Abstract
a). Purpose:
Confocal microscopy and aethesiometry have allowed clinicians to assess the structural and functional integrity of corneal nerves in health and disease. This review summarizes literature on nerves in dry eye disease (DED) and discusses how this data can be applied to DED diagnosis and treatment.
b). Recent findings:
Subjects with DED have a heterogenous symptom and sign profile along with variability in nerve structure and function. Most studies have reported lower nerve density and sensitivity in aqueous tear deficiency, while findings are more inconsistent for other DED subtypes. Examining nerve status, along with profiling symptoms and signs of disease, can help categorize subjects into disease phenotypes (structural and functional patterns) that exist under the umbrella of DED. This, in turn, can guide therapeutic decision-making.
c). Summary:
Due to the heterogeneity in symptoms and signs of DED, corneal nerve evaluations can be valuable for categorizing individuals into disease sub-types and for guiding clinical decision making.
Keywords: Corneal nerves, confocal microscopy, aethesiometry, dry eye disease, phenotype
A. INTRODUCTION
The corneal nerve system derives innervation from the trigeminal nerve and functions in ocular healing and processing of sensory stimuli. Studies examining this system have provided insight into its structure and function in health and disease states. Structural and functional nerve abnormalities have been reported in the setting of several disorders including dry eye disease (DED), a common cause of morbidity in the general population. This review will summarize this data and discusses how the evaluation of nerve status may be better incorporated into the clinical examination of DED.
B. CORNEAL NERVES
1. Structure of corneal nerves
By combining findings of light and electron microscopy studies with later studies using vivo confocal microscopy (IVCM), the morphology of corneal nerves has been studied in detail.[1–3] Today, IVCM is the most popular method of studying nerve structure, with images providing information for diagnosis and measurement of treatment response for several disorders.[4, 5] The benefit of IVCM includes non-invasive imaging at high resolution (1–2 μm laterally, 5–10 μm axially, magnification 600x).[6] Cons also exist, including a small field of view, a need for trained operators, and a lack of built-in quantification software.[7, 8] It is also difficult to scan the same area repeatedly, which must be considered when reviewing studies that evaluated nerve changes over time.
Many IVCM microscopes exist, including slit-scanning (e.g. ConfoScan 4; Nidek), tandem-scanning (e.g. Tandem Scanning; Reston), and laser-scanning (e.g. Heidelberg Retina Tomograph; Heidelberg) instruments. Tandem-scanning instruments have a small aperture (30 μm), narrow depth of field (7–11 μm), and limited magnification, creating difficulty in telling <5 μm objects apart, making them better suited for the anterior stroma rather than nerves. Slit-scanning microscopes have wider apertures (e.g. 300 μm) and a larger depth of field (10–26 μm), allowing for improved resolution. Laser-scanning microscopes offer the highest contrast and resolution for nerves given the pinhole aperture (1 μm) and small depth of field (4–7 μm).
Several nerve parameters have been examined via IVCM, including nerve density (nerves within a defined area [μm/mm2 or mm/mm2], nerve length (density of nerves in a frame [mm/mm2], often used as a proxy for density), nerve count (fibers within a frame), reflectivity (graded 0–4), and tortuosity (twisting, graded on a Likert scale or by tortuosity coefficient).[9] IVCM also allows for morphologic examination of various cell types (epithelial, endothelial, dendritiform cells (DCs)). Dendritiform cells are thought to be antigen presenting cells that are found within the cornea. In non-inflamed states, they are mostly located in the peripheral cornea. In the setting of systemic immune disorders (Sjögren's syndrome, graft-versus-host disease (GVHD) and local inflammation (keratitis), these cells increase in number and size and migraine into the central cornea.[10] DCs are further categorized by maturity or “activation” state. Based on studies in animals,[11–13] when DCs become “activated”, they enlarge, and the number and length of their dendrites increase. A limitation of IVCM is that it is often challenging to compare findings across studies due to resolution differences between microscopes and variability in the reporting of outcome measures.[14, 15]
2. Function of corneal nerves
Corneal nerves are sensory, and several types of sensory fibers exist in the system.[16] The majority of fibers are polymodal nociceptors, which process mechanical, heat, and chemical stimuli. A smaller number of fibers are mechanonociceptors, which identify mechanical stimuli. Finally, a minority of nerves are cold-sensitive receptors, which respond to a decrease in temperature.[17] Sensitivity evaluation is the most common method for assessing nerve function – this can be done qualitatively in clinic with the use of a cotton tip or floss, or quantitatively using an aesthesiometer. The Cochet-Bonnet (CB) aesthesiometer has a nylon filament that contacts the eye; the filament is then retracted until the individual detects the stimulus, providing measurement of a mechanical detection threshold. A lower reading corresponds to a lower sensitivity. The Belmonte instead propels air jets (with or without CO2, at varied intensities and temperatures), allowing testing of mechanical, chemical, thermal, and pain thresholds.[18] A lower reading (lower threshold) corresponds to a higher sensitivity.
Both instruments have limitations. Both devices require that the eye is open during testing. For CB, the operating technician must be weary of proper filament placement and pressure in order to take reproducible measurements. Furthermore, the CB requires sterilization between patients and its filament can be aged by humidity and temperature. Also, the CB has a narrow testing range and many healthy individuals can detect the filament when fully extended. Thus, it is difficult to use this instrument to examine for corneal hypersensitivity.[19] The Belmonte is not commercially available, has a bulky exterior, and requires more time for sensitivity readings. Both instruments have been found to have higher reproducibility in the central cornea compared to the conjunctiva.[20, 21] Finally, various studies have used different protocols (different distances from the cornea, air temperature, locations of testing) make comparisons across the literature, even with the same instrument, challenging.
C. NERVES IN HEALTHY INDIVIDUALS
A handful of studies have described nerve attributes in healthy individuals. One tandem-scanning study of 65 healthy individuals (mean age 46±19 years, range 15–79) reported a mean nerve density of 8,404±2,012 μm/mm2 (range 4,735–14,018 μm/mm2).[22] In comparison, a slit-scanning studies of healthy individuals [n=60 (age <35 or >50 years) reported a nerve density of 14,731±6,056 μm/mm2.[23] Finally, two laser-scanning studies of healthy subjects [n=85 (mean age 38±16 years, range 18–87) and n=106 (mean age 50, range 15–88)] estimated nerve densities at 20,300±6,500 μm/mm2 (range 5,000–35,000 μm/mm2) and 19,000±4,500 μm/mm2 (range 13,400 to 23,400 μm/mm2), respectively.[24, 25] It is important to note the overlap in reported nerve values across studies when examining healthy individuals. Such overlap is expected between different IVCM instruments. However, the overlap remains even when comparing values obtained with one instrument. While this may be due to operator technique or the section of cornea sampled, it may also indicate a heterogeneity in density values in healthy and diseased eyes.
Fewer studies have examined sensitivity in healthy individuals. In an Australian study of 18 healthy subjects (mean age 34.40±8.09 years), detection thresholds on CB and Belmonte were 5.50±0.80 cm and 64.40±29.40 mL/min, respectively. However, 56% of the measurements were beyond the maximum threshold of the CB (individuals felt the stimulus when the thread was at its full length of 6 cm), while all values were within the testing range of the Belmonte.[18] Near identical results were reported in a British study of 17 subjects (mean age = 20.30 years, range = 19–26) where 61% of subjects felt the stimulus at 6 cm, while no threshold reached the max Belmonte stimulus intensity.[26]
D. NERVES IN DED
DED is defined as “a multifactorial disease of the ocular surface characterized by a loss of homeostasis of the tear film, and accompanied by ocular symptoms, in which tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles.”[27] Its pathogenesis is complex, involving a cyclical process of tear osmolarity changes, inflammatory damage, and tear film instability, among others.[28] There exists heterogeneity in DED in terms of phenotypes and underlying mechanisms. DED is often subtyped into aqueous tear deficiency (ATD), evaporative DED, and a mixed sub-type depending on diagnostic findings. Also, DED can occur as an isolated phenomenon or secondary to a systemic disorder, such as Sjögren's syndrome or GVHD.[14, 29] Nerve attributes have also been evaluated in DED, with the additional challenge that different studies used different definitions for the disease.[30] Overall, similar to what was seen in healthy individuals, there was a wide range of nerve parameters with overlapping values between DED and healthy controls. This further demonstrates the heterogeneity of nerve status in individuals with and without eye disease.
1. Structural anomalies in DED
Many studies have examined nerve structure in individuals with DED. Most studies focused on individuals with ATD while a minority examined individuals with evaporative DED. Overall, most studies (using laser-scanning IVCM) found that individuals with ATD (variably defined) had lower nerve densities compared to controls. For example, a French study examined 12 subjects with ATD (irritation, tear instability, staining ≥2, Schirmer ≤10 mm) and 10 controls and found a lower nerve density in the ATD group (9,426±2,640 vs. 15,956±2,431 μm/mm2, p<0.0001).[31] Similar findings were described in a Korean study that examined 40 individuals with ATD (symptoms, tear film break-up time (TBUT) <5 s, Schirmer <10 mm) and 18 controls and reported lower density (9,884±2,548 vs. 12,030±2,203 μm/mm2, p<0.005) and higher tortuosity (3.70±0.50 vs. 1.60±0.60, p<0.001) in the ATD group.[32] Other studies have used nerve counts as a surrogate for nerve density and reported similar results – a Chinese study evaluated 43 subjects with ATD (symptoms, TBUT <10 s, Schirmer <5 mm) and 14 controls and noted a lower nerve count (34.91±8.08 vs. 45.87±4.21 nerves/mm2 [frame = 400×400 μm], p<0.001) and higher tortuosity (3.01±0.49 vs. 1.94±0.46, p<0.001) in the ATD group.[33] Similarly, an Italian study that examined 15 subjects with ATD (definition not provided) and 15 controls reported lower nerve counts in ATD (3.90±0.50 vs. 5.80±1.30 nerves/frame, p<0.001).[34] Contrasting from these findings, a Chinese study that used slit-scanning IVCM on 30 subjects with ATD (symptoms, staining, Schirmer ≤8 mm) reported higher nerve density in the ATD group, but differences between groups were not significant (1,424±610 vs.1,316±665 μm/frame [frame = 340×255 μm], p=0.50).[35]
Fewer studies have examined evaporative DED, but overall, no significant differences in density were found compared to controls using laser-scanning IVCM. An Indian study examined 52 subjects with evaporative DED (high Ocular Surface Disease Index (OSDI) score, low TBUT, normal Schirmer; further specifications not provided) and 43 controls and found no difference in nerve density between the groups (27.20±0.60 vs. 28.60±0.80 nerves/mm2 [frame of 400×400 μm], p>0.05).[36] Another Indian study that examined 47 subjects with evaporative DED (symptoms, TBUT <10s, Schirmer >10 mm) and 33 controls also reported no difference in nerve density (~30.00 nerves/mm2 both groups, p>0.05).[37]
It is not known why individuals with ATD seem to have more nerve abnormalities than individuals with evaporative DED. Hypotheses include distinct pathophysiologic mechanisms for the two diseases that differentially affect corneal nerves, including the close association between inflammation and ATD and the finding that many individuals with ATD (especially in the setting of Sjögren's) have a component of neurotrophic keratitis.[29] Inflammation has been closely linked to corneal nerve dysfunction in other studies – in particular, one study that utilized a mouse model found that exposure of an 'inflammatory soup?' (bradykinin, histamine, prostaglandin E2, serotonin, and ATP) to mouse corneas lead to alterations in firing pattern (paroxysmal discharges and silent periods with stimulation) and waveform morphology (flatter, with lower peak amplitude) of recorded impulses from actively firing cold and polymodal nociceptors.[38]
2. Functional anomalies in DED
Similar to nerve density, corneal sensitivity has been frequently reported to be decreased in ATD. However, studies in mixed DED populations have not replicated these findings. Specifically, the French study above used CB to examine 12 subjects with ATD and 10 controls and reported a lower sensitivity in the ATD group (5.00±0.83 vs. 5.89±0.22 cm, p=0.01).[31] Similarly, an American study that used CB on 10 subjects with ATD (OSDI >20, TBUT ≤6 s, tear meniscus height <220 μm on optical coherence tomography) and 10 healthy controls reported lower sensitivity in the ATD group (3.60±1.65 vs. 5.45±0.83 cm, p<0.05).[39] Hypoesthesia has also been found in studies using Belmonte. A Spanish study that used Belmonte on 10 subjects with ATD (symptoms, Schirmer <10 mm) and 10 controls found hypoesthesia (higher mechanical threshold) in the ATD cohort (134.0±24.0 vs. 78.0±12.0 mL/min, p=0.02).[40] Another Spanish study that used Belmonte on 44 subjects with DED (symptoms, staining, TBUT ≤6s; n=14 with Sjögren's) and 42 controls found hypoesthesia in the DED group [mechanical (152.60±33.80 vs. 109.0±23.30 mL/min, p<0.001), chemical (23.90±4.30 vs. 16.40±3.10 %CO2, p<0.001), heat (+0.34±0.13 vs. +0.26±0.05 °C, p<0.001), cold (-0.14±0.15 vs. −0.05±0.04 °C, p<0.001)].[41] Diverging from these findings, other studies have reported hypersensitivity in DED. Specifically, an American study that used Belmonte on 20 subjects with DED (symptoms, TBUT ≤5 s, staining ≥2) and 20 controls noted hyperesthesia (lower mechanical threshold) vs. the control group (34.60±21.09 vs. 61.50±20.07 mL/min, p<0.05).[42]
3. Animal models of AT
Animal studies support findings of lower density and sensitivity in ATD. One study exposed mice to environmental stress (fan) for 5 hours/day for 3 days and found lower nerve density after the stressor (2,813±762 to 1,898±286 pixels/frame, p=0.01) while tortuosity (0.81±0.33 to 0.96±0.40, p=0.31) and reflectivity (0.83±0.37 to 0.78±0.43, p=0.76) did not change with stress.[43] Another study exposed mice to scopolamine (with a subsequent reduction in tear production) and noted hypoesthesia on CB at 2 weeks compared to controls (2.72±0.30 vs. 3.50±0.40 cm, p<0.0001).[44] Lower sensitivity has also been described in a breed of dogs that spontaneously develop ATD – in a study of West Highland White Terriers, sensitivity was lower in dogs with ATD (discharge, conjunctival hyperemia/chemosis, chronic keratitis, Schirmer <15mm) vs. controls of the same breed via corneal touch threshold [1.40 (range 0.60–2.30) vs. 2.20 (range 1.20–10.30) g/mm2, p=0.07].[45] Unfortunately, animal models of evaporative DED have yet to be examined, making comparisons to human findings difficult.
4. Relationship between structure and function
A number of studies have evaluated relationships between structure and function in DED, with the majority reporting positive relationships, e.g. lower nerve density via laser-scanning IVCM correlating with lower sensitivity. The Chinese study that examined 43 subjects with ATD and 14 controls reported a positive association between density and CB sensitivity (r = 0.38, p=0.04).[33] Similarly, the French study that examined 12 subjects with ATD and 10 controls also reported positive associations between CB sensitivity and density (r = 0.64, p=0.05) and nerve count (r = 0.65, p=0.04).[31] Finally, the Spanish study that examined 10 subjects with ATD and 10 controls found associations between nerve density with mechanical (r = −0.79, p<0.001), chemical (r = −0.80, p<0.001), and thermal cold thresholds on Belmonte (r = −0.63, p<0.001).[40] This indicates a positive association between density and sensitivity.
5. Relationships with symptoms
A common symptom of DED is pain, for which several symptom measures exist. The Ocular Surface Disease Index (pain, vision, triggers, and quality of life [46]) and Dry Eye Questionnaire (DEQ5; dryness, discomfort, and tearing [47]) include a variety of questions regarding painful and non-painful symptoms. The Neuropathic Pain Symptom Inventory modified for the Eye (NPSI-E; pain metrics specific to neuropathic pain [48]) and Ocular Pain Assessment Survey (OPAS [49]) were developed specifically to assess ocular pain. Importantly, some studies correlated nerve metrics with pain-specific questions (e.g. OSDI-discomfort, NPSI-E) while others examined relationships with total questionnaire scores, of which pain is only one component.
Studies in ATD have demonstrated negative relationships between symptoms and nerve parameters, in which higher symptoms in individuals are associated with lower nerve density (via laser-scanning IVCM). For example, an American study examined 22 individuals with Sjögren's DED, 12 with non-Sjögren's DED (symptoms, TBUT <10s, corneal staining), and 7 healthy controls and found a strong negative correlation between density and OSDI (r = −0.91, p<0.001).[50] The Chinese study that examined 43 subjects with ATD and 14 controls also found a negative relationship between nerve length and OSDI, albeit at a lower magnitude (r = 0.27, p=0.02).[33] However, this finding was not reproduced in evaporative DED – the Indian study that examined 52 subjects with evaporative DED and 43 controls did not find relationships between nerve density (r=0.10, p=0.33) or length (r=0.15, p=0.13) with OSDI-discomfort.[36]
Inconsistent relationships have been reported for sensitivity and DED symptoms. A French study examined 30 subjects with post-keratectomy DED (defining specifications not provided), reporting a negative association between CB sensitivity and OSDI (r = −0.65, p<0.01).[51] Conversely, an American study that examined 129 subjects with DED symptoms (DEQ5 score ≥6) noted a significant, but weak negative association between Belmonte thresholds and pain (OSDI: r = −0.18, p=0.04 and r = −0.20, p=0.03; NPSI-E: r = −0.23, p=0.01 and r = −0.21, p=0.02). This translates into a positive relationship between sensitivity and pain as lower thresholds on Belmonte indicate higher sensitivity.[52] It is important to note however that the latter study excluded individuals with Sjögren's, GVHD, and a history of refractive surgery, thus study populations were not similar across studies. Other studies have reported both positive and negative weak associations between CB sensitivity and OSDI in ATD (e.g. r = 0.13 [33], r = 0.20 [39], r = −0.14 [53]; p<0.05 for each).
Overall, lower nerve density has been associated with higher symptoms in individuals with ATD, while inconsistencies have been found across the literature with regards to the relationship between corneal sensitivity and symptoms.
6. Relationships with signs
Studies have also examined relationships between nerve parameters and DED signs. Overall, most studies reported negative relationships between nerve metrics (via laser-scanning IVCM) and corneal staining, while inconsistent relationships were found for TBUT and Schirmer.
For example, the Chinese study that examined 43 subjects with ATD and 14 controls found that nerve density (r = −0.49, p=0.01), nerve length (r = −0.31, p=0.04), nerve count (r = −0.36, p=0.02), nerve reflectivity (r = −0.34, p=0.03), and corneal sensitivity (r = −0.30, p=0.04) negatively correlated with corneal staining, while none of these measures related to TBUT or Schirmer.[33] Similarly, that American study of 10 subjects with ATD found that CB sensitivity negatively correlated with corneal staining (r = −0.46, p<0.01), but not TBUT (r = 0.25, p>0.05).[39] Further supporting these findings, the Spanish study that examined 44 individuals with DED and 42 controls found a positive association between mechanical and chemical Belmonte thresholds and corneal staining (coefficients not provided, p<0.05), but no associations with TBUT and Schirmer.[41] Finally, an American study that examined 403 subjects with DED symptoms (DEQ5≥6; Sjögren's, GVHD, post-refractive patients excluded) found higher staining scores in individuals with corneal hyposensitivity (n=46; defined as Belmonte mechanical threshold ≥145 mL/min) compared to those with normal sensation (n=306) or hypersensitivity (n=50; defined as threshold ≤ 40mL/min (2.40±2.90 vs. 2.10±2.50 vs. 1.40±1.90 respectively, p<0.05 for each), while no differences were noted in TBUT or Schirmer scores between the 3 groups.[54] Other studies, however, reported relationships between nerves and TBUT – an American study that examined corneal nerve density in 4 regions (nasal, temporal, superior, and inferior quadrants) in 46 subjects with DED (defining specifications not provided) found that density correlated with staining (r = −0.42, r = −0.39, r = 0.36, r = −0.47; p<0.001 each) and TBUT (r = 0.57, r = 0.40, r = 0.50, r = 0.58; p<0.05 each) in all four regions, but not to Schirmer.[55]
Overall, individuals with ATD have lower nerve density and sensitivity that relate to a higher degree of corneal staining. In comparison, there are inconsistencies across the literature regarding relationships between nerve parameters and TBUT or Schirmer.
E. NERVE EVALUATIONS IN THE DIAGNOSIS AND TREATMENT OF DED
DED may be understood as an umbrella term, characterized by multiple phenotypes with different symptoms, signs, and nerve findings.[56] This is exemplified by the lack of consistent relationships between symptoms and signs of disease, the lack of a 'gold standard' disease definition, and the overlap of nerve parameters in DED and healthy individuals.[54, 57] Given this variability, nerve evaluations should be incorporated into the work-up of individuals with DED because they can help define and categorize DED phenotypes. This is especially the case for sensitivity, which can help identify contributors to symptoms and signs and provide information on the origin of symptoms.
Nerve definitions as they relate to DED phenotypes:
When describing nerve parameters as they relate to DED, several terminologies arise that must be first defined.
Nociceptive pain: defined as “pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors.”[58] When applied to the ocular surface, nociceptive pain occurs due to any noxious stimuli that triggers a nociceptor response and causes a painful sensation. Tear film abnormalities (e.g. decreased tear production, high or unstable tear osmolarity, presence of inflammatory mediators), environmental factors (e.g. air pollution), abnormal ocular anatomy (e.g. pterygium), or toxicity (e.g. topical glaucoma medications) are common sources of ocular surface nociceptive pain.
Neuropathic pain: defined as “pain caused by a lesion or disease of the somatosensory nervous system.”[58] As such, neuropathic pain stems from an abnormality in the nerves themselves. This can occur due to an abnormality in peripheral sensory neurons (e.g. peripheral neuropathic pain), central neurons (e.g. central neuropathic pain), or both. Hyperalgesia and allodynia are features often seen in individuals with neuropathic pain.[58, 59] Hyperalgesia is defined as “increased or augmented pain response from a stimulus that normally does provoke pain,” while allodynia is defined as “pain due to a stimulus that normally does not provoke pain,” for example pain evoked by light touch to the skin (e.g. cutaneous allodynia). Secondary hyperalgesia is defined as an increase in pain sensitivity when a noxious stimulus is delivered to a region surrounding, but not including the zone of injury (increased pain sensitivity outside of the area of injury or inflammation), and its presence suggests a central component to pain.[60]
Sensitization: defined as “increased responsiveness of nociceptive neurons to their normal input, and/or recruitment of a response to normally subthreshold inputs.”[58] Sensitization is used to describe the changes in nerve function (peripheral or central) that underlie neuropathic pain.
Neurotrophic keratitis (NK): a phenotype that describes decreased sensitivity and corneal epithelial abnormalities (grade 1: corneal staining, grade 2: epithelial defect, grade 3: ulceration or perforation) that may or may not be accompanied by pain.[29, 61]
It is important to remember that the corneal nerve pathway is dynamic and that individuals may have more than one pain type. For example, ongoing nociceptive pain (e.g. inflammatory mediators) may lead to peripheral nerve sensitization, and ongoing peripheral nerve input may lead to centralization of pain.[60] Overall, nerve hypersensitivity tends to manifest as chronic pain (i.e. neuropathic pain) while hyposensitivity often manifests with epithelial changes (i.e. neurotrophic keratitis). However, individuals can have both neurotrophic keratitis and neuropathic pain, as is seen outside the eye in individuals with painful diabetic neuropathy.[62]
Diagnostic tests that can be incorporated into the DED evaluation to evaluate nerve structure and function:
Analysis of ocular and non-ocular symptom profiles: The DED examination begins with symptom assessment, as certain characteristics (e.g. burning, tingling, electricity-like pains, and sensitivity to light and wind) are suggestive of neuropathic etiology.[63] This can be gleaned by examining responses to specific questions within the OSDI (e.g. Q1 - eyes that are sensitive to light?, Q3 - eyes that feel painful or sore?, Q10 - eyes that are uncomfortable during windy conditions? [46]) or by using an ocular pain-specific questionnaire like the NPSI-E.[48] Also, it is important to query for the presence of systemic pain conditions like migraine or fibromyalgia, as pain often travels together.[64] Demonstrating this, an American study on 154 subjects with DED symptoms (DEQ5≥6) found that subjects with multiple comorbid pain syndromes (n=97; mean = 6.2 disorders, 3.8 pain locations) reported more severe ocular symptoms than subjects with fewer syndromes (n=57; mean = 2.5 disorders, 1.1 pain locations) using multiple scales (NPSI-E: 29.0±23.0 vs. 19.0±19.0, p=0.006; OSDI: 44.0±25.0 vs. 29.0±22.0, p<0.0005; DEQ5 13.60±3.70 vs. 11.70±3.90, p=0.004), while tear parameters were similar (TBUT: 8.90±3.80 vs. 9.40±3.60 seconds, p=0.39; corneal staining: 2.20±2.80 vs. 2.20±2.30, p=0.85; Schirmer 13.80±6.60 vs. 14.00±6.20 mm, p=0.87).[65] Similarly, an American study of 250 subjects with DED symptoms (DEQ5≥6) found that subjects with comorbid migraine (n=31) had higher NPSI-E scores (39.39±23.33 vs. 21.86±20.17, p=0.0001), light sensitivity (5.77±3.59 vs. 3.45±3.17, p=0.0001) and wind sensitivity (5.19±3.49 vs. 2.88±3.07, p=0.0001) than those without migraine, but again had similar tear parameters (TBUT: 8.35±3.59 vs. 9.61±5.02 seconds, p=0.39; corneal staining: 1.69±1.93 vs. 2.14±2.56, p=0.53; Schirmer: 14.15±9.04 vs. 12.93±7.32 mm, p=0.56).[66] This suggests that neuropathic mechanisms may contribute to painful DED symptoms in individuals with systemic pain co-morbidities.
Corneal sensitivity: Corneal sensitivity is often qualitatively assessed in the clinical setting with a cotton tip applicator or dental floss, with sensation graded on a 0–3 scale (absent, decreased, normal, increased). Corneal sensation can be evaluated centrally or in various quadrants. Increased or decreased sensitivity suggests an abnormality in the sensory pathway, but cannot determine its origin (i.e. peripheral, central, or both).
Persistent pain after anesthesia: This test assesses pain before and after topical anesthetic placement, such as proparacaine. The test often requires reworking the clinic flow as the provider must assess the patient prior to placement of topical anesthesia. The patient is first asked to grade ocular pain intensity in each eye (range 0–10), after which a drop of topical anesthetic is placed in each eye. Pain intensity (range 0–10) is re-assessed after ~30 seconds (some investigators wait 1–2 min). If pain persists after anesthesia, this suggests a central neuropathic or non-ocular component to pain, as peripheral nociceptors should be quieted by the anesthetic. If pain is eliminated with anesthesia, this suggests a nociceptive or peripheral neuropathic origin to the pain.[67, 68] The test is not informative if the patient does not have pain prior to anesthesia.
Nerve architecture via IVCM: IVCM provides high resolution images of nerves (Figure 1). However, there is no built-in software to quantify nerves, so providers must rely on qualitative assessments. As such, IVCM provides a general feel on nerve density (e.g. reduced vs. normal) and morphology (e.g. no, mild, severe tortuosity). Reduced density and tortuosity have been consistently reported in ATD.[29, 69] One group reported that in individuals with clinically diagnosed peripheral neuropathic pain, some nerves were hyperreflective and abruptly terminated with a swelling at the nerve ending. They termed this finding microneuroma (MN) based on similar findings in animal studies,[70] and found it to be a specific marker for peripheral neuropathic pain (Figure 2).[71] On the other hand, other studies cited that inconsistencies in definitions for terms and use of language, limitations in IVCM imaging, and lack of standardized sampling and reporting may have led to inaccurate classification of physiological nerve characteristics as pathological microneuromas.[72] Supporting this, some researchers have described that nerves near the stroma bend 90° when entering the sub-basal nerve layer and that depending on the cut, this bend may look similar to a MN.[73] Our group evaluated for this confocal feature in 153 subjects with DED symptoms (DEQ5≥6) and did not find correlations between abrupt termination and nerve swelling and other metrics suggestive of neuropathic pain.[69] As such, more research is needed to determine how IVCM images can best be incorporated into the nerve evaluation.
Presence of cutaneous allodynia (e.g. pain to light touch): The presence of cutaneous allodynia can be easily assessed by lightly touching the periocular skin surrounding the eyes and assessing for a pain response.
In addition to evaluating nerves, ocular surface status (TBUT, corneal staining, tear volume/production) and ocular surface anatomy should be examined. Abnormalities in these compartments often manifest as nociceptive sources of pain.
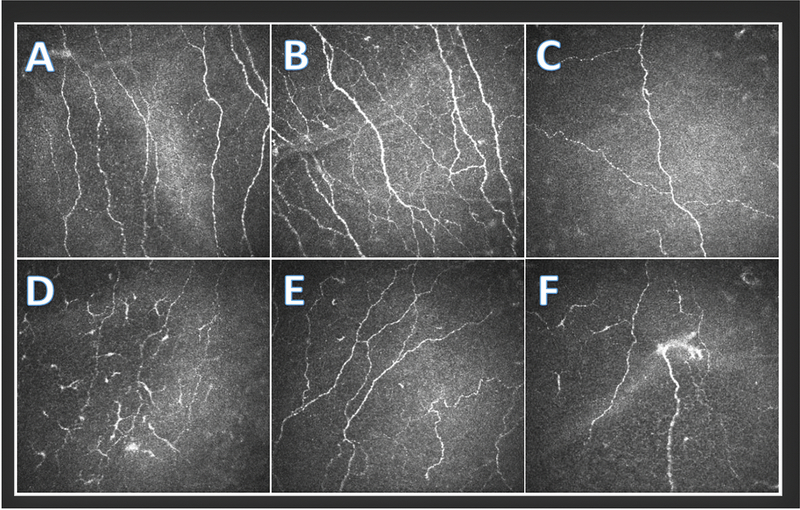
Figure 1.
In Vivo Confocal Microscopy of Sub-basal Nerves (Heidel Retina Tomograph/Cornea Rostock Module by Heidelberg Engineering, Heidelberg, Germany) depicting A) a normal nerve pattern with no dendritic cells, B) increased nerve branching, C) decreased nerve density, D) decreased nerve density and many activated dendritic cells, E) increased nerve tortuosity, and F) decreased nerve density, a probable microneuroma, and a few activated dendritic cells.
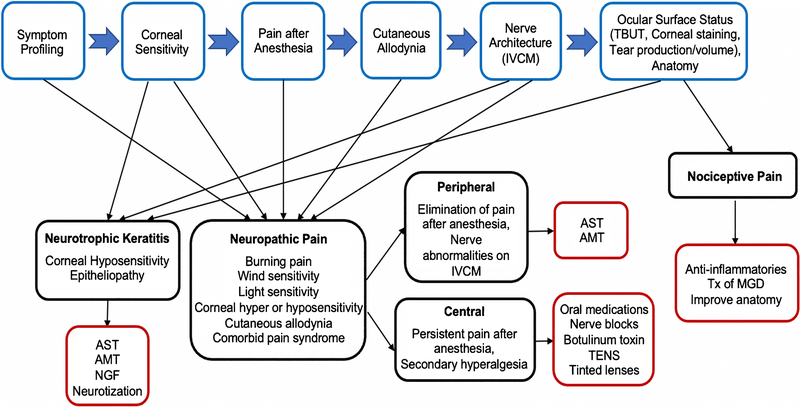
Figure 2.
Phenotyping algorithm based on signs, symptoms, and nerves evaluation and how this can aid in clinical decision-making; with demonstrated examples for neurotrophic, neuropathic, and nociceptive profiles. IVCM=in vivo confocal microscopy; TBUT = tear breakup time; AST=autologous serum tears; AMT=amniotic membrane transplant; NGF=nerve growth factor; TENS=transcutaneous electrical nerve stimulation; Tx=treatment; MGD= meibomian gland dysfunction
2. Sub-categorizing DED phenotypes can aid in guiding treatment algorithms:
The treatment ladder for DED begins by treating any nociceptive sources of pain (rapid TBUT, corneal staining, low tear production) using artificial tears, topical anti-inflammatories, and/or addressing underlying anatomical abnormalities (e.g. meibomian gland dysfunction [MGD], conjunctivochalasis, pterygium).[29] If pain persists despite these approaches, or if certain symptoms (e.g. burning, wind and light sensitivity) or co-morbidities (migraine, fibromyalgia, post-surgical pain) are present, neuropathic pain should be considered (Figure 2).[74] Suspected neuropathic pain based on symptoms should be fully examined with the above-discussed diagnostic processes.
One common phenotype is neurotrophic keratitis (NK), which presents with decreased sensitivity and signs of corneal epitheliopathy (e.g. increased corneal staining). NK is often observed in the setting of diabetes, viral infection, anesthetic abuse, and after neurosurgical procedures.[29, 75] Autologous serum tears (AST) are helpful in treating NK.[75] For example, in a Japanese study of 11 subjects with NK treated with 20% topical AST drops 5–10 times daily, epithelial defects resolved in all eyes within 6–32 days (mean 17.10±8.0 days) and sensitivity improved on CB compared to baseline (3.00±2.29 cm vs. 1.18±1.16 cm, p<0.05).[76] Similarly, in an American study of 6 subjects with NK, subjects treated with autologous plasma for a mean of 4.7 months (range 3–6 months) showed decreased symptoms on OSDI (39.5±11.2 to 16.8±6.0, p=0.003), increased sensitivity on CB (0.90±1.24 to 4.20±1.40 cm, p<0.0001), increased nerve count on laser-scanning IVCM (0.75±0.81 to 4.81±1.98, p=0.0004), and decreased corneal staining (values not provided, p=0.0003) at mean 5 month follow-up (range 3–6 months).[77] Besides AST, amniotic membrane transplantation (AMT) has been used to treat individuals with NK.[78] More recently, recombinant human nerve growth factor (NGF) (Oxervate, Cenegermin, Dompe) was approved for the treatment of NK.[79, 80]. In recalcitrant NK, neurotization is a surgical procedure that can be performed to increase sensation and improve epitheliopathy.[81, 82]
Several treatment options have been studied in individuals with presumed peripheral (corneal) neuropathic pain. Like in NK, AST have been studied in individuals with peripheral neuropathic pain. In 16 individuals with light sensitivity with a presumed neuropathic component (decreased nerve length/count, normal slit-lamp exam), treatment with AST (mean 3.80±0.50 months, range 1–8) decreased pain severity (9.10±0.20 to 3.10±0.30, p<0.0001) and increased nerve count (10.50±1.40 to 15.10±1.60 nerves/frame, p<0.0001).[83] Besides AST, AMT has also been studied in individuals with peripheral pain – an American study of 9 patients who received AMT (mean retention time 6.4 ± 1.1 days) reported reduced pain scores (6.3 ± 0.8 to 1.9 ± 0.6, p = 0.0003) and increased nerve density on laser-scanning IVCM (17,700.9 ± 1315.7 to 21,891.3 ± 2040.5 μm/mm2, p = 0.05).[85] While there is interest, no data are available on the use of recombinant NGF for the treatment of peripheral neuropathic pain. Finally, several agents have been evaluated in animal models and are now making their way to the clinical realm. For example, TRPV1 antagonists mitigated capsaicin (50 μL 0.02%) induced ocular pain in animal models.[86] A clinical trial in humans with post-refractive pain is underway.
In individuals with a suspected central component to pain (persistent pain after anesthesia, cutaneous allodynia, co-morbid fibromyalgia or migraine), oral medications including α2γ ligands (gabapentin or pregabalin), selective serotonin-norepinephrine reuptake inhibitors (e.g. duloxetine), and/or tricyclic antidepressants (e.g. nortriptyline) can be considered.[29] In a case series of 8 subjects with presumed neuropathic ocular pain (pain out of proportion, poor response to topical therapies), gabapentin (starting 300 mg daily, escalation to 600–900 TID) and pregabalin (starting 75 mg daily, escalation to 150 mg BID) led to complete pain relief in 2 subjects (NRS = 0 on a 0–10 scale), marked improvement in 3 subjects (NRS ≤ 2), and slight improvement in 1 subject (NRS = 10 to 7), while 2 subjects had no improvement in pain. The 2 subjects who noted complete pain relief were also on concomitant duloxetine (starting 20 mg, escalation to 60 mg daily).[87] In a British study, 25 subjects with clinically diagnosed peripheral neuropathic pain were treated with nortriptyline (10–25 mg starting dose, escalation to 100 mg daily) which led to lower pain at 4 weeks post-treatment (via NRS; 3.80±2.39 vs. 6.36±2.18, p<0.0001). Overall, 84% of subjects (n = 21) reported pain improvement [28% with >50% improvement (n = 7), 40% with 25–50% improvement (n = 10), and 32% with <25% improvement (n = 8)].[88]
In individuals with cutaneous allodynia who fail or are intolerant to oral medications, nerve blocks may be utilized. This modality entails long-term reversible blockade of depolarizing sodium channels, which prevents generation of action potentials involved in propagating the pain signal, combined with a long-acting corticosteroid to strengthen the effects.[89] A case series reported on outcomes of 11 subjects with presumed neuropathic ocular pain after periocular (supraorbital, supratrochlear, infratrochlear, and infraorbital) nerve blocks (4 mL of 0.5% bupivacaine with 1 mL of 80 mg/mL methylprednisolone acetate). In total, 7 subjects experienced pain relief, lasting from hours to months.[87]
In individuals with co-morbid headache and light sensitivity, migraine treatments can be initiated, such as botulinum toxin A (BoNT-A) or transcutaneous electrical nerve stimulation (TENS).[90, 91] In a study of 76 individuals with chronic migraine who received BoNT-A (100–150 units every 3 months), improvements in photophobia scores were noted following BoNT-A (via Visual Light Sensitivity Questionnaire-8 (VLSQ-8); 3.37 vs. 4.89, p<0.001). Furthermore, dry eye symptoms significantly improved but only in the subset of patients with severe DED symptoms at baseline (DEQ5 score ≥12; n=38) (via DEQ5; 13.80±4.02 vs. 15.40±2.47, p=0.03).[92] Similarly, a study evaluating the efficacy of TENS found that an in-office 30 minute session improved ocular pain in an open-label fashion in 14 individuals with chronic ocular pain. Overall, mean pain intensity was reduced 5 minutes post-vs. pre-treatment (0–10 NRS: right eye 4.54±3.20 to 1.92±2.50, p=0.01; left eye 4.46±3.36 to 2.00±2.38, p=0.01).[93] Tinted lens spectacles that block out specific wavelengths of light (~480 nm) are also helpful in managing individuals with photophobia[94], including those with co-morbid migraine.[95]
Importantly, individuals with ocular pain are often found to have an emotional component to their symptoms.[96] Clinicians must pair with other members of the care team to address the emotional consequences of chronic pain. Cognitive behavioral therapy (CBT) [97, 98], medications, and acupuncture[99] have all been used to target chronic pain-related depression and anxiety for pain conditions outside the eye and thus may be helpful in individual with ocular pain. Finally, all of the therapies listed above can be used concomitantly with traditional DE medications.
F. CONCLUSION
DED is an umbrella term applied to individuals with a wide range of symptoms and signs. The various phenotypic presentations of DED are due, in part, to individual differences in nerve function. Tests that can be used to evaluate nerve status, including structure and function, should be incorporated into the clinical examination of individuals with DED as this can aid in disease sub-typing. This in turn can guide therapeutic decision-making, especially in individuals who do not respond to first-line treatment modalities.
Acknowledgements:
Supported by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Clinical Sciences R&D (CSRD) I01 CX002015 (Dr. Galor) and Biomedical Laboratory R&D (BLRD) Service I01 BX004893 (Dr. Galor), Department of Defense Gulf War Illness Research Program (GWIRP) W81XWH-20-1-0579 (Dr. Galor) and Vision Research Program (VRP) W81XWH-20-1-0820 (Dr. Galor), National Eye Institute R01EY026174 (Dr. Galor) and R61EY032468 (Dr. Galor), NIH Center Core Grant P30EY014801 (institutional) and Research to Prevent Blindness Unrestricted Grant (institutional).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Human & Animal Rights: All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Erie JC, McLaren JW, and Patel SV, Confocal Microscopy in Ophthalmology. American Journal of Ophthalmology, 2009. 148(5): p. 639–646. [DOI] [PubMed] [Google Scholar]
- 2.Müller LJ, et al. , Corneal nerves: structure, contents and function. Experimental Eye Research, 2003. 76(5): p. 521–542. [DOI] [PubMed] [Google Scholar]
- 3.Müller LJ, Pels L, and Vrensen GF, Ultrastructural organization of human corneal nerves. Investigative Ophthalmology & Visual Science, 1996. 37(4): p. 476–488. [PubMed] [Google Scholar]
- 4.Liu YC, Lin MT, and Mehta JS, Analysis of corneal nerve plexus in corneal confocal microscopy images. Neural Regen Res, 2021. 16(4): p. 690–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simsek C, et al. , In Vivo Confocal Microscopy Evaluation in Dry Eye and Related Diseases. Current Ophthalmology Reports, 2019. 7(3): p. 187–195. [Google Scholar]
- 6.Jalbert I, et al. , In vivo confocal microscopy of the human cornea. British Journal of Ophthalmology, 2003. 87(2): p. 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petroll WM and Robertson DM, In Vivo Confocal Microscopy of the Cornea: New Developments in Image Acquisition, Reconstruction, and Analysis Using the HRT-Rostock Corneal Module. The ocular surface, 2015. 13(3): p. 187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaheen BS, Bakir M, and Jain S, Corneal nerves in health and disease. Survey of Ophthalmology, 2014. 59(3): p. 263–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel DV and McGhee CNJ, In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: a review. British Journal of Ophthalmology, 2009. 93(7): p. 853. [DOI] [PubMed] [Google Scholar]
- 10.Mantopoulos D, Cruzat A, and Hamrah P, In Vivo Imaging of Corneal Inflammation: New Tools for Clinical Practice and Research. Seminars in Ophthalmology, 2010. 25(5–6): p. 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiao H, et al. , Topographical and morphological differences of corneal dendritic cells during steady state and inflammation. Ocular immunology and inflammation, 2020. 28(6): p. 898–907. [DOI] [PubMed] [Google Scholar]
- 12.Maruoka S,Inaba M, and Ogata N, Activation of dendritic cells in dry eye mouse model. Investigative ophthalmology & visual science, 2018. 59(8): p. 3269–3277. [DOI] [PubMed] [Google Scholar]
- 13.Schaumburg CS, et al. , Ocular surface APCs are necessary for autoreactive T cell-mediated experimental autoimmune lacrimal keratoconjunctivitis. The Journal of Immunology, 2011. 187(7): p. 3653–3662. [DOI] [PubMed] [Google Scholar]
- 14.Alhatem A,Cavalcanti B, and Hamrah P, In Vivo Confocal Microscopy in Dry Eye Disease and Related Conditions. Seminars in Ophthalmology, 2012. 27(5–6): p. 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Aqaba MA, et al. , Corneal nerves in health and disease. Progress in Retinal and Eye Research, 2019. 73: p. 100762. [DOI] [PubMed] [Google Scholar]
- 16.Yang AY, Chow J, and Liu J, Corneal Innervation and Sensation: The Eye and Beyond. The Yale journal of biology and medicine, 2018. 91(1): p. 13–21. [PMC free article] [PubMed] [Google Scholar]
- 17.Bessou P and Perl ER, Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. Journal of Neurophysiology, 1969. 32(6): p. 1025–1043. [DOI] [PubMed] [Google Scholar]
- 18.Golebiowski B, Papas E, and Stapleton F, Assessing the sensory function of the ocular surface: Implications of use of a non-contact air jet aesthesiometer versus the Cochet–Bonnet aesthesiometer. Experimental Eye Research, 2011. 92(5): p. 408–413. [DOI] [PubMed] [Google Scholar]
- 19.Lum E and Murphy PJ, Effects of ambient humidity on the Cochet–Bonnet aesthesiometer. Eye, 2018. 32(10): p. 1644–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chao C, et al. , Ocular Surface Sensitivity Repeatability with Cochet-Bonnet Esthesiometer. Optometry and Vision Science, 2015. 92(2). [DOI] [PubMed] [Google Scholar]
- 21.Stapleton F, et al. , Corneal and conjunctival sensitivity to air stimuli. British Journal of Ophthalmology, 2004. 88(12): p. 1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erie JC, et al. , The Effect of Age on the Corneal Subbasal Nerve Plexus. Cornea, 2005. 24(6). [DOI] [PubMed] [Google Scholar]
- 23.Patel DV, et al. , Corneal Sensitivity and Slit Scanning In Vivo Confocal Microscopy of the Subbasal Nerve Plexus of the Normal Central and Peripheral Human Cornea. Cornea, 2009. 28(7). [DOI] [PubMed] [Google Scholar]
- 24.Niederer RL, et al. , Age-related differences in the normal humancornea: alaser scanning in vivo confocal microscopy study. British Journal of Ophthalmology, 2007. 91(9): p. 1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parissi M, et al. , Standardized Baseline Human Corneal Subbasal Nerve Density for Clinical Investigations With Laser-Scanning in Vivo Confocal Microscopy. Investigative Ophthalmology & Visual Science, 2013. 54(10): p. 7091–7102. [DOI] [PubMed] [Google Scholar]
- 26.Murphy PJ, et al. , Reliability of the Non-Contact Corneal Aesthesiometer and its comparison with the Cochet–Bonnet aesthesiometer. Ophthalmic and Physiological Optics, 1998. 18(6): p. 532–539. [PubMed] [Google Scholar]
- 27.Craig JP, et al. , TFOS DEWS II Definition and Classification Report. Ocul Surf, 2017. 15(3): p. 276–283. [DOI] [PubMed] [Google Scholar]
- 28.Ganesalingam K, et al. , Molecular evidence for the role of inflammation in dry eye disease. Clinical and Experimental Optometry, 2019. 102(5): p. 446–454. [DOI] [PubMed] [Google Scholar]
- 29.Patel S, et al. , Corneal Nerve Abnormalities in Ocular and Systemic Diseases. Experimental Eye Research, 2020: p. 108284. [DOI] [PubMed] [Google Scholar]
- 30.Kloosterboer A, Dermer HI, and Galor A, Diagnostic tests in dry eye. Expert Review of Ophthalmology, 2019. 14(4–5): p. 237–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Labbé A, et al. , The Relationship between Subbasal Nerve Morphology and Corneal Sensation in Ocular Surface Disease. Investigative Ophthalmology & Visual Science, 2012. 53(8): p. 4926–4931. [DOI] [PubMed] [Google Scholar]
- 32.Choi EY, et al. , Corneal Microstructural Changes in Non- Sjˆgren Dry Eye Using Confocal Microscopy: Clinical Correlation. 2015. 56: p. 680–686. [Google Scholar]
- 33.Labbé A, et al. , Corneal Nerve Structure and Function in Patients With Non-Sjögren Dry Eye: Clinical Correlations. Investigative Ophthalmology & Visual Science, 2013. 54(8): p. 5144–5150. [DOI] [PubMed] [Google Scholar]
- 34.Villani E, et al. , In Vivo Confocal Evaluation of the Ocular Surface Morpho-Functional Unit in Dry Eye. Optometry and Vision Science, 2013. 90(6). [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, et al. , Altered Corneal Nerves in Aqueous Tear Deficiency Viewed by In Vivo Confocal Microscopy. Cornea, 2005. 24(7). [DOI] [PubMed] [Google Scholar]
- 36.Shetty R, et al. , Corneal Dendritic Cell Density Is Associated with Subbasal Nerve Plexus Features, Ocular Surface Disease Index, and Serum Vitamin D in Evaporative Dry Eye Disease. BioMed Research International, 2016. 2016: p. 4369750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khamar P, et al. , Dysregulated Tear Fluid Nociception-Associated Factors, Corneal Dendritic Cell Density, and Vitamin D Levels in Evaporative Dry Eye. Investigative Ophthalmology & Visual Science, 2019. 60(7): p. 2532–2542. [DOI] [PubMed] [Google Scholar]
- 38.Parra A, et al. , Tear fluid hyperosmolality increases nerve impulse activity of cold thermoreceptor endings of the cornea. PAIN®, 2014. 155(8): p. 1481–1491. [DOI] [PubMed] [Google Scholar]
- 39.Rahman EZ, et al. , Corneal Sensitivity in Tear Dysfunction and its Correlation With Clinical Parameters and Blink Rate. American Journal of Ophthalmology, 2015. 160(5): p. 858–866.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bentez-del-Castillo JM, et al. , Relation between Corneal Innervation with Confocal Microscopy and Corneal Sensitivity with Noncontact Esthesiometry in Patients with Dry Eye. Investigative Ophthalmology & Visual Science, 2007. 48(1): p. 173–181. [DOI] [PubMed] [Google Scholar]
- 41.Bourcier T, et al. , Decreased Corneal Sensitivity in Patients with Dry Eye. Investigative Ophthalmology & Visual Science, 2005. 46(7): p. 2341–2345. [DOI] [PubMed] [Google Scholar]
- 42.SadeDePaiva C and Pflugfelder SC, Corneal epith eliopathy of dry eye induces hyperesthesia to mechanical air jet stimulation. American Journal of Ophthalmology, 2004. 137(1): p. 109–115. [DOI] [PubMed] [Google Scholar]
- 43.Simsek C, et al. , Alterations of Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress. Investigative Ophthalmology & Visual Science, 2018. 59(5): p. 1986–1995. [DOI] [PubMed] [Google Scholar]
- 44.Simsek C, et al. , Changes in Murine Subbasal Corneal Nerves After Scopolamine-Induced Dry Eye Stress Exposure. Investigative Ophthalmology & Visual Science, 2019. 60(2): p. 615–623. [DOI] [PubMed] [Google Scholar]
- 45.Leonard BC, et al. , Comprehensive Clinical, Diagnostic, and Advanced Imaging Characterization of the Ocular Surface in Spontaneous Aqueous Deficient Dry Eye Disease in Dogs. Cornea, 2019. 38(12): p. 1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiffman RM, et al. , Reliability and validity of the Ocular Surface Disease Index. Arch Ophthalmol, 2000. 118(5): p. 615–21. [DOI] [PubMed] [Google Scholar]
- 47.Chalmers RL, Begley CG, and Caffery B, Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont Lens Anterior Eye, 2010. 33(2): p. 55–60. [DOI] [PubMed] [Google Scholar]
- 48. Farhangi M, et al. , Modification of the Neuropathic Pain Symptom Inventory for use in eye pain (NPSI-Eye). Pain, 2019. 160(7): p. 1541–1550.30883524 (**) This study is very important because it validated a neuropathic pain questionnaire that can be used in individuals with ocular pain, the NPSI-Eye.
- 49. Qazi Y, et al. , Validity and Reliability of a Novel Ocular Pain Assessment Survey (OPAS) in Quantifying and Monitoring Corneal and Ocular Surface Pain. Ophthalmology, 2016. 123(7): p. 1458–68.27089999 (**) This study is very important because it validated a pain questionnaire that can be used in individuals with ocular pain.
- 50.Tepelus TC, et al. , Correlation between corneal innervation and inflammation evaluated with confocal microscopy and symptomatology in patients with dry eye syndromes: a preliminary study. Graefe's Archive for Clinical and Experimental Ophthalmology, 2017. 255(9): p. 1771–1778. [DOI] [PubMed] [Google Scholar]
- 51.Denoyer A, et al. , Dry Eye Disease after Refractive Surgery: Comparative Outcomes of Small Incision Lenticule Extraction versus LASIK. Ophthalmology, 2015. 122(4): p. 669–676. [DOI] [PubMed] [Google Scholar]
- 52.Spierer O, et al. , Corneal Mechanical Thresholds Negatively Associate With Dry Eye and Ocular Pain Symptoms. Investigative Ophthalmology & Visual Science, 2016. 57(2): p. 617–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liang Q, et al. , Ocular Surface Epithelial Thickness Evaluation in Dry Eye Patients: Clinical Correlations. Journal of Ophthalmology, 2016. 2016: p. 1628469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Galor A, et al. , Corneal Nerve Pathway Function in Individuals with Dry Eye Symptoms. Ophthalmology, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tanaka A, et al. , In Vivo Confocal Microscopy in Patients with Dry Eye Disease Demonstrates Decreased Peripheral Corneal Nerve Density and Correlation to Clinical Signs. Investigative Ophthalmology & Visual Science, 2017. 58(8): p. 3753–3753. [Google Scholar]
- 56.Bron AJ, et al. , Predicted Phenotypes of Dry Eye: Proposed Consequences of Its Natural History. The Ocular Surface, 2009. 7(2): p. 78–92. [DOI] [PubMed] [Google Scholar]
- 57.Villani E, et al. , Imaging Biomarkers for Dry Eye Disease. Eye Contact Lens, 2020. 46 Suppl 2: p. S141–s145. [DOI] [PubMed] [Google Scholar]
- 58.IASP Terminology. 2020; Available from: https://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698.
- 59.Rosenthal P and Borsook D, The Corneal Pain System. Part I: The Missing Piece of the Dry Eye Puzzle*. The Ocular Surface, 2012. 10(1): p. 2–14. [DOI] [PubMed] [Google Scholar]
- 60.Galor A, et al. , Neuropathic pain and dry eye. The ocular surface, 2018. 16(1): p. 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sacchetti M and Lambiase A, Diagnosis and management of neurotrophic keratitis. Clinical ophthalmology (Auckland, N.Z.), 2014. 8: p. 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lockwood A, Hope-Ross M, and Chell P, Neurotrophic keratopathy and diabetes mellitus. Eye, 2006. 20(7): p. 837–839. [DOI] [PubMed] [Google Scholar]
- 63.Kalangara JP, et al. , Characteristics of Ocular Pain Complaints in Patients With Idiopathic Dry Eye Symptoms. Eye Contact Lens, 2017. 43(3): p. 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Galor A, et al. , Neuropathic pain and dry eye. Ocul Surf, 2018. 16(1): p. 31–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Galor A, et al. , Neuropathic Ocular Pain due to Dry Eye is Associated with Multiple Comorbid Chronic Pain Syndromes. The journal of pain, 2016. 17(3): p. 310–318.26606863 (*) This study is important because it evaluated dry eye symptoms and signs in individuals chronic pain.
- 66. Farhangi M, et al. , Individuals with migraine have a different dry eye symptom profile than individuals without migraine. Br J Ophthalmol, 2020. 104(2): p. 260–264.31040130 (*) This study is important because it evaluated dry eye symptoms and signs in individuals with and without migraine.
- 67.Crane AM, et al. , Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. British Journal of Ophthalmology, 2017. 101(9): p. 1238. [DOI] [PubMed] [Google Scholar]
- 68.Rosenthal P and Borsook D, Ocular neuropathic pain. British Journal of Ophthalmology, 2016. 100(1): p. 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Dermer H, et al. , Corneal sub-basal nerve plexus microneuromas in individuals with and without dry eye. British Journal of Ophthalmology, 2021: p. bjophthalmol-2020–317891. (*) This study is important because it did not find a relationship between a specific anatomic findings (termed microneuroma) and ocular pain.
- 70.Rózsa AJ, Guss RB, and Beuerman RW, Neural remodeling following experimental surgery of the rabbit cornea. Investigative Ophthalmology & Visual Science, 1983. 24(8): p. 1033–1051. [PubMed] [Google Scholar]
- 71. Moein H-R, et al. , Visualization of microneuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain? The Ocular Surface, 2020. 18(4): p. 651–656.32663518 (**) This study is very important because it presented an anatomic nerve finding (termed microneuroma) as a potential marker for corneal neuropathic pain.
- 72.Chinnery HR, et al. , Identification of presumed corneal neuromas and microneuromas using laser-scanning in vivo confocal microscopy: a systematic review. British Journal of Ophthalmology, 2021: p. bjophthalmol-2020–318156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stepp MA, et al. , Corneal Epithelial “Neuromas”: A Case of Mistaken Identity? Cornea, 2020. 39(7). (*) This study is important because it provided an alternative explanation to the anatomic finding termed microneuroma.
- 74.Labetoulle M, et al. , Role of corneal nerves in ocular surface homeostasis and disease. Acta Ophthalmologica, 2019. 97(2): p. 137–145. [DOI] [PubMed] [Google Scholar]
- 75.Sacchetti M and Lambiase A, Diagnosis and management of neurotrophic keratitis. Clin Ophthalmol, 2014. 8: p. 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsumoto Y, et al. , Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology, 2004. 111(6): p. 1115–1120. [DOI] [PubMed] [Google Scholar]
- 77.Rao K, Leveque C, and Pflugfelder SC, Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. British Journal of Ophthalmology, 2010. 94(5): p. 584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kruse FE, Rohrschneider K, and Völcker HE, Multilayer amniotic membrane transplantation for reconstruction of deep corneal ulcers. Ophthalmology, 1999. 106(8): p. 1504–1511. [DOI] [PubMed] [Google Scholar]
- 79. Bonini S, et al. , Phase II Randomized, Double-Masked, Vehicle-Controlled Trial of Recombinant Human Nerve Growth Factor for Neurotrophic Keratitis. Ophthalmology, 2018. 125(9): p. 1332–1343.29653858 (*) This study is important because it evaluated the efficacy of recombinant NGF in NK.
- 80. Pflugfelder SC, et al. , Topical Recombinant Human Nerve Growth Factor (Cenegermin) for Neurotrophic Keratopathy: A Multicenter Randomized Vehicle-Controlled Pivotal Trial. Ophthalmology, 2020. 127(1): p. 14–26.31585826 (*) This study is important because it evaluated the efficacy of recombinant NGF in NK.
- 81.Kim JS, Rafailov L, and Leyngold IM, Corneal Neurotization for Postherpetic Neurotrophic Keratopathy: Initial Experience and Clinical Outcomes. Ophthalmic Plastic & Reconstructive Surgery, 9000. Publish Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 82.Terzis JK, Dryer MM, and Bodner BI, Corneal Neurotization: A Novel Solution to Neurotrophic Keratopathy. Plastic and Reconstructive Surgery, 2009. 123(1). [DOI] [PubMed] [Google Scholar]
- 83.Aggarwal S, et al. , Autologous Serum Tears for Treatment of Photoallodynia in Patients with Corneal Neuropathy: Efficacy and Evaluation with In Vivo Confocal Microscopy. The Ocular Surface, 2015. 13(3): p. 250–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ali TK, et al. , Use of Autologous Serum Tears for the Treatment of Ocular Surface Disease From Patients With Systemic Autoimmune Diseases. American Journal of Ophthalmology, 2018. 189: p. 65–70. [DOI] [PubMed] [Google Scholar]
- 85.Morkin MI and Hamrah P, Efficacy of self-retained cryopreserved amniotic membrane for treatment of neuropathic corneal pain. The ocular surface, 2018. 16(1): p. 132–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bates BD, et al. , Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. PAIN, 2010. 149(3): p. 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Small LR, et al. , Oral Gabapentinoids and Nerve Blocks for the Treatment of Chronic Ocular Pain. Eye & Contact Lens, 2020. 46(3). [DOI] [PubMed] [Google Scholar]
- 88.Ozmen MC, et al. , Nortriptyline is Effective in Ameliorating Symptoms of Neuropathic Corneal Pain. Investigative Ophthalmology & Visual Science, 2019. 60(9): p. 4732–4732. [Google Scholar]
- 89.Scholz A, et al. , Complex Blockade of TTX-Resistant Na+ Currents by Lidocaine and Bupivacaine Reduce Firing Frequency in DRG Neurons. Journal of Neurophysiology, 1998. 79(4): p. 1746–1754. [DOI] [PubMed] [Google Scholar]
- 90.Diel RJ, et al. , Botulinum Toxin A for the Treatment of Photophobia and Dry Eye. Ophthalmology, 2018. 125(1): p. 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Johnson M, Transcutaneous Electrical Nerve Stimulation: Mechanisms, Clinical Application and Evidence. Reviews in pain, 2007. 1(1): p. 7–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diel RJ, et al. , Photophobia ands ensations of dryness in patients with migraine occur independent of baseline tear volume and improve following botulinum toxin A injections. British Journal of Ophthalmology, 2019. 103(8): p. 1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sivanesan E, et al. , Noninvasive Electrical Stimulation for the Treatment of Chronic Ocular Pain and Photophobia. Neuromodulation: Technology at the Neural Interface, 2018. 21(8): p. 727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katz BJ and Digre KB, Diagnosis, pathophysiology, and treatment of photophobia. Survey of Ophthalmology, 2016. 61(4): p. 466–477. [DOI] [PubMed] [Google Scholar]
- 95.Wilkins AJ, et al. , Tinted Spectacles and Visually Sensitive Migraine. Cephalalgia, 2002. 22(9): p. 711–719. [DOI] [PubMed] [Google Scholar]
- 96.Patel S, et al. , Dysfunctional Coping Mechanisms Contribute to Dry Eye Symptoms. J Clin Med, 2019. 8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lamb SE, et al. , Group cognitive behavioural treatment for low-back pain in primary care: a randomised controlled trial and cost-effectiveness analysis. The Lancet, 2010. 375(9718): p. 916–923. [DOI] [PubMed] [Google Scholar]
- 98.Otis JD, et al. , A Randomized Controlled Pilot Study of a Cognitive-Behavioral Therapy Approach for Painful Diabetic Peripheral Neuropathy. The Journal of Pain, 2013. 14(5): p. 475–482. [DOI] [PubMed] [Google Scholar]
- 99.Mehra D, Cohen NK, and Galor A, Ocular Surface Pain: A Narrative Review. Ophthalmology and Therapy, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]