Abstract
Objective
Oral squamous cell carcinoma (OSCC) is the most frequent oral malignancy with a poor prognosis, in which tumor-infiltrating immune cells may play a critical role. Therefore, our study aims to screen potential immune cells and immune-related genes for predicting OSCC prognosis.
Methods
A total of 310 OSCC patients with full transcriptional data and clinical characteristics were extracted from the TCGA database. Then, we obtained their abundance of tumor-infiltrating immune cells on TIMER 2.0 and analyzed them using xCell method. Univariate and multivariate Cox regressions were applied successively to identify the immune cells associated with overall survival of OSCC patients. Furthermore, we screened the prognostic genes that related to the identified immune cells and validated their expressions by immunohistochemistry.
Results
CD4+ central memory T (TCM) cell was recognized as the sole independent immune cell correlated with OSCC prognosis (p = 0.0085). A novel nomogram based on CD4+ TCM cell abundance was established for predicting the prognosis of OSCC patients, with calibration plots showing good performance for 1-, 3-, 5-year overall survival. Thirty-four related prognostic genes were screened according to the differential abundance of CD4+ TCM cell infiltration. In immunohistochemistry analysis, DEFB1 showed a significant positive relationship with the density of CD4+ TCM cells (p = 0.0075).
Conclusion
CD4+ central memory T cell was proposed as an independent prognostic biomarker for OSCC patients. DEFB1 might positively regulate the abundance of tumor-infiltrating CD4+ TCM cells, thus improving OSCC prognosis. Our findings may provide a new insight into better prognosis prediction and precise medicine for OSCC.
Keywords: oral squamous cell carcinoma, CD4+ central memory T cell, DEFB1, prognosis biomarker
Introduction
Oral squamous cell carcinoma (OSCC) is an aggressive and insidious malignancy covering the lips or other parts of the oral cavity. It was estimated that about 300,000 new cases were diagnosed as OSCC and about 150,000 cases were died from OSCC in 2020, accounting for almost 2% of new cases and deaths in total 36 cancers.1 Though the modified combination therapy involving surgery resection, chemotherapy and radiotherapy has been routinely adopted for OSCC treatment, its prognosis remains poor with a dismal 5-year survival rate of around 65%.2 Nearly 50% of OSCC patients were observed with lymph node metastasis at diagnosis, whose survival rates even decreased by approximately 50%.3 Immunotherapy, through modifying the immune contexture, has revolutionized cancer treatment.4 Specifically, cancer vaccines, through tumor-specific antigens, could promote antitumor immune responses mediated by T cells.5 Adoptive cell transfer, including chimeric antigen receptor T cells and T-cell receptor engineered T cells, have been demonstrated to generate favorable clinical outcomes in metastatic melanoma and hepatocellular carcinoma.6,7 Blocking immune checkpoint molecules, such as PD-1, PDL-1 and CTLA-4, could improve treatment outcomes and enhance prognosis of OSCC patients with metastasis or local recurrence.8 Combined results have suggested that PD-1 or PDL-1 overexpression might be correlated with adverse prognosis in OSCC patients. Indeed, these sound effects of immunotherapy in cancer treatment are inseparable from the immune cells infiltrated within tumor stroma.
The immune cell infiltration in tumor microenvironment, which involves a group of heterogeneous cells, is greatly complex. These immune cells consist of macrophages, neutrophils, dendritic cells, myeloid-derived suppressor cells and lymphocytes, playing a multifold role in the whole process of tumor initiation, progression and metastasis.9 Multiple mechanisms have been evolved to escape immune surveillance in cancer cells, by which results in the dysfunction of immune cells and abrogation of anti-tumor responses.10 The mass-cytometry-based single-cell atlas of immune infiltration in clear cell renal cell carcinoma delineated immunosuppressive phenotypes in T cell populations, including functionally exhausted T cells and inhibitory Tregs.11 Two clusters of exhausted CD8+ T cells were identified to be associated with poor prognosis by single-cell sequencing in non-small cell lung cancer.12 Interestingly, the transcriptomic landscape of T cell populations profiled a differential proportion of exhausted CD8+ T cells in head and neck squamous cell carcinoma patients.13 These common and specific characteristics shown in different cancer types at single-cell level shed light on the significance of tumor-infiltrating lymphocytes (TILs) in cancer progression, prognosis and personalized immunotherapy.10
Currently, the prognostic value of TILs and related genes has been highly appreciated in several cancer types, including OSCC.14 High densities of either CD3+ T cells at invasion margin or CD8+ T cells at tumor center presented favorable outcomes in OSCC patients.15 CD8+ and CD56+ immune cells were implemented as independent prognostic tools that associated with lymph node metastasis in OSCC patients.16 Conventional methods utilized in these studies, like histochemistry or flow cytometry, can only measure the function of a single tumor-infiltrating immune cell rather than the whole immune microenvironment on cancer progression.17 Increasing studies have identified multiple immune-related genes to uncover the role of immune cells in head and neck squamous cell carcinoma and OSCC patients using bioinformatics.18,19 Four immune-related genetic signatures were defined to predict the overall survival (OS) of OSCC patients.18 However, these researches investigated the correlation between immune cells and cancer prognosis at gene-levels, restraining our understandings to tumor-infiltrating immune cells. Therefore, to investigate the prognostic immune cells in OSCC, this study estimated the abundance of infiltrating immune cells among OSCC patients on the web platform TIMER 2.0 and analyzed its correlation with OSCC prognosis. Prognostic genes related to immune cells were screened and further validated using immunohistochemistry (IHC) analysis.
Materials and Methods
Data Collection and Normalization
Transcriptional data and clinical characteristics of OSCC patients were mined from the TCGA database (https://portal.gdc.cancer.gov/). Primary squamous cell carcinoma located in the alveolar ridge, the base of tongue, buccal mucosa, the floor of mouth, hard palate, lip, oral cavity, and tongue were defined as OSCC. Only the patients with complete clinical data including T stage, N stage, histological grade and survival data could be enrolled in this study. Eventually, 310 OSCC patients were included and their transcriptional data were normalized to transcripts per kilobase million (TPM) for further study.
Estimation of Immune Cell Abundance
Several online tools have been released on TIMER2.0 (http://timer.cistrome.org/), in which we selected xCell method, since it is the most classical method and can evaluate up to 36 kinds of infiltrating immune and stromal cells.20 Infiltration estimation for all TCGA samples was downloaded directly on the estimation page for further study.
Screening of Prognosis Related Immune Cells and Prognostic Model
Univariate Cox regression was used to primarily screen out the potential prognostic immune cells, which were then enrolled in multivariate Cox regression model with other clinical characteristics such as age and gender to identify the independent prognostic immune cells. Accordingly, we established a prognostic model for OSCC patients based on their scores of immune cells and clinical characteristics. Concordance index (C-index) value was used to evaluate the predictive value of this model, while resampling method was further applied to verify the accuracy of this nomogram. On this basis, 310 OSCC patients were divided into low score group (n = 155) and high score group (n = 155) according to the median of the score. Kaplan–Meier survival analysis was used to compare the survival probability between two groups. Finally, hierarchical survival analysis was used to explore the optimal population characteristics for this prognostic model.
Bioinformatics Analysis of Genes Associated with CD4+ TCM Cell Infiltration
Furthermore, to explore the genes related with CD4+ TCM cells, we divided the OSCC patients into “high score group” and “low score group” according to the median of the CD4+ TCM cell score. R software and limma package were used to screen the differentially expressed genes (DEGs) between two groups. |Log(FC)|≥1 and adjust p value<0.05 were defined as a filter to DEGs. All DEGs were presented in the form of volcanic maps by R and ggplot2 package. Gene ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis were carried out according to DEGs by R software, clusterProfiler, org.Hs.eg.db and other related packages as previously described,21 to indicate the possible functions and signal pathways related with “CD4+ TCM cell”.
Sample Collection and Microarray Construction
Briefly, all samples were collected from OSCC patients during surgical resection in the Center of Stomatology and the department of Oral and Maxillofacial Surgery, Xiangya Hospital, Central South University. Then, we gathered their corresponding clinicopathological characteristics and survival information. The staging criteria and histopathological classification were based on the 8th edition of the American Joint Committee on Cancer staging system and the 4th edition of the World Health Organization classification of head and neck tumors, respectively. In total, sixty-one OSCC tissue samples were used to construct two identical sets of tissue microarrays, fifty-eight effective points of which were utilized for IHC analysis in this study. Written consent forms have been obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of School of Life Sciences, Central South University on Nov. 30th, 2020 (NO.2020–1–42).
IHC and Scoring System
Then, we used IHC to explore the expression of immune-related DEGs in clinical OSCC tissues. Both tissue microarrays were deparaffinized and rehydrated before IHC staining. Co-expression of CCR7 and CD45RO is identified as CD4+ TCM cell.22 Therefore, tissue microarray 1 was incubated with diluted primary antibodies, namely CCR7 (1:500, ab253187, Abcam) and CD45RO (1:400, 55618, cell signaling technology). Then the endogenous peroxidase and endogenous AP were inactivated by 3% H2O2 and Klear Dual Enzyme Block E36, respectively. Alkaline phosphatase (AP) and horseradish peroxidase (HRP) double staining detection system (ZSGB-BIO, DS-0004) was used for visualization, in which the CD45RO antigen was stained red while the CCR7 antigen was brown. The primary antibody for tissue microarray 2 was DEFB1 (2 µg/mL, ab14425, Abcam), and it was visualized by HRP single staining detection system (SP-9000, ZSGB-BIO). The method for scoring the expression of DEFB1 has been described in our previous study.23 Fifty-eight OSCC patients were then divided into positive group and negative group according to the density of CD4+ TCM cells, between which the expression of DEFB1 was compared.
Statistical Analysis
All statistical analysis and visualization were done by R script (version 4.0.2), R studio (version 1.3.1056) and related packages as previously stated. Cox regression and Kaplan–Meier method were used for survival analysis. Enumeration data were analyzed by chi-square test. The measurement data were tested for normality firstly. Then, Student t test was used for the data in line with the normal distribution, and the Mann–Whitney test was used for those not in line with the normal distribution. All the statistical analyses were two-tailed, and adjust p value or p value less than 0.05 was defined as significant.
Results
Screening Signature Immune Cell Subsets for Predicting OSCC Prognosis
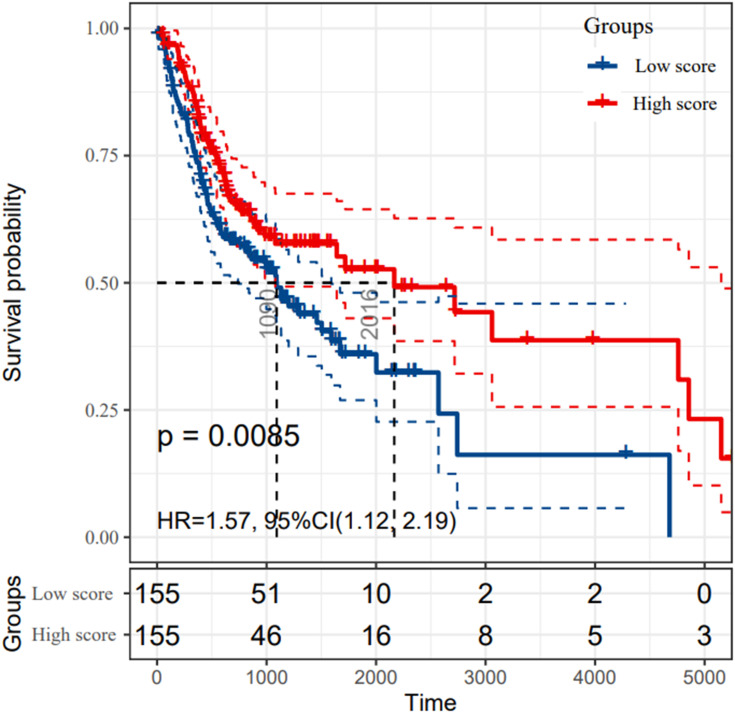
As Table 1 lists, we displayed the clinical and pathologic characteristics of 310 OSCC patients extracted from TCGA database and 58 OSCC patients collected for further IHC analysis. The abundance of each immune cell subset in the TCGA cohort was estimated and analyzed by univariate and multivariate Cox regression. The results of regression model were depicted in Table S1. As a consequence, CD4+ TCM cell was identified as the sole independent immune cell subset for predicting OSCC prognosis. According to the highest score of CD4+ TCM cells, the 310 OSCC patients were divided into “high group” and “low group”. The Kaplan–Meier survival analysis manifested that OS of OSCC patients with low CD4+ TCM cells infiltration were significantly much shorter than those with abundant CD4+ TCM cells infiltration (p = 0.0085) (Figure 1). Hence, our findings suggested CD4+ TCM cell as a novel prognostic marker for OSCC patients.
Table 1.
Clinical Information for OSCC Patients in TCGA Database and Microarrays
| Cohort | TCGA Database | Clinical Tissues for IHC | |
|---|---|---|---|
| N | 310 | 58 | |
| Age, years old | 61.69 ± 12.83 | 51.88 ± 9.27 | |
| Gender | Male | 227 | 51 |
| Female | 103 | 7 | |
| Location | Tongue | 148 | 37 |
| Buccal | 22 | 11 | |
| Others | 140 | 10 | |
| T stage | T1 | 21 | 43 |
| T2 | 104 | 14 | |
| T3 | 84 | 1 | |
| T4 | 114 | 0 | |
| NA (Not available) | 7 | 0 | |
| N stage | N0 | 168 | 39 |
| N1-N3 | 150 | 19 | |
| NA (Not available) | 12 | 0 | |
| Differentiation | Well | 53 | 35 |
| Moderate/Poor | 269 | 23 | |
| NA (Not available) | 8 | 0 |
Abbreviations: OSCC, oral squamous cell carcinoma; IHC, immunohistochemistry.
Figure 1.
The survival rates of oral squamous cell carcinoma (OSCC) patients grouped by CD4+ central memory T cell abundance. The OSCC patients in TCGA cohort were divided into groups with high and low score according to the median abundance of CD4+ central memory T cells. The Kaplan–Meier analysis was used to compare the survival rates of OSCC patients with high and low CD4+ central memory T cell abundance, which was tested by log-rank method.
Establishment of a Prognostic Nomogram Based on Tumor-Infiltrating Immune Cells
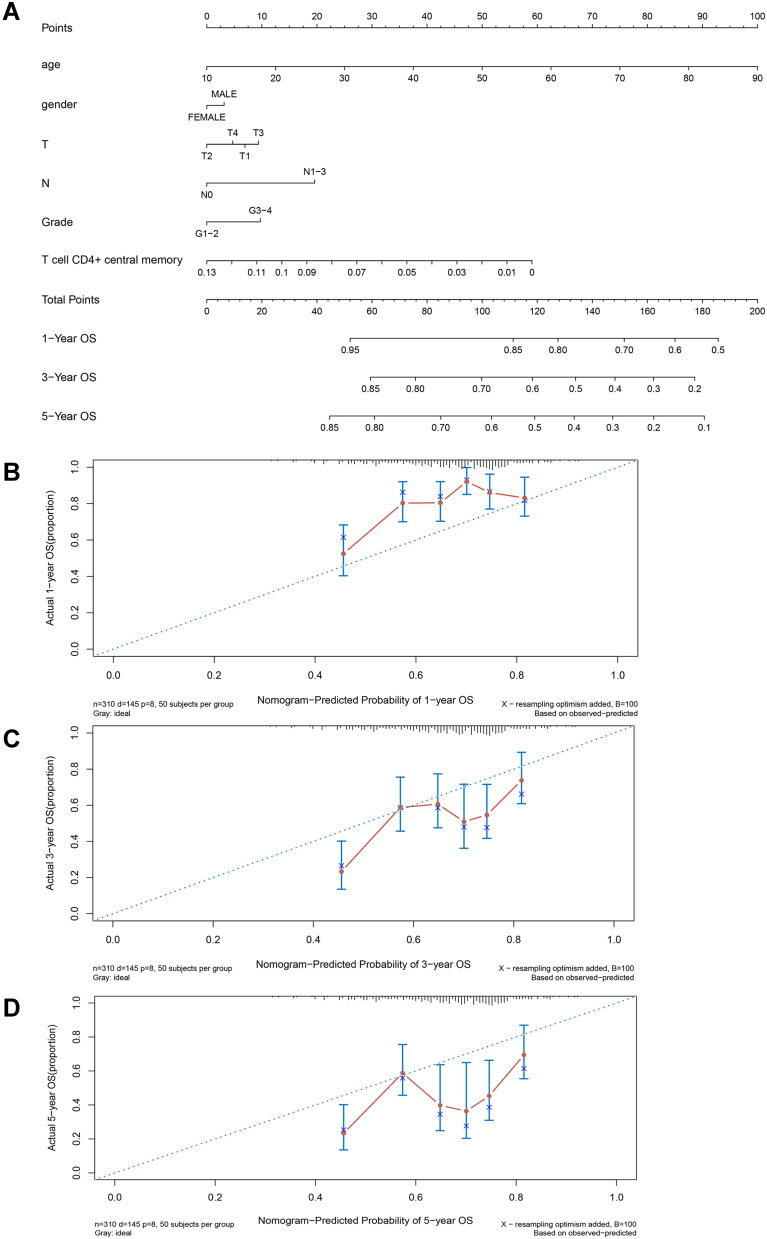
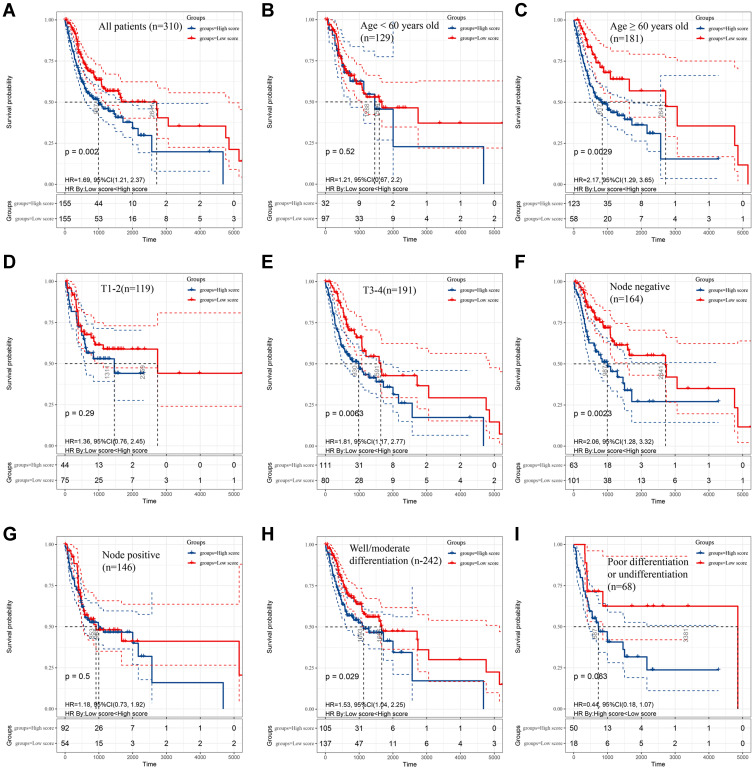
After screening out the prognostic immune cell subset, we constructed a novel prognostic model based on CD4+ TCM cells thus better predicting the survival rates of OSCC patients in 1-, 3- and 5-years. As shown in Figure 2A, variables of age, gender, TNM stage and histological grade were together included in the prognostic nomogram. The concordance index (C-index) of this prediction model was 0.604 (95% confidence interval 0.579–0.629). Additionally, by randomly sampling from the TCGA database, the constructed calibration curves displayed good fitness for 1-, 3-, and 5-year survival rates of OSCC patients between nomogram prediction and the actual outcomes (Figure 2B-D). The Kaplan–Meier survival analysis shown in Figure 3A indicates that patients with low scores calculated by this prediction model significantly lived much longer than those with high scores (p = 0.002). Particularly, further subgroup analysis indicated that the prognostic value of nomogram was more significant for OSCC patients with older age (≥60 years) (p = 0.0029), larger tumor size (T3-4) (p = 0.0063), negative lymph nodes (p = 0.0023) and well/moderate differentiation (p = 0.029) (Figure 3B-I).
Figure 2.
Establishment of predictive nomogram for oral squamous cell carcinoma (OSCC) patients based on age, gender, T sage, N stage, grade and CD4+ central memory T cell abundance in TCGA cohort. (A) Predictive nomogram. Calibration plot of the nomogram with 1-year (B), 3-year (C), and 5-year (D) overall survival of OSCC patients.
Figure 3.
The survival rates of oral squamous cell carcinoma (OSCC) patients grouped by scores calculated by predictive nomogram. The overall survival of all OSCC patients (A), OSCC patients with age < 60 years old (B), age ≥ 60 years old (C), T1-2 stage (D), T3-4 stage (E), negative nodes metastasis (F), positive metastasis (G), well/moderate differentiation (H) and poor differentiation (I). The Kaplan–Meier method was used and p value was calculated by Log rank test.
Identification and Enrichment Analysis of Differentially Expressed Prognostic Immune-Related Genes
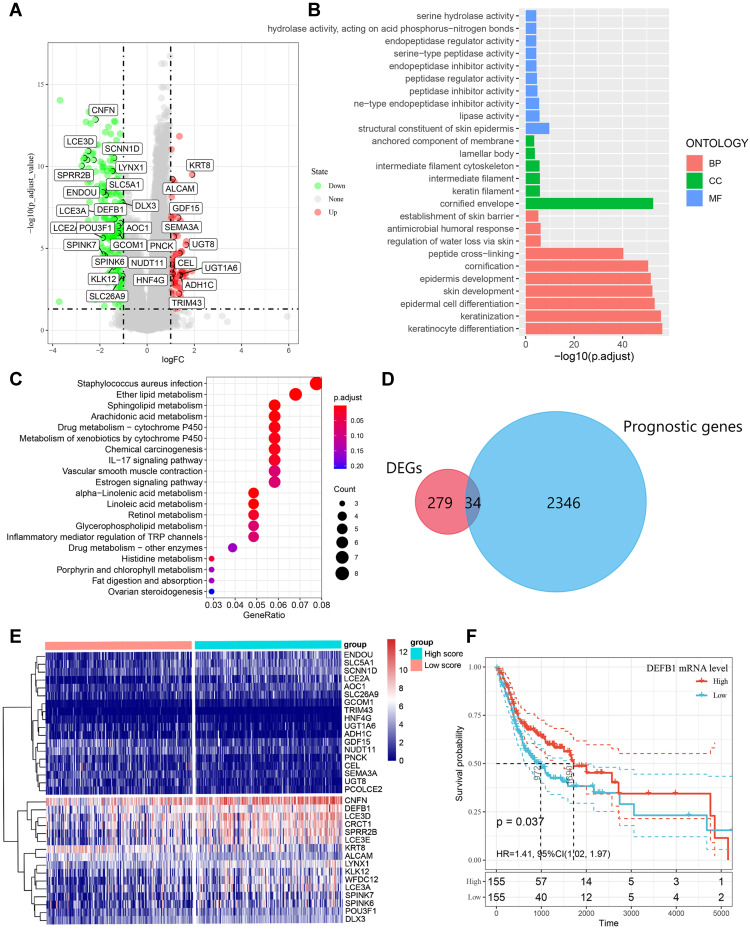
By comparing the DEGs between high and low CD4+ TCM cell score groups, 279 immune-related DEGs were identified with volcano plot analysis and presented in Figure 4A. Both KEGG and GO analysis for pathway enrichment were performed to investigate the potential biological functions of these screened 279 DEGs. As shown in Figure 4B, GO analysis indicated that these DEGs were associated with antimicrobial humoral response, epidermal cell differentiation and keratinization. Chemical carcinogenesis and interleukin (IL)-17 signaling pathway were enriched in the 279 DEGs from KEGG analysis (Figure 4C). Moreover, we screened 2346 genes that correlated with OSCC prognosis from the TCGA database and overlapped them with the pre-identified 279 DEGs through Venn diagram, thus thirty-four candidate prognostic genes were eventually recognized to be related with CD4+ TCM cells (Figure 4D). As shown in Figure 4E, the cluster analysis visually depicted the expression difference and the distribution of these 34 candidate prognostic DEGs between groups with high and low CD4+ TCM cell scores. In addition, we applied Kaplan–Meier survival analysis in the TCGA dataset to validate that DEFB1 was positively correlated with the OS of OSCC patients (Figure 4F). Our study revealed that 34 prognostic genes including DEFB1 might be correlated with the infiltration of CD4+ TCM cells in OSCC.
Figure 4.
Identification of differentially expressed genes (DEGs) that related with CD4+ central memory T cell and functional enrichment analysis. (A) The volcano plot of DEGs between oral squamous cell carcinoma (OSCC) groups with high and low CD4+ central memory T cell abundance. (B)Gene Ontology (GO) enrichment analysis of DEGs related with CD4+ central memory T cell. MF, molecular function; CC, cell component; BP, biological process. (C) Enriched Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways of DEGs related with CD4+ central memory T cell. (D) Venn diagram of the overlapped genes between DEGs related with CD4+ central memory T cell and prognostic genes in OSCC patients. (E) Cluster analysis for prognostic DEGs related with CD4+ central memory T cell OSCC patients from the TCGA cohort. (F) The survival rate of OSCC patients in the TCGA cohort with high and low DEFB1 expression.
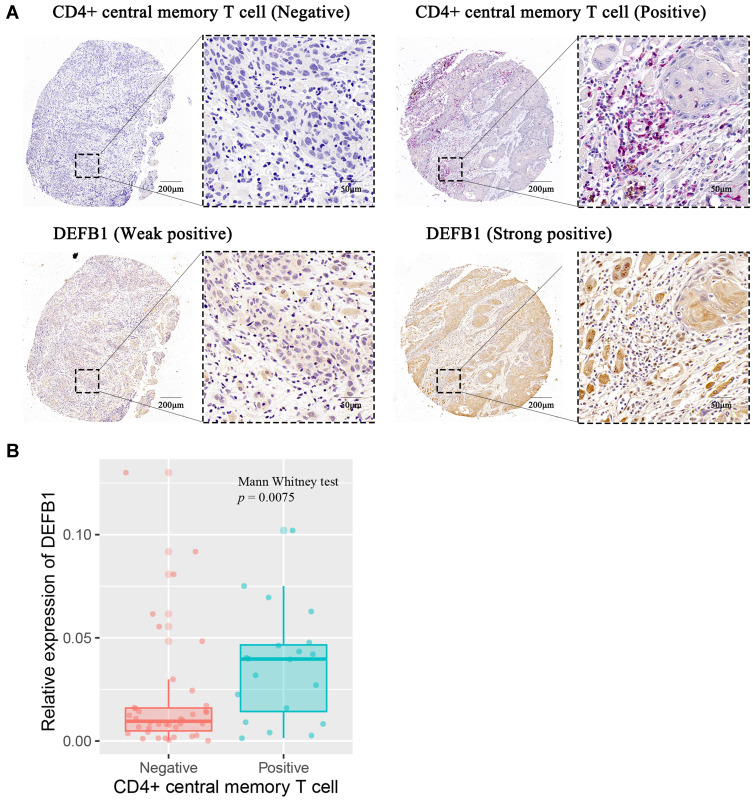
The Expression of CD4+ TCM Cells and DEFB1 in Clinical OSCC Tissues
To further confirm the results concluded by bioinformatics analysis, we chose DEFB1 from the 34 DEGs to detect its expression in 58 clinical OSCC tissues and compared it with corresponding density of tumor-infiltrating CD4+ TCM cells. As shown in Figure 5A, the tumor location with high DEFB1 staining was infiltrated with a high density of CD4+ TCM cells. Quantitatively, our results presented that DEFB1 expression was positively correlated with the abundance of CD4+ TCM cells (p = 0.0075) (Figure 5B). Taken together, our results demonstrated that DEFB1, a prognostic biomarker, was closely associated with tumor-infiltrating CD4+ TCM cells within OSCC tissues.
Figure 5.
The correlation between protein expression level of DEFB1 and infiltrating CD4+ central memory T cells in oral squamous cell carcinoma (OSCC) tissues. (A) Representative images of both high/low expression of DEFB1 and CD4+ central memory T cell at the same location in OSCC tissues. CD4+ central memory T cell was marked with both CD45RO+ (red) and CCR7+ (brown). (B) The correlation between DEFB1 expression and CD4+ central memory T cell infiltration in OSCC tissues. p value was calculated by Mann–Whitney test.
Discussion
Despite immunotherapy, such as adoptive cell transfer and immune checkpoint inhibitors, maintains clinical efficacy, its benefits are still limited in a small fraction of patients.24 Cytotoxic T cells, memory T cells and B cells are beneficial for prolonged survival, while Tregs and neutrophils are associated with poor prognosis.25 Mounting evidenced has suggested that a combination of multiple immune cells is more reliable to reflect the interplays between immune responses and cancer progression. Therefore, it is extremely essential for seeking novel immune cells for better survival stratification and effective immunotherapy in OSCC patients. Our results initially presented the prognostic value of CD4+ TCM cell in OSCC patients and constructed an effective prediction model for clinicians to predict their prognosis preliminarily. Furthermore, we investigated the potential value of CD4+ TCM cell infiltration in OSCC by integrating the transcriptional data in TCGA with bioinformatics. DEFB1 was found to be closely associated with infiltrating CD4+ TCM cells and it was further validated by IHC.
In our study, only CD4+ TCM cell was independently associated with the prognosis of OSCC patients. Any cells expressing CD45RO+ were considered as memory T cells, which are classically divided into effector memory T cells and central memory T cells. They can be activated vigorously by previously encountered antigens and present long-term protection.26 Distinctive with effector memory T cells, central memory T cells, expressing chemokine receptor CCR7, generally recirculate through lymph nodes or secondary lymphoid organs, eventually home to T cell areas of these organs.27 When reactivated, central memory T cells have a considerable capacity to secrete IL-2 and proliferate upon the stimulation of T cell antigen receptors in draining secondary lymphoid.28 After several days of stimulation, plentiful effector T cells migrate to the infection sites, leading to a robust pathogen control.29 Previous studies have manifested that CD45RO+ memory T cells showed a significant prognostic value in malignant tumors, such as gastric cancer and renal cell carcinoma.30,31 Zhou et al proposed to identify memory T cells as independent prognosticator for OSCC patients.32 Increasing CD45RO+ TILs in tumor center could significantly favor their overall and recurrence-free survival. Notably, in a recent study, Vahidi et al detected increased proportions of CD4+ TCM cell populations in breast cancer patients with positive lymphovascular invasion.33 An enhanced percentage of a specific CD4+ TCM cell phenotype in peripheral blood was proposed to potentially predict clinical responses to PD-1 blockade treatment in patients with malignant melanoma.34 Likewise, it was observed that CD4+ TCM cells in peripheral blood were expanded in an anaplastic thyroid cancer patient treated with anti-PD-1 therapy.35 Our KEGG pathway enrichment analysis indicated that IL-17 signaling pathway might be involved in the improved prognosis in OSCC patients with abundant CD4+ TCM cells. Indeed, IL-17, mainly secreted by T helper 17 cells, has been demonstrated to show an elevated expression in OSCC patients and negatively correlated with the OS of head and neck cancer patients.36,37
Furthermore, we identified 34 prognostic genes associated with CD4+ TCM cell in OSCC tissues, including 18 down-regulated genes such as SLC5A1 and PNCK, and 16 up-regulated genes like CNFN and DEFB1 (Figure 4E). Particularly, emerging studies have launched DEFB1 as a tumor suppressor gene that could promote cancer cell apoptosis.38 DEFB1, as one of the most important human β-defensins, was traditionally acknowledged to play an indispensable role in immune response at epithelial surfaces against bacterial infection.39 Decreased DEFB1 gene expression was shown in both OSCC tissues and OSCC cell lines.40,41 Moreover, Han et al have demonstrated DEFB1 to be a favorable prognostic factor for OSCC patients, which could suppress the migration and invasion of OSCC cells but had no influence on their proliferation or apoptosis.42 However, DEFB1, as a link between innate and adaptive immune response, it was still unclear whether its anti-tumor property could be implicated with the infiltrating immune cells in tumor microenvironment. Our analysis implied that DEFB1 expression might be correlated with the abundance of tumor-infiltrating CD4+ TCM cells in OSCC patients.
Therefore, in this study, we determined the expression of DEFB1 in clinical OSCC tissues and evaluated its relationship with the density of CD4+ TCM cells using IHC. Strikingly, a positive correlation was found between DEFB1 gene expression and CD4+ TCM cell abundance. DEFB1 has been predicted to interact with CCR6, a chemokine receptor expressed by specific immune cells such as memory T cells and dendritic cells.43 The high affinity between β-defensins and CCR6 helps in recruiting these immune cells to the site of microbial invasion.44 This may partially explain our results that enriched tumor-infiltrating CD4+ TCM cells were detected in OSCC patients with DEFB1 overexpression. Moreover, it has been reported that the cases without DEFB1 gene expression are easier to generate lymph node metastasis in OSCC patients, which might be associated with MMP-2, RhoA and RhoC.42 Our findings that DEFB1 positively correlated with the abundance of CD4+ TCM cells in OSCC patients may elucidate the potential mechanisms in a new way. The tumor-infiltrating memory T cells has been demonstrated to inhibit tumor metastasis in various cancer types. An increased density of memory T cells in situ colorectal cancer implied a low risk of metastatic invasion45 In lung adenocarcinoma, the infiltration of memory T cells was negatively correlated with lymph node metastasis and prognosis.46 Hence, we postulated that DEFB1 might enhance the tumor-infiltrating CD4+ TCM cells in OSCC patients by which suppressed lymph node metastasis, thus improving the prognosis of OSCC patients. However, further functional experiments in vivo are needed to validate the correlation between DEFB1 expression and tumor-infiltrating CD4+ TCM cells in OSCC.
Conclusion
In summary, our study identified CD4+ TCM cell subset and 34 genes related to tumor-infiltrating CD4+ TCM cells as prognostic biomarkers for OSCC patients. An effective prediction model for estimating the prognosis of OSCC patients was established. We supposed DEFB1 which has been recognized as a prognostic biomarker for OSCC might regulate the abundance of CD4+ TCM cells thus improving OSCC prognosis. These findings may provide new prospects for OSCC prognosis prediction and immunotherapy.
Acknowledgments
This work was supported by the National Natural Science Foundation [81873717], the National Natural Science Foundation [82170973], and the Graduate Students Independently Explore Innovative Projects of Central South University. Also, we appreciated Shanghai Biochip Company, Ltd. for constructing the tissue microarray with us.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding authors upon request.
Ethics Approval Statement
This study was conducted in accordance with the Declaration of Helsinki and has been approved by the Ethics Committee of School of Life Sciences, Central South University on Nov. 30th, 2020 (NO.2020–1–42). All patients have signed informed consent forms before collecting their clinical tissue samples.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Pei J, Liu J, Chen Y, Liu Y, Liao X, Pan J. Relationship between maxillary posterior molar roots and the maxillary sinus floor: cone-beam computed tomography analysis of a western Chinese population. J Int Med Res. 2020;48(6):300060520926896. doi: 10.1177/0300060520926896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arun I, Maity N, Hameed S, et al. Lymph node characteristics and their prognostic significance in oral squamous cell carcinoma. Head Neck. 2021;43(2):520–533. doi: 10.1002/hed.26715 [DOI] [PubMed] [Google Scholar]
- 4.Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306. doi: 10.1038/nrc3245 [DOI] [PubMed] [Google Scholar]
- 5.Butterfield LH. Cancer vaccines. BMJ. 2015;350. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62–68. doi: 10.1126/science.aaa4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang R, Zhang Z, Liu Z, et al. Adoptive cell transfer therapy for hepatocellular carcinoma. Front Med. 2019;13(1):3–11. doi: 10.1007/s11684-019-0684-x [DOI] [PubMed] [Google Scholar]
- 8.Kujan O, van Schaijik B, Farah CS. Immune checkpoint inhibitors in oral cavity squamous cell carcinoma and oral potentially malignant disorders: a systematic review. Cancers. 2020;12(7):1937. doi: 10.3390/cancers12071937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Whiteside TL. Tumor-Infiltrating Lymphocytes in Human Malignancies. RG Landes Company; 1993. [Google Scholar]
- 10.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi: 10.1038/s41423-020-0488-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chevrier S, Levine JH, Zanotelli VRT, et al. An immune atlas of clear cell renal cell carcinoma. Cell. 2017;169(4):736–749. doi: 10.1016/j.cell.2017.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Zhang Y, Zheng L, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24(7):978–985. doi: 10.1038/s41591-018-0045-3 [DOI] [PubMed] [Google Scholar]
- 13.Puram SV, Tirosh I, Parikh AS, et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell. 2017;171(7):1611–1624. doi: 10.1016/j.cell.2017.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barnes TA, Amir E. HYPE or HOPE: the prognostic value of infiltrating immune cells in cancer. Br J Cancer. 2017;117(4):451–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou C, Wu Y, Jiang L, et al. Density and location of CD3+ and CD8+ tumor-infiltrating lymphocytes correlate with prognosis of oral squamous cell carcinoma. J Oral Pathol Med. 2018;47(4):359–367. doi: 10.1111/jop.12698 [DOI] [PubMed] [Google Scholar]
- 16.Fang J, Li X, Ma D, et al. Prognostic significance of tumor infiltrating immune cells in oral squamous cell carcinoma. BMC Cancer. 2017;17(1):1–9. doi: 10.1186/s12885-017-3317-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Che Y, Luo Z, Zhang C, Sun N, Gao S, He J. Immune signature of tumor-infiltrating immune cells predicts the prognosis and therapeutic effects in squamous cell carcinoma. Int Immunopharmacol. 2020;87:106802. [DOI] [PubMed] [Google Scholar]
- 18.Zou C, Huang D, Wei H, et al. Identification of immune-related risk signatures for the prognostic prediction in oral squamous cell carcinoma. J Immunol Res. 2021;2021:6303759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song D, Tian J, Han X, Li X. A model of seven immune checkpoint-related genes predicting overall survival for head and neck squamous cell carcinoma. Eur Arch Oto-Rhino-Laryngol. 2021;1–11. doi: 10.1007/s00405-020-06540-4 [DOI] [PubMed] [Google Scholar]
- 20.Aran D, Hu Z, Butte AJ. xCell: digitally portraying the tissue cellular heterogeneity landscape. Genome Biol. 2017;18(1):220. doi: 10.1186/s13059-017-1349-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu G, Wang LG, Han Y, He QY. clusterProfiler: an R package for comparing biological themes among gene clusters. Omics. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sei JJ, Cox KS, Dubey SA, et al. Effector and central memory poly-functional CD4(+) and CD8(+) T Cells are Boosted upon ZOSTAVAX(®) vaccination. Front Immunol. 2015;6:553. doi: 10.3389/fimmu.2015.00553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu X, Xia K, Xiong H, Su T. G3BP1 may serve as a potential biomarker of proliferation, apoptosis, and prognosis in oral squamous cell carcinoma. J Oral Pathol Med. 2021. doi: 10.1111/jop.13199 [DOI] [PubMed] [Google Scholar]
- 24.Yang Z, Yan G, Zheng L, et al. YKT6, as a potential predictor of prognosis and immunotherapy response for oral squamous cell carcinoma, is related to cell invasion, metastasis, and CD8+ T cell infiltration. OncoImmunology. 2021;10(1):1938890. doi: 10.1080/2162402x.2021.1938890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruni D, Angell HK, Galon J. The immune contexture and Immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer. 2020;20(11):662–680. doi: 10.1038/s41568-020-0285-7 [DOI] [PubMed] [Google Scholar]
- 26.Mackay CR. Dual personality of memory T cells. Nature. 1999;402(6763):3–4. doi: 10.1038/35005503 [DOI] [PubMed] [Google Scholar]
- 27.Rahimi RA, Luster AD. Redefining memory T cell subsets. Trends Immunol. 2020;41(8):645–648. doi: 10.1016/j.it.2020.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pepper M, Jenkins MK. Origins of CD4+ effector and central memory T cells. Nat Immunol. 2011;12(6):467–471. doi: 10.1038/ni.2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Masopust D, Schenkel JM. The integration of T cell migration, differentiation and function. Nat Rev Immunol. 2013;13(5):309–320. doi: 10.1038/nri3442 [DOI] [PubMed] [Google Scholar]
- 30.Wakatsuki K, Sho M, Yamato I, et al. Clinical impact of tumor-infiltrating CD45RO+ memory T cells on human gastric cancer. Oncol Rep. 2013;29(5):1756–1762. doi: 10.3892/or.2013.2302 [DOI] [PubMed] [Google Scholar]
- 31.Hotta K, Sho M, Fujimoto K, et al. Prognostic significance of CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer. 2011;105(8):1191–1196. doi: 10.1038/bjc.2011.368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou C, Li J, Wu Y, Diao P, Yang J, Cheng J. High density of intratumor CD45RO+ memory tumor-infiltrating lymphocytes predicts favorable prognosis in patients with oral squamous cell carcinoma. J Oral Maxillofacial Surg. 2019;77(3):536–545. doi: 10.1016/j.joms.2018.09.039 [DOI] [PubMed] [Google Scholar]
- 33.Vahidi Y, Faghih Z, Talei A-R, Doroudchi M, Ghaderi A. Memory CD4+ T cell subsets in tumor draining lymph nodes of breast cancer patients: a focus on T stem cell memory cells. Cell Oncol. 2018;41(1):1–11. doi: 10.1007/s13402-017-0352-6 [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi Y, Tanemura A, Tada Y, Katayama I, Kumanogoh A, Nishikawa H. Clinical response to PD-1 blockade correlates with a sub-fraction of peripheral central memory CD4+ T cells in patients with malignant melanoma. Int Immunol. 2018;30(1):13–22. doi: 10.1093/intimm/dxx073 [DOI] [PubMed] [Google Scholar]
- 35.Aghajani MJ, Cooper A, McGuire H, et al. Pembrolizumab for anaplastic thyroid cancer: a case study. Cancer Immunol Immunother. 2019;68(12):1921–1934. doi: 10.1007/s00262-019-02416-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JJ, Chang YL, Lai WL, et al. Increased prevalence of interleukin‐17–producing CD4+ tumor infiltrating lymphocytes in human oral squamous cell carcinoma. Head Neck. 2011;33(9):1301–1308. doi: 10.1002/hed.21607 [DOI] [PubMed] [Google Scholar]
- 37.Lee M-H, Chang JT-C, Liao C-T, Chen Y-S, Kuo M-L, Shen C-R. Interleukin 17 and peripheral IL-17-expressing T cells are negatively correlated with the overall survival of head and neck cancer patients. Oncotarget. 2018;9(11):9825. doi: 10.18632/oncotarget.23934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabjerg M, Bjerregaard H, Halekoh U, Jensen BL, Walter S, Marcussen N. Molecular characterization of clear cell renal cell carcinoma identifies CSNK 2A1, SPP 1 and DEFB 1 as promising novel prognostic markers. Apmis. 2016;124(5):372–383. doi: 10.1111/apm.12519 [DOI] [PubMed] [Google Scholar]
- 39.Álvarez ÁH, Velázquez MM, de Oca EPM. Human β-defensin 1 update: potential clinical applications of the restless warrior. Int J Biochem Cell Biol. 2018;104:133–137. doi: 10.1016/j.biocel.2018.09.007 [DOI] [PubMed] [Google Scholar]
- 40.Ghosh SK, McCormick TS, Weinberg A. Human beta defensins and cancer: contradictions and common ground. Front Oncol. 2019;9:341. doi: 10.3389/fonc.2019.00341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Joly S, Compton LM, Pujol C, Kurago ZB, Guthmiller JM. Loss of human β‐defensin 1, 2, and 3 expression in oral squamous cell carcinoma. Oral Microbiol Immunol. 2009;24(5):353–360. doi: 10.1111/j.1399-302x.2009.00512.x [DOI] [PubMed] [Google Scholar]
- 42.Han Q, Wang R, Sun C, et al. Human beta-defensin-1 suppresses tumor migration and invasion and is an independent predictor for survival of oral squamous cell carcinoma patients. PLoS One. 2014;9(3):e91867. doi: 10.1371/journal.pone.0091867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subbiah HV, Babu PR, Subbiah U. In silico analysis of non-synonymous single nucleotide polymorphisms of human DEFB1 gene. Egypt J Med Human Genetics. 2020;21(1):1–9. doi: 10.1186/s43042-020-00110-3 [DOI] [Google Scholar]
- 44.Yang D, Chertov O, Bykovskaia SN, et al. β-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science. 1999;286(5439):525–528. doi: 10.1126/science.286.5439.525 [DOI] [PubMed] [Google Scholar]
- 45.Camus M, Galon J. Memory T-cell responses and survival in human cancer: remember to stay alive. Memory T Cells. 2010;166–177. doi: 10.1007/978-1-4419-6451-9_13 [DOI] [PubMed] [Google Scholar]
- 46.Hu Z, Gu X, Zhong R, Zhong H. Tumor-infiltrating CD45RO+ memory cells correlate with favorable prognosis in patients with lung adenocarcinoma. J Thorac Dis. 2018;10(4):2089. doi: 10.21037/jtd.2018.03.148 [DOI] [PMC free article] [PubMed] [Google Scholar]