Abstract
Purpose
We aimed to examine the association between plasma trimethylamine N-oxide (TMAO), a gut microbial metabolite from dietary phosphatidylcholine, and coronary atherosclerotic burden in patients with type 2 diabetes (T2D).
Methods
In total, 349 patients with T2D were studied, including 70 controls and 279 patients with coronary artery disease (CAD) by coronary angiography. Coronary atherosclerotic burden is quantified by the number of diseased coronary branches and SYNTAX (Synergy between PCI with Taxus and Cardiac Surgery) score. Plasma TMAO levels were determined by UHPLC–MS/MS technique.
Results
The TMAO concentration was significantly higher in the patients with triple vessel disease (TVD) (3.33 [IQR: 1.81–6.65] μM) than those without TVD (2.62 [IQR: 1.50–4.73] μM) (P = 0.015). A similar difference was found between patients with SYNTAX score >22 (3.93 [IQR: 1.81–6.82] μM) and those with SYNTAX score ≤22 (2.54 [IQR: 1.44–4.54] μM) (P = 0.014). TMAO was not significantly correlated with the presence of CAD. Among patients with eGFR <60 mL/min/1.73 m2, the highest tertile of TMAO was significantly associated with TVD (OR = 25.28, 95% CI [2.55–250.33], P = 0.006) and SYNTAX score >22 (OR = 7.23, 95% CI [1.51–34.64], P = 0.013) independent of known risk factors of CAD, compared with lower TMAO tertiles.
Conclusion
TMAO was not independently correlated with the presence of CAD and severity of coronary atherosclerosis in the included population. Nevertheless, the significant association between circulating TMAO and higher coronary atherosclerotic burden was observed in patients with eGFR of lower than 60 mL/min/1.73 m2.
Keywords: trimethylamine N-oxide, coronary artery disease, SYNTAX score, type 2 diabetes
Introduction
With the rising incidence rate of type 2 diabetes (T2D), a series of diabetic complications especially those associated with the cardiovascular system have captured sufficient attention from researchers.1 Despite considerable concerns about traditional risk factors of coronary artery disease (CAD) and modern pharmacotherapy, CAD-associated morbidity and mortality is still approximately 30%.2 Therefore, identifying CAD-T2D novel risk markers can prospectively contribute to the efficient treatment and effective prevention of cardiovascular complication of T2D.
Plentiful studies in both animal models and humans have indicated that the gut microbiota plays an important role in overall metabolism of the host and is associated with cardiometabolic diseases such as T2D.3–8 Notably, trimethylamine N-oxide (TMAO), as a gut microbial metabolite from choline, betaine or L-carnitine considered as the most common phosphatidylcholine, formed in the liver from trimethylamine (TMA), has been found to exert a proatherogenic effect and be relevant to diabetes9 and cardiovascular risk.3,10–12
According to recent studies, plasma TMAO was an independent predictive marker of higher atherosclerosis burden in patients with either ST-segment elevation myocardial infarction (STEMI)13 or stable CAD,14 in which the majority of participants were free from diabetes. So far, the association between TMAO levels and quantified coronary atherosclerotic burden has not been investigated in diabetic patients. In addition, TMAO is cleared by the kidney and linked to renal dysfunction.15,16 Diabetic kidney disease (DKD), one of the most common microvascular complications of diabetes, is the major cause of impaired kidney function in diabetic subjects,17 and may lead to the elevated circulating TMAO. There is no study concentrated on the atherosclerogenetic effect of TMAO in diabetic patients with kidney dysfunction up to the time of writing.
The primary aim of our study is to explore the relationship between plasma TMAO levels and the coronary atherosclerotic burden quantified by the number of diseased major branches of coronary and the SYNTAX score in the patients with T2D. The second objective is to investigate the differences of the above relationship between patients with different renal function levels.
Methods
Participants
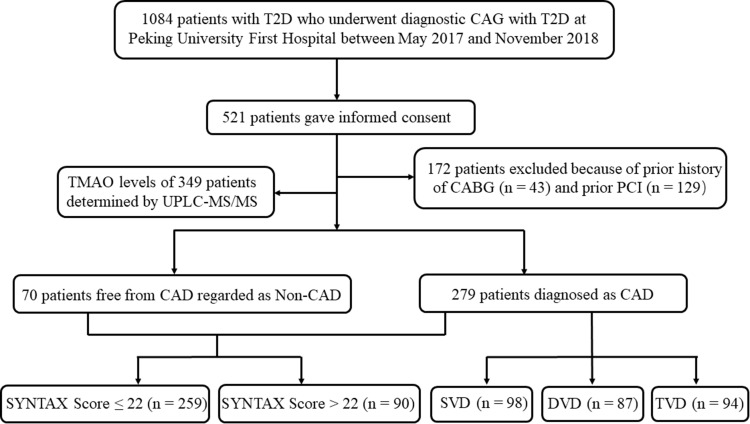
Out of the overall 1084 Chinese patients with T2D who underwent diagnostic coronary artery angiography (CAG) for chest pain, precordial discomfort or positive non-invasive test results (i.e., electrocardiogram suggestive of ischemia, suspicious myocardial perfusion scan, or positive exercise tolerance test) at Peking University First Hospital between May 2017 and November 2018, 521 patients gave informed consent. Afterwards, 172 individuals were excluded for prior history of percutaneous transluminal coronary intervention (PCI) or coronary artery bypass grafting (CABG). A final total of 349 patients with T2D were studied, including 279 cases with CAD defined by diameter stenosis at ≥50% in at least one main branch of coronary (including left anterior descending, left circumflex and right coronary artery), and 70 patients with all main coronary arteries stenosis <50% as Non-CAD (Figure 1).
Figure 1.
Flowchart of coronary atherosclerosis screening and quantification in T2D patients.
Abbreviations: T2D, type 2 diabetes; CAG, coronary arteriography; CABG, coronary artery bypass grafting; PCI, percutaneous transluminal coronary intervention; CAD, coronary artery disease; SVD, single-vessel disease; DVD, dual-vessel disease; TVD, triple-vessel disease.
All the patients were definitively diagnosed as T2D according to Guidelines for the Prevention and Control of Type 2 Diabetes in China (2017 Edition), and received hypoglycemic therapy including insulin and oral drugs under the guidance of doctors.
Ethical Approval of the Study and the Informed Consent
The protocols and the procedures of the study for collecting or handling human blood samples were approved by the Ethics Committee of Peking University First Hospital (No. 2020–103) and registered on Chinese Clinical Trial Registry (No. ChiCTR2000031108). All the included patients gave informed consent. The whole process and all methods of the study were conducted in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans.
Collection and Handling of Human Blood Samples
Peripheral blood samples were collected into vacutainer tubes containing ethylenediaminetetraacetic acid (EDTA) via femoral or radial access at the time of CAG before heparinization and then immediately frozen at −80°C until determination of TMAO.
Determination of Plasma TMAO Levels by LC-MS/MS
Liquid chromatography of Waters I-Class (Waters, Milford, MA, USA) coupled to Waters Xevo TQ-S (Waters, Milford, MA, USA) mass spectrometer with an electrospray ionization (ESI) source in positive ion mode was used to quantify TMAO using multiple reaction monitoring (MRM).
Assessment of Coronary Atherosclerotic Burden
The SYNTAX (SYNergy between percutaneous coronary intervention with TAXus and cardiac surgery) score, an angiographic scoring system, produced from the SYNTAX trial, is generally used to determine the burden and complexity of atherosclerosis in CAD patients.18 The SYNTAX score of each patient was separately graded by the same two interventional cardiologists with extensive experience, blinded to the plasma TMAO levels and any clinical data of patients, operating the SYNTAX score calculator version 2.28 provided from the official website (www.syntaxscore.com/calculator/syntaxscore/frameset.htm). If there was any discrepancy between the readings from the two cardiologists, then the final result would come after the third senior interventional cardiologist checked them. As documented, higher atherosclerosis burden can be identified by the presence of higher SYNTAX score over 22,19,20 then the subjects were divided into two groups according to the SYNTAX trial-defined low (0–22) or intermediate-high (>22) SYNTAX scores.
The diseased vessel was defined as any of the major coronary arteries (including the left anterior descending, the left circumflex branch and the right coronary artery) confirmed as ≥50% stenosis under diagnostic CAG. Subsequently, the patients were also divided based on the number of diseased vessels, into single-vessel disease (SVD), dual-vessel disease (DVD) and triple-vessel disease (TVD). According to previous research, TVD was considered as severe coronary artery disease.21,22 Except for TVD group, the Non-TVD group was comprised of individuals with 0–2 coronary branches affected in this study.
Statistical Analysis
The continuous variables were described as mean ± SD (standard deviation) or medians (interquartile ranges [IQR]) if necessary. The comparison among three groups of different tertiles of TMAO in continuous variables were performed with analysis of variance (ANOVA), and those in categorical variables were analyzed with chi-squared test. The differences between groups with different severity of coronary atherosclerosis (including CAD vs Non-CAD, TVD vs Non-TVD, SYNTAX score ≤22 vs SYNTAX score >22) in TMAO concentrations were conducted using non-parametric test. Univariate and multivariate logistic regression analysis were also used to assess the association between plasma TMAO levels and coronary atherosclerotic burden of patients. Stratified and interaction analyses were conducted according to different eGFR levels (eGFR <60 mL/min/1.73 m2 vs eGFR ≥60 mL/min/1.73 m2). The Empower Stats (www.empowerstats.com, X&Y Solutions, Inc., Boston MA) and R software version 3.6.1 (http://www.R-project.org) were applied in all statistical analysis. Significant statistical difference was accepted at two-tailed P <0.05 in all the above analyses.
Results
Clinical and Laboratory Characteristics of the Participants
As shown in Figure 1, 349 participants were included after strict screening and comprised subgroups with two different grouping methods: the CAD group (n = 279) and Non-CAD group (n = 70); the group with SYNTAX score ≤22 (n = 259) and the group with SYNTAX score >22 (n = 90). Among CAD patients, there were 98 with SVD, 87 with DVD and 94 with TVD.
The included patients were also divided into three subgroups based on tertiles of TMAO levels. Among these three groups with different TMAO levels, there were no significantly statistical differences found in age, sex, BMI, active smoking, history of hyperlipidemia, fasting blood glucose (FBG), hemoglobin A1c (HbA1c), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C) and triglyceride (TG) (P >0.05), however, as expected, significant differences in history of hypertension, diabetes duration, the levels of LDL-C and estimated glomerular filtration rate (eGFR), and the proportion of patients with TVD or SYNTAX score >22 (P <0.05) (Table 1).
Table 1.
Baseline Characteristics of the Study Participants Associated with Plasma Levels of TMAO (Distribution in Tertiles)
| Variables | All n = 349 | Tertiles of TMAO | P-value | ||
|---|---|---|---|---|---|
| Tertile 1 | Tertile 2 | Tertile 3 | |||
| (TMAO <1.96μM) | (1.96 ≤TMAO <4.38μM) | (TMAO ≥4.38μM) | |||
| n = 115 | n = 117 | n = 117 | |||
| Age (y) | 65.3 ± 10.4 | 64.7 ± 11.6 | 65.5 ± 9.4 | 65.6 ± 10.2 | 0.801 |
| Sex, male (n) | 220 (63.0%) | 68 (59.1%) | 76 (65.0%) | 76 (65.0%) | 0.570 |
| BMI (kg/m2) | 26.1 ± 3.6 | 25.8 ± 3.5 | 26.2 ± 3.6 | 26.4 ± 3.7 | 0.320 |
| Active smoking (n) | 169 (48.4%) | 55 (47.8%) | 57 (48.7%) | 57 (48.7%) | 0.988 |
| Hypertension (n) | 256 (73.6%) | 79 (69.3%) | 79 (67.5%) | 98 (83.8%) | 0.009 |
| Hyperlipidemia (n) | 265 (76.1%) | 91 (79.8%) | 85 (72.6%) | 89 (76.1%) | 0.441 |
| Diabetes duration (y) | 10.4 ± 8.9 | 8.6 ± 8.3 | 10.9 ± 9.3 | 11.6 ± 8.8 | 0.018 |
| FBG (mmol/L) | 8.64 ± 4.81 | 8.78 ± 6.73 | 8.66 ± 3.42 | 8.49 ± 3.71 | 0.654 |
| HbA1c (%) | 7.5 ± 1.4 | 7.4 ± 1.4 | 7.6 ± 1.4 | 7.6 ± 1.4 | 0.125 |
| TC (mmol/L) | 3.92 ± 1.07 | 3.99 ± 1.07 | 3.95 ± 1.16 | 3.84 ± 0.98 | 0.771 |
| LDL-C (mmol/L) | 2.24 ± 0.82 | 2.26 ± 0.84 | 2.27 ± 0.90 | 2.18 ± 0.73 | 0.924 |
| HDL-C (mmol/L) | 0.97 ± 0.25 | 1.04 ± 0.29 | 0.97 ± 0.21 | 0.91 ± 0.21 | <0.001 |
| TG (mmol/L) | 1.87 ± 1.32 | 1.83 ± 1.53 | 1.90 ± 1.15 | 1.88 ± 1.27 | 0.528 |
| eGFR (mL/min/1.73 m2) | 74.44 ± 21.65 | 81.36 ± 16.34 | 79.32 ± 15.58 | 62.80 ± 26.31 | <0.001 |
| TMAO (μM) | 2.83 [1.65–5.39] | 1.22 [0.60–1.64] | 2.83 [2.31–3.48] | 6.93 [5.39–9.31] | <0.001 |
| CAD (n) | 279 (79.9%) | 89 (77.4%) | 99 (84.6%) | 91 (77.8%) | 0.301 |
| TVD (n) | 94 (26.9%) | 25 (21.7%) | 28 (23.9%) | 41 (35.0%) | 0.049 |
| SYNTAX score >22 (n) | 90 (25.8%) | 23 (20.0%) | 24 (20.5%) | 43 (36.8%) | 0.004 |
Note: Continuous data are presented as means ± SD or medians (interquartile ranges), and categorical variables are presented as counts (%).
Abbreviations: BMI, body mass index; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; TC, total cholesterol; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; eGFR, estimated glomerular filtration rate; TMAO, trimethylamine N-Oxide; CAD, coronary artery disease; TVD, triple vessel disease.
Plasma Levels of TMAO in Different Subgroups
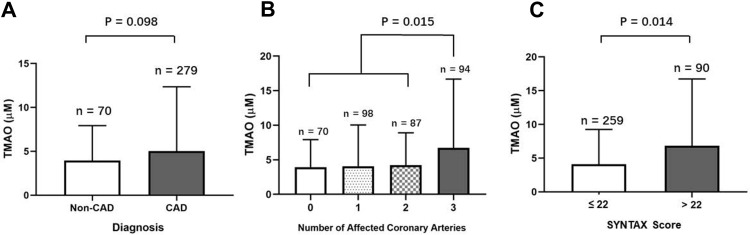
A numerical increase of TMAO concentration was observed in CAD patients (2.82 [IQR: 1.65–5.09] μM) compared with Non-CAD participants (2.32 [IQR: 1.49–5.50] μM) (P = 0.098) (Figure 2A). The plasma TMAO levels were significantly higher in the patients with triple coronary branches disease (3.33 [IQR: 1.81–6.65] μM) compared with those with 0–2 affected coronary branches (2.62 [IQR: 1.50–4.73] μM, P = 0.015) (Figure 2B). A similar difference was found between patients with SYNTAX score >22 (3.93 [IQR: 1.81–6.82] μM) and those with SYNTAX score ≤22 (2.54 [IQR: 1.44–4.54] μM) (P = 0.014) (Figure 2C).
Figure 2.
Plasma levels of TMAO in different subgroups divided by the presence of CAD (A), the number of diseased coronary arteries (B) and the SYNTAX score (C).
Abbreviation: CAD, coronary artery disease.
Evaluation of TMAO as an Associated Factor of Severe Coronary Atherosclerosis
As presented in Table 2, the association between TMAO (as continuous variable or categorical variable in tertiles) and CAD was not significant in Non-adjusted Model and adjusted models (P >0.05). By comparison, the continuous TMAO levels were significantly associated with TVD and SYNTAX score >22 in both Non-adjusted Model (for TVD, OR = 1.05, 95% CI [1.02–1.09], P = 0.004; for SYNTAX score >22, OR = 1.05, 95% CI [1.02–1.09], P = 0.003) and Model I adjusted for age and sex (for TVD, OR = 1.05, 95% CI [1.02–1.09], P = 0.005; for SYNTAX score >22, OR = 1.05, 95% CI [1.02–1.09], P = 0.005). The patients in the tertile with highest TMAO concentration appeared to have an increased risk of TVD and SYNTAX score >22 in comparison with the lowest tertile in Non-adjusted Model (for TVD, OR = 1.94, 95% CI [1.08–3.48], P = 0.026; for SYNTAX score >22, OR = 2.32, 95% CI [1.29–4.20], P = 0.005) and Model I (for TVD, OR = 1.89, 95% CI [1.05–3.40], P = 0.035; for SYNTAX score >22, OR = 2.28, 95% CI [1.25–4.16], P = 0.007). However, the significant association between TMAO levels and severe coronary lesion (including TVD and SYNTAX score >22) in Non-adjusted Model, became insignificant in Model II adjusted for log-eGFR. The OR of TVD and SYNTAX score >22 in Model II with log-eGFR adjusted were 1.01 (95% CI [0.97–1.06], P = 0.506) and 1.01 (95% CI [0.97–1.05], P = 0.526), respectively, taking TMAO concentration as continuous variable. Moreover, the OR of TVD and SYNTAX score >22 in Model II with log-eGFR adjusted were 1.23 ([95% CI 0.65–2.33], P = 0.527) and 1.52 ([95% CI 0.80–2.90], P = 0.201), respectively, in the individuals with highest tertile of TMAO compared with those in lowest tertile of TMAO. The full adjustment for all known risk factors of coronary atherosclerosis (including log-eGFR, age, sex, BMI, active smoking, hypertension, diabetes duration, FBG, HbA1c, LDL-C, HDL-C and TG) in Model III, which covered Non-adjusted Model and Model I–II, exerted negative impact on the crude association between plasma TMAO levels and severe coronary lesion (P >0.05) (Table 2).
Table 2.
Association Between Plasma TMAO Levels and Severity of Coronary Atherosclerosis in Multiple Regression Model
| Outcome | Non-Adjusted Model | Model I | Model II | Model III | |||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | OR (95% CI) | P-value | ||
| CAD | Continuous TMAO (μM) | 1.03 (0.98, 1.09) | 0.247 | 1.03 (0.96, 1.08) | 0.294 | 1.01 (0.98, 1.08) | 0.785 | 1.02 (0.95, 1.09) | 0.577 |
| Tertiles of TMAO | |||||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| T2 | 1.61 (0.83, 3.13) | 0.163 | 1.53 (0.78, 3.01) | 0.220 | 1.54 (0.79, 3.00) | 0.210 | 1.29 (0.57, 2.91) | 0.536 | |
| T3 | 1.02 (0.55, 1.90) | 0.944 | 0.95 (0.50, 1.78) | 0.869 | 0.75 (0.39, 1.46) | 0.399 | 0.69 (0.29, 1.62) | 0.395 | |
| TVD | Continuous TMAO (μM) | 1.05 (1.02, 1.09) | 0.004 | 1.05 (1.02, 1.09) | 0.005 | 1.01 (0.97, 1.06) | 0.506 | 1.01 (0.96, 1.07) | 0.605 |
| Tertiles of TMAO | |||||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| T2 | 1.13 (0.61, 2.09) | 0.691 | 1.09 (0.59, 2.03) | 0.783 | 1.10 (0.59, 2.05) | 0.769 | 0.61 (0.29, 1.30) | 0.234 | |
| T3 | 1.94 (1.08, 3.48) | 0.026 | 1.89 (1.05, 3.40) | 0.035 | 1.23 (0.65, 2.33) | 0.527 | 0.91 (0.41, 2.04) | 0.827 | |
| SYNTAX score >22 | Continuous TMAO (μM) | 1.05 (1.02, 1.09) | 0.003 | 1.05 (1.02, 1.09) | 0.005 | 1.01 (0.97, 1.05) | 0.526 | 1.00 (0.95, 1.06) | 0.893 |
| Tertiles of TMAO | |||||||||
| T1 | 1.00 | 1.00 | 1.00 | 1.00 | |||||
| T2 | 1.03 (0.54, 1.96) | 0.923 | 0.99 (0.52, 1.90) | 0.985 | 1.00 (0.52, 1.91) | 0.999 | 1.03 (0.46, 2.31) | 0.936 | |
| T3 | 2.32 (1.29, 4.20) | 0.005 | 2.28 (1.25, 4.16) | 0.007 | 1.52 (0.80, 2.90) | 0.201 | 1.56 (0.67, 3.64) | 0.298 | |
Notes: Model I: Adjusted for age and sex. Model II: Adjusted for log-eGFR. Model III: Adjusted for all of these variables, including log-eGFR, age, sex, BMI, active smoking, hypertension, diabetes duration, FBG, HbA1c, LDL-C, HDL-C and TG.
Stratified Analysis and Test for Interaction
Since eGFR led to an evident impact on the crude association between TMAO concentration and severity of coronary lesion, the stratified analysis was performed according to different eGFR levels. Patients with lowest and medium tertiles of TMAO levels (T1-2) were used as reference (OR = 1) during the analyses. Among patients with eGFR <60 mL/min/1.73 m2, highest tertile of TMAO was statistically associated with TVD (OR = 25.28, 95% CI [2.55–250.33], P = 0.006) and SYNTAX score >22 (OR = 7.23, 95% CI [1.51–34.64], P = 0.013), even after adjustment for all of these variables, including age, sex, BMI, hypertension, active smoking, diabetes duration, FBG, HbA1c, LDL-C, HDL-C and TG. Nevertheless, there is no significant association between highest tertile of TMAO and serious coronary lesion in patients with eGFR ≥60 mL/min/1.73 m2 in Non-adjusted Model or adjusted models (for TVD, ORN-adj = 0.78, 95% CI [0.39–1.56)], P = 0.489; ORadj = 0.75, 95% CI [0.32–1.78], P = 0.517. For SYNTAX score >22, ORN-adj = 1.37, 95% CI [0.71–2.64], P = 0.341; ORadj = 1.23, 95% CI [0.52–2.90], P = 0.635) (Table 3). Significant interactive effects of eGFR and TMAO concentration on TVD (P for interaction <0.001) and SYNTAX score >22 (P for interaction = 0.040) were observed (Table 3).
Table 3.
Association Between Plasma TMAO and Severity of Coronary Atherosclerosis in Patients with Different eGFR Levels
| Non-Adjusted Model | Adjusted Model | P for Interaction | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |||
| TVD | ||||||
| eGFR <60 mL/min/1.73 m2 (n = 80) | <0.001 | |||||
| Tertiles of TMAO | T1-2 | 1.00 | 1.00 | |||
| T3 | 4.12 (1.59, 10.64) | 0.004 | 25.28 (2.55, 250.33) | 0.006 | ||
| eGFR ≥60 mL/min/1.73 m2 (n = 269) | ||||||
| Tertiles of TMAO | T1-2 | 1.00 | 1.00 | |||
| T3 | 0.78 (0.39, 1.56) | 0.489 | 0.75 (0.32, 1.78) | 0.517 | ||
| SYNTAX score >22 | ||||||
| eGFR <60 mL/min/1.73 m2 (n = 80) | 0.040 | |||||
| Tertiles of TMAO | T1-2 | 1.00 | 1.00 | |||
| T3 | 2.99 (1.18, 7.54) | 0.021 | 7.23 (1.51, 34.64) | 0.013 | ||
| eGFR ≥60 mL/min/1.73 m2 (n = 269) | ||||||
| Tertiles of TMAO | T1-2 | 1.00 | 1.00 | |||
| T3 | 1.37 (0.71, 2.64) | 0.341 | 1.23 (0.52, 2.90) | 0.635 | ||
Note: The adjusted model was adjusted for all of these variables, including age, sex, BMI, hypertension, active smoking, diabetes duration, FBG, HbA1c, LDL-C, HDL-C and TG.
Discussion
In the present study, circulating TMAO levels was not independently associated with the presence of CAD or heavier coronary atherosclerotic burden in T2D patients among a Chinese North population. Nevertheless, we found a significant association between elevated TMAO levels and TVD or SYNTAX score >22 in those T2D patients with eGFR<60 mL/min/1.73 m2, even following the adjustment for traditional risk factors of CAD. The positive correlation between peripheral TMAO level and severity of coronary lesion has been reported in the general population previously,14,23 but our study specifically explored this relationship in T2D patients. Furthermore, for the first time in individuals with T2D, it also has extended to the importance of renal function.
Existing studies have revealed T2D patients present with aberrant gut microbiota, the disruption of gut barrier normal function and an enhancement in gut permeability, which would collectively lead to abnormal production and absorption of TMAO.24–26 The elevated levels of TMAO induced by T2D could accelerate the presence and progression of CAD by promoting foam cell formation, altering cholesterol and bile acid metabolism and activating inflammatory pathways.3,27,28 A small-sized clinical observation study showed that peripheral TMAO levels were remarkably increased in CAD-T2D patients compared with healthy controls, however, the study failed to make the comparison among T2D patients internally and analyze the relationship between TMAO levels and coronary stenosis in those diabetic patients.28 Our study went further and indicated that elevated TMAO concentration is related to more serious coronary atherosclerosis in terms of not only the presence of TVD but also higher SYNTAX score over 22, independent of age and sex in patients with T2D. Hence, it is conceivable that higher TMAO concentrations correlate with increased risks of major adverse cardiovascular events (MACE) and all-cause mortality in population with T2D.29
The extended observations in the study provide a new perspective about the impact of renal function on the association between TMAO levels and the quantified serious coronary lesion. The association mentioned above was dramatically significant in patients with eGFR <60 mL/min/1.73 m2, whether the conventional predictors were adjusted for or not, differing from the results in patients with eGFR ≥60 mL/min/1.73 m2. Our results could support the previous research finding from Missailidis et al. that TMAO level is an independent predictor of mortality in chronic kidney disease (CKD) 3–5 patients (eGFR <60 mL/min/1.73 m2).15 The study by Winther et al., mainly focused on patients with type 1 diabetes (T1D), have recently demonstrated that the association between higher concentrations of plasma TMAO and mortality or cardiovascular events may be mediated through impaired renal function and speculated that TMAO is a kidney function marker or a novel risk factor for micro- and macrovascular complications of diabetes.30 Physiologically, TMAO is excreted in urine31 and circulating TMAO levels increase with the decline of renal function.16 Nevertheless, the consideration that peripheral TMAO may reflect renal function to a certain extent, could not refute the possibility that higher TMAO levels may exacerbate atherosclerogenesis through the pathways correlated with damaging kidney function. In C57BL/6J mice, chronic dietary exposures increasing TMAO directly gives rise to progressive renal fibrosis and kidney dysfunction.32 Among 1434 participants from Framingham Offspring Study, TMAO was identified to be positively related to incident CKD (in 123 cases) after the follow-up for over 8 years.33 Therefore, we inferred the increase of TMAO concentration accompanied by renal dysfunction and these two factors promote mutually in the process of atherosclerogenesis. If the kidney fairly copes with circulating TMAO, the development of atherosclerosis largely depends on known risk factors of CAD. Whereas, when the renal function declines to a certain level, for example, eGFR <60 mL/min/1.73 m2, accumulated TMAO is strongly associated with exacerbation of atherosclerosis, regardless of those conventional cardiovascular predictors. The novel result is that the higher confidence limits of OR value for TVD and SYNTAX score >22 reach up to 250.33 and 34.64, respectively, as a result of dramatical TMAO accumulation in CKD 5 patients with the highest TMAO levels and more severe coronary stenosis.
The production of TMAO is a complex process involving specific gut microbes that metabolize the dietary-derived precursors mainly from red meat or seafood, including choline, betaine and L-carnitine. Reasonably, circulating TMAO levels will become higher with the increase of red meat or seafood in multiple human studies.34–36 However, the effects of diet on TMAO levels are heterogeneous in other investigations. For example, in a German population-based cohort study by Rath et al., while two major bacterial TMA-forming pathways were linked to the TMAO concentration, meat intake did not predict plasma TMAO levels, suggesting the high inter-individual variability in TMAO production.37 Furthermore, Mediterranean diet, a beneficial dietary pattern to patients with cardiovascular disease, contains TMAO-rich seafood but does not affect the level of plasma TMAO.38,39 The results displayed the complexity of relationships between diet and TMAO. Our study was a real-world study among patients with T2D and we did not restrict their red meat or seafood intake, which might better reflect the real situation of these patients. Except for diet, the gut flora composition, which is significantly different between people with T2D and those without T2D, exerts a great impact on TMAO levels.8,40 For example, Larsen et al. found that the abundance of class Clostridia and phylum Firmicutes were distinctly decreased, while class Betaproteobacteria was notably increased in T2D patients.40 Moreover, the ratios of Bacteroidetes to Firmicutes and Bacteroides-Prevotella to C. coccoides-E. rectale are positively correlated with plasma glucose.40 In our study, all the included individuals met diagnostic criteria of T2D, which could reduce the heterogeneity of gut microbiota to some degree.
TMAO, which plays a role in vascular dysfunction and atherosclerosis, may open a preventative and therapeutic avenue ultimately conducive to severe CAD, especially in patients with eGFR <60 mL/min/1.73 m2. The application of prebiotics and probiotics could contribute to favorable alterations of gut microbiota composition. Prebiotics, certain kinds of non-digestible food components, could promote the growth of beneficial bacteria,41 while probiotics require supplementation of specific bacteria strains. Foods containing prebiotics or probiotics could decrease the bacteria which transform certain precursors into TMA, or increase the bacteria capable of depleting TMA or those lacking the indispensable genes of converting carnitine or choline into TMA. Another devised method is using antibiotics especially broad-spectrum antibiotics to change the gut microbiota composition, which could annihilate microbiota capable of transforming a series of dietary precursors, such as choline, betaine and L-carnitine, into TMA. Broad spectrum antibiotics, ciprofloxacin and metronidazole included, could lead to almost complete decrease of peripheral TMAO levels which, however, were detectable again after the withdrawal of antibiotics one month later.11 Besides, the long-term use of antibiotics may not be advisable since it would give rise to multi-drug resistant bacteria strains and microbiota repopulation. Furthermore, antibiotics could not only eliminate harmful bacteria but also affect most beneficial strains.11 Instead of altering the gut microbiota composition, the restriction of carnitine and choline intake is not viable because they are important nutrients for the human body and deficiency in them can give rise to various organ dysfunctions. Choline and its derivatives contribute to stabilizing the cell membrane as phosphatidylcholine and generating certain neurotransmitters such as acetylcholine. Moreover, L-carnitine plays a role in maintaining cardiac and skeletal muscle function and is negatively associated with mortality after acute myocardial infarction and MACE.42 There are also other devised possibilities including the use of certain analogs and the suppression of TMA precursors. In animal models, reducing levels of TMAO by selectively inhibiting microbial TMA production through 3,3-dimethyl-1-butanol (DMB), one kind of structural choline analog, could decrease diet-induced atherosclerosis,43 likewise, the inhibition of TMAO by knocking-out FMO3 could also suppress atherosclerosis.44–46 Thus, a strength of our study was the ability to explore whether the prospective TMAO may serve as a potential therapeutic or prophylactic target of severe coronary lesion by invasive methods, especially for T2D patients with DKD.
The study inevitably has its limitations. Firstly, as a single-center study, the sample size is relatively small, especially the patients with TVD or SYNTAX score >22. Therefore, the selection bias for patients cannot be excluded. Secondly, as a cross-sectional observational study, causality could not be determined in this association. Prospective studies and basic medical research are warranted. Due to the contemporary cohort undergoing diagnostic CAG between 2017–2018, the long-term outcome data are not available yet. Hopefully, we are following up these participants persistently. Finally, despite adjustments for multiple traditional risk factors of CAD in the process of drawing the novel conclusion, other potential confounding factors cannot be completely excluded, such as undiagnosed intestinal diseases and unknown antibiotics application. Nevertheless, we found no abnormal composition of the fecal flora and no parasite based on feces smear.
Conclusion
Elevated plasma TMAO levels were not significantly associated with the presence of CAD and higher coronary atherosclerotic burden in T2D patients included in the present study. However, they were independently correlated with severe coronary atherosclerosis, including TVD and higher SYNTAX score than 22, in patients with eGFR of lower than 60 mL/min/1.73 m2.
Acknowledgments
The authors thank the Department of Cardiology of Peking University First Hospital for the help in the process of collecting blood samples and assessing the coronary stenosis. This work was supported by the National Natural Science Foundation of China (No. 30771033).
Disclosure
All authors declared no conflicts of interest.
References
- 1.Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 2018;17(1):83. doi: 10.1186/s12933-018-0728-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2016 update: a report from the American Heart Association. Circulation. 2016;133(4):e38–360. doi: 10.1161/CIR.0000000000000350 [DOI] [PubMed] [Google Scholar]
- 3.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19(5):576–585. doi: 10.1038/nm.3145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Zhao F, Wang Y, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5(1):14. doi: 10.1186/s40168-016-0222-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tang WH, Wang Z, Li XS, et al. Increased trimethylamine n-oxide portends high mortality risk independent of glycemic control in patients with type 2 diabetes mellitus. Clin Chem. 2017;63(1):297–306. doi: 10.1373/clinchem.2016.263640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schugar RC, Shih DM, Warrier M, et al. The TMAO-producing enzyme flavin-containing monooxygenase 3 regulates obesity and the beiging of white adipose tissue. Cell Rep. 2017;20(1):279. doi: 10.1016/j.celrep.2017.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen HK, Gudmundsdottir V, Nielsen HB, et al. Human gut microbes impact host serum metabolome and insulin sensitivity. Nature. 2016;535(7612):376–381. doi: 10.1038/nature18646 [DOI] [PubMed] [Google Scholar]
- 8.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450 [DOI] [PubMed] [Google Scholar]
- 9.Dambrova M, Latkovskis G, Kuka J, et al. Diabetes is associated with higher trimethylamine N-oxide plasma levels. Exp Clin Endocrinol Diabetes. 2016;124(4):251–256. doi: 10.1055/s-0035-1569330 [DOI] [PubMed] [Google Scholar]
- 10.Senthong V, Wang Z, Li XS, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc. 2016;5(6). doi: 10.1161/JAHA.115.002816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang WH, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368(17):1575–1584. doi: 10.1056/NEJMoa1109400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38(11):814–824. doi: 10.1093/eurheartj/ehw582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dong Z, Liang Z, Guo M, Hu S, Shen Z, Hai X. The association between plasma levels of trimethylamine N-oxide and the risk of coronary heart disease in Chinese patients with or without type 2 diabetes mellitus. Dis Markers. 2018;2018:1578320. doi: 10.1155/2018/1578320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Senthong V, Li XS, Hudec T, et al. Plasma trimethylamine N -oxide, a gut microbe–generated phosphatidylcholine metabolite, is associated with atherosclerotic burden. J Am Coll Cardiol. 2016;67(22):2620–2628. doi: 10.1016/j.jacc.2016.03.546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Missailidis C, Hällqvist J, Qureshi AR, et al. Serum trimethylamine-N-oxide is strongly related to renal function and predicts outcome in chronic kidney disease. PLoS One. 2016;11(1):e0141738. doi: 10.1371/journal.pone.0141738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bain MA, Faull R, Fornasini G, Milne RW, Evans AM. Accumulation of trimethylamine and trimethylamine-N-oxide in end-stage renal disease patients undergoing haemodialysis. Nephrol Dialysis Transplant. 2006;21(5):1300–1304. doi: 10.1093/ndt/gfk056 [DOI] [PubMed] [Google Scholar]
- 17.Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res Clin Pract. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843 [DOI] [PubMed] [Google Scholar]
- 18.Sianos G, Morel MA, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention. 2005;1(2):219–227. [PubMed] [Google Scholar]
- 19.Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet (London, England). 2013;381(9867):629–638. doi: 10.1016/S0140-6736(13)60141-5 [DOI] [PubMed] [Google Scholar]
- 20.Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360(10):961–972. doi: 10.1056/NEJMoa0804626 [DOI] [PubMed] [Google Scholar]
- 21.Zeng M, Yan X, Wu W. Risk factors for revascularization and in-stent restenosis in patients with triple-vessel disease after second-generation drug-eluting stent implantation: a retrospective analysis. BMC Cardiovasc Disord. 2021;21(1):446. doi: 10.1186/s12872-021-02259-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Jiang L, Xu L, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872–882. doi: 10.1177/2047487319826398 [DOI] [PubMed] [Google Scholar]
- 23.Sheng Z, Tan Y, Liu C, et al. Relation of circulating trimethylamine N-oxide with coronary atherosclerotic burden in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2019;123(6):894–898. doi: 10.1016/j.amjcard.2018.12.018 [DOI] [PubMed] [Google Scholar]
- 24.Sanchez-Alcoholado L, Castellano-Castillo D, Jordan-Martinez L, et al. Role of gut microbiota on cardio-metabolic parameters and immunity in coronary artery disease patients with and without type-2 diabetes mellitus. Front Microbiol. 2017;8:1936. doi: 10.3389/fmicb.2017.01936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanase DM, Gosav EM, Neculae E, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM). Nutrients. 2020;12(12). doi: 10.3390/nu12123719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao L, Lou H, Peng Y, Chen S, Fan L, Li X. Elevated levels of circulating short-chain fatty acids and bile acids in type 2 diabetes are linked to gut barrier disruption and disordered gut microbiota. Diabetes Res Clin Pract. 2020;169:108418. doi: 10.1016/j.diabres.2020.108418 [DOI] [PubMed] [Google Scholar]
- 27.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472(7341):57–63. doi: 10.1038/nature09922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ma G, Pan B, Chen Y, et al. Trimethylamine N-oxide in atherogenesis: impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci Rep. 2017;37(2). doi: 10.1042/BSR20160244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Croyal M, Saulnier PJ, Aguesse A, et al. Plasma trimethylamine N-oxide and risk of cardiovascular events in patients with type 2 diabetes. J Clin Endocrinol Metab. 2020;105(7):2371–2380. doi: 10.1210/clinem/dgaa188 [DOI] [PubMed] [Google Scholar]
- 30.Winther SA, Øllgaard JC, Tofte N, et al. Utility of plasma concentration of trimethylamine N-oxide in predicting cardiovascular and renal complications in individuals with type 1 diabetes. Diabetes Care. 2019;42(8):1512–1520. doi: 10.2337/dc19-0048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeisel SH, daCosta KA, Youssef M, Hensey S. Conversion of dietary choline to trimethylamine and dimethylamine in rats: dose-response relationship. J Nutr. 1989;119(5):800–804. doi: 10.1093/jn/119.5.800 [DOI] [PubMed] [Google Scholar]
- 32.Tang WH, Wang Z, Kennedy DJ, et al. Gut microbiota-dependent trimethylamine N-oxide (TMAO) pathway contributes to both development of renal insufficiency and mortality risk in chronic kidney disease. Circ Res. 2015;116(3):448–455. doi: 10.1161/CIRCRESAHA.116.305360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rhee EP, Clish CB, Ghorbani A, et al. A combined epidemiologic and metabolomic approach improves CKD prediction. J Am Soc Nephrol. 2013;24(8):1330–1338. doi: 10.1681/ASN.2012101006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Costabile G, Vetrani C, Bozzetto L, et al. Plasma TMAO increase after healthy diets: results from 2 randomized controlled trials with dietary fish, polyphenols, and whole-grain cereals. Am J Clin Nutr. 2021;114(4):1342–1350. doi: 10.1093/ajcn/nqab188 [DOI] [PubMed] [Google Scholar]
- 35.Li J, Li Y, Ivey KL, et al. Interplay between diet and gut microbiome, and circulating concentrations of trimethylamine N-oxide: findings from a longitudinal cohort of US men. Gut. 2021;gutjnl-2020-322473. doi: 10.1136/gutjnl-2020-322473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamaya R, Ivey KL, Lee DH, et al. Association of diet with circulating trimethylamine-N-oxide concentration. Am J Clin Nutr. 2020;112(6):1448–1455. doi: 10.1093/ajcn/nqaa225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rath S, Rox K, Kleine Bardenhorst S, et al. Higher trimethylamine-N-oxide plasma levels with increasing age are mediated by diet and trimethylamine-forming bacteria. mSystems. 2021;6(5):e0094521. doi: 10.1128/mSystems.00945-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang WHW, Bäckhed F, Landmesser U, Hazen SL. Intestinal microbiota in cardiovascular health and disease: JACC state-of-the-art review. J Am Coll Cardiol. 2019;73(16):2089–2105. doi: 10.1016/j.jacc.2019.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin LE, Djuric Z, Angiletta CJ, et al. A Mediterranean diet does not alter plasma trimethylamine N-oxide concentrations in healthy adults at risk for colon cancer. Food Funct. 2019;10(4):2138–2147. doi: 10.1039/C9FO00333A [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen N, Vogensen FK, van den Berg FW, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5(2):e9085. doi: 10.1371/journal.pone.0009085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang C, Yin A, Li H, et al. Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine. 2015;2(8):968–984. doi: 10.1016/j.ebiom.2015.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mendelsohn AR, Larrick JW. Dietary modification of the microbiome affects risk for cardiovascular disease. Rejuvenation Res. 2013;16(3):241–244. doi: 10.1089/rej.2013.1447 [DOI] [PubMed] [Google Scholar]
- 43.Wang Z, Roberts AB, Buffa JA, et al. Non-lethal inhibition of gut microbial trimethylamine production for the treatment of atherosclerosis. Cell. 2015;163(7):1585–1595. doi: 10.1016/j.cell.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miao J, Ling AV, Manthena PV, et al. Flavin-containing monooxygenase 3 as a potential player in diabetes-associated atherosclerosis. Nat Commun. 2015;6:6498. doi: 10.1038/ncomms7498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shih DM, Wang Z, Lee R, et al. Flavin containing monooxygenase 3 exerts broad effects on glucose and lipid metabolism and atherosclerosis. J Lipid Res. 2015;56(1):22–37. doi: 10.1194/jlr.M051680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Warrier M, Shih DM, Burrows AC, et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015;10(3):326–338. doi: 10.1016/j.celrep.2014.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]