ABSTRACT
Background
Valid biomarkers of fruit and vegetable (FV) intake are needed for field-based nutrition research.
Objectives
To examine criterion-related validity of pressure-mediated reflection spectroscopy as a proxy measure of FV intake, using plasma carotenoids and self-reported FV and carotenoid intake as primary and secondary criterion measures, respectively.
Methods
Healthy adults 18–65 y of age, self-identifying as African American/black (n = 61), Asian (n = 53), white (n = 70), or Hispanic (n = 29), in North Carolina and Minnesota were recruited. Skin carotenoids were assessed via pressure-mediated reflection spectroscopy (Veggie Meter), skin melanin via spectrophotometer, and total plasma carotenoid concentration by HPLC–photodiode array detection. Self-reported carotenoid and FV intake was assessed using a semiquantitative FFQ. Relations between skin carotenoids, plasma carotenoids, FV, and carotenoid intake, with differences by race or ethnicity, age, sex, weight status, cholesterol, and melanin index, were examined by bivariate correlations and adjusted multivariate linear regressions.
Results
The overall unadjusted correlation between skin and total plasma carotenoids was r = 0.71 and ranged from 0.64 (non-Hispanic black) to 0.80 (Hispanic). Correlations between skin carotenoids and self-reported FV intake ranged from 0.24 (non-Hispanic black) to 0.53 (non-Hispanic white), with an overall correlation of r = 0.35. In models adjusted for age, sex, racial or ethnic group, and BMI, skin carotenoids were associated with plasma carotenoids (R2 = 0.55), FV (R2 = 0.17), and carotenoid intake (R2 = 0.20). For both plasma carotenoid and FV measures, associations with skin carotenoids did not vary by race, but these relations did differ by skin melanin—those with lower melanin had a lower correlation between skin and plasma carotenoids.
Conclusions
Reflection spectroscopy–assessed skin carotenoids may be a reasonable alternative to measurement of plasma carotenoids, a biomarker used to approximate FV intake.
Keywords: skin carotenoids, biomarker, fruit and vegetable intake, noninvasive, nutrition assessment, skin tone, melanin
Introduction
For optimal health, the 2020–2025 US Dietary Guidelines recommend 2.5 cup-equivalents of vegetables per day (with a focus on red, orange, and dark-green vegetables) and 2 cup-equivalents of fruit per day (1). Inadequate fruit and vegetable (FV) intake is associated with increased risk of cardiovascular disease (2), metabolic syndrome (3), diabetes (4), cancer (5), and obesity (6). Overall, Americans underconsume FVs (7), and this is particularly pronounced among marginalized communities (8–10). Effective interventions to increase Americans’ FV intake are needed.
To evaluate the effectiveness of FV interventions, and for accurate population monitoring of FV intake, valid, objective measures of FV intake are critical. Self-reported FV intake includes bias and error (11–14). Intervention-related bias, in which intervention participants overreport intake of nutrient-dense foods simply because they are made more aware of healthier dietary patterns, is of particular concern (15). Plasma carotenoid concentrations are not subject to bias and error inherent in self-reported intake measures and are correlated with FV intake (16) and thus may be used as a proxy for FV intake (17). However, expense, participant burden, and requirements for specimen collection, storage, and measurement often make plasma carotenoid concentrations impractical for field use in community settings.
Skin carotenoid measurement has been suggested as a proxy for FV intake assessment, and skin carotenoid measurement has been used to determine intervention effectiveness (18–20). Resonance Raman spectroscopy (RRS)–measured skin carotenoid signal is correlated with total plasma carotenoids (r = 0.62), total carotenoid concentrations in skin biopsy specimens (r = 0.66), and reported FV intake (r = 0.39), indicating concurrent and convergent validity (21). A newer method, pressure-mediated reflection spectroscopy (RS), now offers an economical, commercially available, portable, sensitive measure of skin carotenoids, with reduced hemoglobin interference by blanching the skin with gentle pressure (Veggie Meter; Longevity Link Corp.) (22, 23). Nonetheless, although studies have examined associations between skin carotenoids and plasma/serum carotenoids or self-reported FV intake (24–26), only a limited number have included racially and ethnically diverse populations (27–32), few have had large sample sizes (25, 31, 33, 34), and most validation studies were conducted using RRS instead of RS. Although a limited number of validation studies have used pressure-mediated RS (26, 32, 35), these studies have been small and of limited racial or ethnic diversity.
There are several reasons to validate RS in diverse populations. First, although skin melanin theoretically should not interfere with skin carotenoid measurement (36) and 1 study showed no association between skin melanin and skin carotenoid concentrations (36), greater heterogeneity in skin carotenoid measurement was observed with higher concentrations of skin melanin (36). Although this heterogeneity could be due to multiple causes, whether skin melanin is associated with the precision of the RS skin carotenoid measurement should be explored (36). Second, the extent to which skin carotenoids are reflective of FV intake could vary, based on the carotenoid content of specific FV choices, which are subject to cultural and socioeconomic influences (37, 38). Last, body composition, age, sex, and blood lipid concentrations are associated with carotenoid biodistribution (39), so RS should be validated in a healthy but physiologically diverse group.
Therefore, the purpose of this study was to examine criterion-related validity of RS-assessed skin carotenoids as a proxy for plasma carotenoid concentration (primary criterion measure) and FV intake assessment by FFQ (secondary measure) among a racially and ethnically diverse sample. The primary hypothesis was that RS-assessed skin carotenoid score would be associated with total plasma carotenoid concentrations [the criterion standard biomarker for FV intake (40)] for all racial or ethnic groups (r > 0.5), although the magnitude of associations may differ between groups. We also examined potential moderation of the associations between RS-assessed skin carotenoids with plasma carotenoids and dietary intake by race or ethnicity, sex, age, weight status, percent body fat, plasma cholesterol, and skin melanin.
Methods
Setting and participants
We recruited and enrolled participants for this cross-sectional observational validation study from in and around Greenville, North Carolina, and St. Paul/Minneapolis, Minnesota. These 2 study settings provide variance in UV light exposure, which may affect skin carotenoids, as UV radiation generates free radicals, which can be quenched by carotenoids (41, 42). These 2 settings also provided greater US cultural and dietary diversity. The predeclared primary and secondary endpoints did not change during the course of the research or during post hoc analyses. Any analyses not prespecified are considered exploratory.
Recruitment
In North Carolina, participants were recruited from university listservs, word of mouth, and community locations. Recruitment in Minnesota occurred at the Minnesota State Fair, through the University of Minnesota Driven to Discover Research Facility (http://d2d.umn.edu). In addition, participants were recruited through university listservs, community centers, and neighborhood venues.
Some researchers have speculated that the accuracy of RS-assessed skin carotenoids could differ by skin color. In addition, it is possible that the usefulness of RS-assessed skin carotenoids as a proxy measure for FV intake may vary across racial or ethnic groups due to differences in the types of FVs consumed. Consequently, it is important to validate RS-assessed skin carotenoids in a racially and ethnically diverse sample. Therefore, eligibility criteria included self-identifying with a primary racial or ethnic identity of Hispanic (regardless of race), white, African American/black, or Asian; having a BMI (in kg/m2) 18.5–34.9; being between 18 and 65 y of age; being healthy as determined by health history questionnaire (i.e., no history of chronic disease, including cancer, cardiovascular disease, previous heart attack or stroke, diabetes, chronic kidney disease); not taking lipid-lowering medication; not currently pregnant or lactating; not having vegetable allergies; and not currently dieting or planning to begin a special diet. Inclusion and exclusion criteria were based on factors that affect carotenoid absorption and metabolism (39).
Enrollment
Prescreened participants attended an additional in-person screening to confirm self-reported height and weight. If the confirmed BMI validated eligibility, participants provided informed written consent and proceeded with the study measurements. Participants received a $75 gift card upon completion of study measures. This study was approved by the East Carolina University Institutional Review Board (IRB), which served as the Single IRB of Record for both research sites. All participants were informed about the study, study-related questions were answered, and participants signed informed consent. At East Carolina University, study visits occurred in a research office in the Department of Public Health in the Brody School of Medicine and in the clinic room at the East Carolina Diabetes and Obesity Institute. At the University of Minnesota, study visits occurred at the Epidemiology Clinical Research Center.
Measures
Skin carotenoid assessment
Using pressure-mediated reflection spectroscopy, the RS device (model 716W0224, Veggie Meter; Longevity Link Corp.) uses broadband white light to measure skin carotenoids directly across their spectral range (400–750 nm). Each RS device comes with a standard calibration method that is used for all devices. Research staff were trained to conduct the RS device skin measurements in a cross-site training. Participants were first instructed to wash their hands with soap and water; the fingernail on the right index finger had to be limited in length to allow measurement with the RS device. If a participant had a fingernail that was too long to fit into the device, the participant was asked to cut the fingernail if he or she still wanted to remain eligible for the study. The right index finger was scanned 3 times in rapid succession with the participant taking the finger out and reinserting after a few seconds. The mean of the 3 skin carotenoid score measures was calculated and used in all analyses. The 3 skin carotenoid score measures had a median coefficient of variation 0.047 (IQR: 0.029, 0.078).
Self-reported dietary intake
Participants were asked to self-complete the 152-item Harvard semiquantitative FFQ (SFFQ) based on intake during the past year (43). Details on the development of this SFFQ are provided by Willett et al. (43). This instrument is designed to assess FV intake and carotenoid intake from foods and dietary supplements. Yuan et al. (44) found correlations between the Harvard SFFQ and plasma concentrations of specific carotenoids ranging from 0.63 for lycopene to 0.74 for β-cryptoxanthin, suggesting reasonable validity for estimating carotenoid intake. After SFFQs were completed, they were analyzed by Harvard University using standard methods. A “times/day” variable was created for total fruit, total vegetables, and total FV (including white potatoes), calculated as the sum of the times per day estimates for the FV items. We calculated total dietary carotenoid intake by summing participants’ daily intakes for the following: α-carotene, β-carotene, β-cryptoxanthin, lycopene, and lutein and zeaxanthin. We examined self-reported dietary carotenoid intake with and without supplements, and the amounts were similar; thus, we used the values for dietary carotenoid intake without supplements.
Assessment of plasma biomarkers
Participants fasted for at least 7 h prior to their scheduled clinic visit. A blood specimen was collected by venipuncture by a trained phlebotomist. In brief, 6 mL of whole blood was drawn into labeled EDTA-coated blood collection tubes and centrifuged at 2500 × g for 10 min at 4°C. The plasma was collected for carotenoid, cholesterol, and glucose measures. Samples were stored at –80°C until transferred on dry ice to Eurofins Craft Technologies for carotenoid, cholesterol, and glucose analyses.
Plasma carotenoids
Carotenoids were measured by HPLC with photodiode array detection using modifications of the method reported by Arab et al. (45). After being thawed, 150-μL aliquots of plasma were diluted with 150 μL water containing EDTA and ascorbic acid, deproteinated using 300 μL ethanol-containing tocol (Matreya) as an internal standard and butylated hydroxytoluene as an antioxidant. The samples were extracted twice with hexane, and the combined hexane extract was evaporated under reduced pressure using a centrifugal evaporator. The residue was dissolved in ethyl acetate assisted by vortex mixing, diluted with 90 acetonitrile/10 isopropanol, and ultrasonically agitated for 15 s. A 20-μL volume was injected by refrigerated autosampler held at 20°C. The diode array detector recorded the spectra from 270 to 600 nm. Carotenoids were measured at 450 nm and tocol at 300 nm. The separation was achieved on a C18 column (250 × 4.6 mm, 3 μm) isocratically using a mobile phase of acetonitrile/dioxane 50/50 methanol/isopropanol. Calibrations were performed with neat standards of carotenoids: lutein (Millipore Sigma), zeaxanthin (Millipore Sigma), β-cryptoxanthin, all-trans-lycopene, α-carotene, and trans-β-carotene (Millipore Sigma) using peak areas and corrected for tocol recovery. Standard concentrations were determined spectrophotometrically using Beer's law and corrected for HPLC purity. Total carotenoids were calculated as the sum of trans-lutein, trans-zeaxanthin, cis-lutein/zeaxanthin, α-cryptoxanthin, β-cryptoxanthin, trans-lycopene, cis-lycopene, α-carotene, trans-β-carotene, and cis-β-carotene. These carotenoids are prominent in the normal diet and constitute >85% of blood carotenoids.
Plasma glucose and cholesterol
Plasma glucose and cholesterol were assessed from fasted participants as possible covariates and to provide metabolic health information related to the population being studied. Plasma glucose was measured colorimetrically at 570 nm using an enzyme-coupled assay (Millipore Sigma) on a microplate reader per the manufacturer's instructions, using a glucose colorimetric/fluorometric assay kit (MAK263; Sigma Aldrich). Plasma cholesterol (mg/dL) was measured colorimetrically at 570 nm using an enzyme-coupled assay (Millipore Sigma) on a microplate reader per the manufacturer's instructions, using a cholesterol quantification assay kit from Sigma Aldrich (CS0005).
Potential covariates
Marital status, formal educational attainment, and annual household income were assessed by self-report via questionnaire. Skin melanin, self-reported race or ethnicity, anthropometrics (weight, height, body fat percentage), self-reported UV exposure, and self-reported tobacco exposure were all assessed as possible covariates due to their potential for these variables to confound the relation between plasma and skin carotenoids, as well as self-reported dietary and skin carotenoids. These are described in detail below. Data collection tools are available from the corresponding author upon request.
Skin coloration
A handheld spectrophotometer (CM-700D Spectrophotometer with Skin Analysis Software package v 1.3; Konica Minolta Sensing Americas) was used to quantify melanin index and hemoglobin index with 3 measures on the pad of the right index finger (46–50).
Race and ethnicity
As noted above, self-identifying as 1 of the 4 racial or ethnic groups of interest was an eligibility criterion. Participants were asked, “What do you consider to be your predominant or primary race/ethnicity?”
Anthropometrics
Staff measured height using a Shorr height board (Irwin Shorr) and weight and body composition using a combination scale and bioelectrical impedance device (Tanita DC-430U Body Composition Analyzer). The bioelectrical impedance device assesses body mass and body fat percentage based on known conductive and nonconductive properties of biological tissues. Height and weight were used to calculate BMI. Both BMI and body fat percentages were used as continuous variables in all analyses. BMI and body fat were correlated (r = 0.6), so we did not include both in model adjustment.
UV light exposure
Self-reported UV exposure from tanning beds/booths was assessed using a 4-item questionnaire, based on previously validated items (51). Participants were asked validated sun exposure items (52). Sun exposure was assessed as over 1 h/d on weekends or weekdays (yes/no).
Tobacco use
Tobacco exposure was assessed by a validated questionnaire to determine if the participant had ever smoked at least 100 cigarettes in his or her life (53). Additional questions were related to frequency (daily, some days, not at all) of smoking and use of chewing tobacco, snuff or snus, and e-cigarettes, vape pens, and e-hookahs (54). Tobacco usage was not a significant covariate and therefore was not used in statistical models.
Statistical analysis
We examined between-center differences on demographic, FV intake, carotenoid measures, and potential covariates using either a 2-sample t test or a χ2 test. Due to right-skewness, plasma carotenoid, self-reported FV, and carotenoid intakes were log2-tranformed for correlation and modeling. Pearson correlation coefficients were calculated between RS-assessed skin carotenoids, plasma carotenoids, FV intake, and total carotenoid intake. These associations were also stratified by self-reported race or ethnicity, age (<25, 25–40, ≥40 y), biological sex (male/female), BMI (normal weight, 18.5–24.9; overweight, 25–29.9; obese, 30–34.9), cholesterol (low, <125 mg/dL; normal; high, >200 mg/dL), melanin index (≥ or < the median measure), and hemoglobin (≥ or < the median measure). These variables were hypothesized to influence the correlation between plasma and skin carotenoids. Cohen's criteria (55) for strength of association were used to determine strength of correlations (correlations of 0 < 0.30 were considered small, 0.3 < 0.50 were medium, and 0.50 or greater were large).
Although blood carotenoid concentrations are robust biomarkers of FV intake, as with most dietary components, tissue distribution of carotenoids can be affected by both intrinsic factors, such as BMI, body fat, and age, and extrinsic factors, including lipid consumed with the meal, smoking, alcohol, and medications (42). Thus, covariates under consideration included age (continuous), biological sex (male, female), race or ethnicity, education level (some college or less, 4+ y of college), annual household income (≤$39,999, $40,000–99,999, ≥$100,000), marital status (single/separated/divorced/widowed, married/living with a partner), BMI (continuous), body fat percentage (continuous), smoking status (smoked ≥100 cigarettes in one's life, smoked <100 cigarettes in one's life), sun exposure (> 1 h/d on weekends or weekdays), tanning bed use (yes, no), location (Minnesota, North Carolina), and season (fall, winter, spring). Because melanin and hemoglobin may influence skin carotenoid measurement, we also assessed melanin as a covariate. After an initial backward model selection, we identified age, sex, BMI, total cholesterol, melanin, and hemoglobin as significant covariates (P < 0.10) in addition to race or ethnicity, which we decided to examine a priori. As a result, we examined an unadjusted model, a “field-based model” (i.e., using covariates that can readily be assessed in field settings), with age, sex, race or ethnicity, and BMI as covariates, and a “research-based model” (i.e., using covariates that are more readily obtained in a laboratory-based setting) that added total cholesterol, melanin index, and hemoglobin to the model. The field-based and research-based models are similar to those used by Seguin-Fowler et al. (56). All interactions between covariates and the RS-assessed skin carotenoids were tested and removed if they were not significant (P > 0.10). Finally, an F test was used to compare the 3 nested models. SAS version 9.4 (SAS Institute) was used for all analyses, and a P value of 0.05 was considered statistically significant.
Results
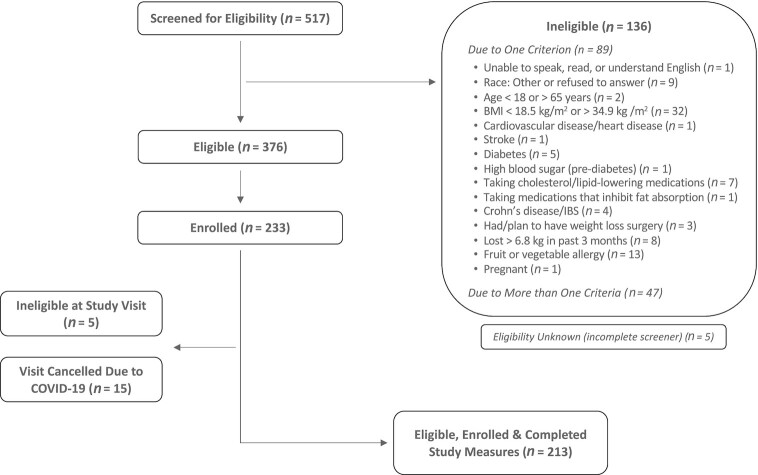
Of those screened (n = 517), 213 (41%) were eligible, were enrolled, and completed study measures. See Figure 1 for the participant flow diagram. Participant characteristics are shown in Table 1. There were 61 African American/black, 53 Asian, 70 white, and 29 Hispanic participants, with a majority being female and college educated. When compared with North Carolina participants, Minnesota participants had higher education levels, had less weekend sun exposure, were more likely to smoke, were older, had lower body fat percentages, and had higher RS-assessed skin carotenoids. Minnesota participants had a mean ± SD fasting blood glucose of 105.1 ± 19.6 mg/dL, and North Carolina participants had a mean ± SD fasting blood glucose of 86.5 ± 10.8 mg/dL, and this was significantly different by site (P < 0.001). An ANOVA test comparing melanin index among the 4 racial or ethnicity groups found those who identified as Asian had a mean ± SD fingertip melanin index of 1.09 ± 0.19; those who identified as Hispanic, 1.06 ± 0.17; those who identified as non-Hispanic black, 1.14 ± 0.17; and those who identified as non-Hispanic white, 1.07 ± 0.16. These differences were not statistically significant. An ANOVA test comparing melanin index among the 4 racial or ethnicity groups found those who identified as Asian had a mean ± SD arm melanin index of 0.88 ± 0.25; those who identified as Hispanic, 0.84 ± 0.21; those who identified as non-Hispanic black, 1.60 ± 0.31; and those who identified as non-Hispanic white, 0.60 ± 0.17. These differences were statistically significant (P < 0.0001).
FIGURE 1.
Participant flow diagram. Two participants did not complete the FFQ. IBS, irritable bowel syndrome.
TABLE 1.
Participant (n = 213) sociodemographics, behavioral variables, and anthropometrics, by site (Minnesota and North Carolina) and total1
| Characteristic | Minnesota (n = 92) | North Carolina (n = 121) | Total (N = 213) | P value |
|---|---|---|---|---|
| Self-identified race or ethnicity | 0.267 | |||
| Non-Hispanic black or African American | 21 (23) | 40 (33) | 61 (29) | |
| Asian | 28 (30) | 25 (21) | 53 (25) | |
| Non-Hispanic white | 30 (33) | 40 (33) | 70 (33) | |
| Hispanic/Latino | 13 (14) | 16 (13) | 29 (14) | |
| Sex | 0.058 | |||
| Female | 59 (64) | 92 (76) | 151 (71) | |
| Male | 33 (36) | 29 (24) | 62 (29) | |
| Marital status | 0.142 | |||
| Single/separated/divorced/widowed | 47 (51) | 74 (61) | 121 (57) | |
| Married/living with a partner | 45 (49) | 47 (39) | 92 (43) | |
| Education | 0.034 | |||
| Some college or less | 19 (21) | 41 (34) | 60 (28) | |
| ≥4 y of college | 73 (79) | 80 (66) | 153 (72) | |
| Annual household income | 0.180 | |||
| $39,999 or less | 31 (36) | 34 (32) | 65 (34) | |
| $40,000–$99,999 | 32 (37) | 52 (50) | 84 (44) | |
| $100,000 or more | 23 (27) | 19 (18) | 42 (22) | |
| Don't know/refused | 6 | 16 | 22 | |
| Ever used a tanning bed or booth with lamps | 0.816 | |||
| Yes | 21 (23) | 26 (22) | 47 (22) | |
| No | 71 (77) | 95 (79) | 166 (78) | |
| Weekday or weekend sun exposure >1 h | ||||
| Yes | 48 (52) | 89 (74) | 137 (64) | 0.001 |
| No | 44 (48) | 32 (26) | 76 (36) | |
| Sun protection usage | 0.810 | |||
| Yes | 57 (62) | 73 (60) | 130 (61) | |
| No | 35 (38) | 48 (40) | 83 (39) | |
| Smoking ≥100 cigarettes in your lifetime | <0.001 | |||
| Yes | 23 (25) | 8 (7) | 31 (15) | |
| No | 69 (75) | 113 (93) | 182 (85) | |
| Age, mean ± SD, y | 36 ± 13.2 | 32 ± 11.8 | 34 ± 12.5 | 0.038 |
| BMI, mean ± SD, kg/m2 | 25.1 ± 4.2 | 25.9 ± 3.8 | 25.6 ± 4.0 | 0.148 |
| Body fat, mean ± SD, % | 25.8 ± 7.8 | 28.0 ± 7.7 | 27.1 ± 7.8 | 0.041 |
| Plasma total cholesterol, mean ± SD, mg/dL | 151 ± 42 | 142 ± 40 | 146 ± 41 | 0.134 |
| Veggie Meter assessed skin carotenoid score, mean ± SD | 363 ± 99 | 286 ± 114 | 319 ± 114 | <0.001 |
| Finger melanin index, mean ± SD | 1.14 ± 0.16 | 1.06 ± 0.17 | 1.09 ± 0.17 | <0.001 |
| Finger hemoglobin index, mean ± SD | 2.57 ± 0.49 | 2.36 ± 0.50 | 2.45 ± 0.51 | 0.003 |
Values are presented as number (%) unless otherwise indicated.
Table 2 includes results of self-reported dietary intake among participants (n = 211) and plasma carotenoids by site and across sites. Compared with North Carolina participants, Minnesota participants had higher self-reported total carotenoid intakes and higher plasma concentrations of total carotenoids, α- and β-cryptoxanthin, β-carotene, and lutein/zeaxanthin.
TABLE 2.
Harvard semiquantitative FFQ (SFFQ) self-reported dietary data among participants (n = 2111) and HPLC plasma-assessed carotenoids by site (Minnesota and North Carolina) and total
| Characteristic | Minnesota (n = 91), mean ± SD | North Carolina (n = 120), mean ± SD | Total (N = 211), mean ± SD | P value |
|---|---|---|---|---|
| SFFQ variables, unit/d | ||||
| Energy, kcal | 1830 ± 681 | 1810 ± 986 | 1820 ± 866 | 0.876 |
| Protein, g | 72.7 ± 31.3 | 74.8 ± 34.4 | 73.9 ± 33.1 | 0.657 |
| Total fat, g | 69.5 ± 31.2 | 70.7 ± 41.6 | 70.2 ± 37.4 | 0.817 |
| Carbohydrates, g | 229 ± 84.9 | 214 ± 133 | 220 ± 115 | 0.320 |
| Total carotenoid intake, μg | 17,000 ± 11,000 | 13,100 ± 9320 | 14,800 ± 10,300 | 0.007 |
| Total fruit and vegetable intake, times/d | 12.8 ± 6.4 | 11.0 ± 7.0 | 11.8 ± 6.8 | 0.048 |
| α-Carotene,2 μg | 1070 ± 1360 | 581 ± 918 | 793 ± 1150 | 0.004 |
| β-Carotene,2 μg | 6650 ± 5360 | 4740 ± 4640 | 5570 ± 5040 | 0.007 |
| β-Cryptoxanthin,2 μg | 178 ± 202 | 122 ± 157 | 146 ± 180 | 0.028 |
| Lycopene,2 μg | 4560 ± 3380 | 3820 ± 2520 | 4140 ± 2940 | 0.085 |
| Lutein and Zeaxanthin,2 μg | 4590 ± 3580 | 3840 ± 3490 | 4160 ± 3540 | 0.129 |
| Plasma measures | ||||
| Total carotenoids, μg/dL | 152 ± 65.3 | 131 ± 51.9 | 140.1 ± 58.9 | 0.012 |
| α- and β-cryptoxanthin, μg/dL | 22.1 ± 15.9 | 16.9 ± 11.1 | 19.2 ± 13.6 | 0.008 |
| Lutein and zeaxanthin, μg/dL | 30.3 ± 11.4 | 26.9 ± 11.7 | 28.4 ± 11.6 | 0.034 |
| Total β-carotene, μg/dL | 42.2 ± 35.1 | 30.8 ± 23.0 | 35.7 ± 29.4 | 0.008 |
| Total α-carotene, μg/dL | 14 ± 10.7 | 9.6 ± 6.9 | 11.5 ± 9.0 | <0.001 |
| Total lycopene, μg/dL | 43.5 ± 16.4 | 46.7 ± 16.8 | 45.3 ± 16.7 | 0.161 |
Two individuals were missing Harvard SFFQ data.
Estimates of dietary carotenoid intake without estimates from supplements are listed. The values were not substantially different with and without supplement data included.
Table 3 shows correlations between RS-assessed skin carotenoids and log2-transformed plasma carotenoids, log2-transformed FV consumption, and log2-transformed dietary carotenoid intake for the total sample and subgroups by age, sex, race or ethnicity, BMI, total cholesterol, or melanin index. There was a statistically significant positive association between total plasma carotenoids and skin carotenoids in the total sample and in each individual racial or ethnic group. There were statistically significant positive associations between self-reported FV intake and skin carotenoid scores in the total sample and each racial or ethnic group. Correlations between skin carotenoids and self-reported dietary carotenoid intake were similar. Correlations between skin and plasma carotenoids were lower among participants with BMI ≥30 compared with <30 and higher among those with high compared with low blood cholesterol concentrations (Table 3). Among those with a lower melanin index (lower than the median melanin index), the correlation between plasma and skin carotenoids was lower than the correlation among those with a higher melanin index (higher than the median melanin index), r = 0.50 compared with 0.75, respectively (Table 3). There was a positive correlation between FV and carotenoid intake among the total sample (Table 3).
TABLE 3.
Pearson correlations (95% CI) between reflection spectroscopy–assessed skin carotenoids and log2-transformed plasma carotenoids, log2-transformed FV consumption, and log2-transformed dietary carotenoid intake for total sample and subgroups1
| Characteristic | n | Total plasma carotenoids | Self-reported FV intake | Self-reported carotenoid intake |
|---|---|---|---|---|
| Total | 213 | 0.712 (0.64, 0.77) | 0.352 (0.23, 0.47) | 0.382 (0.26, 0.49) |
| Minnesota | 92 | 0.662 (0.52, 0.76) | 0.392 (0.20, 0.55) | 0.382 (0.19, 0.54) |
| North Carolina | 121 | 0.732 (0.64, 0.81) | 0.283 (0.10, 0.44) | 0.302 (0.12, 0.45) |
| Non-Hispanic black | 61 | 0.642 (0.46, 0.77) | 0.24 (–0.02, 0.46) | 0.383 (0.14, 0.58) |
| Asian | 53 | 0.732 (0.58, 0.84) | 0.423 (0.16, 0.62) | 0.512 (0.28, 0.69) |
| Non-Hispanic white | 70 | 0.672 (0.51, 0.78) | 0.522 (0.33, 0.68) | 0.363 (0.14, 0.55) |
| Hispanic/Latino | 29 | 0.802 (0.61, 0.90) | 0.36 (–0.02, 0.64) | 0.384 (0.00, 0.66) |
| <25 y old | 59 | 0.702 (0.54, 0.81) | 0.294 (0.04, 0.51) | 0.284(0.02, 0.50) |
| Between 25 and 40 y old | 96 | 0.712 (0.60, 0.80) | 0.362 (0.17, 0.52) | 0.422 (0.24, 0.57) |
| ≥40 y old | 58 | 0.712 (0.55, 0.82) | 0.423 (0.18, 0.61) | 0.422 (0.18, 0.61) |
| Female | 151 | 0.732 (0.65, 0.80) | 0.322 (0.17, 0.46) | 0.362 (0.21, 0.49) |
| Male | 62 | 0.682 (0.51, 0.79) | 0.472 (0.25, 0.65) | 0.472 (0.25, 0.65) |
| Normal weight (BMI <25) | 99 | 0.702 (0.58, 0.79) | 0.372 (0.18, 0.53) | 0.372 (0.19, 0.53) |
| Overweight | 80 | 0.722 (0.59, 0.81) | 0.353 (0.14, 0.53) | 0.323 (0.11, 0.51) |
| Obese (BMI ≥30) | 34 | 0.602 (0.33, 0.78) | 0.31 (–0.03, 0.59) | 0.513 (0.20, 0.72) |
| Low plasma cholesterol (<125 mg/dL) | 70 | 0.722 (0.58, 0.82) | 0.264 (0.03, 0.47) | 0.323 (0.09, 0.52) |
| Normal plasma cholesterol | 118 | 0.742 (0.65, 0.81) | 0.382 (0.21, 0.53) | 0.412 (0.25, 0.55) |
| High plasma cholesterol (≥200 mg/dL) | 23 | 0.782 (0.54, 0.90) | 0.41 (–0.01, 0.70) | 0.28 (–0.15, 0.62) |
| Melanin index <1.068 | 106 | 0.502 (0.34, 0.63) | 0.19 (–0.00, 0.37) | 0.204 (0.00, 0.37) |
| Melanin index ≥1.068 | 107 | 0.752 (0.66, 0.82) | 0.332 (0.15, 0.49) | 0.382 (0.20, 0.53) |
| Hemoglobin index <2.449 | 106 | 0.732 (0.63, 0.81) | 0.322 (0.14, 0.48) | 0.432 (0.26, 0.57) |
| Hemoglobin index ≥2.449 | 107 | 0.682 (0.56, 0.77) | 0.372 (0.20, 0.53) | 0.322 (0.13, 0.48) |
Values are presented as Pearson correlations (95% CIs). A log2-transformation was used to stabilize variances and improve the linearity of the relations. FV, fruit and vegetables.
P < 0.001.
P < 0.01.
P < 0.05.
Table 4 shows general linear models predicting log2-transformed total plasma carotenoids, log2-transformed self-reported FV consumption, and log2-transformed self-reported dietary carotenoid intake using RS-assessed skin carotenoids (per 100 skin carotenoid score units) and covariates. The unadjusted model indicates that skin carotenoid score was positively associated with plasma carotenoid, self-reported FV, and carotenoid intake, with R2 ranging from 0.50 for plasma carotenoids to 0.13 for self-reported FV intake. R2 improved with the “field-based model” (i.e., using covariates that could be readily assessed in field-based settings), which included race or ethnicity, age, sex, and BMI, ranging from 0.55 for plasma carotenoids to 0.17 for self-reported FV intake.
TABLE 4.
General linear models predicting log2-transformed total plasma carotenoids, log2-transformed self-reported FV consumption, and log2-transformed self-reported dietary carotenoid intake using reflection spectroscopy–assessed skin carotenoids (per 100 units) and covariates among 211 participants1
| Model | Independent variables | Total plasma carotenoids | Self-reported FV intake | Self-reported carotenoid intake |
|---|---|---|---|---|
| Unadjusted | R 2 | 0.503 | 0.125 | 0.143 |
| Skin carotenoids | 0.33 (0.29, 0.38) | 0.25 (0.16, 0.34) | 0.30 (0.20, 0.40) | |
| Field based3 | R 2 | 0.5524 | 0.173 | 0.1955 |
| Skin carotenoids | 0.32 (0.27, 0.37) | 0.27 (0.18, 0.37) | 0.31 (0.21, 0.42) | |
| Age | 0.004 (–0.000, 0.009) | 0.007 (–0.002, 0.016) | 0.010 (–0.000, 0.020) | |
| Female | 0.13 (0.02, 0.24) | 0.14 (–0.09, 0.37) | 0.18 (–0.07, 0.44) | |
| Male | Reference | Reference | Reference | |
| Asian | –0.08 (–0.22, 0.06) | –0.16 (–0.44, 0.12) | –0.19 (–0.51, 0.12) | |
| Hispanic | 0.02 (–0.15, 0.19) | 0.35 (–0.00, 0.70) | 0.33 (–0.02, 0.72) | |
| Non-Hispanic black | –0.05 (–0.18, 0.08) | 0.08 (–0.19, 0.35) | 0.12 (–0.18, 0.42) | |
| Non-Hispanic white | Reference | Reference | Reference | |
| BMI | –0.023 (–0.037, –0.009) | –0.005 (–0.034, 0.024) | –0.017 (–0.049, 0.015) | |
| Research based | R 2 | 0.6622 | 0.183 | 0.205 |
| Melanin | 0.31 (–0.46, 1.09) | –0.01 (–1.82, 1.80) | –0.22 (–2.24, 1.79) | |
| Skin carotenoids | 0.004 (–0.22, 0.23) | 0.03 (–0.50, 0.55) | –0.02 (–0.60, 0.57) | |
| Melanin × skin carotenoids | 0.20 (0.01, 0.40) | 0.17 (–0.29, 0.63) | 0.24 (–0.27, 0.75) | |
| Hemoglobin | –0.15 (–0.26, –0.04) | –0.02 (–0.28, 0.23) | –0.10 (–0.39, 0.19) | |
| Age | 0.001 (–0.003, 0.005) | 0.007 (–0.002, 0.016) | 0.009 (–0.001, 0.020) | |
| Female | 0.12 (0.02, 0.22) | 0.15 (–0.08, 0.39) | 0.19 (–0.07, 0.46) | |
| Male | Reference | Reference | Reference | |
| Asian | –0.11 (–0.23, 0.01) | –0.16 (–0.46, 0.13) | –0.22 (–0.54, 0.10) | |
| Hispanic | –0.05 (–0.20, 0.10) | 0.31 (–0.05, 0.67) | 0.28 (–0.13, 0.68) | |
| Non-Hispanic black | –0.19 (–0.33, –0.04) | 0.03 (–0.31, 0.37) | 0.03 (–0.35, 0.40) | |
| Non-Hispanic white | Reference | Reference | Reference | |
| BMI | –0.022 (–0.035, –0.010) | –0.005 (–0.035, 0.024) | –0.017 (–0.050, 0.016) | |
| Plasma total cholesterol | 0.002 (0.001, 0.003) | 0.000 (–0.002, 0.003) | –0.000 (–0.003, 0.003) |
Model R2 and parameter estimates (95% CIs) are reported. A log2-transformation of the outcomes was used to stabilize variances and improve the linearity of the relations. FV, fruit and vegetables.
P < 0.001.
The field-based model includes covariates that are easily measured in a community or field-based setting, including age, sex, race or ethnicity, and BMI. The research-based model included covariates that are more difficult to assess and would require more resources, including those in the field-based model as well as total cholesterol and melanin.
P < 0.01.
P < 0.05 comparing to previous model R2.
Finally, R2 improved even more in the “research-based model’ (i.e., using covariates that can be obtained in a laboratory-based setting, including cholesterol and melanin), ranging from 0.65 for plasma carotenoids to 0.18 for the self-reported FV intake. It is noteworthy that the race or ethnicity × skin carotenoid interaction was not significant in any of the models. There was a significant (P = 0.043) interaction between melanin index and skin carotenoid score for plasma carotenoids, indicating that for those with a higher melanin index, there was a larger β estimate for the association of skin carotenoids with plasma carotenoids compared with those with a lower melanin index. In other words, in participants with higher melanin compared with those with lower melanin, the same difference in skin carotenoid scores predicted a greater difference in plasma carotenoids. When melanin index was included in the model as a dichotomous variable (low compared with high melanin), the interaction was not statistically significant (P = 0.12). The slopes of skin carotenoid scores (per 100 units)/total plasma carotenoids (μmol/L) were 0.22 (0.04) for the low melanin group and 0.30 (0.03) for the high melanin group (data not shown).
Supplemental Table 1 includes correlations between total and specific carotenoids (as measured using the FFQ and plasma carotenoids) and skin carotenoid status and melanin index. It is noteworthy that there was no significant correlation between plasma lycopene and skin carotenoid score, whereas there were significant, positive correlations between all other plasma carotenoid species and skin carotenoid score, ranging from 0.60 to 0.66 (P < 0.001). Supplemental Table 2 demonstrates the correlations between plasma carotenoids and self-reported FV and carotenoid intake, with overall correlations ranging from 0.32 to 0.37.
Discussion
In the current study, we found a correlation of 0.71 between plasma and skin carotenoids among a diverse sample of participants, and our hypothesis that the correlation between plasma total carotenoids and skin carotenoids would be >0.50 was supported. Our findings are in agreement with prior research indicating that RS-assessed skin carotenoids are associated with plasma carotenoids (26, 32, 36), and this relation holds true among all 4 racial or ethnic groups studied. Thus, despite speculation about the inaccuracy of RS-assessed skin carotenoids in individuals of varying races and ethnicities, RS-assessed skin carotenoids and plasma carotenoids were associated with a correlation of >0.50 in all 4 racial or ethnic groups. The correlation between RS-assessed skin carotenoids and plasma carotenoids in the current study is similar to the correlation of 0.68 found in a recent meta-analysis of studies examining the association between RRS and plasma carotenoids (57). To our knowledge, this study is the first of its kind to show associations between skin and plasma carotenoids, as well as between skin carotenoids and self-reported FV intake, do not vary substantially by race or ethnicity. This is important, demonstrating that the Veggie Meter and possibly other skin carotenoid measures can be used in racially and ethnically diverse populations.
When comparing the field-based models (adjusted for age, sex, race or ethnicity, and BMI) and research-based models (adjusting for covariates in the field-based model plus cholesterol and melanin), the R2 increased from 0.55 to 0.65. This suggests that skin carotenoid scores can be used in field-based conditions, similar to what Seguin-Fowler et al. (56) found among a diverse group of children in 4 US states. The field-based model includes covariates that are more easily measured in community-based settings, whereas the research-based model includes additional covariates that are more easily measured in laboratory or clinical settings. The amount of variation in skin carotenoid scores accounted for by these 2 models (R2 = 0.55 and R2 = 0.65 for the field-based and research-based models, respectively) is important for future researchers to consider when designing studies using skin carotenoid scores as an outcome measure. Researchers must weigh the pros and cons of adding the more resource-intense covariates such as cholesterol and melanin to models. Interestingly, the interaction between skin carotenoids and melanin appears to be an important moderator of the association between skin carotenoid score and plasma carotenoids. This finding regarding the interaction between skin carotenoid score and melanin was unexpected. It may be that melanin should be measured if skin carotenoid scores are used as an outcome in evaluations of dietary interventions. However, our findings should be replicated in future studies.
There were overall low to moderate correlations between plasma carotenoids and self-reported FV and carotenoid intake. The correlations between skin carotenoids and self-reported FV and carotenoid intake were 0.35 and 0.38, respectively. This is similar to the correlation between FV intake and plasma carotenoids found by Yuan et al. (44), who found unadjusted Spearman correlation coefficients ranging from ∼0.26 for lycopene to 0.47 for α-carotene. This is also similar to findings using the National Institutes of Health Diet History Questionnaire, in which Dixon et al. (58) found correlations ranging from 0.15 for dietary lutein/zeaxanthin to 0.62 for dietary β-cryptoxanthin. These low to moderate correlations are most likely due to well-documented inaccuracies in dietary recall measures (59–61).
Although there was a significant, positive association between total plasma carotenoids and skin carotenoid scores, as well as a significant, positive correlation between all individual plasma carotenoids and skin carotenoid scores, there was not a correlation between plasma lycopene and skin carotenoid scores. This has important implications for those who may want to use the RS device to examine changes in FV intake and suggests that the RS device may not be as responsive to changes in intake of lycopene or lycopene-rich FV (e.g., tomatoes, watermelon) intake compared with changes in intake of β-carotene or β-carotene–rich FVs (e.g., yellow-orange and dark green FVs).
This study is not without limitations. First, we stopped recruitment early due to the COVID-19 pandemic and thus did not enroll the targeted number of participants in the Hispanic group. This may have reduced power to detect associations and test for between-group differences. Second, although we used an FFQ that has been found to provide carotenoid intake estimates that correlate moderately with plasma carotenoids, we did not use the gold-standard approaches for self-reported diet (24-h dietary recalls or food records) as our criterion dietary measure. SFFQ data tend to present overestimates of health-promoting foods and should only be used to rank participants, not to quantify absolute intake (62–63). Furthermore, because we limited the sample to relatively healthy volunteers, generalizability to populations with chronic disease is limited at this time. Ideally, the study would also include an examination of how reflection spectroscopy works before and after a controlled, dietary intervention. To that end, we are currently conducting a randomized controlled trial of a carotenoid-containing juice to determine the sensitivity of the RS device to changes in intake. In addition, although the measure of melanin was objective, it may be confounded by carotenoids in the skin as well as hemoglobin and bilirubin (64). We did not control for hydration status when measuring body composition, and this is a limitation because bioelectrical impedance is sensitive to hydration status. Finally, skin carotenoid status is limited as a concentration biomarker of FV intake, particularly given that skin carotenoid status cannot detect intake of FVs that do not contain carotenoids (65). Despite these limitations, the study has several strengths, including the use of measurement tools with reasonably good validity and reliability, as well as recruitment from 4 racial or ethnic groups in 2 geographically distinct areas of the United States.
Conclusions
In conclusion, we found that skin carotenoid score correlated with plasma carotenoids, a measure commonly used as a biomarker of FV intake in a large sample of individuals from 4 different racial or ethnic groups. Future studies should focus on elucidating the sensitivity of skin carotenoid measurements to change in FV intake and to carefully define the contributions of individual variables contributing to interindividual variability in skin carotenoid responses to diet. Previous studies have suggested that human variation in carotenoid and lipid metabolism–related genes (66–70) can partially explain interindividual variability in plasma carotenoid concentrations; therefore, future efforts should focus on determining the degree to which skin carotenoid responses to diet may be influenced by interindividual genetic variation. Findings of the current study are supportive of using skin carotenoids as an alternate to plasma carotenoids when it is not feasible to collect and analyze blood. Findings from feeding studies evaluating the sensitivity of skin carotenoids to changes in FV intake on the order of magnitude observed in public health interventions are critical to determining the usefulness of skin carotenoids as an evaluation measure.
Supplementary Material
Acknowledgments
We thank Archana Kaur, Lauren Berg, Abigail Gelineau, Patricia Brophy, Angela Clark, Gabriel Dubis, and Kristina Foyt for assistance and staff at Eurofins Craft Technologies for carotenoid, glucose, and cholesterol analyses.
The authors’ contributions were as follows—SBJP, MNL, and NEM: conceptualized and led the study; SGM, PLC-M, JO, and NJ: led Redcap database creation, data collection, and data entry; QW: analyzed all data; SBJP, NEM, MNL, LH, NH, NEC, and RB: contributed to study design, data collection, and interpretation of results; SBJP: drafted the paper; and all authors: contributed to subsequent drafts of the paper and have reviewed and approved the final version of the paper as submitted.
Notes
This research was supported by the National Heart, Lung, and Blood Institute (NHLBI; grant R01HL142544) and by USDA Agricultural Research Service (CRIS 3092-51000-059-NEW2S; NEM). NIH grant UL1TR000114 from the National Center for Advancing Translational Sciences supported data management. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the USDA or NIH NHLBI.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: FV, fruit and vegetable; IRB, institutional review board; RRS, resonance Raman spectroscopy; RS, reflection spectroscopy; SFFQ, semiquantitative FFQ.
Contributor Information
Stephanie B Jilcott Pitts, Department of Public Health, East Carolina University, Greenville, NC, USA.
Nancy E Moran, USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
Qiang Wu, Department of Biostatistics, East Carolina University, Greenville, NC, USA.
Lisa Harnack, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Neal E Craft, Craft Nutrition Consulting, Elm City, NC, USA.
Neil Hanchard, Department of Molecular and Human Genetics, Baylor College of Medicine, Houston, TX, USA.
Ronny Bell, Department of Social Sciences and Health Policy, Division of Public Health Sciences, Wake Forest School of Medicine, Winston-Salem, NC, USA.
Stacey G Moe, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Nevin Johnson, Department of Public Health, Brody School of Medicine, East Carolina University, Greenville, NC, USA.
Justice Obasohan, School of Medicine, University of North Carolina at Chapel Hill, Chapel Hill, NC, USA.
Pamela L Carr-Manthe, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Melissa N Laska, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN, USA.
Data Availability
Data described in this manuscript will be made available upon request pending institutional review board approval.
References
- 1. US Department of Agriculture, US Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th ed.[Internet]. 2020[cited 22 Feb 2021]. https://www.https//www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf. [Google Scholar]
- 2. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Beydoun MA, Chen X, Jha K, Beydoun HA, Zonderman AB, Canas JA. Carotenoids, vitamin A, and their association with the metabolic syndrome: a systematic review and meta-analysis. Nutr Rev. 2019;77(1):32–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, Sun Q. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies [published correction appears in BMJ 2013;347:f6935]. BMJ. 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aune D, Giovannucci E, Boffetta P, Fadness LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46(3):1029–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He K, Hu FB, Colditz GA, Manson JE, Willett WC, Liu S. Changes in intake of fruits and vegetables in relation to risk of obesity and weight gain among middle-aged women. Int J Obes. 2004;28(12):1569–74. [DOI] [PubMed] [Google Scholar]
- 7. Guenther PM, Dodd KW, Reedy J, Krebs-Smith SM. Most Americans eat much less than recommended amounts of fruits and vegetables. J Am Diet Assoc. 2006;106(9):1371–9. [DOI] [PubMed] [Google Scholar]
- 8. Satia JA. Diet-related disparities: understanding the problem and accelerating solutions. J Am Diet Assoc. 2009;109(4):610–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kirkpatrick SI, Dodd KW, Reedy J, Krebs-Smith SM. Income and race/ethnicity are associated with adherence to food-based dietary guidance among US adults and children. J Acad Nutr Diet. 2012;112(5):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stimpson JP, Nash AC, Ju H, Eschbach K. Neighborhood deprivation is associated with lower levels of serum carotenoids among adults participating in the Third National Health and Nutrition Examination Survey. J Am Diet Assoc. 2007;107(11):1895–902. [DOI] [PubMed] [Google Scholar]
- 11. Kirkpatrick SI, Collins CE, Keogh RH, Krebs-Smith SM, Neuhouser ML, Wallace A. Assessing dietary outcomes in intervention studies: pitfalls, strategies, and research needs. Nutrients. 2018;10(8):1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hebert JR, Hurley TG, Peterson KE, Resnicow K, Thompson FE, Yaroch AL,Ehlers M, Midthune D, Williams GC, Greene GW et al. Social desirability trait influences on self-reported dietary measures among diverse participants in a multicenter multiple risk factor trial. J Nutr. 2008;138(1):226S–34S. [DOI] [PubMed] [Google Scholar]
- 13. Archer E, Blair SN. Implausible data, false memories, and the status quo in dietary assessment. Adv Nutr. 2015;6(2):229–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller TM, Abdel-Maksoud MF, Crane LA, Marcus AC, Byers TE. Effects of social approval bias on self-reported fruit and vegetable consumption: a randomized controlled trial. Nutr J. 2008;7(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harnack L, Himes JH, Anliker J, Clay T, Gittelsohn J, Jobe JB, Ring K, Snyder P, Thompson J, Weber JL. Intervention-related bias in reporting of food intake by fifth-grade children participating in an obesity prevention study. Am J Epidemiol. 2004;160(11):1117–21. [DOI] [PubMed] [Google Scholar]
- 16. Campbell DR, Gross MD, Martini MC, Grandits GA, Slavin JL, Potter JD. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol Biomarkers Prev. 1994;3(6):493–500. [PubMed] [Google Scholar]
- 17. Lampe JW, Huang Y, Neuhouser ML, Tinker LF, Song X, Schoeller DA, Kim S, Raftery D, Di C, Zheng C et al. Dietary biomarker evaluation in a controlled feeding study in women from the Women's Health Initiative cohort. Am J Clin Nutr. 2017;105(2):466–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bayles J, Peterson AD, Jilcott Pitts S, Bian H, Goodell S, Burkholder S, Hegde AV, Stage VC. Food-based Science, Technology, Engineering, Arts, and Mathematics (STEAM) learning activities may reduce decline in preschoolers’ skin carotenoid status. J Nutr Educ Behav. 2021;53(4):343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jilcott Pitts SB, Wu Q, Truesdale KP, Haynes-Maslow L, McGuirt JT, Ammerman A, Bell R, Laska MN. One-year follow-up examination of the impact of the North Carolina healthy food small retailer program on healthy food availability, purchases, and consumption. Int J Environ Res Public Health. 2018;15(12):2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seguin RA, Morgan EH, Hanson KL, Ammerman AS, Jilcott Pitts SB, Kolodinsky J, Sitaker M, Becot FA, Connor LM, Garner JA et al. Farm Fresh Foods for Healthy Kids (F3HK): an innovative community supported agriculture intervention to prevent childhood obesity in low-income families and strengthen local agricultural economies. BMC Public Health. 2017;17(1):306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mayne ST, Cartmel B, Scarmo S, Lin H, Leffell DF, Welch E, Ermakov I, Bhosale P, Bernstein PS, Gellermann W. Noninvasive assessment of dermal carotenoids as a biomarker of fruit and vegetable intake. Am J Clin Nutr. 2010;92(4):794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ermakov I V, Whigham LD, Redelfs AH, Jahns L, Stookey J, Bernstein PS, Gellermann W. Skin carotenoids as biomarker for vegetable and fruit intake: validation of the reflection-spectroscopy based “Veggie Meter.” FASEB J. 2016;30(1_Suppl):403–9. [Google Scholar]
- 23. Ermakov IV, Gellermann W. Dermal carotenoid measurements via pressure mediated reflection spectroscopy. J Biophotonics. 2012;5(7):559–70. [DOI] [PubMed] [Google Scholar]
- 24. Radtke MD, Jilcott Pitts SJ, Jahns L, Firnhaber GC, Loofbourrow BM, Zeng A, Scherr RE. Criterion-related validity of spectroscopy-based skin carotenoid measurements as a proxy for fruit and vegetable intake: a systematic review. Adv Nutr. 2020;11(5):1282–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Morgan EH, Graham ML, Marshall GA, Hanson KL, Seguin-Fowler RA. Serum carotenoids are strongly associated with dermal carotenoids but not self-reported fruit and vegetable intake among overweight and obese women. Int J Behav Nutr Phys Act. 2019;16(1):104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jahns L, Johnson LAK, Conrad Z, Bukowski M, Raatz SK, Jilcott Pitts S, Wang Y, Ermakov IV, Gellermann W. Concurrent validity of skin carotenoid status as a concentration biomarker of vegetable and fruit intake compared to multiple 24-h recalls and plasma carotenoid concentrations across one year: a cohort study. Nutr J. 2019;18(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen LM, Scherr RE, Linnell JD, Ermakov I V, Gellermann W, Jahns L, Keen CL, Miyamoto S, Steinberg FM, Young HM et al. Evaluating the relationship between plasma and skin carotenoids and reported dietary intake in elementary school children to assess fruit and vegetable intake. Arch Biochem Biophys. 2015;572:73–80. [DOI] [PubMed] [Google Scholar]
- 28. Beccarelli LM, Scherr RE, Dharmar M, Ermakov IV, Gellermann W, Jahns L, Linnell JD, Keen CL, Steinberg FM, Young HM et al. Using skin carotenoids to assess dietary changes in students after 1 academic year of participating in the Shaping Healthy Choices Program. J Nutr Educ Behav. 2017;49(1):73–78.e1. [DOI] [PubMed] [Google Scholar]
- 29. Aguilar SS, Wengreen HJ, Dew J. Skin carotenoid response to a high-carotenoid juice in children: a randomized clinical trial. J Acad Nutr Diet. 2015;115(11):1771–8. [DOI] [PubMed] [Google Scholar]
- 30. Aguilar SS, Wengreen HJ, Lefevre M, Madden GJ, Gast J. Skin carotenoids: a biomarker of fruit and vegetable intake in children. J Acad Nutr Diet. 2014;114(8):1174–80. [DOI] [PubMed] [Google Scholar]
- 31. Scarmo S, Henebery K, Peracchio H, Cartmel B, Lin H, Ermakov IV, Gellermann W, Bernstein PS, Duffy VB, Mayne ST. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. Eur J Clin Nutr. 2012;66(5):555–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jilcott Pitts SB, Jahns L, Wu Q, Moran NE, Bell RA, Truesdale KP, Laska MN. A non-invasive assessment of skin carotenoid status through reflection spectroscopy is a feasible, reliable and potentially valid measure of fruit and vegetable consumption in a diverse community sample. Public Health Nutr. 2018;21(9):1664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rerksuppaphol S, Rerksuppaphol L. Carotenoid intake and asthma prevalence in Thai children. Pediatr Rep. 2012;4(1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zidichouski JA, Mastaloudis A, Poole SJ, Reading JC, Smidt CR. Clinical validation of a noninvasive, Raman spectroscopic method to assess carotenoid nutritional status in humans. J Am Coll Nutr. 2009;28(6):687–93. [DOI] [PubMed] [Google Scholar]
- 35. Hill C, O'Brien D, Paschall M, Bersamin A. Validity of reflection spectroscopy as a biomarker of vegetable and fruit intake in a Yu'pik alaska native population (P18-124-19). Curr Dev Nutr. 2019;3(Suppl 1):nzz039. [Google Scholar]
- 36. Ermakov IV, Ermakova M, Sharifzadeh M, Gorusupudi A, Farnsworth K, Bernstein PS, Stookey J, Evans J, Arana T, Tao-Lew L et al. Optical assessment of skin carotenoid status as a biomarker of vegetable and fruit intake. Arch Biochem Biophys. 2018;646(PG-46-54):46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kamphuis CBM, Giskes K, de Bruijn G-J, Wendel-Vos W, Brug J, van Lenthe FJ. Environmental determinants of fruit and vegetable consumption among adults: a systematic review. Br J Nutr. 2006;96(4):635. [PubMed] [Google Scholar]
- 38. Devine CM, Wolfe WS, Frongillo EA, Bisogni CA. Life-course events and experiences: association with fruit and vegetable consumption in 3 ethnic groups. J Am Diet Assoc. 1999;99(3):309–14. [DOI] [PubMed] [Google Scholar]
- 39. Moran NE, Mohn ES, Hason N, Erdman JW, Johnson EJ. Intrinsic and extrinsic factors impacting absorption, metabolism, and health effects of dietary carotenoids. Adv Nutr. 2018;9(4):465–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. Washington (DC): National Academies Press; 2000. [PubMed] [Google Scholar]
- 41. Balić A, Mokos M. Do we utilize our knowledge of the skin protective effects of carotenoids enough?. Antioxidants. 2019;8(8):259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sies H, Stahl W. Carotenoids and UV protection. Photochem Photobiol Sci. 2004;3(8):749–52. [DOI] [PubMed] [Google Scholar]
- 43. Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51–65. [DOI] [PubMed] [Google Scholar]
- 44. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Subar AF, Sampson LK, Willett WC. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Arab L, Cambou MC, Craft N, Wesseling-Perry K, Jardack P, Ang A. Racial differences in correlations between reported dietary intakes of carotenoids and their concentration biomarkers. Am J Clin Nutr. 2011;93(5):1102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pezdirc K, Hutchesson MJ, Whitehead R, Ozakinci G, Perrett D, Collins CE. Fruit, vegetable and dietary carotenoid intakes explain variation in skin-color in young Caucasian women: a cross-sectional study. Nutrients. 2015;7(7):5800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Pezdirc K, Hutchesson MJ, Williams RL, Rollo ME, Burrows TL, Wood LG, Oldmeadow C, Collins CE. Consuming high-carotenoid fruit and vegetables influences skin yellowness and plasma carotenoids in young women: a single-blind randomized crossover trial. J Acad Nutr Diet. 2016;116(8):1257–65. [DOI] [PubMed] [Google Scholar]
- 48. Yun IS, Lee WJ, Rah DK, Kim YO, Park BY. Skin color analysis using a spectrophotometer in Asians. Ski Res Technol. 2010;16(3):311–15. [DOI] [PubMed] [Google Scholar]
- 49. Kikuchi K, Katagiri C, Yoshikawa H, Mizokami Y, Yaguchi H. Long-term changes in Japanese women's facial skin color. Color Res Appl. 2018;43(1):119–29. [Google Scholar]
- 50. Walsh S, Chaitanya L, Breslin K, Muralidharan C, Bronikowska A, Pospiech E, Koller J, Kovatsi L, Wollstein A, Branicki W et al. Global skin colour prediction from DNA. Hum Genet. 2017;136(7):847–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lazovich DA, Stryker JE, Mayer JA, Hillhouse J, Dennis LK, Pichon L, Pagoto S, Heckman C, Olson A, Cokkinides V et al. Measuring nonsolar tanning behavior: indoor and sunless tanning. Arch Dermatol. 2008;144(2):225–30. [DOI] [PubMed] [Google Scholar]
- 52. Glanz K, Yaroch AL, Dancel M, Saraiya M, Crane LA, Buller DB, Manne S, O'Riordan DL, Heckman CJ, Hay J et al. Measures of sun exposure and sun protection practices for behavioral and epidemiologic research. Arch Dermatol. 2008;144(2):217–22. [DOI] [PubMed] [Google Scholar]
- 53. Bondy SJ, Victor JC, Diemert LM. Origin and use of the 100 cigarette criterion in tobacco surveys. Tob Control. 2009;18(4):317–23. [DOI] [PubMed] [Google Scholar]
- 54. National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. 2018 Behavioral Risk Factor Surveillance System. [Internet]. Centers for Disease Control and Prevention; 2018 [cited 29 Jul 2020]. Available from: http://www.cdc.gov/brfss/questionnaires/pdf-ques/2014_BRFSS.pdf. [Google Scholar]
- 55. Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Lawrence Erlbaum; 1988. [Google Scholar]
- 56. Seguin-Fowler R, Hanson K, Marshall G, Belarmino E, Jilcott Pitts SB, Kolodinsky J, Sitaker M, Ammerman A. Fruit and vegetable intake assessed by repeat 24-hr recalls, but not by a dietary screener, is associated with skin carotenoid measurements in children. Nutrients. 2021;13(3):980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jilcott Pitts SB, Johnson NS, Wu Q, Firnhaber GC, Kaur AP, Obasohan J. A meta-analysis of studies examining associations between resonance Raman spectroscopy-assessed skin carotenoids and plasma carotenoids among adults and children. Nutr Rev[epub ahead of print 2 Apr 2021]. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dixon LB, Subar AF, Wideroff L, Thompson FE, Kahle LL, Potischman N. Carotenoid and tocopherol estimates from the NCI Diet History Questionnaire are valid compared with multiple recalls and serum biomarkers. J Nutr. 2006;136:3054–61. [DOI] [PubMed] [Google Scholar]
- 59. Dwyer JT, Coleman KA. Insights into dietary recall from a longitudinal study: accuracy over four decades. Am J Clin Nutr. 1997:65(4):1153S–8S. [DOI] [PubMed] [Google Scholar]
- 60. Dhurandhar NV, Schoeller D, Brown AW, Heymsfield SB, Thomas D, Sorensen TIA, Speakman JR, Jeansonne M, Allison DB; Energy balance measurement: when something is not better than nothing. Energy Balance Measurement Working Group. Int J Obe. 2015;39:1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Freedman LS, Commins JM, Moler JE, Arab L, Baer DJ, Kipnis V, Midthune D, Moshfegh AJ, Neuhouser ML, Prentice RL et al. Pooled results from 5 validation studies of dietary self-report instruments using recovery biomarkers for energy and protein intake. Am J Epidemiol. 2014;180(2): 172–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Krebs-Smith SM, Heimendinger J, Subar AF, Patterson BH, Pivonka E. Estimating fruit and vegetable intake using food frequency questionnaires: a comparison of instruments. Am J Clin Nutr. 1994;59:283s. [Google Scholar]
- 63. Thompson FE, Subar AF. Dietary assessment methodology. In Nutrition in the prevention and treatment of disease. 4th ed. Editors, Coulston AM, Ferruzzi MG, Boushey CJ, Delahanty LM. Academic Press; 2017. [Google Scholar]
- 64. Masuda Y, Yamashita T, Hirao T, Takahashi M. An innovative method to measure skin pigmentation. Skin Res Technol. 2009;15:224–9. [DOI] [PubMed] [Google Scholar]
- 65. Woodside JV, Draper J, Lloyd A, McKinley MC. Use of biomarkers to assess fruit and vegetable intake. Proc Nutr Soc. 2017;76(3):308–15. [DOI] [PubMed] [Google Scholar]
- 66. Zubair N, Kooperberg C, Liu J, Di C, Peters U, Neuhouser ML. Genetic variation predicts serum lycopene concentrations in a multiethnic population of postmenopausal women. J Nutr. 2015;145(2):187–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Borel P, Desmarchelier C, Nowicki M, Bott R. Lycopene bioavailability is associated with a combination of genetic variants. Free Radic Biol Med. 2015;83:238–44. [DOI] [PubMed] [Google Scholar]
- 68. Borel P, Desmarchelier C, Nowicki M, Bott R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary β-carotene bioavailability in healthy men. J Nutr. 2015;145(8):1740–7. [DOI] [PubMed] [Google Scholar]
- 69. Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N. Interindividual variability of lutein bioavailability in healthy men: characterization, genetic variants involved, and relation with fasting plasma lutein concentration. Am J Clin Nutr. 2014;100(1):168–75. [DOI] [PubMed] [Google Scholar]
- 70. Moran NE, Thomas-Ahner JM, Fleming JL, McElroy JP, Mehl R, Grainger EM, Riedl KM, Toland AE, Schwartz SJ, Clinton SK. Single nucleotide polymorphisms in β-carotene oxygenase 1 are associated with plasma lycopene responses to a tomato-soy juice intervention in men with prostate cancer. J Nutr. 2019;149(3):381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in this manuscript will be made available upon request pending institutional review board approval.