Abstract
Background
Hospitalizations with complicated urinary tract infection (cUTI) in the United States have increased. Though most often studied as a subset of cUTI, catheter-associated UTI (CAUTI) afflicts a different population of patients and carries outcomes distinct from non-CA cUTI (nCAcUTI). We examined the epidemiology and outcomes of hospitalizations in these groups.
Methods
We conducted a cross-sectional multicenter study within the 2018 National Inpatient Sample (NIS) database, a 20% stratified sample of discharges from US community hospitals, to explore characteristics and outcomes of patients discharged with a UTI diagnosis. We divided cUTI into mutually exclusive categories of nCAcUTI and CAUTI. We applied survey methods to develop national estimates.
Results
Among 2 837 385 discharges with a UTI code, 500 400 (17.6%, 19.8% principal diagnosis [PD]) were nCAcUTI and 126 120 (4.4%, 63.8% PD) were CAUTI. Though similar in age (CAUTI, 70.1 years; and nCAcUTI, 69.7 years), patients with nCAcUTI had lower comorbidity (mean Charlson, 4.3) than those with CAUTI (mean Charlson, 4.6). Median (interquartile range [IQR]) length of stay (LOS) was 5 (3–8) days in nCAcUTI and 5 (3–9) days in CAUTI. Overall median (IQR) hospital costs were similar in nCAcUTI ($9713 [$5923–$17 423]) and CAUTI ($9711 [$5969–$17 420]). Though low in both groups, hospital mortality was lower in nCAcUTI (2.8%) than in CAUTI (3.4%). Routine discharges home were higher in nCAcUTI (41.5%) than CAUTI (22.1%).
Conclusions
There are >626 000 hospital admissions with a cUTI, comprising ~1.8% of all annual admissions in the United States; 4/5 are nCAcUTI. Because CAUTI is frequently the reason for admission, preventive efforts are needed beyond the acute care setting.
Keywords: epidemiology, hospitalization, outcomes, UTI
Urinary tract infections (UTI) present a substantial challenge to the US health care system. In the early 2000s, >100 000 patients were admitted annually for this condition, and there were >1 million emergency department (ED) visits related to UTIs [1]. An analysis from 2011 suggests that these numbers have increased dramatically, and now ~400 000 hospitalizations occur annually for which UTI is listed as the principal diagnosis and is, thus, the primary reason for hospitalization [2]. Despite the fact that this estimate excludes UTIs that lead to more serious consequences, such as sepsis, the annual cost of hospital care for these patients exceeded a staggering $2.8 billion in 2011.
During the same time frame, rates of antimicrobial resistance have risen sharply. Concurrently, the proportion of UTI admissions meeting criteria for “complicated UTI” (cUTI) has, by definition, grown as well. This has made UTI, and in particular cUTI, into a more complex clinical conundrum [3, 4]. Because timely and appropriate empiric antibiotic coverage is the single most important modifiable risk factor that mitigates infection-related morbidity and costs, shifting antimicrobial susceptibilities have reduced treatment choices and raised the potential for many patients to receive inappropriate empiric therapy, irrespective of the best intentions of clinicians. These concurrent trends are mutually reinforcing in that they further increase costs and strain the health care system [5]. Despite potential policy implications of such growth in volume, the full contemporary burden of cUTI hospitalizations remains poorly understood. We set out to quantify the contemporary epidemiology and hospital outcomes of hospitalizations due to cUTI in the United States.
METHODS
Ethics Statement
Because this study used publicly available fully de-identified data, it was exempt from ethics review under US 45 CFR 46.101(b)4 [6].
Patient Consent
This study does not include factors necessitating patient consent.
Study Design and Patient Population
We conducted a multicenter cross-sectional study of patients admitted to all US acute care hospitals in 2018 with a diagnosis of cUTI. Our case identification approach relied on a slightly modified previously published International Classification of Diseases, Tenth Revision (ICD-10), algorithm and can be found in Supplementary Table 1 [3–5]. Because catheter-associated UTI (CAUTI), though usually considered a cUTI subgroup, afflicts a distinct population of patients and is a known effect modifier of the outcomes, we examined non-catheter-associated complicated UTI (non-CA cUTI) and CAUTI as 2 separate, mutually exclusive groups [4]. As we were interested in the total annual burden of cUTI in the United States, we erred on the side of sensitivity, and thus did not exclude any discharges that our algorithm identified as having this condition. To ground our estimates for cUTI in overall UTI admissions, we used ICD-10 codes for uncomplicated UTI (Supplementary Table 2) and combined them with the numbers of non-CA cUTI and CAUTI to calculate the total annual number of UTI admissions. To quantify the prevalence of resistant pathogens, we used their corresponding ICD-10 codes (Supplementary Table 3). We defined a hospital’s cUTI caseload as a proportion of all admissions at that hospital that fit the cUTI criteria.
Data Source
The NIS approximates a 20% stratified sample of discharges from US community hospitals, excluding rehabilitation and long-term acute care hospitals [7]. It is specifically designed to identify, track, and analyze national trends in health care utilization, access, charges, quality, and outcomes. Covering ~97% of the US population, complex survey methods estimate >35 million annual hospitalizations and aid in developing national estimates for specific conditions listed in the database [7].
The unit of analysis in this database is a discharge and not a patient. Thus, it is not feasible to distinguish rehospitalizations from index hospitalizations for any single patient. In the current manuscript, we use the terms “hospitalization,” “admission,” and “discharge” interchangeably.
Statistical Analyses
We examined demographic, clinical, and hospital characteristics of the individual discharges, as well as their hospital outcomes. We report continuous variables as means with SDs and as medians with interquartile ranges (IQRs) and categorical variables as percentages based on complex survey methods that weight strata provided by NIS. For the rare occasion where a stratum contained a single hospitalization, the stratum was centered at the grand mean rather than the stratum mean. Because the intent of the study was to provide descriptive epidemiology for cUTI hospitalizations in the United States, we did not undertake formal hypothesis testing.
RESULTS
Among 35 527 481 total hospitalizations in the United States in 2018, 2 837 385 had any UTI diagnosis code, with 481 640 (17.0%) having UTI as the principal diagnosis. Of all UTI admissions, 626 520 fit our cUTI definition, of which 500 000 (79.9%) were non-CA cUTI, and the remainder CAUTI.
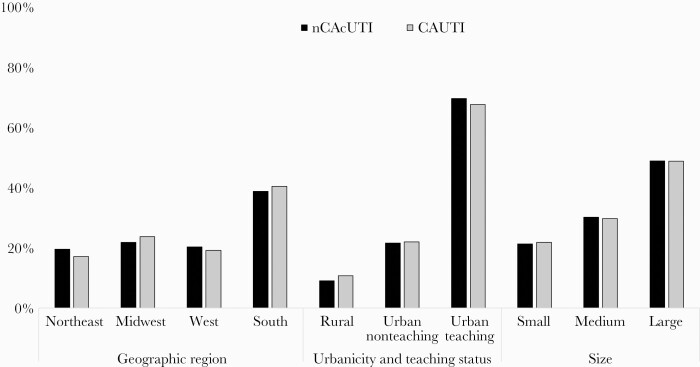
Nearly one-half of all cUTI hospitalizations occurred in large institutions, over two-thirds in urban areas, and a plurality in the Southern United States (Figure 1). The mean hospital cUTI caseload (SD) was 2.1% (1.6% [1.0%] non-CA cUTI and 0.5% [0.4%] CAUTI). In both groups, 70% of the patients were ≥65 years of age, males predominated, and on average each patient suffered from between 4 and 5 comorbidities (Table 1; Supplementary Table 4). Commensurate with the age distribution, Medicare was the most common primary payor in both groups, accounting for >70% of all cases. Together with Medicaid, >80% of all hospitalizations associated with cUTI were covered by a government-sponsored plan.
Figure 1.
Hospital characteristics. Abbreviations: CAUTI, catheter-associated UTI; non-CA cUTI, non-catheter-associated complicated urinary tract infection.
Table 1.
Baseline Characteristics
| non-CA cUTI | non-CA cUTI | CAUTI | CAUTI | |
|---|---|---|---|---|
| No. | %/SD/IQR | No. | %/SD/IQR | |
| 500 400 | 79.87% | 126 115 | 20.13% | |
| Age | ||||
| By group | ||||
| Birth to 17 | 6740 | 1.35% | 350 | 0.28% |
| 18–44 | 37 845 | 7.56% | 9405 | 7.46% |
| 45–54 | 34 310 | 6.86% | 9155 | 7.26% |
| 55–64 | 71 095 | 14.21% | 18 965 | 15.04% |
| 65–84 | 249 475 | 49.86% | 60 275 | 47.79% |
| ≥85 | 100 930 | 20.17% | 27 970 | 22.18% |
| Missing | a | a | ||
| Years | ||||
| Mean | 69.74 | 17.23 | 70.66 | 16.04 |
| Median | 73 | 62–82 | 74 | 62–83 |
| Sex | ||||
| Male | 312 475 | 62.45% | 83 725 | 66.39% |
| Female | 187 920 | 37.55% | 42 390 | 33.61% |
| Missing | a | a | ||
| Race | ||||
| White | 344 295 | 68.80% | 89 445 | 70.92% |
| Black | 60 280 | 12.05% | 17 725 | 14.05% |
| Hispanic | 54 875 | 10.97% | 10 255 | 8.13% |
| Asian or Pacific Islander | 13 595 | 2.72% | 2445 | 1.94% |
| Native American | 2105 | 0.42% | 825 | 0.65% |
| Other/Unknown | 14 280 | 2.85% | 2550 | 2.02% |
| Missing | 10 970 | 2.19% | 2875 | 2.28% |
| Total No. of Elixhauser comorbidities | ||||
| Mean | 4.27 | 2.21 | 4.55 | 2.11 |
| Median | 4 | 3–6 | 4 | 3–6 |
| Admission source | ||||
| ED | 405 090 | 80.95% | 110 645 | 87.73% |
| Non-ED | 95 310 | 19.05% | 15 475 | 12.27% |
| Admission type | ||||
| Nonelective | 456 770 | 91.28% | 121 075 | 96.00% |
| Elective | 40 150 | 8.02% | 4 935 | 3.91% |
| Missing | 480 | 0.10% | 110 | 0.09% |
| Weekend admission | 122 120 | 24.40% | 32 075 | 25.43% |
| cUTI as principal diagnosis | 99 210 | 19.83% | 80 430 | 63.77% |
| Pyelonephritis | 64 305 | 12.85% | 8760 | 6.95% |
| Pathogen | ||||
| ESBL | 10 660 | 2.13% | 3390 | 2.69% |
| CR | 1115 | 0.22% | 400 | 0.32% |
| MDRO | 6340 | 1.27% | 3205 | 2.54% |
| FQ-R | 2380 | 0.48% | 740 | 0.59% |
| Primary expected payer | ||||
| Medicare | 357 690 | 71.48% | 98 325 | 77.96% |
| Medicaid | 47 060 | 9.40% | 12 625 | 10.01% |
| Private | 73 975 | 14.78% | 11 140 | 8.83% |
| Self-pay | 10 850 | 2.17% | 1355 | 1.07% |
| No charge | 910 | 0.18% | 100 | 0.08% |
| Other | 9530 | 1.90% | 2450 | 1.94% |
| Missing | 385 | 0.08% | 125 | 0.10% |
Abbreviations: CAUTI, catheter-associated UTI; CR, carbapenem-resistant; ED, emergency department; ESBL, extended-spectrum beta-lactamase; FQ-R, fluoroquinolone-resistant; HCUP, Healthcare Utilization Project; IQR, interquartile range; MDRO, multidrug-resistant; non-CA cUTI, non-catheter-associated complicated urinary tract infection.
To protect patient privacy, the HCUP prohibits publication of cell sizes with ≤10 discharges.
Over 80% of all admissions originated in the emergency department, and >90% were considered nonelective (Table 1). UTI was the principal diagnosis in 99 210 (19.8%) in the non-CA cUTI group, and in 80 430 (63.8%) in CAUTI. Among those whose cUTI was a secondary diagnosis, sepsis was the most common principal diagnosis in both non-CA cUTI (22.3%) and CAUTI (17.8%) (Supplementary Table 5). Although pyelonephritis was rare in both, it was twice as prevalent in non-CA cUTI (12.8%) as in CAUTI (6.9%). Antimicrobial resistance codes appeared slightly more frequently among admissions with CAUTI than with non-CA cUTI, but were uncommon in both (Table 1).
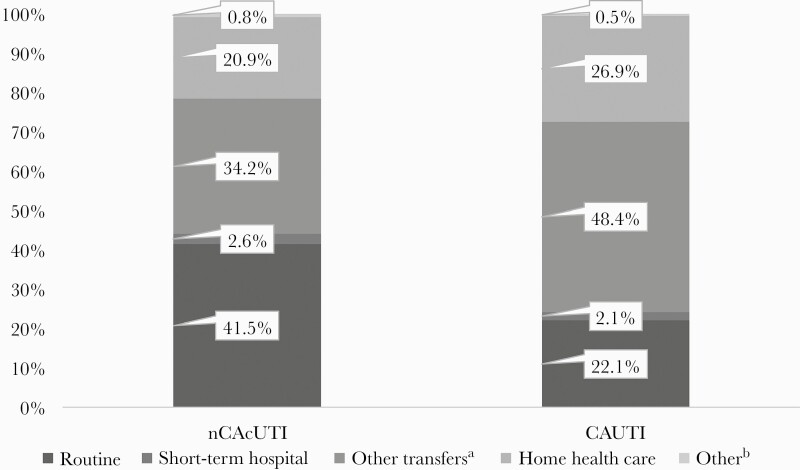
The outcomes of hospitalizations with non-CA cUTI and CAUTI are shown in Table 2 and Figure 2. While mortality overall was low, it was ~20% higher in admissions with CAUTI (3.4%) relative to those with non-CA cUTI (2.8%). Among patients who survived their cUTI hospitalization, the rate of a routine discharge home among those with non-CA cUTI (41.5%) was nearly twice that of CAUTI (22.1%), and the rate of transfers to another medical facility was approximately one-third higher in CAUTI (48.4%) than in non-CA cUTI (34.2%) (Figure 2). Similarly, the proportion of patients discharged with home health care was higher in CAUTI (26.9%) than in non-CA cUTI (20.9%). While median length of stay, charges, and costs were similar in the 2 groups (Table 2), their most common DRGs were different, and therefore so were their median costs (Supplementary Table 6). The most common DRG in the non-CA cUTI, seen in 14.2% of the discharges, was “septicemia or severe sepsis without mechanical ventilation (MV) >96 hours with major complication or comorbidity (MCC),” netting a median reimbursement (IQR) of $12 226 ($7889–$19 216). In contrast, the most frequently reimbursed DRG in the 44.7% of the CAUTI group was “other kidney and urinary tract diagnoses with MCC,” at a median rate (IQR) of $8635 ($5693–$13 718).
Table 2.
Hospital Outcomes
| non-CA cUTI | non-CA cUTI | CAUTI | CAUTI | |
|---|---|---|---|---|
| No. | %/SD/IQR | No. | %/SD/IQR | |
| 500 400 | 79.87% | 126 115 | 20.13% | |
| Hospital mortality | 13 905 | 2.78% | 4330 | 3.43% |
| Length of stay, d | ||||
| Mean | 7.06 | 9.20 | 7.90 | 11.20 |
| Median | 5 | 3–8 | 5 | 3–9 |
| Total charges | ||||
| Mean | $70 076 | $126 687 | $74 149 | $163 791 |
| Median | $39 690 | $21 997–$75 739 | $39 168 | $21 955–$74 765 |
| Total costs | ||||
| Mean | $16 225 | $30 246 | $17 285 | $35 393 |
| Median | $9713 | $5923–$17 423 | $9711 | $5969–$17 420 |
Abbreviations: CAUTI, catheter-associated UTI; IQR = interquartile range; non-CA cUTI, non-catheter-associated complicated urinary tract infection.
Includes skilled nursing facility, intermediate care, and another type of facility.
Figure 2.
Discharge destinations among survivors. aIncludes skilled nursing facility, intermediate care, and another type of facility. bIncludes against medical advice, destination unknown, and missing. Abbreviations: CAUTI, catheter-associated UTI; non-CA cUTI, non-catheter-associated complicated urinary tract infection.
DISCUSSION
We demonstrate that in 2018 across the United States there were >600 000 admissions involving cUTI, representing 1 out of every 500 hospitalizations. We further confirm that non-CA cUTI and CAUTI are different entities, striking populations distinct from each other in characteristics including age, chronic disease burden, gender, and race. It is notable that only 29% of all hospitalizations that include a cUTI have it as the principal diagnosis, with non-CA cUTI being substantially less likely to be the reason for hospitalization than CAUTI. This substantiates our conjecture that the annual national bill for cUTI hospitalizations is far larger than previously reported [2]. Indeed, although certainly inclusive of the costs of the entire hospitalization and not just the increment attributable to the cUTI, based on our data, the total national cost for these hospitalizations in the United States is on the order of $44 billion, of which the vast bulk, $35 billion, is consumed by non-CA cUTI. Furthermore, for individual institutions, unless a patient with CAUTI also has sepsis or requires a surgical procedure, the DRG-allotted hospital LOS is shorter by nearly a full day than an average patient’s actual LOS.
The total contemporary burden of hospitalizations with cUTI is not well defined. Simmering and colleagues analyzed the epidemiology and outcomes of admissions to US hospitals of adult patients with UTI as the principal diagnosis [2]. Using the NIS database from 1998 to 2011, they reported an annual increase in prevalence from 264 404 in 1998 to 436 635 in 2011, costing the US health care system in aggregate $2.8 billion. When inflated to 2021 $US, this translates to an estimated $3.7 billion in direct costs. Although credible as the lower limit of total resources devoted to inpatient UTI treatment, this number includes only those adult patients brought to the wards by the need for inpatient UTI treatment and excludes not only those under 18 years of age but also others whose UTI may have been an inciting event for more serious complications, such as sepsis. This is borne out at least in part by the fact that sepsis and hypertensive heart disease are among the top 10 principal diagnoses in both non-CA cUTI and CAUTI when neither is the primary reason for admission.
A more recent effort to examine UTI epidemiology in a large US database comprised mostly of records from private payors examined the incidence and outcomes of patients specifically with cUTI in either an in- or outpatient setting [8]. In a 5-year sample frame of the study, there were nearly 700 000 cUTI cases, approximately one-fifth of whom were hospitalized. The median cost of each hospitalization was estimated at nearly $10 000. This analysis, however, included limited codes to identify cUTI and, by virtue of the payor mix, overrepresented a population that was younger and had fewer comorbidities than the average cUTI patient, thus limiting the study’s generalizability.
A more inclusive analysis by Lodise and coworkers looked at cUTI admissions through the lens of avoidable hospitalizations. Using the Premier database, they identified >120 000 cUTI hospitalizations between 2013 and 2018 [9]. Defining as avoidable all cUTI hospitalizations that carried low acuity (ie, no evidence of sepsis, low comorbidity burden), they discovered that the rate of potentially avoidable admissions was nearly 20%, incurring on average an LOS of >4 days and costs of $8000. Applying this rate to the total volume of non-CA cUTI and CAUTI in 2018 would suggest that >125 000 cUTI hospitalizations could be avoided, saving nationally as much as $1 billion in hospital costs. Although the authors did not examine this directly, they hypothesized that the likelihood of antimicrobial resistance may play a role in admission decisions, thus potentially consigning patients to a hospitalization simply due to the need for broad-spectrum coverage unavailable to outpatients [9].
While antimicrobial resistance is not a criterion for cUTI in all definitions, it is known to be a common feature of cUTI, which makes empiric treatment challenging and hospital stays more costly [10]. Several investigators have corroborated that if a cUTI is carbapenem-resistant, for example, the mean cost of hospitalization for its treatment is well over $20 000, and much more still when the infection is nosocomial [4, 11–13]. While we rarely identified antimicrobial resistance codes in the current data set, it has been shown in other conditions that administrative data underreport microbiology and that they are likely specific but lack sensitivity [14].
Although a subset of cUTI, CAUTI is a major focus of quality improvement efforts. Our data suggest that efforts to prevent nosocomial CAUTIs may need to be broadened beyond the acute care setting. The fact that nearly two-thirds of all CAUTI discharges carry UTI as the principal diagnosis indicates that only a little over one-third of all CAUTI hospitalizations are potentially the focus of the current panoply of CAUTI preventive efforts. In other words, the challenge of CAUTI to the health care system mainly transpires outside the acute hospital setting. Hence, it would seem far more important to address CAUTI that arises outside the acute care hospital than within it. Because the NIS lacks the “present on admission” (POA) designation, it is not possible to verify this further. However, it is likely that a substantial proportion of these infections were POA. This should be confirmed in a future analysis in a data source that records this information.
Our observations raise further questions. Specifically, why do patients with CAUTI require an admission for the treatment of their UTIs in the absence of severe sepsis or septic shock? Although patients with CAUTI appear older and suffer from more comorbid illnesses than those diagnosed with a non-CA cUTI, the differences are too small to explain the greater need for hospitalization. Furthermore, sepsis complicates CAUTI less frequently than non-CA cUTI. One possible explanation for the difference in need for admission is concern about the prevalence of antimicrobial resistance that requires intravenous antibiotics [15]. Consistent with this hypothesis, despite coding for resistant gram-negative pathogens occurring rarely in the NIS, these codes appeared more commonly in CAUTI than non-CA cUTI. If it is true that hospitalization hinges on susceptibility patterns, then the availability of adequate outpatient regimens may obviate the need for at least some proportion of these admissions. In addition, if confirmed in other studies, CAUTI prevention, and not just its treatment, would require a concerted effort in settings other than acute care.
Our study, although large and highly generalizable, has a number of limitations. Because our case definition relied on administrative coding, there may be misclassification. Although our algorithm to identify cUTI has not been clinically validated, there has been an effort to verify that the administrative coding corresponds faithfully to clinical circumstances. A study funded by the Centers for Medicare and Medicaid Services and carried out by RTI International in the context of monitoring billing practices for certain health care–associated complications found no substantial over- or undercoding of CAUTI in hospital billing practices as recorded in the MedPAR data in 2009–2010 [16]. Indeed, the investigators noted a 94% concordance between clinical and billing records. A small single-center study by Marra and colleagues, on the other hand, reported that ICD-10 coding failed to detect >98% of clinical CAUTIs [17]. The study did not comment on the code’s specificity. No study has addressed coding practices in the setting of non-CA cUTI. Thus, misclassification remains a concern. Another important limitation is our inability to differentiate between initial and repeat hospitalizations. However, given the aim of the study, this did not preclude us from estimating the full burden of hospitalizations with cUTI.
In summary, we have estimated the contemporary volume of hospitalizations associated with cUTIs, comprising both CAUTI and non-CA cUTI, in the United States. We confirm that the patient population is large, diverse, and resource-intensive. One important and novel finding is that a CAUTI is more likely to be the reason for admission than it is to be an incidental or complicating factor. This has implications for both antimicrobial stewardship and quality monitoring. That is, if these UTIs are likely to occur outside an acute care institution, they need to be a focus of prevention efforts in those loci, and not just in the hospital. Similarly, if the central reason for acute admissions for CAUTI is concerns regarding antimicrobial resistance requiring inpatient treatment, it may be prudent to rethink treatment options for these patients. Finally, given that the volume of non-CA cUTIs is quadruple that of CAUTI, non-CAUTI cUTIs deserve increased scrutiny as a target for quality improvement efforts.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This study was supported by a grant from Spero Therapeutics, Cambridge, Massachusetts, USA.
Potential conflicts of interests. M.D.Z. is a consultant to Spero Therapeutics. Her employer, EviMed Research Group, LLC, has received research grant support from Spero Therapeutics. B.H.N.’s employer, OptiStatim, LLC, has received support from EviMed Research Group, LLC. K.S. was an employee of and stockholder in Spero Therapeutics during the conduct of this study. She currently serves as a consultant to Spero. A.F.S. is a consultant to and has received research grant support from Spero Therapeutics. M.D.Z. and A.F.S. have received grant support and/or have served as consultants to Merck, Melinta, Tetraphase, Pfizer, Astellas, Shionogi, The Medicines Company, Lungpacer, and Theravance. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Author contributions. M.D.Z., K.S., and A.F.S. contributed substantially to the study design, data interpretation, and writing of the manuscript. B.H.N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. He contributed substantially to the study design, data analysis, and writing of the manuscript. No persons other than the authors participated in the study or the writing of the manuscript.
Prior presentation. Portions of these data have been accepted for presentation at the IDWeek 2021 annual conference.
Availability of data. This is a public data set available for purchase through the Agency for Healthcare Research and Quality Healthcare Utilization Project division.
References
- 1. Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am J Med 2002; 113:5S–13S. [DOI] [PubMed] [Google Scholar]
- 2. Simmering JE, Tang F, Cavanaugh JE, Polgreen LA, Polgreen PM.. The increase in hospitalizations for urinary tract infections and the associated costs in the United States, 1998-2011. Open Forum Infect Dis 2017; 4:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zilberberg MD, Nathanson BH, Sulham K, Shorr AF.. Antimicrobial susceptibility and cross-resistance patterns among common complicated urinary tract infections in U.S. hospitals, 2013 to 2018. Antimicrob Agents Chemother 2020; 64:e00346–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zilberberg MD, Nathanson BH, Sulham K, et al. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 2017; 17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zilberberg MD, Nathanson BH, Sulham K, et al. 30-day readmission, antibiotics costs and costs of delay to adequate treatment of Enterobacteriaceae UTI, pneumonia, and sepsis: a retrospective cohort study. Antimicrob Resist Infect Control 2017; 6:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. US Department of Health and Human Services Office for Human Research Protections. Human subject regulations decision charts. Available at: https://www.hhs.gov/ohrp/regulations-and-policy/decision-charts/index.html. Accessed 3 February 2021.
- 7. Agency for Healthcare Research and Quality Healthcare Utilization Project. Overview of the national (nationwide) inpatient sample (NIS). Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed 28 June 2021.
- 8. Carreno JJ, Tam IM, Meyers JL, Esterberg E, Candrilli SD, Lodise TP Jr.. Longitudinal, nationwide, cohort study to assess incidence, outcomes, and costs associated with complicated urinary tract infection. Open Forum Infect Dis 2020; 7:XXX–XX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lodise TP, Chopra T, Nathanson BH, Sulham K.. Hospital admission patterns of adult patients with complicated urinary tract infections who present to the hospital by disease acuity and comorbid conditions: how many admissions are potentially avoidable? Am J Infect Control. 2021;49:1528–34. [DOI] [PubMed] [Google Scholar]
- 10. Wagenlehner FME, Bjerklund Johansen TE, Cai T, et al. Epidemiology, definition and treatment of complicated urinary tract infections. Nat Rev Urol 2020; 17:586–6000. [DOI] [PubMed] [Google Scholar]
- 11. McCann E, Sung AH, Ye G, et al. Contributing factors to the clinical and economic burden of patients with laboratory-confirmed carbapenem-nonsusceptible gram-negative urinary tract infections. Clinicoecon Outcomes Res 2020; 12:191–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zilberberg MD, Nathanson BH, Sulham K, Shorr AF.. Multiple antimicrobial resistance and outcomes among hospitalized patients with complicated urinary tract infections in the US, 2013–2018: a retrospective cohort study. BMC Infect Dis 2021; 21:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vallejo-Torres L, Pujol M, Shaw E, et al. RESCUING Study Group and Study Sites. Cost of hospitalised patients due to complicated urinary tract infections: a retrospective observational study in countries with high prevalence of multidrug-resistant gram-negative bacteria: the COMBACTE-MAGNET, RESCUING study. BMJ Open 2018; 8:e020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins TL, Deshpande A, Zilberberg MD, et al. Assessment of the accuracy of using ICD-9 diagnosis codes to identify pneumonia etiology in patients hospitalized with pneumonia. JAMA Netw Open 2020; 3:e207750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomila A, Shaw E, Carratalà J, et al. ; COMBACTE-MAGNET WP5- RESCUING Study. Predictive factors for multidrug-resistant gram-negative bacteria among hospitalised patients with complicated urinary tract infections. Antimicrob Res Infect Control 2018; 7:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Snow CL, Holtzman L, Waters H, et al. Accuracy of coding in the hospital-acquired conditions–present on admission program: final report. RTI project number 0209853.230.001.085. Available at: https://www.cms.gov/medicare/medicare-fee-for-service-payment/hospitalacqcond. Accessed 15 May 2011.
- 17. Marra AR, Alkateri M, Edmond MB.. Catheter-associated urinary tract infection: utility of the ICD-10 metric as a surrogate for the National Healthcare Safety Network (NHSN) Surveillance metric. Infect Control Hosp Epidemiol 2017; 38:506–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.