Abstract
Objective
Cancer patients and survivors may be disproportionately affected by COVID-19. We sought to determine the effects of the pandemic on thyroid cancer survivors’ health care interactions and quality of life.
Methods
An anonymous survey including questions about COVID-19 and the Patient-Reported Outcomes Measurement Information System profile (PROMIS-29, version 2.0) was hosted on the Thyroid Cancer Survivors’ Association, Inc website. PROMIS scores were compared to previously published data. Factors associated with greater anxiety were evaluated with univariable and multivariable logistic regression.
Results
From May 6, 2020, to October 8, 2020, 413 participants consented to take the survey; 378 (92%) met the inclusion criteria: diagnosed with thyroid cancer or noninvasive follicular neoplasm with papillary-like nuclear features, located within the United States, and completed all sections of the survey. The mean age was 53 years, 89% were women, and 74% had papillary thyroid cancer. Most respondents agreed/strongly agreed (83%) that their lives were very different during the COVID-19 pandemic, as were their interactions with doctors (79%). A minority (43%) were satisfied with the information from their doctor regarding COVID-19 changes. Compared to pre-COVID-19, PROMIS scores were higher for anxiety (57.8 vs 56.5; P < .05) and lower for the ability to participate in social activities (46.2 vs 48.1; P < .01), fatigue (55.8 vs 57.9; P < .01), and sleep disturbance (54.7 vs 56.1; P < .01). After adjusting for confounders, higher anxiety was associated with younger age (P < .01) and change in treatment plan (P = .04).
Conclusion
During the COVID-19 pandemic, thyroid cancer survivors reported increased anxiety compared to a pre-COVID cohort. To deliver comprehensive care, providers must better understand patient concerns and improve communication about potential changes to treatment plans.
Key words: thyroid cancer, quality of life, anxiety, physician communication, COVID-19, telehealth
Abbreviations: PROMIS, Patient-Reported Outcomes Measurement Information System; QOL, quality of life; ThyCa, Thyroid Cancer Survivors’ Association, Inc
Introduction
The COVID-19 pandemic has led to rapid changes in our society, economy, and health system that are unprecedented in the modern era. Cancer patients and survivors are particularly affected because they face novel challenges. Patients’ need to obtain cancer care suddenly had to be balanced against the risk of viral exposure in a health care facility as well as resource shortages. Early data in the spring of 2020 suggested that an underlying cancer diagnosis may be associated with an increased risk of death or intensive care unit admission related to COVID-19 infection.1 In a survey by the American Cancer Society Cancer Action Network in March 2020, a third of cancer patients and survivors reported that they were worried about the impact of COVID-19 on their ability to get treatment for their cancer. This concern was particularly acute for those who were in active treatment, of whom nearly half (40%) expressed worry.2
Thyroid cancer currently has an estimated lifetime risk of 1.3% in the United States,3 and its overall incidence has increased 3% annually from 1974 to 2013.4 While thyroid cancer as a whole is associated with a high survival rate, survivors often require extended surveillance and lifelong thyroid hormone supplementation or replacement. Previous studies have shown that thyroid cancer survivors experience physical and psychological challenges associated with reduced self-reported quality of life (QOL)5 that are similar to, or worse than, patients with lung, colorectal, breast, and prostate cancer.6 , 7 In this study, we sought to understand how the COVID-19 pandemic is affecting thyroid cancer patients’ health care interactions and preferences for their care, as well as their QOL and emotional well-being, to better tailor support to patients during and after the current pandemic and potential future crises.
Methods
Survey Design and Participants
An anonymous, web-based, cross-sectional survey was designed to assess the impact of the COVID-19 pandemic on thyroid cancer survivors’ QOL. The target population was thyroid cancer patients and survivors in the United States. The survey consisted of novel questions and the Patient-Reported Outcomes Measurement Information System 29-item (PROMIS-29) profile, version 2.0. PROMIS-29 is a National Institutes of Health–sponsored measure that assesses patient-reported anxiety, depression, fatigue, pain interference, physical function, sleep disturbance, and the ability to participate in social roles and activities. This measure has been validated in a range of patient populations, including those with cancer.8 , 9 All novel survey questions were developed by a multidisciplinary team of clinicians, which included a surgeon and 2 psycho-oncologists. These questions were then pretested once by a team of 18 volunteers, comprised of 7 clinicians, 9 healthy volunteers outside of the medical field, and 2 thyroid cancer survivors. These volunteers were asked to assess novel questions for clarity, readability, logic, and flow, the technical quality of the survey, and the total length of time spent taking the survey, and were individually debriefed over email. Based on responses from pretesting, refinements were incorporated before survey finalization.
The final survey consists of 4 parts. Section 1 includes questions regarding thyroid cancer clinical characteristics, including the type of cancer, stage, and treatment history. Section 2 includes questions about COVID-19 and the impact of the pandemic on respondents’ lives, including whether the respondent and/or close friends or family were diagnosed with COVID-19, whether income or employment changed, how health care interactions changed, and an estimate of the level of worry by the respondent about the pandemic and associated changes. Section 3 consists of the 29-item PROMIS profile, version 2.0. Finally, Part 4 consists of questions regarding demographic information, including age, sex, race, geographic location, education, and employment status. The complete final survey can be found in Supplementary Material.
Data Collection
The study period was from May 6, 2020, to October 8, 2020. The survey was created and the data were collected through Research Electronic Data Capture, a secure web application for building and managing online surveys and databases.10 In collaboration with ThyCa: Thyroid Cancer Survivors’ Association, Inc, the survey was posted as a link on their homepage, www.thyca.org, and promoted in its free email newsletter. ThyCa is a nonprofit organization of thyroid cancer survivors, caregivers, and health care professionals, with over 85 000 subscribers. Responses were anonymously recorded, with a unique identifier code generated automatically for each participant through Research Electronic Data Capture. No tracking data were collected, and all data were self-reported, including demographic and clinical characteristics.
Ethical Considerations
This study was reviewed and approved by our institutional review board. All participants who clicked the survey link were required to read an electronic consent and check a box certifying that they were “at least 18 years old, had read and understood the consent form, and gave their consent freely to participate in the study” before accessing survey questions. No remuneration was provided for study participation.
Data Analysis
Surveys were analyzed only if participants consented to the survey and accessed and answered all 4 sections. Participants were excluded if they reported that they had not been diagnosed with thyroid cancer by a physician. Due to the recent change in classification and their inclusion in the pre-COVID-19 comparison cohort,6 patients with noninvasive follicular neoplasm with papillary-like nuclear features were included, as they may have previously been considered to have cancer. Only participants from within the United States were included in our analysis because of the diverse nature of the impact of COVID-19 internationally as well as the fact that PROMIS validation is US-based. All inclusion and exclusion criteria were determined based on self-reported data.
Data were checked for normality both formally and with visual depictions. Descriptive statistics of survey participants were performed using STATA (Version 14.2, StataCorp) and SAS software. All PROMIS responses were scored using item response theory models via the online HealthMeasures Scoring Service (https://www.assessmentcenter.net/ac_scoringservice). This software converts raw participant responses to a T-score metric, with a mean of 50 representing the mean of the US general population and a standard deviation of 10. For all domains, a higher T-score indicates “more” of the concept being measured, whether positive (eg, more social interaction) or negative (eg, more fatigue). For the domains of anxiety, depression, fatigue, pain interference, and sleep disturbance, higher scores indicated “worse” patient-reported QOL, while in the domains of satisfaction with social participation and physical functioning, higher scores indicated “better” QOL. PROMIS T-scores were compared to previously published data6 from a similarly recruited thyroid cancer cohort before the COVID-19 pandemic and analyzed using the Mann-Whitney U and Wilcoxon signed rank tests. To assess factors associated with increased reported anxiety, univariable and multivariable linear regressions were performed. Significant factors (P < .01) in the univariable analysis were then assessed for multicollinearity, and significant independent variables were included in a multivariable model. All tests were 2-sided and P values < .05 were concluded to be statistically significant. Analyses were performed with SAS software, version 9.4 (SAS Institute).
Results
Response Rate and Participant Characteristics
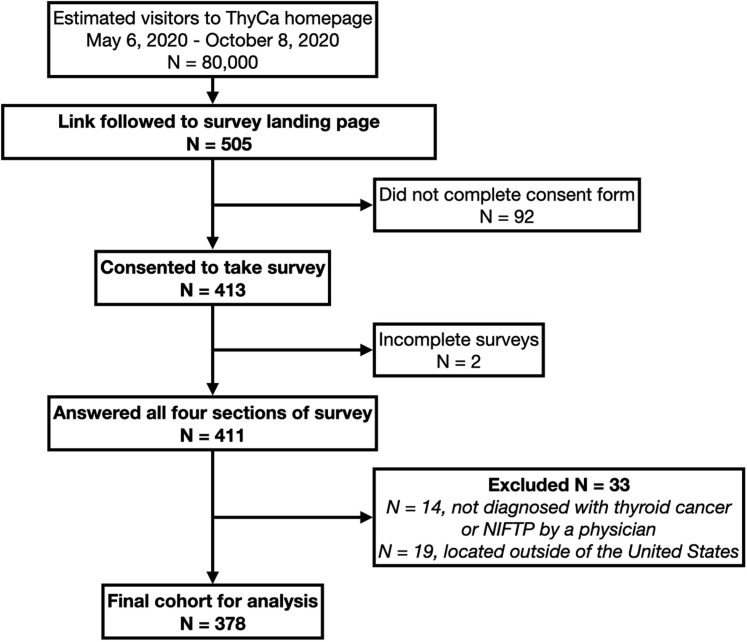
During the study period, the ThyCa website (all pages) had approximately 80 000 visitors (all-comers), and the survey link was followed and the landing page accessed 505 times, for an estimated 0.6% click rate. A total of 413 participants consented to take the survey, and 411 (99.5%) subsequently answered all the survey sections, for an estimated response rate of 0.5%. Surveys were excluded if the respondent had not been diagnosed with thyroid cancer by a physician (N = 14) or if the respondent was located outside of the United States (N = 19), for a total of 378 surveys remaining for analysis (Fig. 1 ). Demographic and cancer characteristics are demonstrated in Table 1 . The majority of participants were women (87%) and White (90%), with papillary thyroid carcinoma (74%). Almost all respondents (97%) had undergone surgery, and 70% had undergone radioactive iodine therapy. At the time of the survey, participants were a mean of 8.3 years from diagnosis; most (N = 299, 80%) had finished treatment or were not planning to undergo treatment, while 76 (20%) were in the middle of or awaiting treatment (7 awaiting surgery, 23 awaiting or undergoing radioactive iodine, 21 awaiting or undergoing chemotherapy or targeted therapy, 1 awaiting or undergoing external beam radiation therapy, and 9 awaiting or undergoing other therapies). The majority (N = 276, 73%) completed the survey within its first month (May 6, 2020, to June 5, 2020).
Fig. 1.
Participant flow diagram.
Table 1.
Participant Demographic and Cancer Characteristics (N = 378)
| Age, y, mean (SD) | 53.0 (12.9) |
| Sex, n (%) | … |
| Female | 335 (88.6) |
| Male | 40 (10.6) |
| Other | 3 (0.79) |
| Race, n (%) | … |
| American Indian/Alaska Native | 4 (1.1) |
| Asian | 11 (3.0) |
| Native Hawaiian/Other Pacific Islander | 1 (0.3) |
| Black/ African American | 4 (1.1) |
| White | 334 (90.3) |
| More than one race | 11 (3.0) |
| Unknown/not reported | 5 (1.4) |
| Ethnicity, n (%) | … |
| Hispanic/Latino | 17 (4.7) |
| Not-Hispanic/Latino | 340 (93.4) |
| Unknown/not reported | 7 (1.9) |
| Urban/rural, n (%) | … |
| Urban area | 170 (47.8) |
| Urban cluster | 121 (34.0) |
| Small town/rural area | 65 (18.3) |
| Distance traveled for thyroid cancer treatment,a n (%) | … |
| Over 50 miles | 97 (25.9) |
| Over 100 miles | 59 (15.8) |
| Education, n (%) | … |
| Less than a high school diploma | 1 (0.3) |
| High school degree or equivalent | 11 (2.9) |
| Some college, no degree | 73 (19.4) |
| Associate degree | 42 (11.1) |
| Bachelor's degree | 118 (31.3) |
| Master's degree | 94 (24.9) |
| Professional degree or doctorate | 38 (10.1) |
| Employment, n (%) | … |
| Employed full-time (≥40 hours per week) | 151 (40.0) |
| Employed part-time (<40 hours per week) | 39 (10.3) |
| Unemployed and currently looking for work | 12 (3.2) |
| Student | 3 (0.8) |
| Retired | 97 (25.7) |
| Homemaker | 28 (7.4) |
| Unable to work | 34 (9.0) |
| Other | 14 (3.7) |
| Insurance status,b n (%) | … |
| No insurance | 3 (0.8) |
| Insured through my or a family member's employer | 236 (62.9) |
| Affordable Care Act plan | 16 (4.3) |
| Other private health insurance (not through an employer) | 12 (3.2) |
| Medicaid | 13 (3.5) |
| Medicare | 60 (16.0) |
| Veterans Health Administration | 2 (0.5) |
| Other | 7 (1.9) |
| Not sure | 26 (6.9) |
| Reported diagnosis or medication for anxiety or depression | 106 (28.0) |
| Cancer type or NIFTPc | … |
| Papillary | 279 (74.4) |
| Follicular | 26 (6.9) |
| Medullary | 41 (10.9) |
| Hürthle cell | 17 (4.5) |
| NIFTP | 6 (1.6) |
| Anaplastic | 1 (0.3) |
| I am not sure | 5 (1.3) |
| Cancer stage c | … |
| I | 101 (28.1) |
| II | 63 (17.6) |
| III | 58 (16.2) |
| IV | 60 (16.7) |
| Unknown/have not been staged | 77 (12.5) |
| Years since diagnosis | … |
| mean (SD) | 8.3 (8.9) |
| Surgical therapy, n (%) | 366 (96.8) |
| Radioactive iodine, n (%) | 265 (70.1) |
| Diagnosed with COVID | 7 (1.9) |
| Family/friend with COVID | 54 (14.3) |
| Someone close who died from COVID | 10 (2.6) |
Abbreviation: NIFTP = Noninvasive follicular thyroid neoplasm with papillary-like nuclear features.
Missing data: a4 missing, b3 missing, c19 missing,
Table 2 compares the characteristics of the study cohort to that of a previously published pre-COVID-19 cohort,6 as well as to national data.4 , 11 Compared to the national data, our survey population was older, more predominately women, more were White, had a lower percentage of papillary and follicular thyroid carcinoma, and had a higher cancer stage. The characteristics were more similar between our cohort and the pre-COVID-19 survey cohort but had statistically significant differences in all but sex and percent of surgical therapy.
Table 2.
Comparison of Survey Cohort to pre-COVID Study Cohort and National Data
| Variable | Present study N = 378 |
Pre-COVID cohorta N = 1743 |
P value | National datab,c N = 77 276b |
P value |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 53 ± 13 | 51 ± 13 | <.01 | 48 ± 16b | <.01 |
| Sex, n (%) | … | … | .9 | … | <.01 |
| Female | 335 (89%) | 1541 (88%) | … | 58 213 (75%)b | … |
| Race, n (%) | … | … | <.01 | … | <.01 |
| White | 334 (90%) | 1654 (95%) | 63 479 (82%) b | … | |
| Cancer type (or NIFTP), n (%) | … | … | <.01 | … | <.01 |
| Papillary | 279 (74%) | 1313 (85%) | … | 64 625 (84%) b | … |
| Follicular | 26 (6.9%) | 97 (6.3%) | … | 8359 (11%) b | … |
| Medullary | 41 (10.9%) | 74 (4.8%) | … | 1685 (2.2%) b | … |
| Hürthle cell | 17 (4.5%) | 42 (2.7%) | … | … | … |
| NIFTP | 6 (1.6%) | 12 (0.8%) | … | … | … |
| Anaplastic | 1 (0.3%) | 7 (0.5%) | … | 975 (1.3%) b | … |
| Cancer stage, n (%) | … | … | .02 | … | <.01 |
| I | 101 (28%) | 522 (30%) | … | 25 580 (67%) b | … |
| II | 63 (18%) | 298 (17%) | … | 2870 (7.6%) b | … |
| III | 58 (16%) | 243 (14%) | … | 4562 (12%) b | … |
| IV | 60 (17%) | 199 (11%) | … | 3045 (8.0%) b | … |
| Unknown/not yet staged | 77 (13%) | 481 (28%) | … | 1881 (5.0%) b | … |
| Surgical therapy, n (%) | 366 (97%) | 1710 (98%) | .1 | … | … |
| Radioactive iodine, n (%) | 265 (70%) | 1366 (78%) | <.01 | 139 238 (50%)c | <.01 |
Abbreviation: NIFTP = Noninvasive follicular neoplasm with papillary-like nuclear features.
P values compared to present study cohort.
Goswami et al.6
Lim et al,4 Surveillance, Epidemiology, and End Results (SEER) data 1974-2013.
Orosco et al,11 SEER 1992-2009 and National Cancer Database 2004-2012, N = 276 558.
General Effects of COVID-19
Most respondents (83%) agreed or strongly agreed that their lives were very different during COVID-19 compared to before. Only 7 (1.9%) had been diagnosed with COVID-19, but an additional 64 (16%) reported symptoms consistent with COVID-19 and suspected an infection. Fifty-four respondents (14%) had a family member or friend diagnosed with COVID, and 10 (3%) had a family member or close friend who had died from COVID. Most respondents (92%) reported a change in access to extended family and non-family social supports. These were predominately mild (46%: continued visits with social distancing and/or regular phone, video, or social media connections) or moderate (40%: lost in-person and remote contact with a few people, but not all supports), although 6% reported losing in-person and remote contact with all supports. A “shelter in place” order was in effect for 288 respondents (73%), and over half (204, 55%) reported some change in family income or employment, with 3% reporting that they were unable to pay bills or meet basic needs.
Effects of COVID-19 on Health care Experience and Preferences
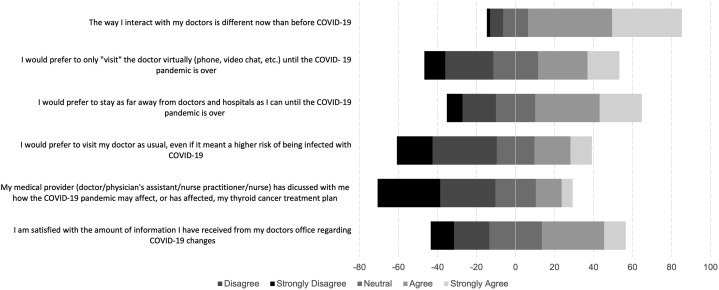
Most participants (300, 80%) reported a change in their access to health care during the pandemic. Many of these changes were reported as mild (37%: eg, appointments moved to telehealth) or moderate (39%: eg, delays or cancelations in appointments or delays in obtaining prescriptions with minimal impact on health), but 4% reported being unable to access needed care which affected their health. A total of 45 respondents (12% of the total cohort, 59% of the 76 undergoing active treatment or awaiting treatment) reported that their thyroid cancer treatment plan had changed due to COVID-19. For 22 of these respondents (49%), the start of treatment was delayed, for 2 (4%), the type of treatment had changed, and 21 (47%) reported it had changed in another way. Most respondents (79%) agreed or strongly agreed that the way they interacted with their doctors was different than before COVID-19, and Figure 2 demonstrates the respondents’ attitudes toward virtual vs in-person doctors’ visits and information regarding COVID-19 changes or effects on treatment plans. Responses were mixed regarding virtual visits (42% preferred virtual, 23% neutral), but the majority of respondents preferred staying away from health care facilities during the pandemic (55% agree/strongly agree). Notably, less than half of respondents (43%) agreed or strongly agreed that they were satisfied with the amount of information from their doctor’s office regarding COVID-19 changes, and only 19% agreed or strongly agreed that their medical provider had discussed how the pandemic may affect or had affected their thyroid cancer treatment plan.
Fig. 2.
Attitudes toward changes in health care delivery during the COVID-19 pandemic.
Effects of COVID-19 on QOL and Well-being
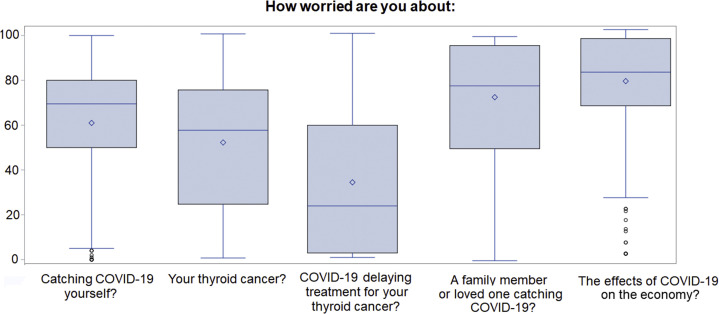
When asked to scale their worry about COVID-related items on a scale of 0-100 (Fig. 3 ), respondents scored their worry about contracting COVID-19 (mean, score 61) higher than worry about their thyroid cancer (mean, 52; P < .001) or about COVID delaying thyroid cancer treatment (mean, 34; P < .001). Respondents were also significantly more worried about a family member or loved one contracting COVID-19 (mean, 73) than they were about contracting it themselves (P < .001). Notably, the highest overall score was given for worry about the effects of COVID-19 on the economy (mean, 77).
Fig. 3.
Sources of worry during the COVID-19 pandemic. Respondents were asked, “Over the past 7 days, how worried have you been about…”
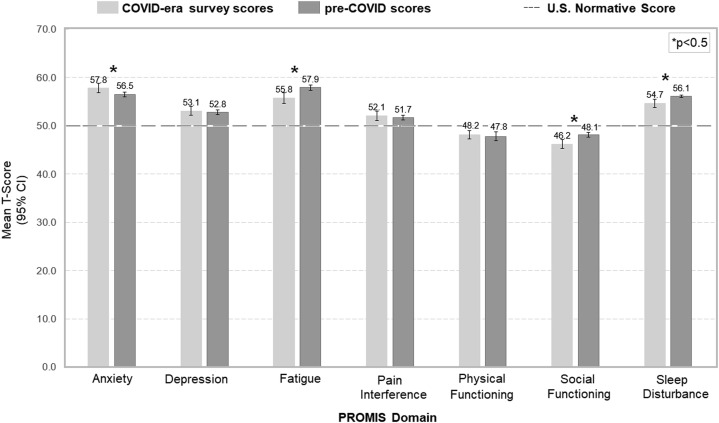
Figure 4 demonstrates the T-scores for the 7 PROMIS domains. All scores were significantly different from the normative score of 50 (P < .05 for all). Compared to previously published QOL data for thyroid cancer patients before the COVID-19 pandemic,6 T-scores were significantly higher in the domain of anxiety (57.8 ± 9.4 vs 56.5 ± 10.9; P < .05) and lower for the ability to participate in social roles and activities (46.2 ± 9.5 vs 48.1 ± 10.1; P < .01). Conversely, during the COVID-19 pandemic, T-scores were significantly lower in the domains of fatigue (55.8 ± 10.7 vs 57.9 ± 11.6; P < .01) and sleep disturbance (54.7 ± 8.2 vs 56.1 ± 4.0; P < .01). Univariable (Table 3 ) and multivariable analyses (Table 4 ) were performed to identify the factors associated with increased scores for anxiety. Upon multivariable analysis, younger age and change in treatment plan during COVID were significantly associated with increased scores for anxiety.
Fig. 4.
Patient-Reported Outcomes Measurement Information System (PROMIS) scores. P values refer to comparison between COVID-era survey scores and pre-COVID scores.
Table 3.
Univariable Analysis of Factors Associated With Increased PROMIS T-scores for Anxiety
| Variable | Unadjusted estimate |
95% CI | P value |
|---|---|---|---|
| Age (continuous or categories) | -0.26 | (-0.33, -0.20) | <.01 |
| Female sex | 4.10 | (1.03, 7.17) | .01 |
| Race (ref: White) | … | … | … |
| Black, American Indian or Alaska native, Asian, Hawaiian | -0.5 | (-3.46, 2.47) | .74 |
| or Pacific Islander, more than one race, unknown, or | |||
| would rather not report | |||
| Ethnicity (ref: not-Hispanic Latino) | |||
| Hispanic/Latino | 5.16 | (0.64-9.69) | .03 |
| Unknown/not reported | 3.53 | (-4.27, 9.62) | .45 |
| Region | … | … | … |
| Small town or rural area (<2500 people) | 1.29 | (-2.49, 2.56) | .98 |
| City (>50 000 people) | 0.99 | (-2.18, 1.73) | .82 |
| Travel distance | … | … | … |
| Over 50 miles | 1.11 | (-1.93, 2.44) | .82 |
| Over 100 miles | 1.34 | (-2.36, 2.89) | .85 |
| Education (ref: master's or professional/doctorate) | … | … | |
| Less or equal to high school or equivalent | 2.83 | (-0.85-10.26) | .10 |
| Some college or associate/bachelor's degree | 1.02 | (-1.24, 2.78) | .45 |
| Employment (ref: full-time) | … | … | … |
| Part-time | -0.71 | (-3.86, 2.44) | .66 |
| Unemployed and currently looking for work | -1.56 | (-6.82, 3.69) | .56 |
| Retired | -6.1 | (-8.35, -3.79) | <.01 |
| Othera | 2.36 | (-0.07, 4.80) | .06 |
| Insurance status (ref: insured through employer or other private insurance) | |||
| Affordable Care Act Plan | -4.25 | (-8.87, 0.38) | .07 |
| Medicaid | 4.35 | (-0.74, 9.45) | .09 |
| Medicare | -4.50 | (-7.08, -1.93) | <.01 |
| Othera | -2.82 | (5.94, 0.29) | .08 |
| Reported diagnosis or medication for anxiety or | |||
| depression | -5.43 | (-7.47, -3.38) | <.01 |
| Cancer type (ref: papillary) | … | … | … |
| Follicular | -1.85 | (-5.63, 193) | .34 |
| Medullary | -2.85 | (-5.93, 0.23) | .07 |
| Follicular | -2.10 | (-6.71, 2.51) | .37 |
| Hürthle cell | -0.26 | (-5.69, 5.18) | .93 |
| Otherb | … | … | … |
| Cancer stage (ref: I) | … | … | … |
| II | -1.41 | (-4.37, 1.55) | .35 |
| III | -2.26 | (-5.30, 0.78) | .14 |
| IV | -5.40 | (-8.40, -2.38) | <.01 |
| Unknown or have not been staged | -0.96 | (-3.74, 1.83) | .50 |
| Years since diagnosis | -0.22 | (-0.32, -0.11) | <.01 |
| Awaiting treatment (ref: finished treatment or not planning additional treatment) | |||
| Currently undergoing treatment or awaiting treatment | 2.51 | (0.02, 4.99) | .05 |
| Treatment change due to COVID (ref: no change) | … | … | |
| Change in treatment plan | 5.8 | (2.46, 9.15) | <.01 |
| Family/friend with COVID | 1.11 | (-1.16, 3.82) | .4243 |
| Someone close who died from COVID | 3.01 | (-3.80, 9.81) | .3793 |
| Satisfied with the amount of information from doctors about COVID (ref: agree or strongly agree) | … | … | … |
| Neutral | -1.18 | (-3.50, 1.15) | .32 |
| Disagree or strongly disagree | 2.28 | (0.02, 4.53) | .05 |
Ref: denotes reference group.
No insurance, Veteran’s Health Administration, Other, Unsure.
Non-invasive follicular neoplasm/anaplastic/uncertain.
Table 4.
Multivariable analysis of factors associated with increased PROMIS scores for anxiety
| Variable | Estimate | 95% CI | P value |
|---|---|---|---|
| Age (continuous or categories) | -0.35 | (-0.54, -0.16) | <.01 |
| Female sex | 2.63 | (4.06, 9.33) | .84 |
| Insurance status (ref: insured/other private) | … | … | |
| Affordable | 0.86 | (-9.52, 11.25) | .87 |
| Medicaid | 3.96 | (-6.53, 14.44) | .45 |
| Medicare | 4.05 | (-3.22, 11.31) | .27 |
| othera | 3.79 | (-2.93, 10.52) | .26 |
| Reported diagnosis or medication for anxiety or | |||
| depression | 2.08 | (-2.81, 6.97) | .40 |
| Years since diagnosis | 0.05 | (-0.21, 0.31) | .69 |
| Radioactive iodine treatment | 2.29 | (-1.56, 6.14) | .24 |
| Treatment change due to COVID (ref: no change) | … | … | |
| Change in treatment plan | 4.46 | (0.09, 8.83) | .04 |
| Satisfied with physician’s communication during COVID | … | … | … |
| Disagree or strongly disagree | -0.64 | (-5.69, 4.40) | .80 |
| Neutral | 0.11 | (-4.39, 4.60) | .96 |
No insurance, Veteran’s Health Administration, Other, Unsure.
Discussion
This study surveyed thyroid cancer survivors about the effects of COVID-19 on their lives, interactions with health care, and QOL and well-being. Previous studies have found that thyroid cancer survivors suffer from significant psychologic and emotional distress, leading to impairments in QOL.12 , 13 QOL scores among thyroid cancer survivors are similar to, or worse than, those of other cancers with more compromised survival, including colon, breast, and gynecologic cancers.6 , 7 Given the overall excellent survival rate observed for most thyroid cancers, most survivors will have many years during which they must cope with the repercussions of their diagnosis and treatment, making it all the more crucial for providers to understand these long-lasting, QOL-altering consequences. In order to assess health-related QOL among US thyroid cancer survivors, Goswami et al6 used the National Institutes of Health–sponsored PROMIS-29 validated health-related QOL instrument through an anonymous online survey in 2017.6 This study, which used a similar recruitment strategy via the Thyroid Cancer Survivor’s Association website and listserv, aimed to survey a similar population of thyroid cancer survivors during the COVID-19 pandemic. The goal was to capture COVID-19-related differences in QOL among thyroid cancer survivors in a before-and-after comparison.
Thyroid cancer survivors during the COVID-19 pandemic scored significantly higher in the domain of anxiety and lower in their ability to participate in social roles and activities than the prepandemic cohort. These differences, though statistically significant, were small and within one standard deviation of the normative mean. The clinical significance of these results is unclear, but notably, there was a similar difference from the pre-COVID score within both domains. The decrease in social roles and activities reported in the survey likely reflects widespread societal changes due to social distancing. Nearly all respondents reported changes in access to social supports, suggesting that these changes may have had a meaningful impact on survivors’ lives and well-being.
The pandemic’s effect on anxiety is more difficult to quantify. The increased anxiety reported may be a reflection of more widespread psychological distress from COVID-19 and quarantine across the general population.14, 15, 16, 17, 18 A national study of the general US population in July 2020 found that rates of depression and anxiety had more than doubled in the United States compared to prepandemic data.19 A multitude of potential causes of pandemic-related psychological distress have been suggested, including economic instability, fear of COVID infection, and media exposure.17 , 20 The state of uncertainty, a common condition during the pandemic, may have also negatively influenced mental health and QOL during the pandemic. Uncertainty has been shown to initiate a brain-body pathway linking cognitive stress to the autonomic nervous system and a physiologic stress response.21 This, in turn, has been shown to have negative effects on the clinical outcomes of cancer patients.22 In our sample, younger age was independently associated with increased anxiety—an association that has also been demonstrated in the general US population.19 , 20 While the causes of this trend remain unknown, the pandemic may have particularly affected anxiety and stress among the young due to greater economic vulnerability, increased childcare responsibilities, and/or greater reliance on social or community networks.
A change in treatment plan during COVID was also significantly associated with increased anxiety in our cohort. Our cohort had a mean of 8.3 years from diagnosis, and only 20% were undergoing or awaiting treatment. Therefore, the number of affected patients was small (N = 45) and may not be generalizable. However, the effects of COVID-19 on the management of thyroid cancer patients are wide-ranging and have been well-described.23, 24, 25 At the forefront of these changes was a rapid conversion from office visits to telemedicine in order to limit travel and potential exposure for both patients and health care providers.26 , 27 Most participants in our survey reported a change in the way they interacted with their doctors during the pandemic, and most, in fact, preferred to stay away from health care facilities. However, less than 1 in 5 respondents had a discussion with their health care provider about how the pandemic had affected or may affect their thyroid cancer treatment, and less than half were satisfied with the amount of information they had received from their doctor’s office regarding COVID-19 changes. This represents an important opportunity for improvement for providers, who should seek to enhance and promote patient communication, particularly via virtual or digital health platforms when limited by social distancing requirements.
Challenges to communication during COVID-19 almost certainly include the acuity of the pandemic and the early lack of reliable data and guidelines. Providers themselves may have been unsure how best to counsel patients early in 2020 when much remained unknown. Moreover, the telemedicine format may have contributed additional challenges. Telemedicine became a key tool for social distancing during the pandemic and was facilitated by the US Centers for Medicare & Medicaid Services’s expansion of coverage for telehealth services to Medicare beneficiaries in March 2020.28 In our survey, most participants reported that they preferred virtual visits. However, the virtual format fundamentally changes interpersonal communication. In a survey of endocrinologists during COVID-19, providers reported difficulty connecting with patients on an emotional level via telemedicine.26 This connection is essential, as doctor-patient communication may be one of the best tools to alleviate anxiety and fear among our patients. Studies before the COVID-19 pandemic have highlighted the importance of attentive and empathetic doctor-patient communication in reducing emotional distress in cancer patients,29 and during the pandemic, clear patient-centered communication was found to buffer the adverse psychological effects of the fear of COVID-19 among cancer patients.30 As telemedicine has quickly become a fundamental part of the practice of medicine, it is crucial that providers work toward improving digital health systems and facilitating communication.
In this survey, thyroid cancer survivors reported decreased sleep disturbance and decreased fatigue compared to pre-COVID-19 QOL data. Again, these differences were small, and their clinical significance is unclear, but this finding was surprising, particularly in the setting of exacerbated anxiety, as poor sleep quality is closely linked to depression and anxiety.31, 32, 33 Fatigue is common among thyroid cancer survivors and persists long after the diagnosis and initial treatment.34 One explanation for the lasting effects is “cancer-related fatigue,” which is defined by the National Comprehensive Cancer Network as a “distressing, persistent, subjective sense of physical, emotional, and/or cognitive tiredness or exhaustion related to cancer or cancer treatment that is not proportional to recent activity and interferes with usual functioning.”35 The cause remains unknown, but hypotheses include dysregulation of serotonin, cytokines, hypothalamic-pituitary-adrenal axis, muscle metabolism/ATP, or circadian rhythm disruption.36 Current literature regarding the effect of COVID-19 on sleep suggests divergent findings. Some studies have reported a high prevalence of sleep problems during the pandemic, while others point to possible benefits on sleep patterns resulting from lockdown and the absence of typical work or school schedules.37 , 38 Pandemic-related changes to sleep patterns or lifestyle may affect cancer-related fatigue and could be an important focus of further research.
This study has several limitations. All data were self-reported and anonymous, and there was no mechanism to prevent the same participant from completing multiple surveys. As respondents were recruited from the ThyCa website and email listserv, all respondents had access to the internet and were either already involved in or seeking information or support from ThyCa. This suggests a high level of engagement in their thyroid cancer care and may suggest a higher socioeconomic status and/or education level, which introduced selection bias into our cohort. Our low response rate may have been a reflection of our lack of targeted recruitment: the link was posted on the ThyCa website and was included once in a regular newsletter; no additional recruitment strategies were employed. However, our estimated response rate may be an underestimate, as the website is freely accessible and web traffic may not accurately reflect potential participants. Additionally, site visitors seeking specific information (eg, support group information or the low iodine cookbook) may not have visited the main homepage where the survey was linked.
There were significant differences in the characteristics of our cohort when compared with national data (Table 2), including older age, higher female and White predominance, less papillary and more medullary thyroid cancer, higher stage, and a higher proportion of those undergoing radioactive iodine ablation. Therefore, our sample may not accurately reflect the views of all thyroid cancer survivors and may overrepresent later-stage patients or those with more aggressive disease when compared to national data. This cohort did have more similar demographic and clinical characteristics to the pre-COVID-19 study than to national data, but there were statistically significant differences in age, race, cancer type, cancer stage, and the proportion of people undergoing radioactive iodine therapy, which may have affected our QOL data. Due to survey anonymity, there is no way to know whether the cohort captured by this survey overlaps with the pre-COVID-19 study’s cohort, and therefore differences in answers may not reflect predominately pandemic-related changes.
The effects of COVID-19 on thyroid cancer survivors are only beginning to be elucidated, and it may be years before we understand the wide-reaching and long-term ramifications. Mental health and psychological distress are important components of QOL, and providers must recognize stressors for patients and establish frameworks for support. While vaccination numbers continue to rise in the United States, uncertainty remains regarding new strains and emerging variants, and the pandemic continues to affect our patients’ lives and interactions with health care.
Conclusions
The findings of this study contribute to a better understanding of thyroid cancer survivors’ experience during COVID-19 and their sources of worry and psychological stress. Compared to prepandemic data, survey respondents had statistically higher anxiety and decreased ability to participate in social roles and activities. Respondents reported the highest worry scores about the effects of COVID on the economy and about family or loved ones becoming infected. Despite changes in their lives and interactions with health care providers, most respondents had not had conversations with their medical providers about how COVID-19 may affect their treatment, and less than half were satisfied with the information they received from their doctors. This highlights an important opportunity for providers to improve communication during times of crisis in order to reduce uncertainty and alleviate anxiety for patients.
Acknowledgment
We would like to thank ThyCa: Thyroid Cancer Survivors’ Association, Inc for their assistance with survey development and participant recruitment.
Disclosure
Drs Graves, Goyal, Levin, Nuño, Kim, Campbell, Shen, Gosnell, Roman, and Duh have no multiplicity of interest to disclose. Dr Sosa is a member of the Data Monitoring Committee of the Medullary Thyroid Cancer Consortium Registry supported by GlaxoSmithKline, Novo Nordisk, Astra Zeneca, and Eli Lilly. Industry research support was received from Exelixis and Eli Lilly. Dr Suh is a consultant for Prescient Surgical, Medtronic, and Iota Biosciences.
Supplementary Material
References
- 1.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Pandemic Impact on Cancer Patients and Survivors: Survey Findings Summary. American Cancer Society. https://www.fightcancer.org/sites/default/files/National%20Documents/Survivor%20Views.COVID19%20Polling%20Memo.Final_.pdf
- 3.American Cancer Society | Cancer Facts & Statistics American Cancer Society | Cancer Facts & Statistics. http://cancerstatisticscenter.cancer.org/
- 4.Lim H., Devesa S.S., Sosa J.A., Check D., Kitahara C.M. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. doi: 10.1001/jama.2017.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschebrook-Kilfoy B., James B., Nagar S., et al. Risk factors for decreased quality of life in thyroid cancer survivors: initial findings from the North American Thyroid Cancer Survivorship Study. Thyroid. 2015;25(12):1313–1321. doi: 10.1089/thy.2015.0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goswami S., Mongelli M., Peipert B.J., Helenowski I., Yount S.E., Sturgeon C. Benchmarking health-related quality of life in thyroid cancer versus other cancers and United States normative data. Surgery. 2018;164(5):986–992. doi: 10.1016/j.surg.2018.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Applewhite M.K., James B.C., Kaplan S.P., et al. Quality of life in thyroid cancer is similar to that of other cancers with worse survival. World J Surg. 2016;40(3):551–561. doi: 10.1007/s00268-015-3300-5. [DOI] [PubMed] [Google Scholar]
- 8.Cella D., Riley W., Stone A., et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179–1194. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cook K.F., Jensen S.E., Schalet B.D., et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102. doi: 10.1016/j.jclinepi.2015.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research Electronic Data Capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Orosco R.K., Hussain T., Noel J.E., et al. Radioactive iodine in differentiated thyroid cancer: a national database perspective. Endocr Relat Cancer. 2019;26(10):795–802. doi: 10.1530/ERC-19-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tagay S., Herpertz S., Langkafel M., et al. Health-related Quality of Life, depression and anxiety in thyroid cancer patients. Qual Life Res. 2006;15(4):695–703. doi: 10.1007/s11136-005-3689-7. [DOI] [PubMed] [Google Scholar]
- 13.Husson O., Haak H.R., Buffart L.M., et al. Health-related quality of life and disease specific symptoms in long-term thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol. 2013;52(2):249–258. doi: 10.3109/0284186X.2012.741326. [DOI] [PubMed] [Google Scholar]
- 14.Holmes E.A., O’Connor R.C., Perry V.H., et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–560. doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lim X.Y., Yap A.C., Mahendran R., Yu J. The interplay between anxiety, fear, protective behaviors, compassion, and resilience among older adults during a COVID-19 lockdown: a structural equation modeling study. Transl Behav Med. 2021;11(5):1172–1178. doi: 10.1093/tbm/ibaa143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernández R.S., Crivelli L., Guimet N.M., Allegri R.F., Pedreira M.E. Psychological distress associated with COVID-19 quarantine: Latent profile analysis, outcome prediction and mediation analysis. J Affect Disord. 2020;277:75–84. doi: 10.1016/j.jad.2020.07.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher M.W., Zvolensky M.J., Long L.J., Rogers A.H., Garey L. The Impact of COVID-19 experiences and associated stress on anxiety, depression, and functional impairment in American adults. Cognit Ther Res. 2020:1–9. doi: 10.1007/s10608-020-10143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fitzpatrick K.M., Drawve G., Harris C. Facing new fears during the COVID-19 pandemic: the State of America’s mental health. J Anxiety Disord. 2020;75:102291. doi: 10.1016/j.janxdis.2020.102291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khubchandani J., Sharma S., Webb F.J., Wiblishauser M.J., Bowman S.L. Post-lockdown depression and anxiety in the USA during the COVID-19 pandemic. J Public Health (Oxf) 2021;43(2):246–253. doi: 10.1093/pubmed/fdaa250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holingue C., Badillo-Goicoechea E., Riehm K.E., et al. Mental distress during the COVID-19 pandemic among US adults without a pre-existing mental health condition: Findings from American trend panel survey. Prev Med. 2020;139:106231. doi: 10.1016/j.ypmed.2020.106231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peters A., McEwen B.S., Friston K. Uncertainty and stress: Why it causes diseases and how it is mastered by the brain. Prog Neurobiol. 2017;156:164–188. doi: 10.1016/j.pneurobio.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Lutgendorf S.K., Sood A.K., Antoni M.H. Host factors and cancer progression: biobehavioral signaling pathways and interventions. J Clin Oncol. 2010;28(26):4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang V.H.M., Gild M., Glover A., Clifton-Bligh R., Robinson B.G. Thyroid cancer in the age of COVID-19. Endocr Relat Cancer. 2020;27(11):R407–R416. doi: 10.1530/ERC-20-0279. [DOI] [PubMed] [Google Scholar]
- 24.Smulever A., Abelleira E., Bueno F., Pitoia F. Thyroid cancer in the Era of COVID-19. Endocrine. 2020;70(1):1–5. doi: 10.1007/s12020-020-02439-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Medas F., Ansaldo G.L., Avenia N., et al. Impact of the COVID-19 pandemic on surgery for thyroid cancer in Italy: nationwide retrospective study. Br J Surg. 2021;108(4):e166–e167. doi: 10.1093/bjs/znab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chablani S.V., Sabra M.M. Thyroid cancer and telemedicine during the COVID-19 pandemic. J Endocr Soc. 2021;5(6):bvab059. doi: 10.1210/jendso/bvab059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Klain M., Nappi C., Maurea S., et al. Management of differentiated thyroid cancer through nuclear medicine facilities during Covid-19 emergency: the telemedicine challenge. Eur J Nucl Med Mol Imaging. 2021;48(3):831–836. doi: 10.1007/s00259-020-05041-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Royce T.J., Sanoff H.K., Rewari A. Telemedicine for cancer care in the time of COVID-19. JAMA Oncol. 2020;6(11):1698–1699. doi: 10.1001/jamaoncol.2020.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zachariae R., Pedersen C.G., Jensen A.B., Ehrnrooth E., Rossen P.B., von der Maase H. Association of perceived physician communication style with patient satisfaction, distress, cancer-related self-efficacy, and perceived control over the disease. Br J Cancer. 2003;88(5):658–665. doi: 10.1038/sj.bjc.6600798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rossi A.A., Marconi M., Taccini F., Verusio C., Mannarini S. From fear to hopelessness: the buffering effect of patient-centered communication in a sample of oncological patients during COVID-19. Behav Sci (Basel) 2021;11(6):87. doi: 10.3390/bs11060087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Casagrande M., Favieri F., Tambelli R., Forte G. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12–20. doi: 10.1016/j.sleep.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rajkumar R.P. Sleep, physical activity and mental health during the COVID-19 pandemic: complexities and opportunities for intervention. Sleep Med. 2021;77:307–308. doi: 10.1016/j.sleep.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rezaei M., Khormali M., Akbarpour S., Sadeghniiat-Hagighi K., Shamsipour M. Sleep quality and its association with psychological distress and sleep hygiene: a cross-sectional study among pre-clinical medical students. Sleep Sci. 2018;11(4):274–280. doi: 10.5935/1984-0063.20180043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Husson O., Nieuwlaat W.A., Oranje W.A., Haak H.R., van de Poll-Franse L.V., Mols F. Fatigue among short- and long-term thyroid cancer survivors: results from the population-based PROFILES registry. Thyroid. 2013;23(10):1247–1255. doi: 10.1089/thy.2013.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mock V., Atkinson A., Barsevick A., et al. NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park) 2000;14(11A):151–161. [PubMed] [Google Scholar]
- 36.Ebede C.C., Jang Y., Escalante C.P. Cancer-related fatigue in cancer survivorship. Med Clin North Am. 2017;101(6):1085–1097. doi: 10.1016/j.mcna.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Jahrami H., BaHammam A.S., Bragazzi N.L., Saif Z., Faris M., Vitiello M.V. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi: 10.5664/jcsm.8930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alfonsi V., Gorgoni M., Scarpelli S., et al. COVID-19 lockdown and poor sleep quality: Not the whole story. J Sleep Res. 2021;30(5):e13368. doi: 10.1111/jsr.13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.