant difference was observed between the CR1 and CR2 groups with respect to OS, DFS and relapse incidence. However, in univariate analysis, intermediate and adverse cytogenetics at diagnosis (p<0.001) and male patients (p=0.039) were associated with higher rates of relapse (Table 3). Only cytogenetics at diagnosis remained a significant risk factor for relapse in multivariate analysis (HR 2.58 for intermediate risk and HR 10.32 for adverse risk, p<0.001) (Table 4). Ultimately, 52 patients (19.5%) died of disease relapse. Of note, of the 16 pts who were found to have MRD at time of transplant, 5 relapsed and 4 of these died of disease.
Table 3.
Correlation of relapse and NRM with patient, disease, and transplant characteristics
| Relapse % (95% CI) | NRM % (95% CI) | ||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| 1 year | 3 year | p-value | 1 year | 3 year | p-value | ||
| Overall | 17 (12, 21) | 21 (17, 27) | 15 (11, 20) | 21 (16, 26) | |||
|
| |||||||
| CR status | 0.693 | 0.404 | |||||
| CR1 | 18 (13, 23) | 23 (17, 29) | 14 (9, 19) | 19 (14, 25) | |||
| CR2 | 14 (7, 23) | 18 (10, 28) | 21 (12, 32) | 27 (17, 39) | |||
|
| |||||||
| Age | 0.811 | 0.015 | |||||
| <50 | 21 (13, 29) | 24 (16, 32) | 8 (4, 14) | 15 (9, 23) | |||
| 50–59 | 13 (7, 21) | 19 (11, 28) | 18 (11, 27) | 23 (14, 32) | |||
| ≥60 | 15 (8, 23) | 21 (13, 31) | 22 (14, 32) | 27 (18, 37) | |||
|
| |||||||
| Patient gender | 0.039 | 0.009 | |||||
| Female | 11 (7, 18) | 16 (10, 23) | 20 (13, 27) | 28 (21, 37) | |||
| Male | 21 (15, 28) | 26 (19, 33) | 12 (7, 18) | 15 (9, 21) | |||
|
| |||||||
| Match of 8 | 0.467 | 0.014 | |||||
| ≤7 | 12 (5, 22) | 16 (7, 27) | 24 (13, 36) | 33 (21, 46) | |||
| 8 | 18 (13, 23) | 23 (17, 29) | 13 (9, 18) | 18 (13, 24) | |||
|
| |||||||
| Conditioning Regimen | 0.295 | 0.006 | |||||
| Chemotherapy | 12 (8, 18) | 18 (12, 25) | 19 (13, 26) | 26 (19, 34) | |||
| TBI-based | 21 (15, 29) | 26 (18, 34) | 11 (6, 17) | 15 (9, 22) | |||
|
| |||||||
| HCT-CI | 0.662 | 0.034 | |||||
| Low | 14 (7, 24) | 22 (13, 33) | 13 (6, 22) | 16 (8, 26) | |||
| Intermediate | 16 (10, 25) | 20 (12, 29) | 12 (6, 20) | 17 (10, 25) | |||
| High | 18 (11, 26) | 22 (15, 30) | 20 (13, 27) | 28 (20, 36) | |||
|
| |||||||
| Etiology | 0.625 | 0.053 | |||||
| De novo | 19 (14, 25) | 23 (17, 30) | 15 (10, 20) | 17 (12, 24) | |||
| Secondary | 13 (6, 22) | 19 (11, 29) | 17 (10, 27) | 26 (16, 37) | |||
| Therapy - related | 8 (1, 23) | 16 (5, 33) | 16 (5, 33) | 32 (15, 51) | |||
|
| |||||||
| MRD | 0.482 | 0.796 | |||||
| Negative | 16 (12, 21) | 21 (16, 27) | 15 (11, 20) | 21 (16, 27) | |||
| Positive | 19 (4, 41) | 25 (7, 48) | 19 (4, 41) | 19 (4, 41) | |||
|
| |||||||
| Cytogenetic risk profile | < 0.001 | 0.074 | |||||
| Favourable | 4 (0, 16) | 7 (1, 21) | 4 (0, 16) | 11 (3, 26) | |||
| Intermediate | 10 (6, 15) | 14 (9, 19) | 18 (13, 25) | 25 (19, 32) | |||
| Adverse | 38 (26, 49) | 46 (34, 58) | 13 (6, 22) | 16 (8, 26) | |||
|
| |||||||
| Donor/patient gender | 0.359 | 0.915 | |||||
| match | 18 (12, 25) | 23 (16, 30) | 17 (11, 23) | 21 (15, 28) | |||
| mismatch | 15 (9, 22) | 20 (13, 27) | 14 (9, 21) | 21 (15, 29) | |||
Abbreviations: HCT-CI, Hematopoietic Cell Transplantation Comorbidity Index; MRD, minimal residual disease
Table 4.
Multivariate analysis model of risk factors for transplantation outcomes in all patients.
| Variables | NRM* | Relapse* | OS | DFS | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age (years) | 0.109 | ||||||||
| <50 | Reference | ||||||||
| ≥50 | 1.66 (0.88–3.12) | ||||||||
|
| |||||||||
| Patient gender | 0.01 | 0.194 | |||||||
| Female | Reference | Reference | |||||||
| Male | 0.53 (0.32–0.86) | 1.41 (0.83–2.4) | |||||||
|
| |||||||||
| Conditioning regimen | 0.318 | ||||||||
| Chemotherapy | Reference | ||||||||
| TBI-based | 0.73 (0.39–1.36) | ||||||||
|
| |||||||||
| HCT-CI | 0.106 | 0.059 | 0.065 | ||||||
| Low | Reference | Reference | Reference | ||||||
| Intermediate | 1.1 (0.52 −2.33) | 0.93 (0.54 −1.58) | 0.83 (0.5 −1.38) | ||||||
| High | 1.84 (0.91–3.7) | 1.48 (0.9–2.44) | 1.3 (0.83–2.14) | ||||||
|
| |||||||||
| Match of 8 | 0.009 | ||||||||
| ≤7 | Reference | ||||||||
| 8 | 0.48 (0.28–0.81) | ||||||||
|
| |||||||||
| Cytogenetic risk profile | <0.001 | <0.001 | <0.001 | ||||||
| Favorable | Reference | Reference | Reference | ||||||
| Intermediate | 2.58 (0.61–10.9) | 3.75 (1.37–10.28) | 3.14 (1.27–7.78) | ||||||
| Adverse | 10.3 (2.47–43.02) | 6.12 (2.19–17.1) | 5.92 (2.34–14.94) | ||||||
Abbreviations: HR, hazard ratio
cause-specific cox proportional hazards model
Non-relapse mortality and causes of death
Of the entire cohort, 68 patients died without prior relapse for a cumulative incidence of NRM at 1 and 3-years of 15% (95% CI: 11–20) and 21% (95% CI: 16–26), respectively. Causes of NRM included infection (n=36), GVHD (n=12), graft failure (n=2), organ toxicities (n=8), recurrence of previous solid malignancies (n=3), secondary malignancies (n=3) and other causes (n=4). Four patients died of EBV lymphoproliferative disorders, all between 6 and 12 months post-transplant. Of 21 patients dying prior to day 100, most (n=18) succumbed to infections, with bacterial infections (n=10) being the leading cause. An additional 7 patients succumbed to early non-bacterial infections, (4 adenovirus, 1 CMV, 2 toxoplasmosis with 1 patient dying from multi-organ-failure without an identified pathogen.
Univariate analysis of NRM is shown in Table 3. Variables that were associated with increased risk of NRM were: ie ≥ 50 years (p=0.015), <8/8 HLA match (p=0.014), female gender (p=0.009), patients receiving non TBI conditioning (p=0.006) and high HCT-CI (p=0.034). There was no difference in NRM between patients in CR1 and CR2. Multivariate analysis showed that <8/8 HLA match (p=0.009) and female gender (p=0.01) remained independently associated with NRM (Table 4).
Female patients had a higher NRM (20% at 1 year and 28% at 3 years) in comparison to males (12% at 1 year and 15% at 3 years, p=0.009). However, this difference in NRM was driven by the subset of females receiving the non-TBI regimen where the NRM at 1 and 3 years was 28% and 39%, respectively, compared to 12% and 16% in the males receiving a non-TBI regimen. NRM in both males and females receiving the TBI containing regimen were comparable to that seen in males receiving the non-TBI regimen. Patient, donor, and graft characteristics, conditioning variables, and causes of death were examined to determine the etiology of the increased NRM in the non-TBI female subset but no definitive etiology was identified. In the subset of patients who received the non-TBI regimen, there was no difference observed between the CR1 and CR2 groups. There was a strong trend towards a higher NRM among secondary or therapy-related AML patients as compared to de novo (p=0.053).
Discussion
This study shows that patients with AML in CR1 or CR2 undergoing myeloablative ex vivo TCD allo-HSCT had an OS and a DFS comparable to that reported after unmodified transplants (35–40) with a much lower incidence of acute and chronic GVHD and without a higher relapse rate. We also confirmed that there was no significant difference in OS, DFS and relapse incidence between patients transplanted in CR1 or CR2.
The use of TCD transplants for the treatment of patients with AML has been stifled by concerns of a higher relapse risk. This concern was primarily based upon extrapolation from historical results in CML patients undergoing TCD transplants (41). The majority of publications describing results of TCD transplants have reported on a variety of hematologic diseases. Those focused on patients with AML have been very limited and are primarily derived from this center. Our first study, published 20 years ago, reported outcomes of 31 patients with de novo AML in CR1 and 8 in CR2 who received TCD bone marrow transplants from HLA-identical sibling donors. The estimated probability of DFS at 4 years for the CR1 patients was 77% with a median follow-up of 56 months. For those transplanted in CR2, the DFS probability estimate at 3 years was 50% with a median follow up of 48 months. The median age for CR1 patients was 37 and for CR2 patients, 33 years, with a range of 15–55 years. Relapse rate was 3.2% at 4 years and 12.5% at 3 years for CR1 and CR2 patients, respectively. In this relatively young group, NRM was 19.4% and 37.5% for CR1 and CR2, respectively. Notably, there was no acute grade II-IV GVHD and the incidence of chronic GVHD was 3% (14).
In the current era, supportive care and advanced cell separation techniques have altered the landscape of HSCT. The median age of transplant candidates has increased. Greater numbers of unrelated donors are being used and the most common stem cell source is GCSF mobilized PBSC. In addition, enrichment of stem cells and depletion of lymphocytes is now accomplished by automated immunomagnetic bead selection of CD34+ progenitors, a process which depletes the majority of accessory cells. This method has replaced the more labor intensive lectin-based or antibody-based separation techniques that were primarily developed to deplete T-cells.
More recently, in a collaborative effort with MD Anderson Cancer Center (MDACC), we compared transplant outcomes of 115 patients with AML in CR1 undergoing TCD transplants at MSKCC with 181 comparable patients who received unmodified allografts at MDACC (19) over the same time period. In this older group of patients, the median age was 52 and 48 years, respectively for the MSKCC vs MDACC patients. Donors were HLA matched related or unrelated in 77% at MSKCC and 92% at MDACC. At MSKCC, 23% of the patients received HLA non-identical grafts vs. 8% at MDACC. Grafts were bone marrow in 7% vs 32% and PBSC in 93% vs. 68% (MSKCC vs MDACC). There was no statistically significant difference in OS, RFS or incidence of relapse between the two centers. However, there was a significantly lower incidence of acute and chronic GVHD (p=0.005 and p<0.001, respectively) in those who received the TCD grafts (19).
In the present study, we report our expanded experience of 266 consecutive patients who received TCD HCT for AML in CR1 or CR2 from 2001 to 2014. The characteristics of patients transplanted in CR1 are similar to those analyzed in the MSKCC-MDACC comparative study (19). However, the present study includes 126 patients who received transplants depleted of T-cells by the CliniMACS device instead of the ISOLEX separator which is no longer in use. As shown in Table 1, the patients transplanted in CR1 were almost exclusively those with an intermediate or high risk cytogenetic profile. In contrast, a third of those transplanted in CR2 had had favorable characteristics at diagnosis but relapsed and were referred for transplant only after achieving CR2. Patients in CR2 were significantly more likely to receive an HLA-non-identical graft. Despite a higher co-morbidity index in the CR2 patients, the TBI based conditioning was used in 58% of CR2 patients versus 42% for CR1 patients (p=0.03). The criteria to use the non-TBI regimen in the 2nd CR patients was the same as for the CR1 patients, i.e., patients with treatment related AML, prior MDS, age>60 yrs. It is notable that the number of CR2 patients from 2010 until 2014 was far less (15 patients) compared to the number of CR2 patients from the start of the study until 2009 (51 patients). Therefore, the majority of the 2nd CR transplants were performed prior to our change in policy due to a higher incidence of renal dysfunction in the patients receiving full dose TBI and may therefore have accounted for this difference.
As shown in Tables 2 and 3, results in the expanded CR1 cohort are similar to those reported in the earlier two center study (19), with 3 yr OS and DFS of 63% and 58%, respectively, compared to 57% and 58% in the two-center study. Strikingly, in this study, the OS (54%), DFS (51%) and cumulative incidence of relapse (18%) at 5 years for patients transplanted in CR2 did not differ significantly from those demonstrated by patients transplanted in CR1. Consistent with previous reports (14–23), the present study confirms the low cumulative incidences of acute and chronic GVHD following the CD34 selection techniques employed. This resulted in CRFS rates almost identical to DFS rates at 3 and 5 years.
In this study, as in our own and other prior studies of TCD HCT applied to the treatment of AML, the incidence of relapse post transplant for patients transplanted in CR1 (14,18,19,42) is no higher than the relapse rate reported after unmodified HLA-matched transplants administered after myeloablative conditioning (10,35,43–45). The low number of residual alloreactive donor T cells infused and the time required for reconstitution of donor T cells matured from lymphoid precursors makes it unlikely that either of these mechanisms contribute to the low incidence of relapse seen at a median of 8 months for CR1 and 6 months for CR2. In the current patient cohort, for example, the median dose of T-cells administered was 2 × 10 3/kg. On the other hand, NK cells recover within 3 weeks of a CD34+ selected TCD graft (46), and their expression of specific KIRs in both HLA disparate and certain HLA-matched hosts correlated with a reduced risk of relapse (47–49). To what degree NK cells or other components of the innate immune system contribute to the low overall relapse rates remains to be determined.
One of the most significant prognostic factors for long term success of transplantation for patients with AML in CR1 is the cytogenetic profile at diagnosis (10,35,43–45). In this study, the OS and DFS rates for patients exhibiting intermediate cytogenetic features (64% and 61%) or adverse features (45% and 38%) compare favorably with OS and DFS rates reported for unmodified grafts in younger patient (43,44). However, for such patients, transplanted either in CR1 or CR2, relapse was still the most significant cause of treatment failure. Differences in NRM between these cytogenetic risk categories or between CR1 and CR2 were not statistically significant. It is recognized that the alterations in gene expression induced by adverse cytogenetics have thus far been difficult to target. Since TCD grafts can abrogate the risk of GvHD without immunosuppressive drug prophylaxis, they provide a unique platform for innovative strategies. These include immunomodulating drugs and biologics as well as immunotherapeutic effectors selectively targeting determinants differentially expressed by clonogenic AML cells to eradicate residual disease and prevent relapse.
In our study cohort, 62% of patients were older than 50 years and the majority of patients were considered high risk based on diagnosis, disease status, and HCT-CI. However, the NRM rates were similar to those observed in our previous reports of TCD BM and PBSC transplants which included a larger proportion of younger patients (14,15,20,50). The NRM seen before day 100 was mostly attributed to infection, most of which were in the early peri-transplantation period and bacterial in nature. Therefore, they could not be attributed to delayed immune reconstitution. Since this study spanned 14 yrs, the methods for viral monitoring changed dramatically over this time period, in particular for CMV and EBV. HHV-6 was difficult to identify in the early years of this study but became available later on as pcr technology advanced. Therefore, to give exact reactivation rates of this specific cohort over the 14 yr period is difficult. However, evaluation of the incidence of double stranded DNA viruses after ex vivo CD34+ selected HSCT for patients with AML and MDS over a period from early 2012 through December 2014, with prospective monitoring by quantitative PCR assays for CMV, ADV, HHV-6, and EBV in whole blood or plasma have been reported (56). In a cohort of 176 patients, the cumulative incidences for CMV, HHV-6, ADV, and EBV viremia were 44%, 61%, 7%, and 16%, respectively. Overall, viremia by dsDNA viruses occurred in 85% of TCD HCT recipients by day +100 and 33% of pts experienced >=2 viremias by day +180. Despite the high incidence of reactivation, only 5 patients in our study cohort died of viral infections.
Although several variables demonstrated an association with NRM in univariate analysis, only HLA allelelic mismatches and female gender remained significant variables in the multivariate analysis. The significantly increased risk of NRM observed for ≤7/8 matched donors is consistent with the increased risk of NRM that has also been observed following HLA non-identical unmodified transplants. In the latter, however, this increased NRM risk has usually been ascribed to the need for more intensive posttransplant immumosuppression to prevent or treat GVHD (51). Alternatively, since transplant-derived donor T-cells specific for latent-viruses such as CMV and adenovirus are usually specific for epitopes presented by only 1–3 HLA alleles, they may be ineffective in HLA non-identical recipients if their restricting allele is not shared by the patient (52,53).
In this expanded cohort, the increased risk of NRM previously reported in females conditioned with busulfan, melphalan and fludarabine (19) was again observed, and was significant both in univariate and multivariate analysis. Conversely, in univariate but not multivariate analyses, the risk of relapse was increased in male transplant recipients. As a result, ultimate OS and DFS for female and male patients did not differ. Nevertheless, these findings suggest that gender differences in either the intrinsic sensitivity of patient cells to the chemotherapeutic agents used (54) or gender differences in the pharmacokinetics and/or pharmacodynamics of these drugs may contribute to both toxicity and tumor eradication. In one prior study evaluating predicted and actual pharmacokinetics of busulfan (1.6 mg/kg I.V./day × 2) followed by a fixed dose of melphalan (140 mg/m2×1) administered to patients receiving autologous transplants for lymphoma or myeloma, actual AUCs for busulfan significantly exceeded predicted levels in females but not males in univariate analysis. However, this was not detected in multivariate analysis and overall toxicities did not differ (55). However, drugs and dose intensities administered to these patients prior to referral for transplantation are quite different from those given to AML patients. Planned prospective studies of the pharmacokinetics of busulfan, melphalan, fludarabine, and ATG may clarify to what degree gender affects their pharmacokinetics in this setting and to what degree this is correlated with NRM and ultimate outcome.
As with all retrospective studies there are inherent limitations to this study. First, there is the potential for selection bias in the type of conditioning (TBI vs. chemotherapy-based) administered. As noted above, the criteria to use a chemotherapy-based regimen were based upon the pt’s age; diagnosis of treatment related AML and secondary AML from MDS; prior renal dysfunction; etc. Although there were no significant differences in outcomes of OS or DFS based upon the conditioning regimen, there were differences in other outcomes in subgroups, such as the increased NRM seen in females treated with the all-chemotherapy regimen. Particular protocols at a given point in time have also led to eligible patients undergoing transplant with TBI regimens. We did not see the difference in outcomes between patients undergoing TBI conditioning versus busulfan-based conditioning as reported by Copelan et al. (25). Therefore, we cannot recommend one cytoreductive regimen over the other based upon our data. This would likely require a prospective randomized trial comparing the two regimens. Second, the study period was 14 years during which time supportive care advanced, especially with respect to infectious disease prophylaxis, monitoring, and treatment. Nevertheless, our comparison of outcomes in the group of patients who received an Isolex CD34 selected graft (before 2010) and those who received a CliniMACs graft (most done from 2009 onward), revealed no statitiscally significant difference between the two time periods. Third, the number of patients in the CR2 group was significantly lower than that for CR1 reflecting the increasing proportion of patients with high risk forms of AML referred early for transplantation. However, the study has several distinctive and informative attributions: First, the large number of patients included in the expanded study with a median follow-up of > 6 years. Second, this large retrospective analysis confirms the findings of the earlier two center study comparing TCD to unmodified transplants for patients with AML in CR1 (19) demonstrating that these TCD grafts are associated with a similarly low relapse rate and a low incidence of acute and chronic GVHD. Thus, this study further dispels the misconception of higher relapse rates with ex vivo TCD transplantation. The study also shows strikingly similar findings in the patients transplanted for AML in CR2. A randomized, multi-center phase III trial of calcineurin inhibitor free interventions for prevention of GVHD (BMT CTN protocol 1301; NCT02345850) has just recently completed accrual. This study is evaluating three strategies for GVHD prophylaxis: the CD34 ex-vivo TCD vs post-transplant cyclophosphamide vs standard CNI/methotrexate. With chronic CRFS as the primary endpoint, this large study of over 300 patients will hopefully identify the optimal GVHD prevention strategy among the three arms.
Future trials need to focus on reducing NRM and relapse, both of which remain significant obstacles to the success of all transplants. To that end, we have embarked on an alpha-beta T cell depletion strategy in the setting of a reduced intensity transplant. This trial is currently underway at our institution. In addition, given the low incidence of both acute and chronic GVHD without immunosuppressive drug therapy seen in our TCD transplants, they provide a unique platform for strategies employing drugs and biologicals, as well as donor derived immunotherapeutic effectors.
Highlights.
Ex-vivo TCD HSCT in AML CR 1 & 2 results in comparable OS & DFS as with unmodified HSCT
Transplant outcomes are similar in patients with AML CR1 & 2 following TCD HSCT
Risk of relapse after Ex-vivo TCD HSCT is no higher than after unmodified HSCT
Ex-vivo TCD HSCT results in significantly lower GvHD rates compared to unmodified HSCT
Acknowledgments
JM analyzed the data and wrote the manuscript; IC analyzed the data; PH analyzed the data and edited the manuscript; MAM coordinated collection of the data and edited the manuscript; JB, HC-M, PD, GK, M-AP, DP, CS, BS, RT, JWY, SAG, collected data and edited the manuscript, RJO wrote the manuscript; AAJ and EBP analyzed the data and wrote the manuscript and EBP coordinated collection of the data. We gratefully acknowledge the expert care provided to our patients by the fellows, advanced practice practitioners, and nurses of Memorial Sloan-Kettering Cancer Center.
Financial disclosure: This research was supported in part by National Institutes of Health Grant P01 CA2376, the Sawaris Family Research Fund, the Bergstein Family Fund. I.C. was supported in part by Fundación Leucemia y Linfoma, Beca “Marcos Fernandez” 2012. J.M. was supported by the ASBMT-GETH International Scholarship Program-2013 grant.
Footnotes
Conflict of interest: There are no conflicts of interest to report with respect to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Longo DL, Döhner H, Weisdorf DJ, Bloomfield CD. Acute Myeloid Leukemia [Internet]. Vol. 373, New England Journal of Medicine. 2015. [cited 2016 Nov 26]. pp. 1136–52. Available from: 10.1056/NEJMra1406184 [DOI] [PubMed] [Google Scholar]
- 2.Gupta V, Tallman MS, Weisdorf DJ. Allogeneic hematopoietic cell transplantation for adults with acute myeloid leukemia: myths, controversies, and unknowns. Blood. American Society of Hematology; 2011. Feb 24;117(8):2307–18. [DOI] [PubMed] [Google Scholar]
- 3.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009. Jun 10;301(22):2349–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010. Sep 16;116(11):1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. American Society of Hematology; 2010. Jan 21;115(3):453–74. [DOI] [PubMed] [Google Scholar]
- 6.Estey EH. Acute myeloid leukemia: 2014 update on risk-stratification and management. Am J Hematol. 2014. Nov;89(11):1063–81. [DOI] [PubMed] [Google Scholar]
- 7.Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. 2012. Oct;9(10):579–90. [DOI] [PubMed] [Google Scholar]
- 8.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. 2016. Jan 7;127(1):62–70. [DOI] [PubMed] [Google Scholar]
- 9.Forman SJ, Rowe JM. The myth of the second remission of acute leukemia in the adult. Blood. 2013. Feb 14;121(7):1077–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Østgård LSG, Lund JL, Nørgaard JM, Nørgaard M, Medeiros BC, Nielsen B, et al. Impact of Allogeneic Stem Cell Transplantation in First Complete Remission in Acute Myeloid Leukemia: A National Population-Based Cohort Study. Biol Blood Marrow Transplant. 2018. Feb;24(2):314–23. [DOI] [PubMed] [Google Scholar]
- 11.Versluis J, Hazenberg CLE, Passweg JR, van Putten WLJ, Maertens J, Biemond BJ, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. The Lancet Haematology. 2015. Oct;2(10):e427–36. [DOI] [PubMed] [Google Scholar]
- 12.Cornelissen JJ, Versluis J, Passweg JR, van Putten WLJ, Manz MG, Maertens J, et al. Comparative therapeutic value of post-remission approaches in patients with acute myeloid leukemia aged 40–60 years. Leukemia. Nature Publishing Group; 2015. May;29(5):1041–50. [DOI] [PubMed] [Google Scholar]
- 13.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol. 2017. Apr 10;35(11):1154–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998. Feb 1;91(3):1083–90. [PubMed] [Google Scholar]
- 15.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007. Dec 15;110(13):4552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro-Malaspina H, Jabubowski AA, Papadopoulos EB, Boulad F, Young JW, Kernan NA, et al. Transplantation in Remission Improves the Disease-Free Survival of Patients with Advanced Myelodysplastic Syndromes Treated with Myeloablative T Cell-Depleted Stem Cell Transplants from HLA-Identical Siblings. Biology of Blood and Marrow Transplantation. Elsevier; 2008. Apr;14(4):458–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perales M-A, Jenq R, Goldberg JD, Wilton AS, Lee SSE, Castro-Malaspina HR, et al. Second-line age-adjusted International Prognostic Index in patients with advanced non-Hodgkin lymphoma after T-cell depleted allogeneic hematopoietic SCT. Bone Marrow Transplantation. 2010. Sep;45(9):1408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devine SM, Carter S, Soiffer RJ, Pasquini MC, Hari PN, Stein A, et al. Low risk of chronic graft-versus-host disease and relapse associated with T cell-depleted peripheral blood stem cell transplantation for acute myelogenous leukemia in first remission: results of the blood and marrow transplant clinical trials network protocol 0303. Biol Blood Marrow Transplant. 2011. Sep;17(9):1343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayraktar UD, de Lima M, Saliba RM, Maloy M, Castro-Malaspina HR, Chen J, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013. Jun;19(6):898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamari R, Chung SS, Papadopoulos EB, Jakubowski AA, Hilden P, Devlin SM, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graftversus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015. Dec;21(12):2106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobbs GS, Hamdi A, Hilden PD, Goldberg JD, Poon ML, Ledesma C, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplantation. 2015. Apr;50(4):493–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jakubowski AA, Petrlik E, Maloy M, Hilden P, Papadopoulos E, Young JW, et al. T-Cell Depletion as an Alternative Approach for Patients >55 Years Undergoing Allogeneic Stem Cell Transplantation as Curative Therapy for Hematologic Malignancies. Biology of Blood and Marrow Transplantation. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barba P, Hilden P, Devlin SM, Maloy M, Dierov D, Nieves J, et al. Ex Vivo CD34+ Selected T-Cell Depleted (TCD) Peripheral Blood Stem Cell Grafts for Allogeneic Hematopoietic Stem Cell Transplantation in Acute Leukemia and Myelodysplastic Syndrome is Associated with Low Incidence of Acute and Chronic Graft-Versus-Host Disease (GVHD) and High Treatment Response. Biology of Blood and Marrow Transplantation. Elsevier Inc; 2016. Dec 22;:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glezerman IG, Jhaveri KD, Watson TH, Edwards AM, Papadopoulos EB, Young JW, et al. Chronic kidney disease, thrombotic microangiopathy, and hypertension following T cell-depleted hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2010. Jul;16(7):976–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Copelan EA, Hamilton BK, Avalos B, Ahn KW, Bolwell BJ, Zhu X, et al. Better leukemia-free and overall survival in AML in first remission following cyclophosphamide in combination with busulfan compared with TBI. Blood. American Society of Hematology; 2013. Dec 5;122(24):3863–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jakubowski AA, Small TN, Young JW, Kernan NA, Castro-Malaspina H, Hsu KC, et al. T cell depleted stem-cell transplantation for adults with hematologic malignancies: sustained engraftment of HLA-matched related donor grafts without the use of antithymocyte globulin. Blood. 2007. Dec 15;110(13):4552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadopoulos EB, Carabasi MH, Castro-Malaspina H, Childs BH, Mackinnon S, Boulad F, et al. T-cell-depleted allogeneic bone marrow transplantation as postremission therapy for acute myelogenous leukemia: freedom from relapse in the absence of graft-versus-host disease. Blood. 1998. Feb 1;91(3):1083–90. [PubMed] [Google Scholar]
- 28.Kernan NA, Flomenberg N, Collins NH, O’Reilly RJ, Dupont B. Quantitation of T Lymphocytes in Human Bone Marrow by a Limiting Dilution Assay. . Transplantation. 1985. Sep;40(3):317–22. [DOI] [PubMed] [Google Scholar]
- 29.Keever-Taylor CA, Devine SM, Soiffer RJ, Mendizabal A, Carter S, Pasquini MC, et al. Characteristics of CliniMACS® System CD34-enriched T cell-depleted grafts in a multicenter trial for acute myeloid leukemia-Blood and Marrow Transplant Clinical Trials Network (BMT CTN) protocol 0303. Biol Blood Marrow Transplant. 2012. May;18(5):690–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Döhner H, Estey EH, Amadori S, Appelbaum FR, Büchner T, Burnett AK, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Vol. 115, Blood. 2010. pp. 453–74. [DOI] [PubMed] [Google Scholar]
- 31.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. American Society of Hematology; 2005. Oct 15;106(8):2912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. Br J Haematol. 1997. Jun;97(4):855–64. [DOI] [PubMed] [Google Scholar]
- 33.Filipovich AH, Weisdorf D, Pavletic S, Socié G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graftversus-host disease: I. Diagnosis and staging working group report. Biology of Blood and Marrow Transplantation. Elsevier; 2005. Dec;11(12):945–56. [DOI] [PubMed] [Google Scholar]
- 34.Copelan E, Casper JT, Carter SL, van Burik J-AH, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biology of Blood and Marrow Transplantation. Elsevier; 2007. Dec;13(12):1469–76. [DOI] [PubMed] [Google Scholar]
- 35.Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. American Society of Hematology; 2007. May 1;109(9):3658–66. [DOI] [PubMed] [Google Scholar]
- 36.Burnett AK, Wheatley K, Goldstone AH, Stevens R, Hann I, Hills RK. Long-term results of the MRC AML10 trial. Clin Adv Hematol Oncol. 2006. Jun;4(6):445–51. [PubMed] [Google Scholar]
- 37.Jourdan E, Boiron J-M, Dastugue N, Vey N, Marit G, Rigal-Huguet F, et al. Early allogeneic stem-cell transplantation for young adults with acute myeloblastic leukemia in first complete remission: an intent-to-treat long-term analysis of the BGMT experience. Journal of Clinical Oncology. 2005. Oct 20;23(30):7676–84. [DOI] [PubMed] [Google Scholar]
- 38.Suciu S, Mandelli F, de Witte T, Zittoun R, Gallo E, Labar B, et al. Allogeneic compared with autologous stem cell transplantation in the treatment of patients younger than 46 years with acute myeloid leukemia (AML) in first complete remission (CR1): an intention-to-treat analysis of the EORTC/GIMEMAAML-10 trial. Blood. American Society of Hematology; 2003. Aug 15;102(4):1232–40. [DOI] [PubMed] [Google Scholar]
- 39.Gorin NC, Labopin M, Fouillard L, Meloni G, Frassoni F, Iriondo A, et al. Retrospective evaluation of autologous bone marrow transplantation vs allogeneic bone marrow transplantation from an HLA identical related donor in acute myelocytic leukemia. A study of the European Cooperative Group for Blood and Marrow Transplantation (EBMT). Bone Marrow Transplantation. 1996. Jul;18(1):111–7. [PubMed] [Google Scholar]
- 40.Burnett AK, Goldstone A, Hills RK, Milligan D, Prentice A, Yin J, et al. Curability of patients with acute myeloid leukemia who did not undergo transplantation in first remission. J Clin Oncol. 2013. Apr 1;31(10):1293–301. [DOI] [PubMed] [Google Scholar]
- 41.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990. Feb 1;75(3):555–62. [PubMed] [Google Scholar]
- 42.Wagner JE, Thompson JS, Carter SL, Lancet NK, 2005. Unrelated Donor Marrow Transplantation Trial. Effect of graft-versus-host disease prophylaxis on 3-year disease-free survival in recipients of unrelated donor bone marrow (T-cell Depletion Trial): a multi-center, randomised phase II-III Trial. Lancet 2005: 366: 733–41. [DOI] [PubMed] [Google Scholar]
- 43.Cornelissen JJ, Gratwohl A, Schlenk RF, Sierra J, Bornhauser M, Juliusson G, et al. The European LeukemiaNet AML Working Party consensus statement on allogeneic HSCT for patients with AML in remission: an integrated-risk adapted approach. Nat Rev Clin Oncol. Nature Publishing Group; 2012. Oct;9(10):579–90. [DOI] [PubMed] [Google Scholar]
- 44.Cornelissen JJ, Blaise D. Hematopoietic stem cell transplantation for patients with AML in first complete remission. Blood. American Society of Hematology; 2016. Jan 7;127(1):62–70. [DOI] [PubMed] [Google Scholar]
- 45.Ossenkoppele GJ, Janssen JJWM, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica. Haematologica; 2016. Jan 1;101(1):20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Keever CA, Small TN, Flomenberg N, Heller G, Pekle K, Black P, et al. Immune reconstitution following bone marrow transplantation: comparison of recipients of T-cell depleted marrow with recipients of conventional marrow grafts. Blood. 1989. Apr;73(5):1340–50. [PubMed] [Google Scholar]
- 47.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002. Mar 15;295(5562):2097–100. [DOI] [PubMed] [Google Scholar]
- 48.Hsu KC, Keever-Taylor CA, Wilton A, Pinto C, Heller G, Arkun K, et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood. American Society of Hematology; 2005. Jun 15;105(12):4878–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller JS, Cooley S, Parham P, Farag SS, Verneris MR, McQueen KL, et al. Missing KIR ligands are associated with less relapse and increased graft-versus-host disease (GVHD) following unrelated donor allogeneic HCT. Blood. American Society of Hematology; 2007. Jun 1;109(11):5058–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jakubowski AA, Small TN, Kernan NA, Castro-Malaspina H, Collins N, Koehne G, et al. T Cell–Depleted Unrelated Donor Stem Cell Transplantation Provides Favorable Disease-Free Survival for Adults with Hematologic Malignancies. Biology of Blood and Marrow Transplantation. Elsevier; 2011. Sep 1;17(9):1335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saber W, Opie S, Rizzo JD, Zhang M-J, Horowitz MM, Schriber J. Outcomes after matched unrelated donor versus identical sibling hematopoietic cell transplantation in adults with acute myelogenous leukemia. Blood. American Society of Hematology; 2012. Apr 26;119(17):3908–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Doubrovina E, Oflaz-Sozmen B, Prockop SE, Kernan NA, Abramson S, Teruya-Feldstein J, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood. 2012. Mar 15;119(11):2644–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Reilly RJ, Prockop S, Hasan AN, Koehne G, Doubrovina E. Virus-specific T-cell banks for “off the shelf” adoptive therapy of refractory infections. Bone Marrow Transplantation. Nature Publishing Group; 2016. Sep;51(9):1163–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Huang Y. Pharmacogenomics of Sex Difference in Chemotherapeutic Toxicity. CDDT. Bentham Science Publishers; 2007. Jun 1;4(1):59–68. [DOI] [PubMed] [Google Scholar]
- 55.Willcox A, Wong E, Nath C, Janson B, Harrison SJ, Hoyt R, et al. The pharmacokinetics and pharmacodynamics of busulfan when combined with melphalan as conditioning in adult autologous stem cell transplant recipients. Ann Hematol. Springer Berlin Heidelberg; 2018. Jul 26;97(12):2509–18. [DOI] [PubMed] [Google Scholar]
- 56.Huang Y-T, Kim SJ Lee YJ, Burack D, Nichols P, Maloy M, et al. Co-Infections by Double Stranded DNA Viruses after Ex-Vivo T cell-Depleted CD34+ Selected Hematopoietic Cell Transplantation. Biol of Blood and Marrow Transplantation. 2017. 23: 1759–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]