Abstract
The prenatal environment, and in particular, the maternal-fetal immune environment, has emerged as a targeted area of research for central nervous system (CNS) diseases with neurodevelopmental origins. Converging evidence from both clinical and preclinical research indicates that changes in the maternal gestational immune environment can alter fetal brain development and increase the risk for certain neurodevelopmental disorders. Here we focus on the translational potential of one prenatal animal model –the maternal immune activation (MIA) model. This model stems from the observation that a subset of pregnant women who are exposed to infection during pregnancy have an increased risk of giving birth to a child who will later be diagnosed with a neurodevelopmental disorder, such as autism spectrum disorder (ASD) or schizophrenia (SZ). The preclinical MIA model provides a system in which to explore causal relationships, identify underlying neurobiological mechanisms, and, ultimately, develop novel therapeutic interventions and preventative strategies. In this review, we will highlight converging evidence from clinical and preclinical research that links changes in the maternal-fetal immune environment with lasting changes in offspring brain and behavioral development. We will then explore the promises and limitations of the MIA model as a translational tool to develop novel therapeutic interventions. As the translational potential of the MIA model has been the focus of several excellent review articles, here we will focus on what is perhaps the least well developed area of MIA model research –novel preventative strategies and therapeutic interventions.
Keywords: Animal models, neurodevelopmental disorders, neuroimmunology
I. Developmental origins of health and disease
Over the past four decades, the gestational environment has emerged as a critical window of development that can have long-lasting influences on offspring health. Studies initiated in the 1970s first explored the impact of maternal undernutrition experienced during the Dutch Famine of 1944–45 on the health outcomes of individuals exposed to these adverse conditions during specific gestational periods (1–3). Results from the Dutch Famine Birth Cohort linked starvation during pregnancy with long-lasting changes in offspring health (4–6) and influenced the emerging theory of “fetal programming” that was introduced by David Barker in the 1980s. The fetal origins hypothesis (often referred to as Barker’s hypothesis) stemmed from additional epidemiological observations linking fetal undernutrition with subsequent low birth weights, and increased risk for coronary heart disease in adulthood (7–10). Barker’s initial observations formed the foundation of what would later be known as the Developmental Origins of Health and Disease (DOHaD) model that was expanded to encompass a broad range of prenatal factors that may have lasting consequences on offspring development (11–14).
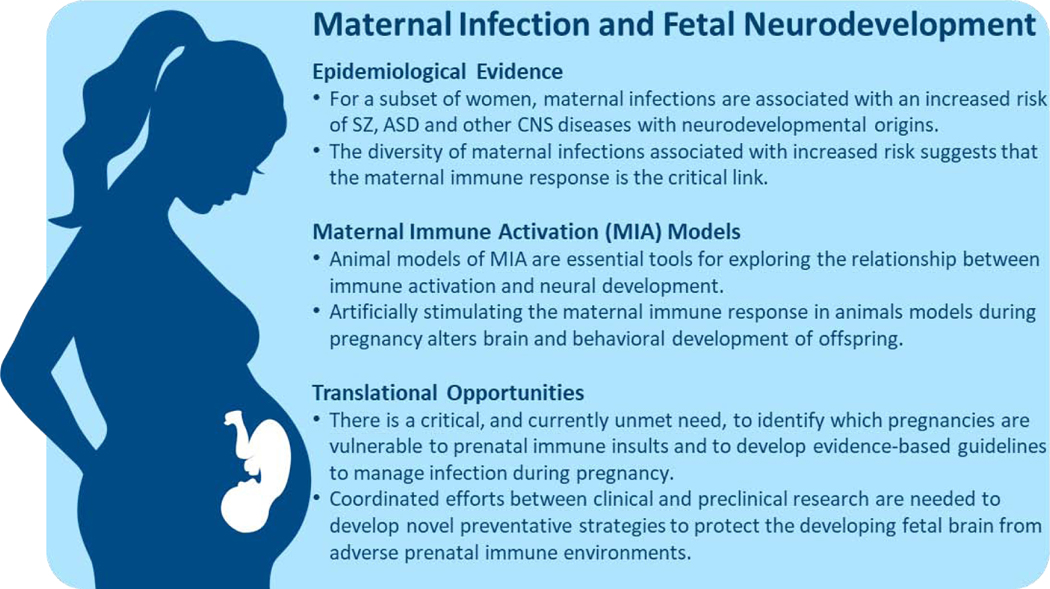
One of the central themes that arose from the DOHaD approach is that the rapid development and plasticity required to build a complex organism during gestation makes the developing fetus vulnerable to adverse intrauterine events. From a neurodevelopmental perspective, the finely orchestrated events required for the billions of cells undergoing neurogenesis, migration, differentiation, synapse formation and apoptosis render the developing fetal brain uniquely vulnerable to environmental insults (15). While it is not surprising that the fetal brain is susceptible to extremely adverse conditions, such as maternal starvation, recent evidence suggests that more subtle changes in the maternal-fetal immune environment can also have a significant impact on neurodevelopment (16–18). Indeed, immune signaling molecules play critical roles in all stages of the typically developing fetal brain (19, 20) and perturbations of the gestational immune environment may have long lasting effects on offspring neurodevelopment (21, 22). In this review, we focus on one change in the maternal-fetal immune environment associated with an increased risk of offspring neurodevelopmental disorders –the maternal response to infections during pregnancy. After a brief summary of the rapidly growing epidemiological and animal model research linking maternal infection with altered fetal brain development, we will explore efforts to develop evidence-based therapeutic interventions and preventative strategies to mitigate the deleterious effects of the maternal response to infection on fetal brain development (Fig 1.).
Figure 1.
Converging evidence from epidemiological studies in humans and preclinical model systems highlights the maternal-fetal immune environment and a potentially modifiable risk factor for neurodevelopmental disorders.
II. Epidemiological evidence linking maternal infection and neurodevelopmental disorders
In the United States, approximately 6 million women become pregnant annually, resulting in approximately 4 million births per year (23). Healthy pregnancies require a “fine-tuned and highly regulated” balance between maternal immune activation needed to maintain a pathogen-free, non-inflammatory environment paired with a unique state of tolerance to avoid rejection of the semiallogeneic fetal-placental unit (24). Given that the majority of women report experiencing at least one infection during pregnancy (25), most fetuses will experience changes in the maternal-fetal immune environment at some point during gestation. Cytokines that are elevated in response to infection, such as interleukin-6 (IL-6) can cross the placenta (26–28) and are thought to act on placental cells to stimulate the downstream production of other immune mediators in the fetal compartment (29). It is essential to emphasize that the vast majority of women who experience infection during pregnancy will go on to have healthy offspring. However, for a subset of women, maternal infection may serve as a “disease primer” into an altered trajectory of fetal brain development that, in combination with other genetic and environmental factors, may ultimately result in a child who will be later diagnosed with a neurodevelopmental disorder (30). While we focus on the two neurodevelopmental disorders more strongly associated with maternal infection, SZ and ASD (31), emerging evidence has also established links with other offspring CNS disorders, including depression, ADHD and bipolar depression (22).
Schizophrenia.
Early evidence linking the prenatal immune environment with SZ stemmed from the observation that births during the winter and spring months was associated with an increased risk of SZ (reviewed in, (32)). More recent research utilizing large birth cohorts and/or biological evaluation on maternal infection has strengthened the association between maternal infection and increased risk for offspring SZ (33). Birth cohort studies have reported increased SZ risk following gestational exposure to a variety of maternal infections, including rubella (34), respiratory infection (35), influenza (35), herpes simplex virus type 2 (36, 37), cytomegalovirus (38), toxoplasmosis (39, 40), genital–reproductive infection (41), and bacterial infections (42). Several studies have also utilized maternal serum or other biobank specimens to identify markers of infection/inflammation that correlate with SZ risk, including influenza antibodies (43), alterations in maternal cytokines (44–46), maternal serum CRP (47), and levels of maternal complement components (48). Neuroimaging and behavioral studies comparing prenatally exposed and unexposed cohorts of individuals with SZ provide further evidence that in-utero exposure to infection may result in a unique trajectory of altered neurodevelopment. Individuals with SZ who were prenatally exposed to infection exhibit a characteristic pattern of volumetric brain changes paired with deficits in both executive function and working memory that differ from individuals with SZ who were not prenatally exposed (49–52). Emerging evidence also highlights the role of gene x environment interactions when considering prenatal risk factors for SZ (53, 54). For example, an association was found between prenatal exposure to kidney infection and increased risk of offspring SZ, but only for mothers with a family history of psychosis (55). Likewise, in a large Swedish cohort study of nearly two million individuals, infection during pregnancy was associated with an increased risk of offspring SZ only among mothers with a history of psychiatric disease (56). Recent evidence also highlights the importance of gestational timing, as high maternal proinflammatory cytokine concentrations in early (but not late) pregnancy were associated with increased risk of offspring psychosis (57). Collectively, these studies highlight the need to understand the relationship between the timing and intensity of maternal infection, as well as genetic susceptibility in order to identify women who are most vulnerable to inflammatory exposures during pregnancy.
Autism spectrum disorder (ASD).
As with SZ, initial evidence linking maternal infection with increase ASD risk was primarily based on case studies following prenatal exposure to infectious agents, such as rubella or cytomegalovirus (58–63). More recent epidemiological studies have provided additional evidence supporting this association, though results vary depending on the type of infectious agent, the timing of the gestational exposure, and intensity of the maternal immune response. Moreover, the association between maternal infection and ASD risk is likely complicated by additional genetic and environmental risk factors, which may be associated with different ASD subtypes (64, 65). In spite of these challenges, several consistent findings have emerged. A large population-based study of children born in Denmark between 1980–2005 found no overall association between maternal infection diagnosis and ASD over the course of the entire pregnancy, but did report a nearly threefold increased risk for ASD following hospitalization for viral infection in the first trimester as well as an increased risk following hospitalization for bacterial infections in the second trimester (66). Self-report data obtained from a subset of the Denmark population study also failed to detect an association between common infections during pregnancy and an increased risk of ASD (67), though influenza exposure was specifically associated with a nearly twofold risk of ASD and febrile episodes greater than one week were associated with a nearly threefold increase. A study from Kaiser Permanente Research Northern California found that although overall occurrence of maternal influenza was not associated with an increased risk of having a child with ASD, episodes of fever during pregnancy, particularly with no anti-fever medication, was associated with an increased risk of ASD (68). A subsequent study found that maternal infections diagnosed in a hospital setting, presumably associated with more severe infections, were associated with an increased risk of ASD, while infections diagnosed in outpatient settings were not (69). Likewise, a study of a large Swedish cohort reported that inpatient diagnosis of any infection at any time during pregnancy was associated with an increased risk of offspring ASD regardless of the type of infectious agents (i.e. viral, bacterial, other) (70). A more recent study of the Swedish cohort also found an association between maternal infection and ASD risk that was present whether the maternal infection was severe (i.e., sepsis, pneumonia, pyelonephritis, meningitis, influenza, and chorioamnionitis) or more moderate (i.e., urinary tract infection) (71). This is further supported by a recent study large multi-site study reporting that women who had an infection during the second trimester of pregnancy accompanied by a fever were more likely to have children with ASD (72). Collectively, these ASD risk studies suggest that a common biological pathway, such as elevated maternal cytokines, may be the key link between various infections and aberrant fetal brain development. Although additional research is needed, the above findings suggest that more severe infection accompanied by a robust inflammatory response increases the risk of ASD in the child, and this phenomenon is not dependent of the pathogen itself, but rather the maternal response to the pathogen, and the time point during pregnancy in which it occurs. Quantification of cytokines, chemokines, and other inflammatory markers obtained from archived maternal sera (73, 74) and amniotic fluid (75, 76) lends further support to the link between maternal infection and increased ASD risk. Additional research is needed as not all studies have found positive associations between inflammatory markers and offspring ASD (77).
Gaps in our knowledge.
The current epidemiological data suggest that, at least for a subset of women, exposure to infection during pregnancy may increase the risk of offspring SZ, ASD, or other CNS diseases with neurodevelopmental origins. There are, however, many remaining questions. Which women are most vulnerable to prenatal infections? What makes a specific pregnancy more likely to have an elevated risk of altered neurodevelopment? If the maternal immune response is the common denominator, which cytokines, chemokines, etc. alter the developing fetal brain? How does the timing and magnitude of the maternal immune response influence the effects? At what point do we have enough epidemiological evidence to guide public health policy on management of infection during pregnancy? What novel therapeutic approaches could be utilized to minimize the long-term effects of prenatal infection on offspring brain and behavioral development? Given that the genetic, ecological, and behavioral diversity of humans is so remarkably heterogeneous, preclinical models of maternal infection are essential for testing causality, identifying molecular mechanisms, and developing novel therapeutic interventions and preventative strategies. Below we briefly summarize the promises and limitations of one of the most widely used preclinical models to study the effect of prenatal immune challenges on offspring neurodevelopment.
III. Preclinical maternal immune activation (MIA) models
The diversity of prenatal infectious agents associated with increased risk of neurodevelopmental disorders suggests that activation of the maternal immune system is the key link between maternal infections and altered fetal brain development. This maternal immune activation (MIA) hypothesis has been tested in animal model systems by activating the immune system during pregnancy and quantifying changes in offspring brain and behavioral development that parallel features of human CNS disorders. Although many immune activating agents are available, here we focus the two most commonly used models used to stimulate robust maternal immune responses. The first, polyinosinic:polycytidylic acid (Poly IC), is a synthetic double-stranded RNA molecule recognized by the pattern recognition receptor, toll-like receptor (TLR)3, which specifically recognizes double stranded RNA, the genetic information for many viruses. The second, lipopolysaccharide (LPS), the cell-wall component of gram-negative bacteria, has been used to mimic infection in many animal studies because it initiates a well-characterized immune response via the activation of TLR4 (78, 79). Rodent offspring born to Poly IC or LPS injected dams exhibit changes in brain and behavioral phenotypes that bear resemblance to human disorders, including both ASD and SZ (80, 81). Excellent reviews summarizing the effects of prenatal immune challenge on brain and behavioral development are available (for reviews, (82–86)). In the current review, we provide a brief update on the strengths and challenges associated with this complex model system and then highlight translational opportunities with a focus on prenatal intervention.
MIA model strengths.
There are several advantages to the use of the MIA model in understanding the basis of immune dysregulation and its effects on neurodevelopment. This area of research has understandably attracted investigators with expertise in several animal model species including mouse, rat, ferret and nonhuman primate. Such model diversity allows the comparison of MIA downstream effects on evolutionarily conserved behavioral and biological outcome measures (87–89). Across species, offspring born to MIA-treated dams exhibit alterations in brain and behavioral development relevant to human neurodevelopmental and neuropsychiatric disease and supported the initial interpretation of the MIA model as an animal model “of” ASD or SZ (90). As the use of animals in mental health research has evolved from a validity-based to a hypothesis-based utility approach (91), the MIA model has been highlighted in recently released NIH-guidelines (NOT-MH-19–053) a model “for” examining the effects of maternal inflammation on neural systems relevant to multiple neurodevelopmental conditions. This subtle, but important difference, in the use of objective and more precise language is consistent with emerging consensus among leaders in the MIA model field who have emphasized that MIA model serves as a valuable tool to explore the impact of prenatal immune challenge on offspring neurodevelopment that may be relevant to understanding the underlying neural mechanisms of a number of CNS diseases (30, 92).
MIA model limitations.
Although the MIA model is recognized as a powerful translational tool to explore the effects of prenatal immune challenge on fetal neurodevelopment, comparisons between animal models and clinical disorders must proceed with caution. Indeed, developing valid animal models to study complex human brain diseases, such SZ and ASD, poses a major obstacle to preclinical research efforts (93, 94). The MIA model is also faced with unusual variability in methodological approaches that make it challenging to compare across studies, thereby raising concerns related to the rigor and reproducibility of the model (95). Despite challenges related to methodological variability, MIA-treated offspring behavioral deficits in pre-pulse inhibition and social development have remained highly reproducible across laboratories (See Fig 1 from (95)). Moreover, factors that influence MIA model offspring outcomes undoubtedly influence the immune system of the dam and/or offspring, including choice of immune activation compounds (i.e., vendor, dose, route of delivery, timing and intensity of the immune response), the selection of animal models (i.e., species, strain, vendor), as well as husbandry and rearing (i.e., vivarium practices, housing, caging systems, etc.). The authors of a recent MIA model consensus paper (95) provide reporting guidelines to improve the model and conclude that, “the emerging view in our field is that we should not continue to ignore the details of how each lab generates their models but rather, we should embrace and explore those details becau se they may reveal critical information about the specific combination of conditions that cause risk.” It is also critical to remember that maternal infections in humans do not always cause disease in offspring, thus the MIA model can be used to determine why some pregnancies are susceptible, while others are resilient to adverse intrauterine conditions. In this sense, the diversity in phenotypic consequences of MIA may be viewed as a strength of the model, rather than a weakness.
Gaps in our knowledge.
Animal models of MIA have demonstrated a causal relationship between prenatal immune challenge and the neuropathological and behavioral abnormalities consistent with a range of neurodevelopmental disorders. However, the vast majority of MIA models have evaluated the effect of prenatal immune challenge in isolation, rather than in combination with other etiologically relevant risk factors. What role does genetic susceptibility play in determining which pregnancies are at risk? Do other postnatal factors (i.e., second hits) influence MIA offspring outcomes? For example, when combining MIA with genetic risk factors relevant to SZ (96–98) or ASD (99, 100), certain aspects of the MIA model behavioral phenotype are exacerbated. Emerging MIA model evidence suggests that genetic and environmental factors interact to cause sex-specific effects (101). Likewise, exposure to aversive postnatal events, including maternal care by a surrogate mother exposed to an immune challenge during gestation (102–104) or exposure to juvenile stress (105), also amplify outcome measures of the mouse MIA model. Finally, there is recent evidence to suggest that the consequences of MIA may be far reaching, as disruptions in brain and behavior have been shown to be transmitted transgenerationally in rodent models (106, 107). It is not known if transgenerational transmission also occurs in primate MIA models, though this would have far reaching implications for our understanding of prenatal immune challenge and human disease (108). The challenge for the next generation of this powerful model is to integrate multiple etiologically relevant “hits” while improving the overall reproducibility of the model. As the translational potential of the MIA model has been the focus of several excellent review articles (92, 109, 110), here we will focus on what is perhaps the least well developed area of MIA model research –prenatal preventative strategies.
IV. Translational opportunities and the prenatal immune environment
Although exposure to infection is very common, there is remarkably limited scientific information for pregnant women and their providers on how and whether to treat infectious diseases during pregnancy. This lack of information stems in part from the exclusion of pregnant and lactating women from clinical research, which has been motivated in part by concern about possible harms of medication use during pregnancy or lactation. In 2017, the Task Force on Research Specific to Pregnant Women and Lactating Women (PRGLAC) was established to provide advice and guidance to the United States Secretary of Health and Human Services on activities related to identifying and addressing gaps in knowledge and research on safe and effective therapies for pregnant women and lactating women. Their 2018 report (111) included an analysis of published scientific evidence on therapies in pregnant women and lactating women based on research articles published over the last 10 years (Appendix VI). The analysis of infectious disease studies found that “There is very limited scientific information for pregnant women and lactating women and their providers on how and whether to treat infectious diseases during pregnancy and lactation” and that “Few original research publications assessed the impact of untreated infection.” While therapeutic interventions targeting the prenatal environment must proceed with caution, there is growing appreciation that the maternal-fetal immune environment is modifiable and presents an opportunity to protect at-risk pregnancies (112). By some estimates, elimination of common maternal infections could substantially reduce the number of SZ cases (32). Although it is not feasible to eliminate maternal infections, it is entirely plausible that reducing the incidence of exposure and/or developing policies to manage infection during pregnancy could reduce the incidence of several neurodevelopmental disorders. This will, however, require coordinated efforts between clinical research and basic science to generate evidence-based guidelines. Below we highlight three opportunities for therapeutic interventions and preventative strategies to explore in the MIA model: (i) Predicting risk and resilience, (ii) Prenatal interventions and (iii) Postnatal interventions.
Predicting risk and resilience.
The majority of women exposed to viral and bacterial infections during pregnancy give birth to neurotypical offspring. Thus, there is a critical and currently unmet, need to understand which pregnancies are most vulnerable to prenatal immune challenge, which are resilient, and how to intervene. The MIA model system is emerging as a powerful translational tool to explore the mechanisms underlying risk and resilience (113). MIA models have experimentally manipulated combinations of timing of the exposure, the type of immune activation (viral versus bacterial, acute versus chronic), other environmental exposures and co-morbidities (such as stress and diet), and intensity and duration of the maternal immune response. As described in previous reviews, each of these factors may individually, or in combination, determine the nature of brain and behavioral alterations that manifest in offspring (for reviews, (92, 109, 110). Here we highlight fetal sex and the interaction with placental functioning that has recently emerged as an important factor in predicting risk and resilience in the MIA model. Although sex has been understudied in the MIA model (114), recent evidence indicates male and female MIA offspring exhibit sex-specific changes in brain and behavioral development (115–117). Females were initially described as less effected and considered resilient, though emerging studies suggest that females exhibit a unique trajectory of neurodevelopment following prenatal immune challenge. For example, a recent mouse LPS model found that while males are more severely impacted by prenatal immune disruption by several measures, females exposed to the same insult exhibit a unique set of vulnerabilities and developmental consequences that is not present in males (118). Sex-specific placental and fetal proinflammatory responses may provide mechanistic insight into the differences in male and female offspring (119), including sex-specific responses of many immune genes to both metabolic and inflammatory stress (120). Given that healthy fetal development is dependent on nutrient and oxygen transfer from the mother (121), sex-specific changes in placental growth and vasculature function are emerging as critical areas of future research. Moreover, emerging evidence suggests that environmental enrichment protects placental functioning at the time of a maternal stressor and provide potential therapeutic intervention opportunities (122). The MIA model will continue to provide a testbed to explore which pregnancies are most vulnerable to prenatal immune challenge, which gestational time points are most susceptible to perturbation of immune homeostasis, and how to best manage the maternal immune response during pregnancy to prevent deleterious effects on fetal brain development.
MIA model prenatal interventions.
Although anti-inflammatory interventions may provide a valuable strategy for the prevention on neurodevelopmental abnormalities associated with maternal infection, relatively few MIA models have explored prenatal interventions designed to mitigate the maternal immune response and protect the developing fetal brain (Table 1). Pioneering work by the late Dr. Paul Patterson first demonstrated that elevations in maternal IL-6 are necessary and sufficient to induce MIA-related alterations in brain and behavioral development (123). In this model, co-administration of an anti-IL-6 antibody prevented MIA-associated deficits in prepulse inhibition, latent inhibition, exploration, and social interaction and normalizes alterations in gene expression in adult offspring brains. Although MIA models have focused on maternal IL-6 as a key cytokine associated with brain and behavioral impairments in offspring (124), emerging evidence highlights a potential role for other cytokines, including IL-17. Treating dams with an IL-17a blocking antibody after MIA injections prevents some MIA-associated offspring behavioral abnormalities, though pretreatment with this antibody prior to MIA induction may have greater therapeutic potential (125). Recent MIA models have explored the role of the purinergic ion channel P2X7 (P2rx7) in inflammatory conditions, demonstrating that that genetic or pharmacological inhibition of both maternal and offspring P2X7 receptors could reverse the MIA-induced changes in brain and behavioral development (126). Although these prenatal interventions studies provide important insight into biological mechanisms underlying the MIA model, these are not likely candidates for therapeutic interventions in humans.
Table 1.
Summary of MIA model prenatal intervention studies.
| Author Year Journal |
Species Strain Sex |
MIA Protocol MIA Validation |
Intervention | Offspring Behavior Outcomes | Offspring Brain Outcomes |
|---|---|---|---|---|---|
| Alizadeh 2020 Behav Brain Res |
Wistar rats | GD 15, 16 LPS 0.5 mg/kg Cytokine validation: No |
Zinc supplementation (30mg/kg) administered through pregnancy via gavage Treatment groups: CON/VEH, CON/ZINC, MIA/VEH, MIA/ZINC |
Y maze MIA<CON (males only) Zinc supplementation restored the alterations in working memory in MIA male rats |
MIA males exhibited moderate decrease in GAD67 expression level in the male pups Zinc supplementation restored GAD67 mRNA level in the male rats |
| Vuillermot 2019 Molecular Autism |
C57BL/6N mice | GD9 Poly(I:C) [Sigma] 5 mg/kg i.v./restraint Cytokine validation: Yes |
Maternal administration of the active VitD hormone, 1,25OHD followed immediately by MIA Treatment groups: CON/VEH, CON/VITD, MIA/VEH, MIA/VITD |
Pre-pubertal (PND30–40) EPM – no differences Social Approach MIA<CON, prevented by VitD Marble Burying MIA<CON, prevented by VitD Fear Conditioning MIA<CON, prevented by VitD |
N/A |
| Luan 2018 Scientific Reports |
C57BL/6N mice | GD9 Poly(I:C) [Sigma] 5mg/kg Subcutaneous Cytokine validation: No |
Maternal administration of the active VitD hormone, 1,25OHD (400ng/kg/2ml) followed immediately by MIA Treatment groups: CON/VEH, CON/VITD, MIA/VEH, MIA/VITD |
PPI: MIA/CON = MIA/VITD AMPH Induced Locomotor Activity: MIA>CON, prevented by VitD |
CON/VITD, MIA/VEH and MIA/VITD had decreased mesDA progenitors at GD11. CON/VEH<CON/VITD mature mesDAs. VitD increased mature mesDA number. CON/VEH<MIA/VEH post-mitotic mesDAs, prevented by VitD. |
| Wang 2019 Autism Research |
C57BL/6J mice | GD12.5 Poly(I:C) [Sigma] 20 mg/kg i.p. Cytokine validation: Yes |
Oral probiotic administration formula (1.5g Probiotics Sachet Children’s Formula/100mL water) from E0.5 to PND21 Average dose intake was 1.5675 × 107 cfu Bifidobacteria and 5.28 × 108 cfu Lactobacillus helveticus per 24hr. Treatment groups: CON/VEH, CON/PRO, MIA/VEH, MIA/PRO |
3-chamber SD: MIA<CON time sniffing, prevented by probiotics Self Grooming: MIA>CON time spent grooming, prevented by probiotics Forced Swim Test: MIA>CON immobility, prevented by probiotics Open-Field/EPM: MIA<CON time spent in center/open arms, prevented by probiotics |
Probiotic groups had decreased cytokine levels. MIA<CON PFC PV+ neurons, prevented by probiotics. MIA<CON GABA and glutamate levels, prevented by probiotics. |
| Choi 2016 Neuroimmunology |
Mice WT and RORγt-TKO (proinflammatory T-cell KO specific to IL-17a) |
GD 12.5, 14.5 Poly(I:C) [Sigma] Cytokine validation: Yes |
Maternal administration of IL-17 antibodies to WT and RORγ-tTKO mice Administration of IL-17 directly to dams during pregnancy Treatment groups: CON/VEH, CON/αIL-17, MIA/VEH, MIA/αIL-17, RORγt-TKO/VEH, RORγt-TKO/MIA |
USVs: MIA>CON Marble Burying: MIA>CON Social Approach: MIA<CON social interaction percentage MIA+IL-17a antibody = CON phenotype MIA+RORγt-TKO = CON phenotype |
MIA and IL-17a direct administration both cause cortical abnormalities (protrusions). MIA/αIL-17 and RORγt-TKO/MIA mice appeared similar to controls |
| Smith 2008 Neuroscience |
C57BL/6J mice, IL-6 KO mice | GD 12.5 Poly(I:C) [Sigma] 20 mg/kg Cytokine validation: Yes |
Dams were injected with IL-6, IFNγ, or vehicle. Treatment groups: CON/VEH, MIA/VEH, MIA/αIL-6, MIA/αIFNγ, MIA/αIL-1β |
PPI: MIA<CON, prevented by αIL-6 and IL-6 KO Social Interaction: MIA<CON, prevented by αIL-6 and IL-6 KO Open Field: MIA<CON center time, prevented by αIL-6 and IL-6 KO Lateral Inhibition: αIFNγ>αIL-6 |
MIA rats with neutralizing antibodies are more genetically similar to CON/VEH rats than MIA/VEH rats. |
| Labrousse 2018 Brain, Behavior and Immunity |
C57BL/6J mice, only male offspring behavior | GD 17 LPS [Sigma] 0.12 μg/mouse/100μL i.p. Cytokine validation: Yes |
Dams were placed on diets with either deficient or balanced levels of n-3 PUFAs. Pups were continued on the same diet postnatally until adult behavior was taken. Treatment groups: CON/BAL, CON/DEF, MIA/BAL, MIA/DEF |
Y Maze: MIA<CON time in novel arm MIA/DEF could not discriminate familiar from novel Novel Object Recognition: MIA<CON time exploring novel object *These effects were not seen when mice did not continue dietary intervention postnatally |
DEF rats had less n-3 and more n-6 PUFAs. MIA decreased DHA and n-3 PUFAs. MIA<CON cFos+ cells in DG and CA1 regions of hippocampus, post Y-maze trial 2. DEF pups had fewer cFos+ cells than BAL pups. Microglia phenotypes and IL-6 receptor levels were unaffected by MIA or diet. PCA revealed negative relationship between n-3 PUFA levels and proinflammatory cytokine levels. |
| Cui K 2009 Schizophrenia Research |
Sprague Dawley rats, only male offspring | GD 15, 16 (midgestation group) or GD 18, 19 (late-gestation group) Mid group dose 100 μg/kg LPS [Sigma]. Late group dose 50 μg/kg LPS [Sigma]. |
Dams were given Ibuprofen at 25 mg/kg shortly after MIA in some cohorts. All dams were injected with BrdU and NeuN markers to the dentate gyrus to assess cell proliferation and survival both immediately following and weeks from LPS exposure. Treatment groups: CON/VEH, MID/VEH, MID/IBU, LATE/VEH, LATE/IBU |
N/A | Group 1: BrdU injection 4hrs post MIA, collection 4 weeks later. MID<LATE BrdU+ cells. Group 2: BrdU injection PND14/60, collection 2 hours later. MID<CON BrdU+ cells at PND14. No effect seen at PND60. Group 3: BrdU injection PND14/60, collection 4 weeks later. MID=LATE<CON BrdU+ cells. No effect seen at PD60. IBU prevented increase in body temperature from MIA. Pretreating dams with IBU did not prevent MIA phenotypes. |
Other prenatal intervention models have utilized dietary interventions as a potential means of reducing the maternal inflammatory response. MIA-treated offspring born to dams treated with oral probiotics during pregnancy did not develop MIA-induced behavioral deficits, including repetitive behaviors, depression and anxiety-like behaviors, and social deficits, and did not exhibit a reduction in parvalbumin positive neurons or GABA in the prefrontal cortex (127). Likewise, co-administration of the vitamin D hormone (1,25OHD, VITD) at the time of MIA-induction prevents behavioral deficits in associative learning, stereotyped digging and social interaction in juvenile offspring (128), and abnormal dopaminergic phenotypes (129). Although VitD plays a modulatory role in inflammatory responses (130), co-administration of VitD at the time of Poly IC injection did not reduce the maternal inflammatory cytokine profiles in blood samples collected four hours later. Additional research is needed to understand the therapeutic mechanisms associated with this particular prenatal VitD intervention. The authors also acknowledge that 1,25OHD as the active VitD hormone cannot be used in pregnancy due to its potential hypercalcaemic effects on the developing fetus and suggest future studies with the safe-to-use dietary form of VitD, cholecalciferol. Another dietary intervention that may have higher translational utility is the omega-3 (n-3) polyunsaturated fatty acids (PUFAs) diet. PUFAs, specifically docosahexaenoic acid (DHA), have been demonstrated to be essential for fetal development, including neuronal, retinal, and immune functions (131). In humans, low maternal DHA during gestation has been associated with reduced visual function and abnormalities in cognition and behavior, such as lower scores on tests of cognitive function (132–136). Although prenatal DHA supplementation reduced preterm birth and improved visual attention in infancy (137), there were no consistent long-term benefits observed later in childhood (138). DHA also has robust anti-inflammatory properties and has been demonstrated to inhibit IL-6 production (139), which has been shown to be a driving factor of pathology in previous MIA models (123). A recent MIA model compared animals assigned to a DHA-deficient (low n-3 PUFA) versus a DHA-sufficient diet administered to the dam throughout gestation and to the offspring (140). The DHA-enriched diet prevented MIA-induced changes in offspring behavior and reduced adult levels of IL-6. However, it is not known if the preventative effects of the DHA diet were driven by the prenatal reduction of maternal inflammatory response, the postnatal benefits of a DHA enriched diet, or both. Emerging evidence from a rat MIA model highlights the importance of considering sex as a biological variable as only male MIA exposed offspring exhibited impairments in working memory and reductions in glutamate decarboxylase 67 (GAD67) mRNA levels in frontal cortex that were restored by zinc supplementation during pregnancy (141). Collectively, these studies suggest that reduction of intrauterine inflammation may provide an opportunity to mitigate the deleterious effects of maternal infection on the developing fetal brain.
MIA model postnatal interventions.
The majority of MIA treatment models have focused on postnatal therapeutic interventions of offspring. For example, antipsychotic drug administration delivered to immature MIA-exposed rodent offspring attenuates the emergence of brain and behavioral abnormalities associated with SZ (80, 142, 143). Other laboratories have explored postnatal anti-inflammatory interventions. For example, periadolescent treatment with celecoxib, a cyclo-oxygenase-2 (COX-2) inhibitor, prevented MK801-induced hyperactivity in MIA-treated rats (144) and MIA mouse offspring treated postnatally with antipurinergic therapy (145, 146) or the gut bacterium Bacteroides fragilis (147) exhibit improved behavioral outcomes relevant to ASD phenotypes. Recent mouse MIA models have explored maternal T helper 17 cell and IL-17 pathways as opportunities to intervene in the prenatal environment (125, 148, 149). Finally, a growing number of postnatal dietary intervention papers have yielded promising results. For example, postnatal dietary (omega) n-3 polyunsaturated fatty acid (PUFA) supplementation prevents brain, behavioral, and epigenetic abnormalities in MIA-treated offspring (150, 151), while a PUFA dietary deficiency exacerbates behavioral changes in MIA-treated offspring (152). Likew ise, treating MIA-exposed juvenile offspring with either a ketogenic diet or glucoraphanin to reverses certain MIA-induced behavioral phenotypes (153, 154). Collectively, these studies highlight the ability to intervene in the neuroimmune postnatal environment and improve MIA-treated offspring outcomes. However, not all behaviors are “rescued” by postnatal interventions (i.e, social behavior deficits persisted in the microbiome-treated MIA offspring (147)), suggesting a need for additional preventative strategies that target earlier developmental time points. Emerging gene x environment MIA models have begun to explore the molecular and neural mechanisms of potential therapeutic interventions, and the potential of inflammatory cytokine production to ameliorate social behavior deficits by directly affecting neuronal activity in the central nervous system (155). Likewise, inconclusive results have been reported in recent MIA model studies that includes postnatal exposure to Delta(9)-tetrahydrocannabinol (THC) cannabidiol (CBD) (156–159) and will require further investigation.
Gaps in our knowledge.
We are at the earliest stages of utilizing the MIA model to explore preventative strategies, and acknowledge the challenges of developing safe and effective preventative interventions. Emerging evidence from human studies links maternal IL-6 levels during pregnancy with a variety of neurobehavioral outcomes, including functional connectivity, fiber tract integrity, and amygdala volume, as well as cognitive and behavioral development (160–163). These studies highlight the importance of the intrauterine immune environment and suggest that manipulation of maternal cytokines could have long-term consequences on offspring neurodevelopment. Indeed, elevated maternal IL-13 has been associated with hyperactivity and inattention eight years after birth (164). Although lasting changes in offspring immune function have been reported in nonhuman primate MIA models (165), additional research is needed to understand the relationship between maternal cytokines and fetal brain development in order to establish biomarkers that predict risk for offspring neurodevelopmental disorders. Can maternal cytokine levels be used to identify offspring at risk for neurodevelopmental and neuropsychiatric disorders? Beyond IL-6, which maternal cytokines influence fetal brain development? Which gestational time points are most must vulnerable? How can we develop evidence-based guidelines to safely and effectively manage maternal immune response during pregnancy? Can neuroimaging studies in infancy predict risk for neurodevelopmental disorders? Integrated research efforts between patient populations and animal models are needed to address critical questions.
IV. Conclusions and Future Directions
Although the prenatal environment is viewed as a time of vulnerability for neurodevelopmental disorder-related insults, it is also a time when preventative strategies and therapeutic interventions may be most effective. Exposure to infection during pregnancy (or the subsequent management of the maternal immune response) is a potentially modifiable risk factor. Given that millions of pregnant women are exposed to infection each year, even a small decrease in risk could have a significant public health effect on the prevalence of offspring neurodevelopmental disorders. There is a critical, and currently unmet, need to develop evidence-based guidelines to manage infection during pregnancy. One such area of management might be through maternal diet and remediation of severe and prolonged fevers. The overarching theme to this area of research is that the infectious agent matters only in as much as the level of the maternal immune response and the gestational timing of exposure. A critical next step is to identify the underpinnings of this differential response to infection to better prevent and treat maternal immune dysregulation during pregnancy.
Acknowledgments
This work was supported by the UC Davis Conte Center (NIMH; P50MH106438). We thank collaborators of the UC Davis Conte Center for conversations on the topic, Simone Grant for assistance with the literature review in Table 1 and Anurupa Kar for assistance in preparing the manuscript. Correspondence should be addressed to Dr. Melissa Bauman, University of California, Davis, The MIND Institute, 2805 50th Street, Sacramento 95817. mdbauman@ucdavis.edu
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.El Hajj N, Schneider E, Lehnen H, Haaf T. Epigenetics and life-long consequences of an adverse nutritional and diabetic intrauterine environment. Reproduction. 2014;148(6):R111–20. Epub 2014/09/05. doi: 10.1530/REP-14-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lumey LH, Stein AD. In utero exposure to famine and subsequent fertility: The Dutch Famine Birth Cohort Study. Am J Public Health. 1997;87(12):1962–6. Epub 1998/02/07. doi: 10.2105/ajph.87.12.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20(3):345–52. Epub 2005/05/17. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Stein Z, Susser M, Saenger G, Marolla F. Nutrition and mental performance. Science. 1972;178(4062):708–13. Epub 1972/11/17. doi: 10.1126/science.178.4062.708. [DOI] [PubMed] [Google Scholar]
- 5.ZA S, M S, G S, F M. Famine and Human Development: The Dutch Hunger Winter of 1944–1945. New York: Oxford University Press; 1975. [Google Scholar]
- 6.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295(7):349–53. Epub 1976/08/12. doi: 10.1056/NEJM197608122950701. [DOI] [PubMed] [Google Scholar]
- 7.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7. Epub 2007/04/21. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 8.Barker DJ, Gluckman PD, Godfrey KM, Harding JE, Owens JA, Robinson JS. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341(8850):938–41. Epub 1993/04/10. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 9.Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet. 1986;1(8489):1077–81. Epub 1986/05/10. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- 10.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2(8663):577–80. Epub 1989/09/09. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- 11.O’Donnell KJ, Meaney MJ. Fetal Origins of Mental Health: The Developmental Origins of Health and Disease Hypothesis. Am J Psychiatry. 2017;174(4):319–28. Epub 2016/11/15. doi: 10.1176/appi.ajp.2016.16020138. [DOI] [PubMed] [Google Scholar]
- 12.Swanson JM, Entringer S, Buss C, Wadhwa PD. Developmental origins of health and disease: environmental exposures. Semin Reprod Med. 2009;27(5):391–402. Epub 2009/08/28. doi: 10.1055/s-0029-1237427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–68. Epub 2009/08/28. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gillman MW, Barker D, Bier D, Cagampang F, Challis J, Fall C, Godfrey K, Gluckman P, Hanson M, Kuh D, Nathanielsz P, Nestel P, Thornburg KL. Meeting report on the 3rd International Congress on Developmental Origins of Health and Disease (DOHaD). Pediatr Res. 2007;61(5 Pt 1):625–9. Epub 2007/04/07. doi: 10.1203/pdr.0b013e3180459fcd. [DOI] [PubMed] [Google Scholar]
- 15.Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, Nemeroff CB, Reyes TM, Simerly RB, Susser ES, Nestler EJ. Early life programming and neurodevelopmental disorders. Biological psychiatry. 2010;68(4):314–9. doi: 10.1016/j.biopsych.2010.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estes ML, McAllister AK. Immune mediators in the brain and peripheral tissues in autism spectrum disorder. Nat Rev Neurosci. 2015;16(8):469–86. doi: 10.1038/nrn3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox-Edmiston E, Van de Water J. Maternal Anti-Fetal Brain IgG Autoantibodies and Autism Spectrum Disorder: Current Knowledge and its Implications for Potential Therapeutics. CNS drugs. 2015;29(9):715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patterson PH. Maternal infection and immune involvement in autism. Trends in molecular medicine. 2011;17(7):389–94. Epub 2011/04/13. doi: 10.1016/j.molmed.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64(1):61–78. Epub 2009/10/21. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Garay PA, McAllister AK. Novel roles for immune molecules in neural development: implications for neurodevelopmental disorders. Frontiers in synaptic neuroscience. 2010;2:136. doi: 10.3389/fnsyn.2010.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estes ML, McAllister AK. Maternal immune activation: Implications for neuropsychiatric disorders. Science. 2016;353(6301):772–7. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knuesel I, Chicha L, Britschgi M, Schobel SA, Bodmer M, Hellings JA, Toovey S, Prinssen EP. Maternal immune activation and abnormal brain development across CNS disorders. Nat Rev Neurol. 2014;10(11):643–60. doi: 10.1038/nrneurol.2014.187. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Johnson T, Sahin L, Tassinari MS, Anderson PO, Baker TE, Bucci-Rechtweg C, Burckart GJ, Chambers CD, Hale TW, Johnson-Lyles D, Nelson RM, Nguyen C, Pica-Branco D, Ren Z, Sachs H, Sauberan J, Zajicek A, Ito S, Yao LP. Evaluation of the Safety of Drugs and Biological Products Used During Lactation: Workshop Summary. Clinical pharmacology and therapeutics. 2017;101(6):736–44. doi: 10.1002/cpt.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang F, Zheng Q, Jin L. Dynamic Function and Composition Changes of Immune Cells During Normal and Pathological Pregnancy at the Maternal-Fetal Interface. Frontiers in immunology. 2019;10:2317. doi: 10.3389/fimmu.2019.02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collier SA, Rasmussen SA, Feldkamp ML, Honein MA, National Birth Defects Prevention S. Prevalence of self-reported infection during pregnancy among control mothers in the National Birth Defects Prevention Study. Birth defects research Part A, Clinical and molecular teratology. 2009;85(3):193–201. doi: 10.1002/bdra.20540. [DOI] [PubMed] [Google Scholar]
- 26.Zaretsky MV, Alexander JM, Byrd W, Bawdon RE. Transfer of inflammatory cytokines across the placenta. Obstet Gynecol. 2004;103(3):546–50. [DOI] [PubMed] [Google Scholar]
- 27.Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11(1):47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- 28.Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290(5):R1345–56. [DOI] [PubMed] [Google Scholar]
- 29.Hauguel-de Mouzon S, Guerre-Millo M. The placenta cytokine network and inflammatory signals. Placenta. 2006;27(8):794–8. doi: 10.1016/j.placenta.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Meyer U. Prenatal poly(i:C) exposure and other developmental immune activation models in rodent systems. Biological psychiatry. 2014;75(4):307–15. doi: 10.1016/j.biopsych.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 31.Meyer U, Feldon J, Dammann O. Schizophrenia and autism: both shared and disorder-specific pathogenesis via perinatal inflammation? Pediatr Res. 2011;69(5 Pt 2):26R–33R. doi: 10.1203/PDR.0b013e318212c196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167(3):261–80. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khandaker GM, Zimbron J, Lewis G, Jones PB. Prenatal maternal infection, neurodevelopment and adult schizophrenia: a systematic review of population-based studies. Psychological medicine. 2013;43(2):239–57. doi: 10.1017/S0033291712000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown AS, Cohen P, Harkavy-Friedman J, Babulas V, Malaspina D, Gorman JM, Susser ES. A.E. Bennett Research Award. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biological psychiatry. 2001;49(6):473–86. [DOI] [PubMed] [Google Scholar]
- 35.Brown AS, Schaefer CA, Wyatt RJ, Goetz R, Begg MD, Gorman JM, Susser ES. Maternal exposure to respiratory infections and adult schizophrenia spectrum disorders: a prospective birth cohort study. Schizophrenia bulletin. 2000;26(2):287–95. doi: 10.1093/oxfordjournals.schbul.a033453. [DOI] [PubMed] [Google Scholar]
- 36.Buka SL, Cannon TD, Torrey EF, Yolken RH, Collaborative Study Group on the Perinatal Origins of Severe Psychiatric D. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biological psychiatry. 2008;63(8):809–15. doi: 10.1016/j.biopsych.2007.09.022. [DOI] [PubMed] [Google Scholar]
- 37.Mortensen PB, Pedersen CB, Hougaard DM, Norgaard-Petersen B, Mors O, Borglum AD, Yolken RH. A Danish National Birth Cohort study of maternal HSV-2 antibodies as a risk factor for schizophrenia in their offspring. Schizophr Res. 2010;122(1–3):257–63. doi: 10.1016/j.schres.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 38.Borglum AD, Demontis D, Grove J, Pallesen J, Hollegaard MV, Pedersen CB, Hede mand A, Mattheisen M, investigators G, Uitterlinden A, Nyegaard M, Orntoft T, Wiuf C, Didriksen M, Nordentoft M, Nothen MM, Rietschel M, Ophoff RA, Cichon S, Yolken RH, Hougaard DM, Mortensen PB, Mors O. Genome-wide study of association and interaction with maternal cytomegalovirus infection suggests new schizophrenia loci. Mol Psychiatry. 2014;19(3):325–33. doi: 10.1038/mp.2013.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mortensen PB, Norgaard-Pedersen B, Waltoft BL, Sorensen TL, Hougaard D, Torrey EF, Yolken RH. Toxoplasma gondii as a risk factor for early-onset schizophrenia: analysis of filter paper blood samples obtained at birth. Biological psychiatry. 2007;61(5):688–93. doi: 10.1016/j.biopsych.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Brown AS, Schaefer CA, Quesenberry CP Jr., Liu L, Babulas VP, Susser ES Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am J Psychiatry. 2005;162(4):767–73. doi: 10.1176/appi.ajp.162.4.767. [DOI] [PubMed] [Google Scholar]
- 41.Babulas V, Factor-Litvak P, Goetz R, Schaefer CA, Brown AS. Prenatal exposure to maternal genital and reproductive infections and adult schizophrenia. Am J Psychiatry. 2006;163(5):927–9. doi: 10.1176/ajp.2006.163.5.927. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen HJ, Mortensen EL, Reinisch JM, Mednick SA. Association between prenatal exposure to bacterial infection and risk of schizophrenia. Schizophrenia bulletin. 2009;35(3):631–7. doi: 10.1093/schbul/sbn121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004;61(8):774–80. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- 44.Buka SL, Tsuang MT, Torrey EF, Klebanoff MA, Wagner RL, Yolken RH. Maternal cytokine levels during pregnancy and adult psychosis. Brain, behavior, and immunity. 2001;15(4):411–20. doi: 10.1006/brbi.2001.0644. [DOI] [PubMed] [Google Scholar]
- 45.Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004;161(5):889–95. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- 46.Allswede DM, Buka SL, Yolken RH, Torrey EF, Cannon TD. Elevated maternal cytokine levels at birth and risk for psychosis in adult offspring. Schizophr Res. 2016;172(1–3):41–5. doi: 10.1016/j.schres.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 47.Canetta S, Sourander A, Surcel HM, Hinkka-Yli-Salomaki S, Leiviska J, Kellendonk C, McKeague IW, Brown AS. Elevated maternal C-reactive protein and increased risk of schizophrenia in a national birth cohort. Am J Psychiatry. 2014;171(9):960–8. doi: 10.1176/appi.ajp.2014.13121579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Severance EG, Gressitt KL, Buka SL, Cannon TD, Yolken RH. Maternal complement C1q and increased odds for psychosis in adult offspring. Schizophr Res. 2014;159(1):14–9. doi: 10.1016/j.schres.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown AS, Vinogradov S, Kremen WS, Poole JH, Bao Y, Kern D, McKeague IW. Association of maternal genital and reproductive infections with verbal memory and motor deficits in adult schizophrenia. Psychiatry research. 2011;188(2):179–86. doi: 10.1016/j.psychres.2011.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166(6):683–90. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown AS, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Penner JD, Kochetkova A, Kern D, Schaefer CA. Prenatal infection and cavum septum pellucidum in adult schizophrenia. Schizophr Res. 2009;108(1–3):285–7. doi: 10.1016/j.schres.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellman LM, Deicken RF, Vinogradov S, Kremen WS, Poole JH, Kern DM, Tsai WY, Schaefer CA, Brown AS. Structural brain alterations in schizophrenia following fetal exposure to the inflammatory cytokine interleukin-8. Schizophr Res. 2010;121(1–3):46–54. doi: 10.1016/j.schres.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karl T, Arnold JC. Schizophrenia: a consequence of gene-environment interactions? Frontiers in behavioral neuroscience. 2014;8:435. doi: 10.3389/fnbeh.2014.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Os J, Rutten BP, Poulton R. Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophrenia bulletin. 2008;34(6):1066–82. doi: 10.1093/schbul/sbn117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166(9):1025–30. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- 56.Blomstrom A, Karlsson H, Gardner R, Jorgensen L, Magnusson C, Dalman C. Associations Between Maternal Infection During Pregnancy, Childhood Infections, and the Risk of Subsequent Psychotic Disorder--A Swedish Cohort Study of Nearly 2 Million Individuals. Schizophrenia bulletin. 2016;42(1):125–33. doi: 10.1093/schbul/sbv112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Allswede DM, Yolken RH, Buka SL, Cannon TD. Cytokine concentrations throughout pregnancy and risk for psychosis in adult offspring: a longitudinal case-control study. Lancet Psychiatry. 2020. Epub 2020/02/09. doi: 10.1016/S2215-0366(20)30006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chess S. Autism in children with congenital rubella. Journal of autism and childhood schizophrenia. 1971;1(1):33–47. [DOI] [PubMed] [Google Scholar]
- 59.Desmond MM, Wilson GS, Melnick JL, Singer DB, Zion TE, Rudolph AJ, Pineda RG, Ziai MH, Blattner RJ. Congenital rubella encephalitis. Course and early sequelae. J Pediatr. 1967;71(3):311–31. [DOI] [PubMed] [Google Scholar]
- 60.Deykin EY, MacMahon B. Viral exposure and autism. American journal of epidemiology. 1979;109(6):628–38. [DOI] [PubMed] [Google Scholar]
- 61.Ivarsson SA, Bjerre I, Vegfors P, Ahlfors K. Autism as one of several disabilities in two children with congenital cytomegalovirus infection. Neuropediatrics. 1990;21(2):102–3. doi: 10.1055/s-2008-1071471. [DOI] [PubMed] [Google Scholar]
- 62.Markowitz PI. Autism in a child with congenital cytomegalovirus infection. Journal of autism and developmental disorders. 1983;13(3):249–53. [DOI] [PubMed] [Google Scholar]
- 63.Sweeten TL, Posey DJ, McDougle CJ. Brief report: autistic disorder in three children with cytomegalovirus infection. Journal of autism and developmental disorders. 2004;34(5):583–6. [DOI] [PubMed] [Google Scholar]
- 64.Frazier TW, Thompson L, Youngstrom EA, Law P, Hardan AY, Eng C, Morris N. A twin study of heritable and shared environmental contributions to autism. Journal of autism and developmental disorders. 2014;44(8):2013–25. doi: 10.1007/s10803-014-2081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Szatmari P, White J, Merikangas KR. The use of genetic epidemiology to guide classification in child and adult psychopathology. International review of psychiatry. 2007;19(5):483–96. doi: 10.1080/09540260701563619. [DOI] [PubMed] [Google Scholar]
- 66.Atladottir HO, Thorsen P, Ostergaard L, Schendel DE, Lemcke S, Abdallah M, Parner ET. Maternal infection requiring hospitalization during pregnancy and autism spectrum disorders. Journal of autism and developmental disorders. 2010;40(12):1423–30. doi: 10.1007/s10803-010-1006-y. [DOI] [PubMed] [Google Scholar]
- 67.Atladóttir HÓ, Henriksen TB, Schendel DE, Parner ET. Autism after infection, febrile episodes, and antibiotic use during pregnancy: an exploratory study. Pediatrics. 2012;130(6):e1447–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zerbo O, Iosif AM, Walker C, Ozonoff S, Hansen RL, Hertz-Picciotto I. Is maternal influenza or fever during pregnancy associated with autism or developmental delays? Results from the CHARGE (CHildhood Autism Risks from Genetics and Environment) study. Journal of autism and developmental disorders. 2013;43(1):25–33. doi: 10.1007/s10803-012-1540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, Croen LA. Maternal Infection During Pregnancy and Autism Spectrum Disorders. Journal of autism and developmental disorders. 2015;45(12):4015–25. doi: 10.1007/s10803013-2016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee BK, Magnusson C, Gardner RM, Blomstrom A, Newschaffer CJ, Burstyn I, Karlsson H, Dalman C. Maternal hospitalization with infection during pregnancy and risk of autism spectrum disorders. Brain, behavior, and immunity. 2015;44:100–5. doi: 10.1016/j.bbi.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Haddad BJS, Jacobsson B, Chabra S, Modzelewska D, Olson EM, Bernier R, Enquobahrie DA, Hagberg H, Ostling S, Rajagopal L, Adams Waldorf KM, Sengpiel V. Long-term Risk of Neuropsychiatric Disease After Exposure to Infection In Utero. JAMA psychiatry. 2019. doi: 10.1001/jamapsychiatry.2019.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Croen LA, Qian Y, Ashwood P, Zerbo O, Schendel D, Pinto-Martin J, Daniele Fallin M, Levy S, Schieve LA, Yeargin-Allsopp M, Sabourin KR, Ames JL. Infection and Fever in Pregnancy and Autism Spectrum Disorders: Findings from the Study to Explore Early Development. Autism research : official journal of the International Society for Autism Research. 2019. doi: 10.1002/aur.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Goines PE, Croen LA, Braunschweig D, Yoshida CK, Grether J, Hansen R, Kharrazi M, Ashwood P, Van de Water J. Increased mid-gestational IFN-gamma, IL-4, and IL-5 in women giving birth to a child with autism: a case-control study. Molecular Autism. 2011;2(1):13–40. Epub August 2, 2011. doi: 10.1186/2040-2392-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jones KL, Croen LA, Yoshida CK, Heuer L, Hansen R, Zerbo O, DeLorenze GN, Kharrazi M, Yolken R, Ashwood P, Van de Water J. Autism with intellectual disability is associated with increased levels of maternal cytokines and chemokines during gestation. Mol Psychiatry. 2016. Epub 2016/05/25. doi: 10.1038/mp.2016.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid chemokines and autism spectrum disorders: an exploratory study utilizing a Danish Historic Birth Cohort. Brain, behavior, and immunity. 2012;26(1):170–6. [DOI] [PubMed] [Google Scholar]
- 76.Abdallah MW, Larsen N, Grove J, Nørgaard-Pedersen B, Thorsen P, Mortensen EL, Hougaard DM. Amniotic fluid inflammatory cytokines: potential markers of immunologic dysfunction in autism spectrum disorders. The World Journal of Biological Psychiatry. 2013;14(7):528–38. [DOI] [PubMed] [Google Scholar]
- 77.Egorova O, Myte R, Schneede J, Hagglof B, Bolte S, Domellof E, Ivars A’roch B, Elgh F, Ueland PM, Silfverdal SA. Maternal blood folate status during early pregnancy and occurrence of autism spectrum disorder in offspring: a study of 62 serum biomarkers. Mol Autism. 2020;11:7. doi: 10.1186/s13229-020-0315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arsenault D, St-Amour I, Cisbani G, Rousseau LS, Cicchetti F. The different effects of LPS and poly I:C prenatal immune challenges on the behavior, development and inflammatory responses in pregnant mice and their offspring. Brain, behavior, and immunity. 2014;38:77–90. doi: 10.1016/j.bbi.2013.12.016. [DOI] [PubMed] [Google Scholar]
- 79.Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neuroscience and biobehavioral reviews. 2009;33(7):1061–79. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophrenia bulletin. 2010;36(3):607–23. Epub 2008/10/11. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Careaga M, Murai T, Bauman MD. Maternal Immune Activation and Autism Spectrum Disorder: From Rodents to Nonhuman and Human Primates. Biological psychiatry. 2017;81(5):391–401. doi: 10.1016/j.biopsych.2016.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain, behavior, and immunity. 2010;24(6):881–97. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 83.Meyer U, Feldon J. Neural basis of psychosis-related behaviour in the infection model of schizophrenia. Behav Brain Res. 2009;204(2):322–34. doi: 10.1016/j.bbr.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Patterson PH. Immune involvement in schizophrenia and autism: etiology, pathology and animal models. Behav Brain Res. 2009;204(2):313–21. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 85.Piontkewitz Y, Arad M, Weiner I. Tracing the development of psychosis and its prevention: what can be learned from animal models. Neuropharmacology. 2012;62(3):1273–89. doi: 10.1016/j.neuropharm.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 86.Bergdolt L, Dunaevsky A. Brain changes in a maternal immune activation model of neurodevelopmental brain disorders. Progress in neurobiology. 2019;175:1–19. doi: 10.1016/j.pneurobio.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stewart AM, Kalueff AV. Developing better and more valid animal models of brain disorders. Behav Brain Res. 2015;276:28–31. doi: 10.1016/j.bbr.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 88.Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1(1):9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ryan AM, Berman RF, Bauman MD. Bridging the species gap in translational research for neurodevelopmental disorders. Neurobiol Learn Mem. 2019;165:106950. Epub 2018/10/23. doi: 10.1016/j.nlm.2018.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Willner P. The validity of animal models of depression. Psychopharmacology (Berl). 1984;83(1):1–16. [DOI] [PubMed] [Google Scholar]
- 91.Gordon JA. A Hypothesis-Based Approach: The Use of Animals in Mental Health Research. NIMH Director’s. Message [Internet]. 2019. [Google Scholar]
- 92.Brown AS, Meyer U. Maternal Immune Activation and Neuropsychiatric Illness: A Translational Research Perspective. Am J Psychiatry. 2018;175(11):1073–83. doi: 10.1176/appi.ajp.2018.17121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nestler EJ, Hyman SE. Animal models of neuropsychiatric disorders. Nat Neurosci. 2010;13(10):1161–9. doi: 10.1038/nn.2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tordjman S, Drapier D, Bonnot O, Graignic R, Fortes S, Cohen D, Millet B, Laurent C, Roubertoux PL. Animal models relevant to schizophrenia and autism: validity and limitations. Behav Genet. 2007;37(1):61–78. doi: 10.1007/s10519-006-9120-5. [DOI] [PubMed] [Google Scholar]
- 95.Kentner AC, Bilbo SD, Brown AS, Hsiao EY, McAllister AK, Meyer U, Pearce BD, Pletnikov MV, Yolken RH, Bauman MD. Maternal immune activation: reporting guidelines to improve the rigor, reproducibility, and transparency of the model. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2019;44(2):245–58. doi: 10.1038/s41386-018-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Abazyan B, Nomura J, Kannan G, Ishizuka K, Tamashiro KL, Nucifora F, Pogorelov V, Ladenheim B, Yang C, Krasnova IN, Cadet JL, Pardo C, Mori S, Kamiya A, Vogel MW, Sawa A, Ross CA, Pletnikov MV. Prenatal interaction of mutant DISC1 and immune activation produces adult psychopathology. Biological psychiatry. 2010;68(12):1172–81. doi: 10.1016/j.biopsych.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lipina TV, Zai C, Hlousek D, Roder JC, Wong AH. Maternal immune activation during gestation interacts with Disc1 point mutation to exacerbate schizophrenia-related behaviors in mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(18):7654–66. doi: 10.1523/JNEUROSCI.0091-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.O’Leary C, Desbonnet L, Clarke N, Petit E, Tighe O, Lai D, Harvey R, Waddington JL, O’Tuathaigh C. Phenotypic effects of maternal immune activation and early postnatal milieu in mice mutant for the schizophrenia risk gene neuregulin-1. Neuroscience. 2014;277:294–305. Epub 2014/06/28. doi: 10.1016/j.neuroscience.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 99.Wu WL, Adams CE, Stevens KE, Chow KH, Freedman R, Patterson PH. The interaction between maternal immune activation and alpha 7 nicotinic acetylcholine receptor in regulating behaviors in the offspring. Brain, behavior, and immunity. 2015;46:192–202. doi: 10.1016/j.bbi.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ehninger D, Sano Y, de Vries PJ, Dies K, Franz D, Geschwind DH, Kaur M, Lee YS, Li W, Lowe JK, Nakagawa JA, Sahin M, Smith K, Whittemore V, Silva AJ. Gestational immune activation and Tsc2 haploinsufficiency cooperate to disrupt fetal survival and may perturb social behavior in adult mice. Mol Psychiatry. 2012;17(1):62–70. doi: 10.1038/mp.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schaafsma SM, Gagnidze K, Reyes A, Norstedt N, Mansson K, Francis K, Pfaff DW. Sex-specific gene-environment interactions underlying ASD-like behaviors. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(6):1383–8. Epub 2017/01/25. doi: 10.1073/pnas.1619312114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Meyer U, Nyffeler M, Schwendener S, Knuesel I, Yee BK, Feldon J. Relative prenatal and postnatal maternal contributions to schizophrenia-related neurochemical dysfunction after in utero immune challenge. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(2):441–56. Epub 2007/04/20. doi: 10.1038/sj.npp.1301413. [DOI] [PubMed] [Google Scholar]
- 103.Richetto J, Calabrese F, Meyer U, Riva MA. Prenatal versus postnatal maternal factors in the development of infection-induced working memory impairments in mice. Brain, behavior, and immunity. 2013;33:190–200. doi: 10.1016/j.bbi.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 104.Schwendener S, Meyer U, Feldon J. Deficient maternal care resulting from immunological stress during pregnancy is associated with a sex-dependent enhancement of conditioned fear in the offspring. Journal of neurodevelopmental disorders. 2009;1(1):15–32. doi: 10.1007/s11689-008-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, Winter C, Riva MA, Mortensen PB, Schedlowski M, Meyer U. Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science. 2013;339(6123):1095–9. doi: 10.1126/science.1228261. [DOI] [PubMed] [Google Scholar]
- 106.Pollak DD, Weber-Stadlbauer U. Transgenerational consequences of maternal immune activation. Seminars in cell & developmental biology. 2019. doi: 10.1016/j.semcdb.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 107.Pollak DD, Weber-Stadlbauer U. Transgenerational consequences of maternal immune activation. Seminars in cell & developmental biology. 2020;97:181–8. Epub 2019/06/25. doi: 10.1016/j.semcdb.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 108.Taouk L, Schulkin J. Transgenerational transmission of pregestational and prenatal experience: maternal adversity, enrichment, and underlying epigenetic and environmental mechanisms. Journal of developmental origins of health and disease. 2016;7(6):588–601. doi: 10.1017/S2040174416000416. [DOI] [PubMed] [Google Scholar]
- 109.Conway F, Brown AS. Maternal Immune Activation and Related Factors in the Risk of Offspring Psychiatric Disorders. Frontiers in psychiatry. 2019;10:430. doi: 10.3389/fpsyt.2019.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gumusoglu SB, Stevens HE. Maternal Inflammation and Neurodevelopmental Programming: A Review of Preclinical Outcomes and Implications for Translational Psychiatry. Biological psychiatry. 2019;85(2):107–21. doi: 10.1016/j.biopsych.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 111.TASK FORCE ON RESEARCH SPECIFIC TO PREGNANT WOMEN AND LACTATING WOMEN https://www.nichd.nih.gov/sites/default/files/2018-09/PRGLAC_Report.pdf: 2018.
- 112.Gata-Garcia A, Diamond B. Maternal Antibody and ASD: Clinical Data and Animal Models. Frontiers in immunology. 2019;10:1129. doi: 10.3389/fimmu.2019.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Meyer U. Neurodevelopmental Resilience and Susceptibility to Maternal Immune Activation. Trends in neurosciences. 2019;42(11):793–806. doi: 10.1016/j.tins.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 114.Coiro P, Pollak DD. Sex and gender bias in the experimental neurosciences: the case of the maternal immune activation model. Transl Psychiatry. 2019;9(1):90. Epub 2019/02/16. doi: 10.1038/s41398-019-0423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kowash HM, Potter HG, Edye ME, Prinssen EP, Bandinelli S, Neill JC, Hager R, Glazier JD. Poly(I:C) source, molecular weight and endotoxin contamination affect dam and prenatal outcomes, implications for models of maternal immune activation. Brain, behavior, and immunity. 2019;82:160–6. doi: 10.1016/j.bbi.2019.08.006. [DOI] [PubMed] [Google Scholar]
- 116.Carlezon WA Jr., Kim W, Missig G, Finger BC, Landino SM, Alexander AJ, Mokler EL, Robbins JO, Li Y, Bolshakov VY, McDougle CJ, Kim KS Maternal and early postnatal immune activation produce sex-specific effects on autism-like behaviors and neuroimmune function in mice. Scientific reports. 2019;9(1):16928. doi: 10.1038/s41598-019-53294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gogos A, Sbisa A, Witkamp D, van den Buuse M. Sex differences in the effect of maternal immune activation on cognitive and psychosis-like behaviour in Long Evans rats. The European journal of neuroscience. 2020. doi: 10.1111/ejn.14671. [DOI] [PubMed] [Google Scholar]
- 118.Braun AE, Carpentier PA, Babineau BA, Narayan AR, Kielhold ML, Moon HM, Shankar A, Su J, Saravanapandian V, Haditsch U, Palmer TD. “Females Are Not Just ‘Protected’ Males”: Sex-Specific Vulnerabilities in Placenta and Brain after Prenatal Immune Disruption. eNeuro. 2019;6(6). doi: 10.1523/ENEURO.0358-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Allard MJ, Giraud A, Segura M, Sebire G. Sex-specific maternofetal innate immune responses triggered by group B Streptococci. Scientific reports. 2019;9(1):8587. doi: 10.1038/s41598-019-45029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barke TL, Money KM, Du L, Serezani A, Gannon M, Mirnics K, Aronoff DM. Sex modifies placental gene expression in response to metabolic and inflammatory stress. Placenta. 2019;78:1–9. doi: 10.1016/j.placenta.2019.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Weckman AM, Ngai M, Wright J, McDonald CR, Kain KC. The Impact of Infection in Pregnancy on Placental Vascular Development and Adverse Birth Outcomes. Frontiers in microbiology. 2019;10:1924. doi: 10.3389/fmicb.2019.01924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nunez Estevez KJ, Rondon-Ortiz AN, Nguyen JQT, Kentner AC. Environmental influences on placental programming and offspring outcomes following maternal immune activation. Brain, behavior, and immunity. 2020;83:44–55. doi: 10.1016/j.bbi.2019.08.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development 123. through interleukin-6. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(40):10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wu WL, Hsiao EY, Yan Z, Mazmanian SK, Patterson PH. The placental interleukin-6 signaling controls fetal brain development and behavior. Brain, behavior, and immunity. 2017;62:11–23. doi: 10.1016/j.bbi.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Choi GB, Yim YS, Wong H, Kim S, Kim H, Kim SV, Hoeffer CA, Littman DR, Huh JR. The maternal interleukin-17a pathway in mice promotes autism-like phenotypes in offspring. Science. 2016;351(6276):933–9. doi: 10.1126/science.aad0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Horvath G, Otrokocsi L, Beko K, Baranyi M, Kittel A, Fritz-Ruenes PA, Sperlagh B. P2X7 Receptors Drive Poly(I:C) Induced Autism-like Behavior in Mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2019;39(13):2542–61. doi: 10.1523/JNEUROSCI.1895-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang X, Yang J, Zhang H, Yu J, Yao Z. Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism research : official journal of the International Society for Autism Research. 2019;12(4):576–88. doi: 10.1002/aur.2079. [DOI] [PubMed] [Google Scholar]
- 128.Vuillermot S, Luan W, Meyer U, Eyles D. Vitamin D treatment during pregnancy prevents autism-related phenotypes in a mouse model of maternal immune activation. Mol Autism. 2017;8:9. doi: 10.1186/s13229-017-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Luan W, Hammond LA, Vuillermot S, Meyer U, Eyles DW. Maternal Vitamin D Prevents Abnormal Dopaminergic Development and Function in a Mouse Model of Prenatal Immune Activation. Scientific reports. 2018;8(1):9741. doi: 10.1038/s41598-018-28090-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wobke TK, Sorg BL, Steinhilber D. Vitamin D in inflammatory diseases. Frontiers in physiology. 2014;5:244. doi: 10.3389/fphys.2014.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bozzatello P, Brignolo E, De Grandi E, Bellino S. Supplementation with Omega-3 Fatty Acids in Psychiatric Disorders: A Review of Literature Data. Journal of clinical medicine. 2016;5(8). Epub 2016/07/30. doi: 10.3390/jcm5080067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hibbeln JR, Davis JM, Steer C, Emmett P, Rogers I, Williams C, Golding J. Maternal seafood consumption in pregnancy and neurodevelopmental outcomes in childhood (ALSPAC study): an observational cohort study. Lancet. 2007;369(9561):578–85. Epub 2007/02/20. doi: 10.1016/s0140-6736(07)60277-3. [DOI] [PubMed] [Google Scholar]
- 133.Oken E, Osterdal ML, Gillman MW, Knudsen VK, Halldorsson TI, Strom M, Bellinger DC, Hadders-Algra M, Michaelsen KF, Olsen SF. Associations of maternal fish intake during pregnancy and breastfeeding duration with attainment of developmental milestones in early childhood: a study from the Danish National Birth Cohort. The American journal of clinical nutrition. 2008;88(3):789–96. Epub 2008/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Oken E, Radesky JS, Wright RO, Bellinger DC, Amarasiriwardena CJ, Kleinman KP, Hu H, Gillman MW. Maternal fish intake during pregnancy, blood mercury levels, and child cognition at age 3 years in a US cohort. American journal of epidemiology. 2008;167(10):1171–81. Epub 2008/03/21. doi: 10.1093/aje/kwn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Georgieff MK, Innis SM. Controversial nutrients that potentially affect preterm neurodevelopment: essential fatty acids and iron. Pediatr Res. 2005;57(5 Pt 2):99r–103r. Epub 2005/04/09. doi: 10.1203/01.pdr.0000160542.69840.0f. [DOI] [PubMed] [Google Scholar]
- 136.Schuchardt JP, Huss M, Stauss-Grabo M, Hahn A. Significance of long-chain polyunsaturated fatty acids (PUFAs) for the development and behaviour of children. European journal of pediatrics. 2010;169(2):149–64. Epub 2009/08/13. doi: 10.1007/s00431-009-1035-8. [DOI] [PubMed] [Google Scholar]
- 137.Colombo J, Gustafson KM, Gajewski BJ, Shaddy DJ, Kerling EH, Thodosoff JM, Doty T, Brez CC, Carlson SE. Prenatal DHA supplementation and infant attention. Pediatr Res. 2016;80(5):656–62. Epub 2016/11/02. doi: 10.1038/pr.2016.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Colombo J, Shaddy DJ, Gustafson K, Gajewski BJ, Thodosoff JM, Kerling E, Carlson SE. The Kansas University DHA Outcomes Study (KUDOS) clinical trial: long-term behavioral follow-up of the effects of prenatal DHA supplementation. The American journal of clinical nutrition. 2019;109(5):1380–92. doi: 10.1093/ajcn/nqz018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Trebble T, Arden NK, Stroud MA, Wootton SA, Burdge GC, Miles EA, Ballinger AB, Thompson RL, Calder PC. Inhibition of tumour necrosis factor-alpha and interleukin 6 production by mononuclear cells following dietary fish-oil supplementation in healthy men and response to antioxidant co-supplementation. The British journal of nutrition. 2003;90(2):405–12. Epub 2003/08/12. [DOI] [PubMed] [Google Scholar]
- 140.Weiser MJ, Mucha B, Denheyer H, Atkinson D, Schanz N, Vassiliou E, Benno RH. Dietary docosahexaenoic acid alleviates autistic-like behaviors resulting from maternal immune activation in mice. Prostaglandins, leukotrienes, and essential fatty acids. 2016;106:27–37. doi: 10.1016/j.plefa.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 141.Alizadeh F, Davoodian N, Kazemi H, Ghasemi-Kasman M, Shaerzadeh F. Prenatal zinc supplementation attenuates lipopolysaccharide-induced behavioral impairments in maternal immune activation model. Behav Brain Res. 2020;377:112247. doi: 10.1016/j.bbr.2019.112247. [DOI] [PubMed] [Google Scholar]
- 142.Piontkewitz Y, Assaf Y, Weiner I. Clozapine administration in adolescence prevents postpubertal emergence of brain structural pathology in an animal model of schizophrenia. Biological psychiatry. 2009;66(11):1038–46. Epub 2009/09/04. doi: 10.1016/j.biopsych.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 143.Piontkewitz Y, Arad M, Weiner I. Risperidone administered during asymptomatic period of adolescence prevents the emergence of brain structural pathology and behavioral abnormalities in an animal model of schizophrenia. Schizophrenia bulletin. 2011;37(6):1257–69. doi: 10.1093/schbul/sbq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zavitsanou K, Lim CK, Purves-Tyson T, Karl T, Kassiou M, Banister SD, Guillemin GJ, Weickert CS. Effect of maternal immune activation on the kynurenine pathway in preadolescent rat offspring and on MK801-induced hyperlocomotion in adulthood: amelioration by COX-2 inhibition. Brain, behavior, and immunity. 2014;41:173–81. doi: 10.1016/j.bbi.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 145.Naviaux RK, Zolkipli Z, Wang L, Nakayama T, Naviaux JC, Le TP, Schuchbauer MA, Rogac M, Tang Q, Dugan LL. Antipurinergic therapy corrects the autism-like features in the poly (IC) mouse model. Plos One. 2013;8(3):e57380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Naviaux J, Schuchbauer M, Li K, Wang L, Risbrough V, Powell S, Naviaux R. Reversal of autism-like behaviors and metabolism in adult mice with single-dose antipurinergic therapy. Translational psychiatry. 2014;4(6):e400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, Codelli JA, Chow J, Reisman SE, Petrosino JF, Patterson PH, Mazmanian SK. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155(7):1451–63. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kim S, Kim H, Yim YS, Ha S, Atarashi K, Tan TG, Longman RS, Honda K, Littman DR, Choi GB, Huh JR. Maternal gut bacteria promote neurodevelopmental abnormalities in mouse offspring. Nature. 2017;549(7673):528–32. doi: 10.1038/nature23910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shin Yim Y, Park A, Berrios J, Lafourcade M, Pascual LM, Soares N, Yeon Kim J, Kim S, Kim H, Waisman A, Littman DR, Wickersham IR, Harnett MT, Huh JR, Choi GB. Reversing behavioural abnormalities in mice exposed to maternal inflammation. Nature. 2017;549(7673):482–7. doi: 10.1038/nature23909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Li Q, Leung YO, Zhou I, Ho LC, Kong W, Basil P, Wei R, Lam S, Zhang X, Law AC, Chua SE, Sham PC, Wu EX, McAlonan GM. Dietary supplementation with n-3 fatty acids from weaning limits brain biochemistry and behavioural changes elicited by prenatal exposure to maternal inflammation in the mouse model. Transl Psychiatry. 2015;5:e641. doi: 10.1038/tp.2015.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Basil P, Li Q, Gui H, Hui TCK, Ling VHM, Wong CCY, Mill J, McAlonan GM, Sham PC. Prenatal immune activation alters the adult neural epigenome but can be partly stabilised by a n-3 polyunsaturated fatty acid diet. Transl Psychiatry. 2018;8(1):125. doi: 10.1038/s41398-018-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Labrousse VF, Leyrolle Q, Amadieu C, Aubert A, Sere A, Coutureau E, Gregoire S, Bretillon L, Pallet V, Gressens P, Joffre C, Nadjar A, Laye S. Dietary omega-3 deficiency exacerbates inflammation and reveals spatial memory deficits in mice exposed to lipopolysaccharide during gestation. Brain, behavior, and immunity. 2018;73:427–40. doi: 10.1016/j.bbi.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 153.Matsuura A, Ishima T, Fujita Y, Iwayama Y, Hasegawa S, Kawahara-Miki R, Maekawa M, Toyoshima M, Ushida Y, Suganuma H, Kida S, Yoshikawa T, Iyo M, Hashimoto K. Dietary glucoraphanin prevents the onset of psychosis in the adult offspring after maternal immune activation. Scientific reports. 2018;8(1):2158. doi: 10.1038/s41598-018-20538-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruskin DN, Murphy MI, Slade SL, Masino SA. Ketogenic diet improves behaviors in a maternal immune activation model of autism spectrum disorder. Plos One. 2017;12(2):e0171643. doi: 10.1371/journal.pone.0171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Reed MD, Yim YS, Wimmer RD, Kim H, Ryu C, Welch GM, Andina M, King HO, Waisman A, Halassa MM, Huh JR, Choi GB. IL-17a promotes sociability in mouse models of neurodevelopmental disorders. Nature. 2020;577(7789):24953. doi: 10.1038/s41586-019-1843-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lecca S, Luchicchi A, Scherma M, Fadda P, Muntoni AL, Pistis M. Delta(9)-Tetrahydrocannabinol During Adolescence Attenuates Disruption of Dopamine Function Induced in Rats by Maternal Immune Activation. Frontiers in behavioral neuroscience. 2019;13:202. doi: 10.3389/fnbeh.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Osborne AL, Solowij N, Babic I, Lum JS, Huang XF, Newell KA, Weston-Green K. Cannabidiol improves behavioural and neurochemical deficits in adult female offspring of the maternal immune activation (poly I:C) model of neurodevelopmental disorders. Brain, behavior, and immunity. 2019;81:574–87. doi: 10.1016/j.bbi.2019.07.018. [DOI] [PubMed] [Google Scholar]
- 158.Osborne AL, Solowij N, Babic I, Lum JS, Newell KA, Huang XF, Weston-Green K. Effect of cannabidiol on endocannabinoid, glutamatergic and GABAergic signalling markers in male offspring of a maternal immune activation (poly I:C) model relevant to schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2019;95:109666. doi: 10.1016/j.pnpbp.2019.109666. [DOI] [PubMed] [Google Scholar]
- 159.Jimenez Naranjo C, Osborne AL, Weston-Green K. Effect of cannabidiol on muscarinic neurotransmission in the pre-frontal cortex and hippocampus of the poly I:C rat model of schizophrenia. Progress in neuro-psychopharmacology & biological psychiatry. 2019;94:109640. doi: 10.1016/j.pnpbp.2019.109640. [DOI] [PubMed] [Google Scholar]
- 160.Spann MN, Monk C, Scheinost D, Peterson BS. Maternal Immune Activation During the Third Trimester Is Associated with Neonatal Functional Connectivity of the Salience Network and Fetal to Toddler Behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2018;38(11):2877–86. doi: 10.1523/JNEUROSCI.227217.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rudolph MD, Graham AM, Feczko E, Miranda-Dominguez O, Rasmussen JM, Nardos R, Entringer S, Wadhwa PD, Buss C, Fair DA. Maternal IL-6 during pregnancy can be estimated from newborn brain connectivity and predicts future working memory in offspring. Nat Neurosci. 2018;21(5):765–72. doi: 10.1038/s41593-018-0128-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Rasmussen JM, Graham AM, Entringer S, Gilmore JH, Styner M, Fair DA, Wadhwa PD, Buss C. Maternal Interleukin-6 concentration during pregnancy is associated with variation in frontolimbic white matter and cognitive development in early life. NeuroImage. 2018. doi: 10.1016/j.neuroimage.2018.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Graham AM, Rasmussen JM, Rudolph MD, Heim CM, Gilmore JH, Styner M, Potkin SG, Entringer S, Wadhwa PD, Fair DA, Buss C. Maternal Systemic Interleukin-6 During Pregnancy Is Associated With Newborn Amygdala Phenotypes and Subsequent Behavior at 2 Years of Age. Biological psychiatry. 2018;83(2):109–19. doi: 10.1016/j.biopsych.2017.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Thurmann L, Herberth G, Rolle-Kampczyk U, Roder S, Borte M, von Bergen M, Lehmann I, Trump S. Elevated Gestational IL-13 During Fetal Development Is Associated With Hyperactivity and Inattention in Eight-Year-Old Children. Frontiers in immunology. 2019;10:1658. doi: 10.3389/fimmu.2019.01658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rose DR, Careaga M, Van de Water J, McAllister K, Bauman MD, Ashwood P. Long-term altered immune responses following fetal priming in a non-human primate model of maternal immune activation. Brain, behavior, and immunity. 2017;63:60–70. doi: 10.1016/j.bbi.2016.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]