Abstract
We found a region of the zebrafish pallium that shows selective activation upon change in the numerosity of visual stimuli. Zebrafish were habituated to sets of small dots that changed in individual size, position, and density, while maintaining their numerousness and overall surface. During dishabituation tests, zebrafish faced a change in number (with the same overall surface), in shape (with the same overall surface and number), or in size (with the same shape and number) of the dots, whereas, in a control group, zebrafish faced the same stimuli as during the habituation. Modulation of the expression of the immediate early genes c-fos and egr-1 and in situ hybridization revealed a selective activation of the caudal part of the dorso-central division of the zebrafish pallium upon change in numerosity. These findings support the existence of an evolutionarily conserved mechanism for approximate magnitude and provide an avenue for understanding its underlying molecular correlates.
Keywords: Approximate Number System, Danio rerio, immediate early genes, number cognition, numerousness, quantity discrimination
Introduction
What underlies the ability to deal with numbers and where did it come from? It has been argued that our ability to accurately represent the number of objects in a set (numerosity), and to carry out numerical comparisons and arithmetic, developed from an evolutionarily conserved system for approximating numerical magnitude, the so-called Approximate Number System (ANS; Dehaene 1997; Feigenson et al. 2004; Gallistel and Gelman 2000).
The cellular processes and neurocircuitry underlying the operating of the ANS remain to be fully defined; however, subregions of the parietal and prefrontal cortex of human and nonhuman primates have been identified as plausible candidates (Viswanathan and Nieder 2013, 2020; Nieder 2016; Piazza and Eger 2016). In nonhuman primates, single-cell recordings identified neurons that exhibit the expected ANS response with a peak of activity to one quantity and a progressive drop-off in activity as the quantity becomes more distant from the preferred one, in a way that obeys Weber’s Law (Nieder and Miller 2003; Nieder and Merten 2007). Similar to the “number neurons” that can be detected in the prefrontal cortex and the ventral intraparietal area in monkeys’ brains, neurons with ANS responses have been identified in crows (Ditz and Nieder 2015, 2016), within the nidopallium caudolaterale (NCL), a brain region that has been argued to be equivalent, though likely not homologous, to the mammalian prefrontal cortex.
A variety of studies have documented nonsymbolic numerical competence in a variety of other vertebrate species ranging from nonprimate mammals (Perdue et al. 2012; Utrata et al. 2012; Abramson et al. 2013; Bánszegi et al. 2016) to several species of birds (Pepperberg 2006; Rugani et al. 2009, 2013; Ditz and Nieder 2016; see for general reviews, Butterworth et al. 2017; Ferrigno and Cantlon 2017 and references therein; Vallortigara 2014, 2017; Nieder 2019). Note that mammals possess a laminated cortex and birds have been shown to possess in their nonlaminated pallium circuits organized in lamina-like and column-like entities (Stacho et al. 2020). However, other animals that lack a laminated cortex, such as amphibians (Krusche et al. 2010; Stancher et al. 2015), reptiles (Gazzola et al. 2018; Miletto Petrazzini et al. 2018), and fish (Stancher et al. 2013; Agrillo et al. 2017), show numerical abilities. Interestingly, in all these taxonomic groups (i.e., fish, amphibians, reptiles, birds, and mammals), numerosity discrimination exhibits a ratio-dependent signature, which is in accordance with the Weber’s law. This might suggest some deep homology in the underlying genetic mechanisms or maybe evolutionary convergence. In order to test the hypothesis of a conserved ANS, a mechanistic, bottom-up approach is needed, with a focus on exploring the neural underpinnings of cognitive features of numerosity and the genes that control them. Use of zebrafish could be key to such a research, for in recent years, it has become established as a developmental and behavioral genetic model species.
Zebrafish have been successfully used for comparative studies of numerosity using conditioning (Agrillo et al. 2017; Potrich et al. 2019), free choice (Pritchard et al. 2001; Potrich et al. 2015; Seguin and Gerlai 2017), and habituation/dishabituation (Messina et al. 2020) experiments. Variation in the expression of specific immediate early genes (IEGs; Lau et al. 2011; Sumbre and de Polavieja 2014) associated with dishabituation to visual numerosity in the overall telencephalon of zebrafish has been reported (Messina et al. 2020). Here, we refine and extend such analyses and reveal for the first time a specific region involved in numerical discrimination in the telencephalon of zebrafish.
The zebrafish telencephalon is composed by two main regions: a dorsal region, called pallium, and a ventral region, named subpallium (Northcutt 1981, 1995; Nieuwenhuys and Meek 1990). These macroscopical subdivisions can be subdivided into several pallial regions, including the central part of the “area dorsalis telencephali” (Dc), the medial part of the area dorsalis telencephali (Dm), and the lateral part of the area dorsalis telencephali (Dl) (Nieuwenhuys 2009; Ganz et al. 2014), and into subpallial nuclei, such as the “area ventralis telencephali” (V; Ganz et al. 2012). Each has specific molecular signatures.
In our study, zebrafish were first presented (habituation) with a set of elements (small dots) that changed in individual size, position, and density from trial to trial, but remained constant in their numerousness and in the overall areas subtended by the stimuli. Then, a novel visual stimulus was shown (dishabituation) involving controlled changes in different groups of animals: in numerosity, in shape, or in size. In a control group, the stimulus remained unchanged. Zebrafish were then sacrificed, their brains were dissected in Dc, Dm, Dl, and V, and processed for quantitative polymerase chain reaction (qPCR) analyses of the expression of c-fos and egr-1. The results were validated by subsequent in situ hybridization assays.
Materials and Methods
Ethical Regulations
Experimental procedures complied with the European Legislation for the Protection of Animals used for Scientific Purposes (Directive 2010/63/EU) and were approved by the Scientific Committee on Animal Health and Animal Welfare (Organismo Preposto al Benessere Animale, OPBA) of the University of Trento and by the Italian Ministry of Health (Protocol n. 893/2018-PR and Protocol n. 135/2020-PR).
Animals
Two hundred and fifty wild-type mixed-strain male 9-month-old zebrafish were used for the behavioral procedures. Eighty of them were randomly selected for qPCR experiments and 80 for in situ hybridization assays. Zebrafish were housed in 3.5-L plastic tanks in an automated aquarium system (ZebTEC Benchtop, Tecniplast) and were kept separated in groups of 10 individuals based on sex. They were reared in standard conditions (28 °C, light/dark cycle of 12 h/12 h); feeding was provided three times per day using dry food in accordance with guidelines.
Habituation–Dishabituation Experiment
Apparatus and Stimuli
The setup was the same as in Messina et al. (2020), and it consisted of a white plastic arena (40 × 60 × 30 cm) inside of which were placed five rectangular smaller tanks (20 × 6.5 × 20 cm, see Fig. 1A) raised 15 cm from the base of the arena, each one housing a single animal. The tanks were made of a white plastic material (Poliplak) on the four sides, with a white mesh (grid 0.1 mm thick) forming the base, allowing for good water circulation. The water in each tank (8 cm in height) was maintained at a constant temperature of 26 °C and was kept clean by a pump and a filter system (Micro Jet Filter MCF 40). The apparatus was lit by two LED strips and a webcam (Microsoft LifeCam Studio) recorded fish behavior from above (50 cm) the setup.
Figure 1 .
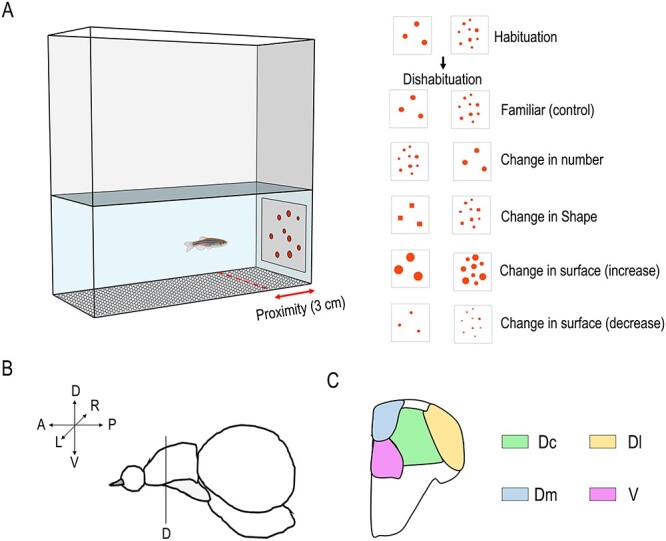
Experimental design. (A) Apparatus and stimuli used for the habituation and dishabituation phases. Scheme of the lateral view (B) of zebrafish telencephalon with a cross-section of telencephalic nuclei (C) tested for molecular biology analyses. Dc, dorsal-central; Dl, dorsal-lateral; Dm, dorsal-medial; V, subpallium.
The stimuli (Fig. 1A) used for the habituation and dishabituation phases were cards (6 × 6 cm) glued on white plastic panels (20 × 6 cm). For the habituation phase, each stimulus depicted a group of three or nine red/orange (RGB: 252, 72, 11) dots on a white background. For each numerosity, a set of nine stimuli configurations was used. Among the different configurations, the spatial dispositions of the dots and the size of each dot (range: 4–11 mm) were randomized. The overall cumulative area of the stimuli (sum of the dots’ areas) was equalized (1.58 cm2) among the different stimuli configurations and the two different numerosities. The visual angle range calculated on the furthest position to the stimulus was 1.15–3.15 °, which is well within the spatial resolution range by zebrafish (Haug et al. 2010 report a minimum separable angle of 0.57 °).
For the dishabituation phase, new sets of nine stimuli were used. The novel stimuli comprised: a change in number (from three to nine dots or vice versa) keeping the same overall surface area; a change in shape (from dots to squares) maintaining the number and the overall area unmodified; and a change in size (increasing or decreasing three times the overall dots’ surface area; in this way, contour length was changed as well) keeping the shape and number unmodified. In each dishabituation stimulus, the spatial distribution of the dots was randomly changed so as to modify continuously density and convex hull as well as the size of each single element.
Procedure
Two days before the starting of the experiment, the fish were singly inserted in the apparatus tank in order to acclimatize the animals to the novel environment and reduce the stress connected to isolation. Fish remained in the tank for the entire duration of the experiment, which lasted for 5 days. During the habituation phase, at the beginning of each trial, one panel depicting three or nine dots (depending on the habituation condition) was introduced in one of the two shortest sides of the tank, followed by the release of a small morsel of food (1–1.2 mm) in proximity to the stimulus (after a delay of 30 s). The stimulus remained in the tank for 2 min after the food delivery and then it was removed. After an inter-trial time of 5 min, a new trial started on the opposite side of the tank with a new panel depicting a different dot configuration (but with the same numerosity). Each fish received 12 daily trials, divided in three sessions of four trials each. Among the 12 trials, the configuration of the habituation stimuli was randomized. On the fifth day, fish performed only the first habituation session (four trials). After that, fish were left in their tanks for 5 h before the dishabituation test. This delay was to allow the IEG expression to return to the baseline level before the test.
The dishabituation phase consisted of a single trial in which a novel test stimulus was presented to the fish. Before the test, fish were randomly assigned to the five different dishabituation groups that included a change of numerosity (from three to nine dots or vice versa, but the same overall area), a change in shape (from dots to squares, but with the same number and overall area), two changes of areas (increasing or decreasing the dots’ surface area, but depicting the same number and shape), or a control condition (same stimulus as used in the habituation phase). In the test trial, the panel was introduced along one of the shortest sides and remained in the tank for the 30 s of test. No food was provided during this test trial. Fish were then sacrificed 30 min after the end of the dishabituation test and their brains were collected.
As a behavioral measure, we analyzed the time spent in proximity of the stimulus (3-cm area) in the 30 s after the stimulus appearance. An absolute proportion of time was calculated by comparing the dishabituation trial (“test time”) with the previous habituation session (average of the four trials, “habituation time”) performed on the same day, using the following formula:
 |
The use of an absolute value for the proportion of time allowed us to detect a behavioral difference between the dishabituation and habituation phases irrespective of whether fish tended to approach or to avoid the novel stimulus compared with the familiar one.
As further behavioral measures, we also considered 1) the number of entries in the proximity area, 2) the numbers of the fish’ head contacts with the stimulus, and 3) the number of turns (180 °) in front of the stimulus in the proximity area. All these behaviors were computed by comparing the number of occurrences during the dishabituation test trial with the corresponding previous habituation trial (the first of the four trials) performed on the same day.
Tissue Preparation: Brain Dissection and Total RNA Extraction
Thirty minutes after the end of the dishabituation phase, fish were sacrificed in a bath of ice-cold phosphate-buffered saline solution (PBS; Fisher Bioreagents); their brains were dissected and embedded for later cryosectioning in optimum cutting temperature (OCT, Tissue-Tek OCT Sakura; Sakura Finetek), frozen, and stored at −20 °C. Fifty-micrometer coronal sections of the brains were prepared using a cryostat (Leica CM 1860 UV; Leica Biosystems). Each section was uncurled with fine brushes, put onto a glass slide (Super-Frost Plus; ThermoFisher Scientific), and stored at −20 °C. Selected brain areas (central part of the area dorsalis telencephali [Dc], medial part of the area dorsalis telencephali [Dm], lateral part of the area dorsalis telencephali [Dl], and the area ventralis telencephali [V]; Fig. 1B,C) were punched out (Li et al. 2018) using 10-μL pipette tips, and their total ribonucleic acid (RNA) was extracted (Arcturus Picopure RNA isolation Kit; ThermoFisher Scientific) according to manufacturer’s instructions. Finally, the purity (A260/A280 and A260/230 values) and the concentration of collected total RNAs were assessed using the Nanodrop spectrophotometer (Nanodrop OneC; ThermoFisher Scientific). Reverse transcription was performed using the SuperScriptTM VILO cDNA Synthesis Kit (Invitrogen, ThermoFisher Scientific) according to manufacturer’s instructions.
qPCR
qPCR experiments were performed in order to analyze the expression of c-fos (NM_205569), egr-1 (NM_131248), and 18S ribosomal RNA (18S) (NM_173234)—which was used as reference gene—and of the molecular markers emx2, emx3, prox1, eomesa, dlx2a, dlx5a. Specific primer pairs were commercially synthesized (Sigma-Aldrich/Merck; see Table 1). qPCR assays were performed in triplicate reactions using the PowerUp SYBR Green Master Mix (2X) and were run in a CFX96 Real-Time System (Bio-Rad). The ΔCq method was used for expression quantification (Messina et al. 2020). Data were normalized on the expression of the 18S reference gene (ΔCq), and the relative expression (to the reference gene) of each target was calculated.
Table 1.
Primers used for qPCR experiments
| Gene | Primer name | Primer sequence | Product size (bp) | Efficiency (E, %) | Accession ID |
|---|---|---|---|---|---|
| c-fos | For Rev |
GTATTACCCGCTCAACCAGAC TCCAGTAACCCTCATTTTGGG |
200 pb | 99.1 | 394198 |
| egr-1 | For Rev |
AGTTTGATCACCTTGCTGGAG AACGGCCTGTGTAAGATATGG |
110 pb | 108.1 | 30498 |
| 18S | For Rev |
TCGCTAGTTGGCATCGTTTATG CGGAGGTTCGAAGACGATCA |
85 pb | 93 | 100037361 |
| emx-2 | For Rev |
GGACTCGTTTCGTTTCCTTG GGACTCGTTTCGTTTCCTTG |
199 pb | 94.4 | 30537 |
| emx-3 | For Rev |
TTCACTCCATCATCGGGTTC GCGTTTGACGAATTGGAGTC |
145 pb | 93.5 | 30536 |
| eomesa | For Rev |
CTTATTGATCTCCGCCTTGC TATTGGTGCTTTCGGAGGAC |
147 pb | 99.4 | 64603 |
| prox1a | For Rev |
TTACGAAGACGCTGTGATGC AATGGTGAAAGGCACTCCTG |
195 pb | 92.0 | 30679 |
| dlx2a | For Rev |
TTCAGCCACCACTTCATCAC AACAGTGTCACGCCCAAATC |
193 pb | 95.0 | 30574 |
| dlx5a | For Rev |
TCATACTCCACAGCGTATCACC AGTAAATGGTTCGGGGCTTC |
148 pb | 90.0 | 30569 |
Note: For, forward primer; Rev: reverse primer.
In Situ Hybridization
In situ hybridization assays were performed to determine the localization of the expression of egr-1 in the zebrafish brain nuclei. RNA probes necessary for the detection of the egr-1 mRNA transcripts were created from total brain cDNA by PCR amplification (using the Phusion High-Fidelity PCR Master Mix with HF Buffer; ThermoFisher Scientific), followed by precipitation and quantification (Nanodrop OneC; ThermoFisher Scientific). Primers for cDNA amplification were as follows: SP6-egr1-forward ATTTAGGTGACACTATAGTCTGTTCAGCCTGGTGAGTG, T7-egr1-reverse TAATACGACTCACTATAGTGGAGACCGGAGAAGGGTAAG. DIG-labeled (Digoxigenin-11-UTP/DIG RNA Labeling Mix, Merk) single-stranded RNA probes were prepared following standard protocols.
The 20-μm brain slices were fixed in 4% paraformaldehyde (Carlo Erba Reagents), rinsed in PBS, and hybridized with egr1 probes in a humidified chamber at 65 ° overnight. Then, slides were washed in formamide/SSC solution (formamide: Invitrogen, ThermoFisher Scientific; 20× SSC, saline-sodium citrate buffer: Gibco, ThermoFisher Scientific) at 65 °C and in MAB solution (maleic acid buffer; Sigma-Aldrich/Merck) at room temperature. After being treated with a blocking solution (composed of Fetal Bovine Serum, Euroclone), blocking reagent (Roche, MAB), the glass slides were incubated with an anti-DIG-AP antibody (Anti-Digoxigenin-AP, Fab fragments; Merck) overnight in a humidified chamber. Slides were treated with BCIP/NBT substrate of alkaline phosphatase (BCIP/NBT Ready-To-Use Substrate; SERVA) and were kept in the dark until the colorimetric reaction reached the expected point. Finally, the slides were mounted using Fluoroschield with DAPI (Sigma-Aldrich/Merck) and were analyzed under a microscope (Observer.Z1, ZEISS) using a 20× objective and a digital camera (Zeiss AxioCam MRc 5).
Using a single-blind procedure (the operator did not know what training the fish underwent), we counted egr-1-positive cells by sampling three different rostro-caudal region of Dc according to section 60 (rostral, Dc1), section 85 (medial, Dc2), and section 98 (caudal, Dc3) of a topological atlas of the neuroanatomy of the zebrafish brain (Rupp et al. 1996). Estimated density was reported as number of counted egr-1-positive cells (dark-blue dots) normalized on the surface of the relative Dc-counted regions in each slice. We used the ZEN Imaging software (Zeiss) for the counting of cells. egr1-positive cells were digitally marked using the event marker of the ZEN software, which then provided the total number of positive cells as the output.
Statistical Analysis
Statistical analyses on behavior, qPCR, and egr1-positive cells count data were performed using the Statistical Package for the Social Sciences (IBM SPSS Statistics; IBM).
On the behavioral data, an arcsin transformation was used, as recommended for data represented as proportions. An analysis of variance (ANOVA) was performed with “habituation” and “test” as between-subjects factors.
Data for qPCR were analyzed with two-way ANOVAs (applying the Greenhouse–Geisser correction to adjust for the lack of sphericity) using habituation (habituation with either three or nine dots) and the type of test (familiar [control condition, no change with respect to the habituation phase], number, shape, surface area increase, and surface area decrease) as between-subject factors and telencephalic nuclei (Dc, Dl, Dm, and V) as a within-subject factor. LSD post hoc tests with Bonferroni correction for multiple comparisons were used for pairwise comparisons.
Data for egr1-positive cells count were acquired by in situ hybridization using two-way ANOVAs by comparing and applying LSD post hoc tests with Bonferroni corrections for multiple comparisons.
Results
Behavior
Proportions of time spent close to the familiar or changed (dishabituated) stimulus is shown in Figure 2. The ANOVA with habituation (three or nine elements) and test (no change [familiar, control group], change in number, change in shape, change in surface area [increase], and change in area [decrease]) revealed a significant main effect of the test (F(4, 240) = 2.880, P = 0.023, η2p = 0.046) but not of the habituation (F(1, 240) = 0.477, P = 0.490, η2p = 0.002) and of the interaction between habituation and test (F(4, 240) = 0.070, P = 0.991, η2p = 0.001). An ANOVA limited to the conditions with a change at test (in number, in shape, and in surface areas) did not reveal any statistically significant heterogeneity among conditions (test: F(3, 192) = 0.523, P = 0.667, η2p = 0.008; habituation: F(1, 192) = 0.195, P = 0.659, η2p = 0.001; habituation × test: F(3, 192) = 0.049, P = 0.986, η2p = 0.001). Significant differences, when the familiar (no change) condition was compared with that of the change in number (t(98) = −2.766, P = 0.007, Cohen’s d = 0.178) and change in areas (increase, t(98) = −2.901, P = 0.005, Cohen’s d = 0.186; decrease, t(98) = −3.386, P = 0.001, Cohen’s d = 0.157), were observed, whereas with the change in shape, the effect only approached the conventional level of significance (t(98) = −1.888, P = 0.062, Cohen’s d = 0.170). Results are shown in Figure 2 (collapsed for the two habituation conditions, i.e., habituation with three and nine dots since no significant difference between the two types of habituation was observed; separate graphs for the two conditions are however shown in the Supplementary Fig. S1). The other behavioral measures (see Supplementary Fig. S2 for details) also revealed similar effects.
Figure 2 .
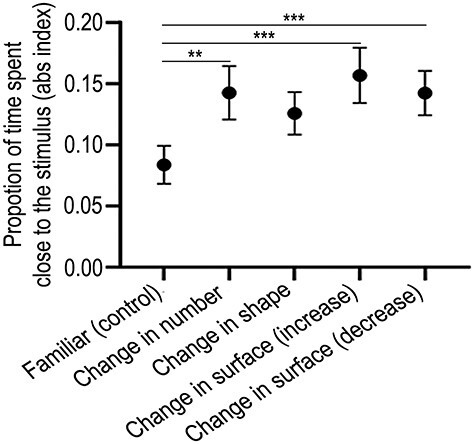
Behavioral data. Results of the dishabituation test expressed as the absolute proportion of time spent near the stimulus. Group means with standard error of mean (SEM) are shown. (**P < 0.01; ***P < 0.005; see text for details of statistics).
Molecular Signature Analyses for Dc, Dl, Dm, and V
In order to assess whether the dissection of the telencephalic nuclei of interest was effective, the expressions of molecular signatures specific for Dc, Dl, Dm, and V were measured. The nuclei under investigation are characterized by the expression of some molecular markers (Ganz et al. 2012, 2014). As reported in the literature, we found that in our samples Dc was primarily characterized by the expression of emx2, emx3, and eomesa; Dl by the expression of emx3, prox1, and eomesa; whereas, emx3 alone was highly expressed in Dm; V was characterized by the expression of dlx2a and dlx5a, with low eomesa mRNA levels (Supplementary Fig. S3).
IEG Expression
Since c-fos and egr-1 are characterized by distinct expression pathways, separate ANOVAs were performed for the two IEGs, with habituation (habituation with either three or nine dots) and type of test (familiar [control condition, no change with respect to the habituation phase], number, shape, surface area increase, and surface area decrease) as between-subject factors and with telencephalic nuclei (Dc, Dl, Dm, and V) as a within-subject factor.
Since the overall ANOVA revealed a main effect of the test (F(4, 70) = 5.646, P = 0.001, η2p = 0.211) and an interaction between telencephalic nuclei and habituation (F(2.555, 178.880) = 2.918, P = 0.044, η2p = 0.042) for c-fos, and a main effect of the telencephalic nuclei (F(2.705, 189.319) = 22.083, P < 0.0001, η2p = 0.281) and an interaction between telencephalic nuclei and test (F(10.818, 189.319) = 2.307, P = 0.012, η2p = 0.150) for egr-1, in the subsequent analyses, we considered test and habituation separately for the distinct telencephalic nuclei (see Supplementary Tables S1-S5 for the complete ANOVAs).
Central Part of the Area Dorsalis Telencephali (Dc)
For c-fos (see Fig. 3, leftmost column), a comparison between familiar (no change) and change in numerosity revealed a significant test × habituation interaction (F(1, 28) = 25.789, P = 0.0001, η2p = 0.479). Change in numerosity from three to nine resulted in an increase in c-fos expression (P = 0.0001), whereas change from nine to three resulted in a decrease (P = 0.005).
Figure 3.
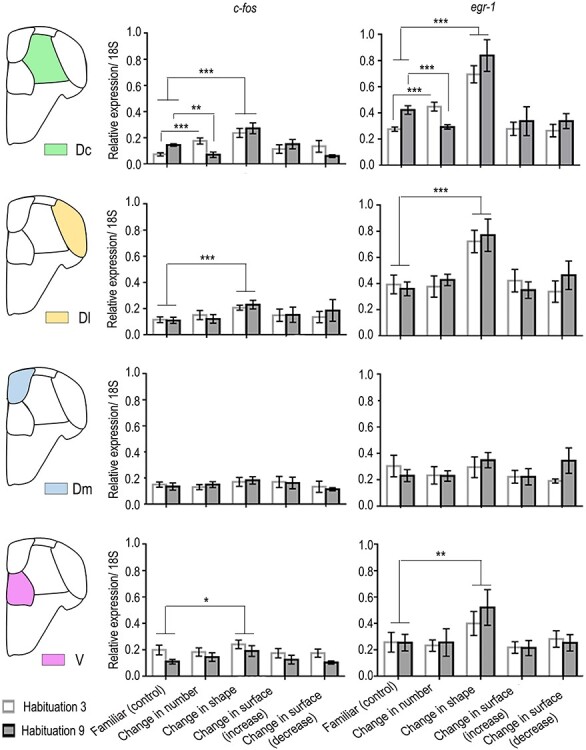
IEG quantification. qPCR results for the relative expression of c-fos and egr-1 in the central part of area dorsalis telencephali (Dc), in the lateral part of area dorsalis telencephali (Dl), in the medial part of area dorsalis telencephali (Dm), and in the “ventral subpallium” (V) for the different test conditions. Group means with SEM are shown. (*P < 0.05; **P < 0.005; ***P < 0.000; see text for details of statistics).
A comparison between familiar (no change) and change in shape revealed only a main effect of the test (F(1, 28) = 26.417, P = 0.0001, η2p = 0.485), with a general increase in c-fos expression as a result of the change in shape.
A comparison between familiar (no change) and change in surface area did not reveal any significant main effect of the test (F(2, 42) = 0.823, P = 0.446), but there was a significant test × habituation interaction (F(2, 42) = 3.717, P = 0.033, η2p = 0.150). The interaction, however, was limited to the decrease in surface area condition (F(1, 28) = 9.077, P = 0.005, η2p = 0.245).
For egr-1 (Fig. 3, rightmost column), a comparison between familiar (no change) and change in numerosity revealed a significant test × habituation interaction (F(1, 28) = 35.905, P = 0.0001, η2p = 0.562). Similarly to c-fos, change in numerosity from three to nine resulted in an increase in egr-1 expression (P = 0.0001), whereas change from nine to three in a decrease (P = 0.001).
A comparison between familiar (no change) and change in shape revealed only a main effect of the test (F(1, 28) = 35.219, P = 0.0001, η2p = 0.557), with an increase in erg-1 expression irrespective of habituation with three or nine elements.
A comparison between familiar (no change) and change in size did not reveal any significant main effect or interaction.
Overall, the results suggested that the central part of the area dorsalis telencephali (Dc) responded to the change in numerosity and shape. One intriguing result with the change in numerosity was that IEG expression was modulated differently by the direction of change, with an increase when numerosity increased (from three to nine) and with a decrease when numerosity decreased (from nine to three). Given that we were interested in the ability of zebrafish to notice a change irrespective of the direction of the behavioral response, we considered the absolute value in, shown in Figure 2. Prompted by IEG results, we reconsidered behavioral data in relative values (Supplementary Fig. S4) for both time spent and number of head contacts with the stimulus. We found that no interaction with habituation condition (three vs. nine) was observed for changes in shape/surfaces (time spent: F(3, 192) = 0.348 n.s.) number of contacts: (F(3, 185) = 0.191 n.s.; Supplementary Fig. S4), whereas for change in numerosity, there was a trend for time spent (F(1, 96) = 3.591 P = 0.061, η2p = 0.036) and a striking effect for number of contacts (F(1,94) = 8.249 P = 0.005, η2p = 0.081). Given that IEG analyses were done only on a small subset of behaviorally tested fish, any direct correlation was prevented. Nonetheless, the results suggest that modulation of IEG closely parallels approach (from small to large numerosity) versus avoidance (from large to small numerosity) responses. On the basis of the pattern of connectivity of Dc with motor areas, we speculate in the Discussion section that higher/lower activation of neurons in Dc could be associated with a higher/lower motor execution, such as approaching or avoiding.
Lateral Part of the Area Dorsalis Telencephali (Dl)
For c-fos (Fig. 3, leftmost column), no significant effects were observed, whereas only a significant main effect of test was observed for egr-1 (F(4, 70) = 6.531, P = 0.0001, η2p = 0.297), clearly due to the change in shape.
The results suggested that the lateral part (Dl) of the zebrafish dorsal pallium was not involved in quantity estimation (number and size) but only (though limited to egr-1 expression) in the detection of change in shape.
Medial Part of the Area Dorsalis Telencephali (Dm)
No significant main effects or significant interactions were apparent for either of the two IEGs (Fig. 3). The results suggested that the medial part (Dm) of the zebrafish dorsal telencephalon did not show any relevant regulation of neural activity following the different types of changes.
Area Ventralis Telencephali (V)
The ANOVA for c-fos revealed only a significant main effect of habituation (F(1, 70) = 10.052, P = 0.002, η2p = 0.131). A significant main effect of the test was detected for both c-fos (F(4, 70) = 3.159, P = 0.019, η2p = 0.081) and egr-1 (F(4, 70) = 5.487, P = 0.001, η2p = 0.195), which was limited to the change in shape (Fig. 3). The results suggested that the area ventralis telencephali (V) was not involved in quantity estimation in zebrafish, either discrete (numerosity) or continuous (surface area), but only in shape.
Counting of Egr-1-Positive Cells
qPCR showed that Dc was the only area that showed modulation of expression of both c-fos and egr-1 to a change in numerosity. However, it also showed modulation of response to change in shape. We thus looked at the spatial location of egr-1-positive cells that respond to numerosity and shape along the rostro-caudal axis of Dc using in situ hybridization (Fig. 4). (We did not show c-fos positive cells due to the weakness of the detected signal in our experiments.)
Figure 4.
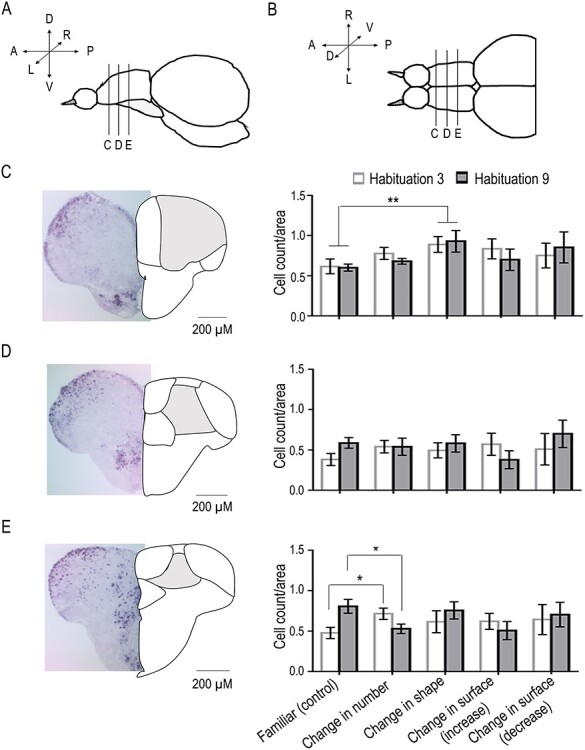
In situ hybridization analysis of egr-1. Mean number of egr-1-positive cells in three different rostro-caudal regions of Dc. Scheme of lateral (A) and dorsal (B) views of zebrafish telencephalon with results for the selected rostral (C), medial (D), and caudal (E) slices in the different test conditions. (Group means with SEM are shown. *P < 0.05; **P < 0.005; see text for details of statistics).
An ANOVA was run in order to evaluate the percentage of egr-1-expressing cells in the three Dc slices along a rostro-caudal position. The ANOVA revealed a significant main effect of the rostro-caudal position of the slice (F(2, 140) = 42.360, P < 0.0001, η2p = 0.377) and a significant interaction of the rostro-caudal position with the test (F(16, 140) = 1.819, P = 0.034, η2p = 0.172).
In particular, as shown in Figure 4C, in the most rostral regions, a comparison between familiar (no change) and change in shape revealed a significant main effect of the test (F(1, 28) = 9.422, P = 0.005, η2p = 0.251). No significant main effect or significant interaction was observed in the medial region of Dc (Fig. 4D). In the most caudal region of Dc (Fig. 4E), a significant interaction between habituation and test (F(2, 56) = 12.907, P = 0.001, η2p = 0.316) was observed, with an increase (P = 0.026) or a decrease (P = 0.011) in cell count depending on whether the change in the stimulus consisted of an increase or a decrease in numerosity.
Thus, in situ hybridization results suggested that the most rostral parts of Dc were responsive to a change in shape, whereas the most caudal parts were responsive to a change in numerosity.
Discussion
The habituation–dishabituation design of the stimulus presentation allows the disentanglement of the effect of changing stimulus numerosity, stimulus shape, and stimulus size.
The results of qPCR experiments showed that different regions of the zebrafish telencephalon differentially expressed c-fos and egr-1 depending on the kind of change in the stimulus. The central part of the area dorsalis telencephali (Dc), the lateral part of the area dorsalis telencephali (Dl), and the area ventralis telencephali (V) were all affected by changes in shape. Note, however, that changes in shape likely represented different aspects of the stimulus, such as shape in itself and spatial aspects. The DI area in teleosts has been proposed to be homologous to the hippocampal formation of mammals and birds (Rodríguez et al. 2002; Teles et al. 2015); thus, perhaps the modulation of response to shape in DI reflects the selectivity to change in the spatial characteristics of the stimulus (note that, in this area, selectivity in expression to change in shape was apparent only for egr-1 but not for c-fos).
Selectivity of response to numerosity was confined to Dc only. This area also responded to shape, but in situ hybridization showed that the rostral part only responded to shape, whereas the most caudal parts only responded to numerosity. More precisely, we found that a larger number of egr-1-expressing neurons was seen in fish habituated with three dots and tested with nine, and a smaller number was seen in those habituated with nine dots and tested with three, suggesting that the increased or decreased expression of egr-1 mRNA in qPCR experiments was probably due to a larger or smaller number of activated neurons recruited during the dishabituation phase in fish facing the numerosity change. This pattern of response is reminiscent of properties of neurons in the lateral intraparietal area of monkeys’ brain that showed increased or decreased activity as a function of the number of elements entering their receptive fields, thus encoding the number of elements in a visual array in a monotonic manner (Roitman et al. 2007).
Most interestingly, in Dc modulation of IEG, responses by direction of change (increase with change from small to large numbers and decrease with change from large to small numbers) seemed to parallel similar increase and decrease in behavioral measures (time spent and number of contacts with the stimuli). Hodological studies (Yamamoto and Ito 2005; Yamamoto et al. 2007), Harvey-Girard et al. 2012, Ito and Yamamoto 2009) showed that Dc constitutes the major descending pathway of the fish pallia; down to midbrain (including optic tectum) and medulla oblongata (areas involved in the downstream control of the spinal cord). Higher activation of neurons in Dc could thus be associated with a higher motor execution, such as approaching or avoiding. Depending on the behavioral (motor) execution of the subjects, therefore, we may expect dichotomized IEG patterns so that approaching fish could differ from avoiding fish as it is shown in association with the direction of change in numerosity.
Selectivity of response to change in surface areas were also limited to only Dc area, however, it appeared to be quite small and variable, depending on whether the change involved an increase or a decrease and whether it applied to large (nine) or small (three) numerosities and then only for c-fos and not for egr-1. Considering that some theoretical accounts of number cognition assume that dealing with discrete (countable) numerosities is one aspect of a more general system dealing with magnitude (either discrete or continuous, see, e.g., Gallistel, 1989; Walsh 2003; and for empirical evidence, see, e.g., De Corte et al. 2017; Bortot et al. 2020), one would expect clear and parallel responsivity to changes in discrete (numerosities) and continuous (surface area) quantities. It could be, however, that the lack of control for distance of seeing made absolute size estimation difficult for zebrafish. Alternatively, it may be that processing for continuous magnitude is done mainly at the level of the tectum (which has been shown to be responsive for changes in surface area in habituation/dishabituation experiments; Messina et al. 2020) and that the telencephalon is mainly involved with discrete quantities.
In the mammalian brain the main area involved in numerosity, cognition is the posterior part of the parietal cortex (Viswanathan and Nieder 2013, 2020; Nieder 2016; Piazza and Eger 2016). Activation of the prefrontal cortex is also observed, but in single-cell recording experiments, it usually occurs with a latency of about 30 ms, suggesting a later stage of processing (Viswanathan and Nieder 2020). In the avian brain, only single-cell recording experiments are available as of yet, and they suggest that the NCL in crows contains number neurons similar to those recorded in the mammalian/prefrontal cortex (Ditz and Nieder 2015, 2016; Nieder 2016, 2017). The possible homology/homoplasy relationship of NCL with regions of the mammalian brain is at present uncertain. Functionally, NCL appears to be a sort of avian equivalent of the prefrontal cortex (Güntürkün 2005), but there are also striking differences (e.g., an apparent lack of direct connection between the NCL and the hippocampal formation). Thus, our finding of a highly selective role of the most caudal parts of Dc in numerosity responsiveness in zebrafish is exciting in terms of the possible similarities of this region with equivalent or homologous regions in the mammalian and avian brains.
There is some (though admittedly not unanimous) consensus that Dl of teleosts is homologous to the medial pallium of tetrapods (i.e., hippocampal formation), Dm to ventral pallium (pallial amygdala), and Dc to dorsal pallium (note that the mammalian isocortex is one example of the many outcomes of the evolution of vertebrate dorsal pallium; Tosches and Laurent 2019). Harvey-Girard et al. (2012), in particular, hypothesized a homology of Dc with efferent layers V and VI of mammalian isocortex. No data are, however, currently available to dissect anatomically or functionally different parts of Dc, such as the most caudal and rostral regions.
It is worth stressing that although we identified a small portion of the zebrafish pallium which selectively responds to numerosity, it is unlikely that it would be the only one. Even in mammals, multiple regions in the brain, for example, the parietal cortex and the prefrontal cortex, are involved in the processing of numerosity (review in Lorenzi et al. 2021). We examined here only the dorsal part of the pallium but not the ventral part (which is ca. more than 30% of the overall structure). Indirect evidence for a possible role of this ventral region (in which nuclei are less well characterized than their dorsal counterparts) comes from previous evidence that IEG expression to the overall pallium showed selectivity to numerosity but with reverse patterns of up- and downregulations with respect to the direction of change (i.e., upregulation for changes from small to large numerosities, though in this case, changes in size also affected IEG expression; see Messina et al. 2020). Obviously, IEG expression cannot reveal the specificity of the role of excitatory and inhibitory neurons; nonetheless, this evidence strongly suggests that there could be selectivity to numerosity (albeit related to other continuous quantities) also in the nuclei of the ventral pallium. This will certainly deserve further research.
In sum, our results offer evidence that the central part of area dorsalis telencephali (Dc) may be a pallial structure of the zebrafish brain most involved in cognitive processes such as shape and numerosity recognition.
Authors’ contributions
A.M, D.P., C.H.B., S.E.F., and G.V. conceived and designed the experiments. A.M., D.P., and I.S. performed the experiments. A.M., D.P., I.S., V.A.S., C.H.B., S.E.F., and G.V. analyzed and interpreted the data. G.V. contributed the reagents/materials. All authors contributed to the manuscript writing.
Supplementary Material
Contributor Information
Andrea Messina, Center for Mind/Brain Sciences, University of Trento, Rovereto 38068, Italy.
Davide Potrich, Center for Mind/Brain Sciences, University of Trento, Rovereto 38068, Italy.
Ilaria Schiona, Center for Mind/Brain Sciences, University of Trento, Rovereto 38068, Italy.
Valeria Anna Sovrano, Center for Mind/Brain Sciences, University of Trento, Rovereto 38068, Italy; Department of Psychology and Cognitive Science, University of Trento, Rovereto 38068, Italy.
Scott E Fraser, Michelson Center for Convergent Bioscience, University of Southern California, Los Angeles CA 90089, USA.
Caroline H Brennan, School of Biological and Chemical Sciences, Queen Mary University of London, London E1 4NS, UK.
Giorgio Vallortigara, Center for Mind/Brain Sciences, University of Trento, Rovereto 38068, Italy.
Funding
Human Frontiers Research Grant (HFSP Research Grant RGP0008/2017 to C.H.B., S.E.F., and G.V.); PRIN 2017 Grant (2017PSRHPZ to G.V.); Leverhulme Grant (RPG-2016-143 to C.H.B.); European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement No. 833504-SPANUMBRA to G.V.).
Notes
We thank Tommaso Pecchia, Grazia Gambardella, Roberta Guidolin, and Ciro Petrone for technical and administrative support. The authors would like to thank Jose Torres-Perez and Eva Sheardown for their useful comments and suggestions. We are also indebted with an anonymous reviewer who suggested us to look at direction of behavioral responses to make sense of IEG expression modulation with direction of change in numerosity. Conflict of Interest: None declared.
Data availability
Data are available in a submitted supplementary file.
References
- Abramson JZ, Hernández-Lloreda V, Call J, Colmenares F. 2013. Relative quantity judgments in the beluga whale (Delphinapterus leucas) and the bottlenose dolphin (Tursiops truncatus). Behav Processes. 96:11–19. [DOI] [PubMed] [Google Scholar]
- Agrillo C, Miletto Petrazzini ME, Bisazza A. 2017. Numerical abilities in fish: a methodological review. Behav Processes. 141:161–171. [DOI] [PubMed] [Google Scholar]
- Bánszegi O, Urrutia A, Szenczi P, Hudson R. 2016. More or less: spontaneous quantity discrimination in the domestic cat. Anim Cogn. 19:879–888. [DOI] [PubMed] [Google Scholar]
- Bortot M, Stancher G, Vallortigara G. 2020. Transfer from number to size reveals abstract coding of magnitude in honeybees. iScience. 23(5):101122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B, Gallistel CR, Vallortigara G. 2017. Introduction: the origins of numerical abilities. Philos Trans R Soc B Biol Sci. 373(1740):20160507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Corte BJ, Navarro VM, Wasserman EA. 2017. Non-cortical magnitude coding of space and time by pigeons. Curr Biol. 27:1264–1265. [DOI] [PubMed] [Google Scholar]
- Dehaene S. 1997. The number sense: how the mind creates mathematics. New York: Oxford University Press. [Google Scholar]
- Ditz HM, Nieder A. 2015. Neurons selective to the number of visual items in the corvid songbird endbrain. Proc Natl Acad Sci USA. 112:7827–7832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditz HM, Nieder A. 2016. Numerosity representations in crows obey the Weber-Fechner law. Proc Biol Sci. 283:20160083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigenson L, Dehaene S, Spelke E. 2004. Core systems of number. Trends Cogn Sci. 8:307–314. [DOI] [PubMed] [Google Scholar]
- Ferrigno S, Cantlon JF. 2017. Evolutionary constraints on the emergence of human mathematical concepts. In: Kaas J, editor. Evolution of nervous systems. 2nd ed. Oxford: Elsevier, pp. 511–521. [Google Scholar]
- Gallistel CR, Gelman II. 2000. Non-verbal numerical cognition: from reals to integers. Trends Cogn Sci. 4:59–65. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. 1989. Animal cognition: the representation of space, time and number. Ann Rev Psychol. 40:155–189. [DOI] [PubMed] [Google Scholar]
- Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M, Brand M. 2012. Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J Comp Neurol. 520:633–655. [DOI] [PubMed] [Google Scholar]
- Ganz J, Kroehne V, Freudenreich D, Machate A, Geffarth M, Braasch I, Kaslin J, Brand M. 2014. Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Res. 3:308. [DOI] [PMC free article] [PubMed]
- Gazzola A, Vallortigara G, Pelliteri-Rosa D. 2018. Continuous and discrete quantity discrimination in tortoises. Biol Lett. 14:20180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güntürkün O. 2005. The avian ‘prefrontal cortex’ and cognition. Curr Opin Neurobiol. 15:686–693. [DOI] [PubMed] [Google Scholar]
- Harvey-Girard E, Giassi AC, Ellis W, Maler L. 2012. Organization of the gymnotiform fish pallium in relation to learning and memory: IV. Expression of conserved transcription factors and implications for the evolution of dorsal telencephalon. J Comp Neurol. 520:3395–3413. [DOI] [PubMed] [Google Scholar]
- Haug MF, Biehlmaier O, Mueller KP, Neuhauss SCF. 2010. Visual acuity in larval zebrafish: behavior and histology. Front Zool. 7:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Yamamoto N. 2009. Non-laminar cerebral cortex in teleost fishes? Biol Lett. 5:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusche P, Uller C, Dicke U. 2010. Quantity discrimination in salamanders. J Exp Biol. 213:1822–1828. [DOI] [PubMed] [Google Scholar]
- Lau BYB, Mathur P, Gould GG, Guo S. 2011. Identification of a brain center whose activity discriminates a choice behavior in zebrafish. Proc Natl Acad Sci. 108:2581–2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CY, Hofmann HA, Harris ML, Earley RL. 2018. Real or fake? Natural and artificial social stimuli elicit divergent behavioural and neural responses in mangrove rivulus, Kryptolebias marmoratus. Proc Biol Sci. 14:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi E, Perrino M, Vallortigara G. 2021. Numerosities and other magnitudes in the brains: a comparative view. Front Psychol. 12:641994. doi: 10.3389/fpsyg.2021.641994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina A, Potrich D, Schiona I, Sovrano VA, Fraser SE, Brannon CH, Vallortigara G. 2020. Response to change in the number of visual stimuli in zebrafish: a behavioural and molecular study. Sci Rep. 10:5769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletto Petrazzini ME, Bertolucci C, Foà A. 2018. Quantity discrimination in trained lizards (Podarcis sicula). Front Psychol. 9:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A, Merten KA. 2007. A labeled-line code for small and large numerosities in the monkey prefrontal cortex. J Neurosci. 27:5986–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A, Miller EK. 2003. Coding of cognitive magnitude: compressed scaling of numerical information in the primate prefrontal cortex. Neuron. 37:149–157. [DOI] [PubMed] [Google Scholar]
- Nieder A. 2016. The neuronal code for number. Nat Rev Neurosci. 17:366–382. [DOI] [PubMed] [Google Scholar]
- Nieder A. 2017. Evolution of cognitive and neural solutions enabling numerosity judgements: lessons from primates and corvids. Philos Trans R Soc B Biol Sci. 373:20160514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieder A. 2019. A brain for numbers: the biology of the number instinct. Cambridge (MA): MIT Press. [Google Scholar]
- Nieuwenhuys R, Meek J. 1990. The telencephalon of actinopterygian fishes. In: Jones EG, Peters A, editors. Comparative structure and evolution of cerebral cortex, part I, cerebral cortex. Boston (MA): Springer, pp. 31–73. [Google Scholar]
- Nieuwenhuys R. 2009. The forebrain of actinopterygians revisited. Brain Behav Evol. 73:229–252. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. 1981. Evolution of the telencephalon in non-mammals. Ann Rev Neurosci. 4:301–350. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. 1995. The forebrain of gnathostomes: in search of a morphotype. Brain Behav Evol. 46:275–288. [DOI] [PubMed] [Google Scholar]
- Pepperberg IM. 2006. Grey parrot numerical competence: a review. Anim Cogn. 9:77–391. [DOI] [PubMed] [Google Scholar]
- Perdue BM, Talbot CF, Stone AM, Beran MJ. 2012. Putting the elephant back in the herd: elephant relative quantity judgments match those of other species. Anim Cogn. 15:955–961. [DOI] [PubMed] [Google Scholar]
- Piazza M, Eger E. 2016. Neural foundations and functional specificity of number representations. Neuropsychologia. 83:257–273. [DOI] [PubMed] [Google Scholar]
- Potrich D, Rugani R, Sovrano VA, Regolin L, Vallortigara G. 2019. Use of numerical and spatial information in ordinal counting by zebrafish. Sci Rep. 9:18323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potrich D, Sovrano VA, Stancher G, Vallortigara G. 2015. Quantity discrimination by zebrafish (Danio rerio). J Comp Psychol. 129:388–393. [DOI] [PubMed] [Google Scholar]
- Pritchard VL, Lawrence J, Butlin RK, Krause J. 2001. Shoal choice in zebrafish, Danio rerio: the influence of shoal size and activity. Anim Behav. 62:1085–1088. [DOI] [PubMed] [Google Scholar]
- Rodríguez F, Lopez JC, Vargas JP, Gomez Y, Broglio C, Salas C. 2002. Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J Neurosci. 22:2894–2903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman JD, Brannon EM, Platt ML. 2007. Monotonic coding of numerosity in macaque lateral intraparietal area. PLoS Biol. 5(8):e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugani R, Fontanari L, Simoni E, Regolin L, Vallortigara G. 2009. Arithmetic in newborn chicks. Proc Biol Sci. 276:2451–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugani R, Vallortigara G, Regolin L. 2013. Numerical abstraction in young domestic chicks (Gallus gallus). PLoS One. 8(6):e65262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupp B, Wullimann MF, Reichert H. 1996. The zebrafish brain: a neuroanatomical comparison with the goldfish. Anat Embryol. 194:187–203. [DOI] [PubMed] [Google Scholar]
- Seguin D, Gerlai R. 2017. Zebrafish prefer larger to smaller shoals: analysis of quantity estimation in a genetically tractable model organism. Anim Cogn. 20:813–821. [DOI] [PubMed] [Google Scholar]
- Stacho M, Herold C, Rook N, Wagner H, Axer M, Amunts K, Güntürkün O. 2020. A cortex-like canonical circuits in the avian forebrain. Science. 369:1585. [DOI] [PubMed] [Google Scholar]
- Stancher G, Rugani R, Regolin L, Vallortigara G. 2015. Numerical discrimination by frogs (Bombina orientalis). Anim Cogn. 18:219–229. [DOI] [PubMed] [Google Scholar]
- Stancher G, Sovrano VA, Potrich D, Vallortigara G. 2013. Discrimination of small quantities by fish (redtail splitfin, Xenotoca eiseni). Anim Cogn. 16:307–312. [DOI] [PubMed] [Google Scholar]
- Sumbre G, de Polavieja GG. 2014. The world according to zebrafish: how neural circuits generate behavior. Front Neural Circuits. 8:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teles MC, Almeida O, Oliveira RF. 2015. Social interactions elicit rapid shifts in functional connectivity in the social decision-making network of zebrafish. Proc Biol Sci. 282:1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosches MA, Laurent G. 2019. Evolution of neuronal identity in the cerebral cortex. Curr Opin Neurobiol. 56:199–208. [DOI] [PubMed] [Google Scholar]
- Utrata E, Virányi Z, Range F. 2012. Quantity discrimination in wolves (Canis lupus). Front Psychol. 16:505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallortigara G. 2014. Foundations of number and space representations in non-human species. In: Geary DC, Bearch DB, Mann K, editors. Evolutionary origins and early development of number processing. New York: Elsevier, pp. 35–66. [Google Scholar]
- Vallortigara G. 2017. An animal’s sense of number. In: Adams JW, Barmby P, Mesoudi A, editors. The nature and development of mathematics. Cross disciplinary perspective on cognition, learning and culture. New York: Routledge, pp. 43–65. [Google Scholar]
- Viswanathan P, Nieder A. 2013. Neuronal correlates of a visual “sense of number” in primate parietal and prefrontal cortices. Proc Natl Acad Sci USA. 110:11187–11192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan P, Nieder A. 2020. Spatial neuronal integration supports a global representation of visual numerosity in primate association cortices. J Cogn Neurosci. 32:1184–1197. [DOI] [PubMed] [Google Scholar]
- Walsh V. 2003. A theory of magnitude: common cortical metrics of time, space and quantity. Trends Cogn Sci. 7:483–488. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ito H. 2005. Fiber connections of the anterior preglomerular nucleus in cyprinids with notes on telencephalic connections of the preglomerular complex. J Comp Neurol. 491:212–233. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Ishikawa Y, Yoshimoto M, Xue HG, Bahaxar N, Sawai N, Yang CY, Ozawa H, Ito H. 2007. A new interpretation on the homology of the teleostean telencephalon based on hodology and a new eversion model. Brain Behav Evol. 69:96–104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a submitted supplementary file.


