Abstract
COVID-19 monoclonal antibodies revolutionized the treatment for eligible patients who have tested positive for SARS CoV-2 infection in an ambulatory setting. In this short report, we describe our experience assisting in the distribution of monoclonal antibodies in Arkansas during the summer surge of the delta variant.
Keywords: COVID 19, monoclonal antibodies
Coronavirus disease 2019 (COVID-19) monoclonal antibodies have revolutionized treatment for eligible patients who test positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in an ambulatory setting. Early treatment is key to preventing progression to severe disease and hospitalization. In November 2020, the Food and Drug Administration (FDA) issued Emergency Use Authorization (EUA) of 2 monoclonal antibody products: bamlanivimab and the combination of casirivimab and imdevimab (REGEN-CoV) for the treatment of mild to moderate COVID-19 illness in persons 12 years of age or older at high risk of progressing to severe disease [1, 2]. Subsequently, in February 2021, etesevimab received FDA EUA for use in combination with bamlanivimab [3]. The use of casirivimab/imdevimab or bamlanivimab/etesevimab decreases the risk of hospital admission or 28-day mortality by 70% [4]. These products were purchased by the US Department of Health and Human Services (HHS) and Assistant Secretary for Preparedness and Response (HHS/ASPR) and supplied to infusion clinics throughout the country in coordination with state and local health jurisdictions. In this short report, we describe our experience assisting in the distribution of monoclonal antibodies in Arkansas during the summer surge of the delta variant.
Arkansas is a predominantly rural state, with 62 of the 75 counties meeting the US Census Bureau’s definition of “rural.” There are only 8 metropolitan areas with a population of >50 000 in the state [5]. In addition, the population has a high rate of comorbidities such as obesity, diabetes, and cardiovascular disease and has low COVID-19 vaccination rates. As such, a large proportion of the state’s population is at high risk of severe complications from COVID-19.
Arkansas was one of the earliest states to see a surge in COVID-19 cases related to the delta variant in summer 2021. The Arkansas Department of Health (ADH) assisted in the distribution of monoclonal antibodies between November 2020 and February 2021, before HHS switched to a “pull” system. Now, the product is supplied directly to infusion locations. In late June, the ADH started holding regular stakeholder calls with existing infusion sites in response to the increasing number of cases. During these calls, we provided information on changes in EUA indications, administration, and supply. During the summer, there was steadily increasing interest in monoclonal antibody therapy for a few reasons. First, educational outreach through different medical and pharmacy societies continued to increase the referral base and attendance at the infusion site calls. Second, as the evidence from clinical trials accumulated, the FDA made several revisions to the original EUAs expanding the criteria used to determine high-risk status in patients. Furthermore, based on pharmacokinetic data, REGEN-CoV was authorized to be given by subcutaneous route in addition to intravenous infusion. Finally, in July 2021, postexposure prophylaxis received authorization for use in specific high-risk settings. These rapidly evolving changes, along with information and resources, were shared on our monoclonal webpage. The webpage also included a prominently displayed map of all infusion sites throughout the state with contact information, which was updated daily with new sites. Over the summer, this culminated with the utilization of monoclonal antibodies in Arkansas increasing by >1600%, while comparatively the caseload of COVID-19 increased by 840% (Table 1).
Table 1.
Monoclonal Antibody Utilization in Arkansas June–September 2021a
| Date | Monoclonal Antibody Utilization, No. of Patient Courses/Week | 7 Day Rolling Average of COVID-19 Cases |
|---|---|---|
| 6/16/2021 | 161 | 247 |
| 6/23/2021 | 95 | 273 |
| 6/30/2021 | 306 | 402 |
| 7/7/2021 | 307 | 448 |
| 7/14/2021 | 507 | 1023 |
| 7/21/2021 | 571 | 1173 |
| 8/4/2021 | 1493 | 2105 |
| 8/11/2021 | 1975 | 2339 |
| 8/25/2021 | 2404 | 2242 |
| 9/1/2021 | 2719 | 2076 |
| 9/7/2021 | 2542 | 1775 |
| 9/15/2021 | 2658 | 1659 |
| 9/22/2021 | 2514 | 1315 |
Abbreviation: COVID-19, coronavirus disease 2019.
Data supplied through tele-tracking.
The ADH developed a unique team focused on monoclonal antibody uptake, known as the “Mab Squad.” This team directly contacted high-risk patients and connected them with infusion sites throughout the state. The squad drew from local APRNs and quickly expanded to 6 teams functioning 7 days a week. Each team included at least 1 logistics navigator, who assisted individuals with overcoming transportation barriers. Notably, the Mab Squad recognized that one of the bottlenecks to treatment was having a referring physician. As a result, the ADH contracted with a large physician group to provide telehealth services to patients who did not have an established primary care provider in order to remove this barrier to early treatment.
Due to the state’s rural nature, having locations in each county within easy driving distance was key to early access. The availability of a subcutaneous route of administration offered a unique advantage. This allowed for an expanded pool of health care workers eligible to provide this treatment, especially in the context of severe nursing shortages. The Arkansas state legislature passed Act 406, which allowed pharmacists to prescribe and administer vaccines and immunizations. Monoclonal antibody therapy, as passive immunization, was determined to be under the scope of this legislation. Several pharmacy locations provided monoclonal antibody therapy in rural locations enabled through a partnership with the State Board of Pharmacy and the Arkansas Pharmacists Association. The 62 “rural” counties with 44% of the population grew to have 83 infusion sites, and the other 13 counties with 56% of the population have 87 sites (P = .21). The amendment of the PREP Act by the ASPR in September 2021 now provides liability protection to licensed pharmacists and pharmacy technicians in addition to an alternate pathway for setting up pharmacy locations in other states [6].
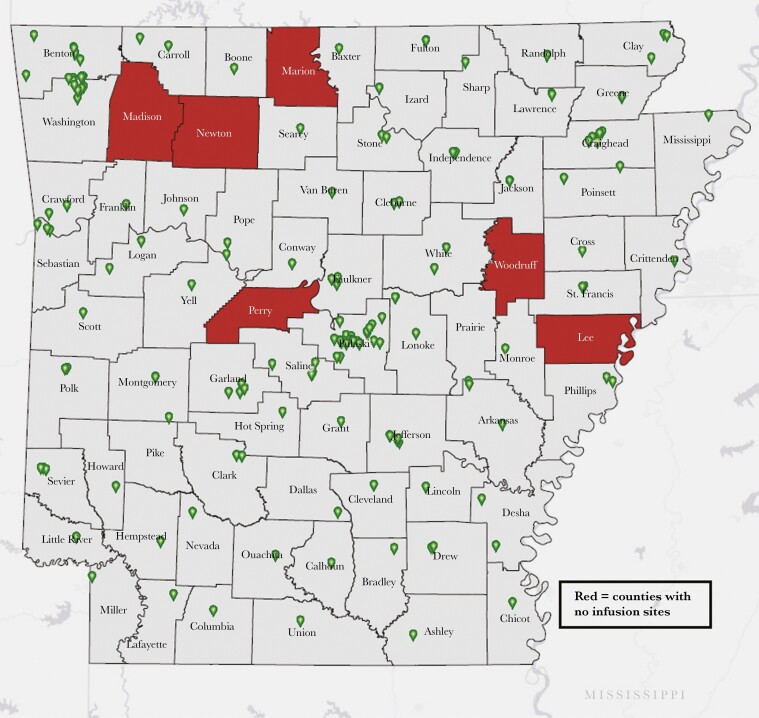
On September 13, 2021, the HHS reverted to a state-coordinated allocation system for monoclonal antibodies in response to the national surge in COVID-19 infections. State health departments did not have significant advance notice of this shift. As a result, they faced the challenge of allocating therapy equitably while maintaining supply to existing clinics with scheduled patients. In Arkansas, we decided on certain basic guiding principles of allocations. We decided to prioritize supply to existing infusion clinics to ensure continuity of patient care. In addition, we also determined that in pursuit of our plan to have an extensive network of local infusion clinics, we would also allocate a small amount to every new clinic that wanted to set up a monoclonal infusion site. Therefore, we preferentially allocated bamlanivimab/etesevimab to the larger hospital-associated infusion clinics and REGEN-CoV to the smaller infusion sites. At this writing, this strategy culminated in a total of 173 infusion sites in 69 out of 75 counties within Arkansas (Figure 1). Throughout the surge, we continued having open, transparent weekly calls with all stakeholders where we explained our weekly allocation decisions.
Figure 1.
Monoclonal antibody therapy site locations as reported to the Arkansas Department of Health—November 3, 2021.
Real-world data from Arkansas have shown that early monoclonal antibody therapy is both safe and highly effective in treating COVID-19 infection [7]. We believe that innovative approaches will be needed to overcome barriers (eg, using telehealth services for patient evaluation followed by subcutaneous therapy in local pharmacies or home infusions for those with transportation or ambulatory issues). Newer monoclonal antibody therapies are in the pipeline and may offer more options to patients and providers in the face of a rapidly evolving virus. Our experience has shown that collaborative agreements between primary care practices and local pharmacies may be a model that allows care to be available closer to the patient’s home. As our current delta surge subsides, we expect the demand for monoclonal antibody therapy to reduce significantly. However, we must remain ever vigilant against future surges and newer viral variants and be prepared to reactivate our existing infrastructure of infusion clinics, pharmacies, and home infusions. Monoclonal antibody therapy has changed our paradigm from “test and isolate” to “test and treat,” putting more tools into the hands of clinicians to improve patient and population outcomes due to COVID-19.
Acknowledgments
We would like to thank Scott Alsbrook for making Figure 1, Kelley Garner for assistance with statistical analysis, and Chychy Smith for assistance with Mab Squad. We also thank Naveen Patil and Jose Romero for manuscript review and comments.
Financial support. None.
Disclaimer. The views expressed in this paper are not necessarily those of the Arkansas Department of Health.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Patient consent. This study did not include factors necessitating patient consent.
References
- 1. An EUA for bamlanivimab—a monoclonal antibody for COVID-19. JAMA 2021; 325:880–1. [DOI] [PubMed] [Google Scholar]
- 2. An EUA for casirivimab and imdevimab for COVID-19. Med Lett Drugs Ther 2020; 62:201–2. [PubMed] [Google Scholar]
- 3. An EUA for bamlanivimab and etesevimab for COVID-19. Med Lett Drugs Ther 2021; 63:49–50. [PubMed] [Google Scholar]
- 4. Dougan M, Nirula A, Azizad M, et al. Bamlanivimab plus etesevimab in mild or moderate Covid-19. N Engl J Med 2021; 385:1382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. US Census. Guide to 2010 Census state and local geography – Arkansas. Available at: https://www.census.gov/library/visualizations/interactive/2020-population-and-housing-state-data.html. Accessed 13 October 2021.
- 6. Federal Register. Available at: https://www.federalregister.gov/documents/2021/09/14/2021-19790/ninth-amendment-to-declaration-under-the-public-readiness-and-emergency-preparedness-act-for-medical. Accessed 14 October 2021.
- 7. Abusalem L, Wood C, Crescencio JCR, Dare RK.. Risk factor analysis for hospital admission following severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) monoclonal antibody treatment. Paper presented at: IDWeek; 29 September–3 October, 2021; Virtual. Poster 519. [Google Scholar]