ABSTRACT
Background
Recent studies showed that eating behaviors such as disinhibition, emotional and external eating, and snacking mediate genetic susceptibility to obesity. It remains unknown if diet quality and intake of specific food groups also mediate the genetic susceptibility to obesity.
Objective
This study aimed to assess if diet quality and intakes of specific food groups mediate the association between a polygenic risk score (PRS) for BMI and BMI and waist circumference (WC). We hypothesized that poor diet quality, high intakes of energy-dense food groups, and low intakes of nutrient-dense food groups mediate the genetic susceptibility to obesity.
Methods
This cross-sectional study included 750 participants (56.3% women, aged 41.5 ± 14.9 y, BMI 27.8 ± 7.5 kg/m2) from the Quebec Family Study. A PRSBMI based on >500,000 genetic variants was calculated using LDpred2. Dietary intakes were assessed with a 3-d food record from which a diet quality score (i.e. Nutrient Rich Food Index 6.3) and food groups were derived. Mediation analyses were conducted using a regression-based and bootstrapping approach.
Results
The PRSBMI explained 25.7% and 19.8% of the variance in BMI and WC, respectively. The association between PRSBMI and BMI was partly mediated by poor diet quality (β = 0.33 ± 0.12; 95% CI: 0.13, 0.60), high intakes of fat and high-fat foods (β = 0.46 ± 0.16; 95% CI: 0.19, 0.79) and sugar-sweetened beverages (β = 0.25 ± 0.14; 95% CI: 0.05, 0.60), and low intakes of vegetables (β = 0.15 ± 0.08; 95% CI: 0.03, 0.32), fruits (β = 0.37 ± 0.12; 95% CI: 0.17, 0.64), and dairy products (β = 0.17 ± 0.09; 95% CI: 0.02, 0.37). The same trends were observed for WC.
Conclusions
The genetic susceptibility to obesity was partly mediated by poor diet quality and intakes of specific food groups. These results suggest that improvement in diet quality may reduce obesity risk among individuals with high genetic susceptibility and emphasize the need to intervene on diet quality among these individuals.
Keywords: genetic susceptibility to obesity, polygenic risk score, obesity, diet quality, dietary intakes, mediation
Introduction
Obesity results from a complex interplay between genetic and environmental factors. It is estimated that 40–75% of the variation in BMI is explained by genetic factors (1–3) and nearly 1000 single nucleotide polymorphisms (SNPs), explaining ∼6% of the variance in BMI, have been identified through genome-wide association studies (4).
The study of gene-environment interaction in obesity is traditionally based on the concept of moderation (or effect modification) in which the effects of genes (exposure) on obesity (outcome) are examined in groups of individuals stratified based on an environmental factor. An alternative approach that can be used to characterize the interplay between genetic and environmental factors in obesity is mediation analysis. In contrast to moderation, mediation analysis is used to assess the extent to which the effects of an exposure (genes) on an outcome (obesity) are explained by a given set of mediators (e.g. dietary factors). Although moderation and mediation are interdependent concepts, the assessment of mediation is motivated by understanding the causal pathways whereby an exposure leads to an outcome with the aim of intervening on the mediator to improve the outcome (5).
Despite extensive evidence supporting the existence of gene-diet interaction in obesity (6–11), relatively few studies have used mediation analysis to identify potential mediators of genetic susceptibility to obesity. A previous study by our group using data from the Quebec Family Study (QFS) showed that disinhibition and susceptibility to hunger, both internally or in response to external food cues, mediated the genetic susceptibility to obesity in adults (12). Other studies in adults (13–17) and children (18, 19) have provided evidence for the role of eating behavior or appetite-related traits in mediating genetic susceptibility. In addition, a recent study found that disinhibition was a key mediator of the association between genetic susceptibility and weight gain in midlife adults (13). To the best of our knowledge, only 1 study explored whether diet quality mediated the genetic susceptibility to obesity, assessed with a polygenic risk score (PRS), and found that a score of diet quality based on a short (14-item) FFQ did not show any mediating effect (17). However, an eating pattern labeled infrequent and unhealthy eating was found to mediate the genetic susceptibility to obesity (17). Whether diet quality and intakes of specific food groups are potential mediators of the genetic susceptibility to obesity remains to be investigated.
In the present study, we investigated the mediating effect of diet quality and specific food groups, derived from a 3-d food record, on the genetic susceptibility to obesity, using BMI and waist circumference (WC) as measures of obesity and a PRS for BMI incorporating whole-genome based variants irrespective of their genome-wide significance. This PRS represents a more powerful approach to capture genetic risk (4, 20). We hypothesized that the genetic susceptibility to obesity is mediated by a poor diet quality, high intakes of energy-dense foods, and low intakes of nutrient-dense foods.
Methods
Study design and participants
Participants of this cross-sectional study are from phases 2 (1989–1997) and 3 (1998–2002) of the QFS (NCT03355729). Participants are French-Canadians from the greater Quebec City area recruited from different media. Additional details on the QFS have been previously published (21, 22). The current study excluded participants without genotype data, aged <18 y, with a diagnosis of type 1 or type 2 diabetes, and without BMI data (Supplemental Figure 1). Participants without WC measurement were excluded from WC analyses. The analyses therefore included 750 participants for BMI and 748 participants for WC. For participants with longitudinal data on phases 2 and 3, the sample selection favored the data collection phase with less missing data relevant to the analyses and no exclusion criteria, or favored phase 2 when there were no exclusion criteria and no difference in the number of missing data between the 2 phases of data collection. The QFS was approved by the Research Ethics Committee of Université Laval and all participants signed an informed consent.
Anthropometric measurements
Anthropometric measurements including weight, height, and WC were measured following standardized procedures (23). BMI was calculated as weight divided by height squared (kg/m2).
Genotyping and PRS
Genome-wide genotyping of participants was performed using the Illumina 610-Quad Chip, as previously described (24). A PRS for BMI, representing an individual's genetic susceptibility to obesity, was derived using LDpred2, a computational algorithm for polygenic scoring that uses the genome-wide genotype profile of participants and relevant genome-wide association study (GWAS) data (25). The PRSBMI was calculated using the summary statistics of the most recent Genetic Investigation of ANthropometric Traits (GIANT) Consortium and UK Biobank meta-analysis of BMI in over 700,000 individuals (4). The LDpred2 tool implemented in R considers the effect size estimates of all variants and accounts for linkage disequilibrium between variants to derive a whole-genome PRS composed of independent variants (25). The PRS used in the present study included 523,101 SNPs.
Dietary assessment
Dietary intakes were assessed with a 3-d food record on 2 weekdays and 1 weekend day (26). All participants received instructions from a registered dietitian on how to complete the food record and measure the portions of food consumed. The registered dietitian subsequently verified every food record with the participant to ensure its accuracy. Energy and nutrient intakes were assessed based on the 2010 version of the Canadian Nutrient File (27).
Diet quality was assessed with the Nutrient Rich Food (NRF) Index 6.3, which measures the nutritional quality of each food in the diet and can be applied to food, meal, and daily diet (28, 29). This index was chosen because the serving size of each food necessary to calculate commonly used diet quality indices such as the Healthy Eating Index (HEI) (30, 31) was not available in the database. The NRF6.3 was also chosen because missing data on vitamin E for 18.3% of food items did not allow use of the NRF9.3. Although the NRF6.3 performs slightly lower than the NRF9.3 to predict the HEI, the NRF6.3 still explains an adequate proportion (i.e. over 35%) of the variance in HEI (28). For each food, the NRF6.3 is calculated as the sum of the proportion of reference daily values (DV) provided by 100 kcal for 6 nutrients to encourage (i.e. protein, fiber, vitamin A, vitamin C, calcium, and iron), minus the sum of the proportion of reference DV provided by 100 kcal for 3 nutrients to limit (i.e. SFAs, sodium, and added or total sugars) (28, 29). The 6 nutrients to encourage are based on the US FDA's definition of “healthy” foods, and the 3 nutrients to limit are based on the FDA and other authoritative sources (29). For each food, each nutrient could not exceed 100% of its reference DV to avoid overvaluing foods that provide very large amounts of some specific nutrients. Total sugars were used in the present study because added sugars were not available in the database. Reference DV for total sugars was set to 100 g, as per Health Canada's Table of DV for nutrition labeling (32). To reflect total diet quality, each food's NRF6.3 score was weighted according to their proportion of total energy intake (%TEI) for each day and a mean NRF6.3 score for the 3 collection days was calculated.
Food items from the food record were classified into food groups mainly based on similarity (e.g. fruits, dairy products) or their macronutrient content (e.g. fat and high-fat foods). Details about food group classification have been presented elsewhere (33). The current analysis included 13 food groups selected based on their positive or negative association with weight gain, obesity, or cardiometabolic health (Supplemental Table 1) (34, 35). Intakes of food groups are expressed in %TEI.
Assessment of covariates
Information on age, sex (men, 0; women, 1), menopausal status (yes, 1; no, 0), current smoking status (yes, 1; no, 0), and current dieting status (yes, 1; no, 0) were collected through questionnaires. The plausibility of self-reported energy intake (rEI) was assessed using the method described by Huang et al. (36) where under- and overreporters of energy intake are defined as those having a ratio of rEI to predicted energy requirements (pERs) that deviates > ± 1 SD calculated from a formula that accounts for measurement error in rEI and pERs. In the present study, the within-individual CV for rEI was 25.0%, the number of days of dietary assessment was 3, and the CV for pERs was 19.1%. The CV accounting for day-to-day variation and measurement error for objective measurement of total energy expenditure (mTEE) was set at 8.2%, as detailed elsewhere (37). pERs were assessed using equations developed by the National Academy of Medicine (38). As an objective measure of physical activity level was not available for all participants, it was assumed that participants were sedentary, as previously done (39). To account for skewness of energy intake, the ± 1 SD CIs were exponentiated using a multiplicative factor of 1 (40). The resulting CIs were 0.78–1.29, meaning that under- and overreporters of energy intake were defined as those having a ratio of rEI to pERs <0.78 and >1.29, respectively. The reporting status (i.e. underreporters, plausible reporters, and overreporters) was considered in the analyses by creating 2 indicator variables representing underreporting (yes, 1; no, 0) and overreporting (yes, 1; no, 0) (41).
Statistical analysis
Statistical analyses were performed using SAS studio version 3.8. Sex differences on participant characteristics, anthropometric measurements, PRSBMI, and dietary intakes were assessed using Student's t-tests and chi-square tests. Linear regression models were used to assess the association between the PRSBMI and BMI or WC. Mediation analyses were conducted to assess if diet quality (NRF6.3) and food groups mediate the association between the PRSBMI and BMI or WC. These analyses were conducted with model 4 of the Process macro, version 3.3, for SAS (42). The Process macro is an ordinary least square regression path analysis modeling tool that uses percentile bootstrap CIs to assess the mediation or indirect effect (i.e. dietary intakes) through which an independent variable (i.e. PRSBMI) influences a dependent variable (i.e. BMI or WC). The present analyses used 5000 bootstrap samples. The total effect (c) is defined as the association between the PRSBMI and BMI or WC and the direct effect (c') represents the association between the PRSBMI and BMI or WC when controlling for the mediator (i.e. dietary intakes). The a and b paths represent the associations between the independent variable (i.e. PRSBMI) and the mediator (i.e. dietary intakes), and between the mediator and the dependent variable (i.e. BMI or WC) while controlling for the independent variable (i.e. PRSBMI), respectively. The percentage of mediation was calculated as a ratio of indirect effect to total effect (i.e. [ab/c]*100). To support the mediation model, in which the PRSBMI is hypothesized to influence BMI and WC through dietary intakes, we used bivariate genetic analyses to examine the extent to which shared genetic effects and nonshared environmental effects underlie the covariation between BMI and dietary intakes. A positive or negative genetic correlation implies that the effects of genes underlying the 2 traits are in the same or opposite direction, respectively. These analyses were performed taking into account the family structure of QFS using SOLAR Eclipse version 8.4.1 (43). Mediation analyses and genetic correlations were adjusted for all covariates presented previously, as these variables are known to influence BMI, WC, or dietary intakes.
Results
Participant characteristics
The sample of this study included 422 women and 328 men, with a mean age of 41.5 ± 14.9 y (range 18.1–75.7 y) and a mean BMI and WC of 27.8 ± 7.5 kg/m2 (range 16.8–64.9 kg/m2) and 88.8 ± 18.1 cm (range 57.9–164.5 cm), respectively (Table 1). A total of 26.4% of the sample had obesity (BMI ≥30 kg/m2) and 29.6% of the sample had abdominal obesity defined as a WC ≥88 cm for women and ≥102 cm for men.
TABLE 1.
Characteristics of the 750 participants from the Quebec Family Study1
| Total | Men | Women | P 2 | |
|---|---|---|---|---|
| Sex, n (%) | — | 328 (43.7) | 422 (56.3) | 0.0006 |
| Age, y | 41.5 ± 14.9 | 41.9 ± 15.3 | 41.2 ± 14.6 | 0.53 |
| BMI, kg/m2 | 27.8 ± 7.5 | 27.6 ± 6.6 | 27.8 ± 8.1 | 0.71 |
| Waist circumference,3 cm | 88.8 ± 18.1 | 94.4 ± 17.0 | 84.4 ± 17.8 | <0.0001 |
| Menopaused,4 n (%) | 126 (17.1) | — | 126 (30.7) | — |
| Dieting,5 n (%) | 44 (5.9) | 12 (3.7) | 32 (7.6) | 0.02 |
| Smoking,5 n (%) | 157 (21.0) | 64 (19.5) | 93 (22.1) | 0.39 |
| Reporting status,6 n (%) | 0.96 | |||
| Underreporters | 86 (11.5) | 37 (11.3) | 49 (11.6) | |
| Plausible reporters | 503 (67.1) | 219 (66.8) | 284 (67.3) | |
| Overreporters | 161 (21.5) | 72 (22.0) | 89 (21.1) | |
| Polygenic risk score | 0.29 ± 0.36 | 0.29 ± 0.35 | 0.29 ± 0.37 | 0.82 |
| Energy intake, kcal/d | 2346 ± 681 | 2702 ± 685 | 2068 ± 534 | <0.0001 |
| Diet quality | ||||
| Nutrient Rich Food Index 6.3 | 10.2 ± 6.7 | 9.4 ± 6.3 | 10.7 ± 7.0 | 0.0008 |
| Food groups, %TEI | ||||
| Whole vegetables | 1.9 ± 2.1 | 1.6 ± 2.0 | 2.1 ± 2.1 | 0.002 |
| Fruits7 | 3.5 ± 3.7 | 2.9 ± 3.4 | 4.1 ± 3.8 | <0.0001 |
| 100% fruit juices | 2.4 ± 3.2 | 2.3 ± 3.0 | 2.4 ± 3.2 | 0.58 |
| Dairy products | 10.4 ± 6.4 | 9.7 ± 6.4 | 10.9 ± 6.4 | 0.008 |
| Milk | 5.2 ± 4.5 | 5.2 ± 4.7 | 5.2 ± 4.4 | 0.97 |
| Yogurt | 0.9 ± 2.0 | 0.6 ± 1.5 | 1.2 ± 2.3 | <0.0001 |
| Cheese | 4.2 ± 4.5 | 3.8 ± 4.5 | 4.5 ± 4.5 | 0.03 |
| Processed meats | 3.3 ± 4.1 | 3.8 ± 4.5 | 3.0 ± 3.7 | 0.01 |
| Plant-based protein foods | 2.1 ± 3.5 | 2.2 ± 3.8 | 2.0 ± 3.1 | 0.44 |
| Nuts and seeds | 1.8 ± 3.1 | 1.9 ± 3.6 | 1.6 ± 2.8 | 0.24 |
| Sugar-sweetened beverages | 2.6 ± 4.5 | 2.7 ± 3.9 | 2.5 ± 4.8 | 0.62 |
| Sugar and sugary foods | 15.7 ± 9.4 | 16.0 ± 9.6 | 15.4 ± 9.3 | 0.38 |
| Fat and high-fat foods | 14.1 ± 9.6 | 14.2 ± 9.8 | 14.0 ± 9.5 | 0.71 |
Data are mean ± SD or n (%), %TEI, percentage or total energy intake.
P values for sex differences based on Student's t-test or chi-square test.
n = 748, women n = 420.
n = 738, women n = 410.
n = 749, women n = 421.
Underreporters, rEI/pER <0.78; plausible reporters, 0.78 ≤ rEI/pER ≤1.29; overreporters, rEI/pER >1.29.
Excluding fruit juices.
rEI, reported energy intake; pER, predicted energy requirement.
Associations between PRSBMI, BMI, and WC
The PRSBMI explained 25.7% of the variance in BMI (β = 10.56 ± 0.66; 95% CI: 9.27, 11.85, P < 0.0001) and 19.8% of the variance in WC (β = 22.53 ± 1.66; 95% CI: 19.27, 25.79, P < 0.0001), respectively.
Dietary mediators of the association between PRSBMI and BMI
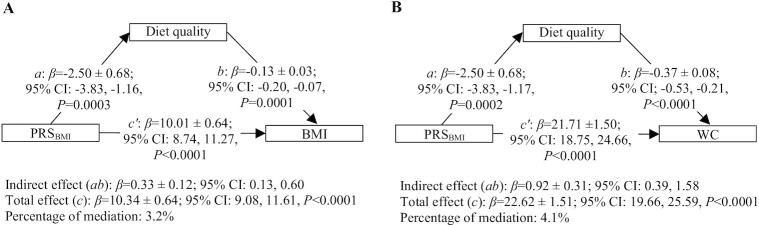
Results from the mediation analyses showed that a poor diet quality partly mediated the association between the PRSBMI and BMI (β = 0.33 ± 0.12; 95% CI: 0.13, 0.60) (Figure 1A). Among food groups, high intakes of sugar-sweetened beverages (SSBs) (β = 0.25 ± 0.14; 95% CI: 0.05, 0.60) and fat and high-fat foods (β = 0.46 ± 0.16; 95% CI: 0.19, 0.79) partly mediated the association between the PRSBMI and BMI (Table 2). Low intakes of whole vegetables (β = 0.15 ± 0.08; 95% CI: 0.03, 0.32), fruits excluding fruit juices (β = 0.37 ± 0.12; 95% CI: 0.17, 0.64), and dairy products (β = 0.17 ± 0.09; 95% CI: 0.02, 0.37), particularly milk (β = 0.13 ± 0.08; 95% CI: 0.01, 0.30) and yogurt (β = 0.12 ± 0.06; 95% CI: 0.02, 0.25) also partially mediated this association. The total effect (c), representing the association between the PRSBMI and BMI (β = 10.34 ± 0.64; 95% CI: 9.08, 11.61, P < 0.0001), was slightly reduced by these mediators, resulting in the percentage of mediation varying between 1.2 and 4.4%.
FIGURE 1.
Mediating effect of diet quality on the association between genetic susceptibility to obesity (PRSBMI) and obesity measures (BMI and WC) in the Quebec Family Study.1 1Values are β-coefficients ± SEs. n = 738 for BMI analysis and n = 737 for WC analysis. Analyses are performed on complete cases resulting in the exclusion of n = 12 participants with missing data on menopausal (n = 12), dieting (n = 1), or smoking status (n = 1) from analysis related to BMI and the exclusion of n = 11 participants with missing data on menopausal status from analysis related to WC. Mediation analyses are conducted using the Process Macro v. 3.3 for SAS that uses percentile bootstrap CIs to assess the mediating or indirect effect through which the PRSBMI influences BMI (Figure 1A) or WC (Figure 1B). 95% CI for indirect effect are estimated through 5000 bootstrap samples. Mediation models are adjusted for age, sex (men, 0; women, 1), current dieting status (yes, 1; no, 0) menopausal status (yes, 1; no, 0), current smoking (yes, 1; no, 0) status, and misreporting of dietary intakes [(underreporting, yes, 1; no, 0) and (overreporting, yes, 1; no, 0)]. Diet quality assessed by the Nutrient Rich Food Index 6.3 (NRF6.3). a, association between the PRSBMI and diet quality (mediator); b, association between diet quality (mediator) and BMI or WC adjusted for PRSBMI; total effect (c), association between the PRSBMI and BMI or WC without adjustment for diet quality (mediator); direct effect (c'), association between the PRSBMI and BMI or WC adjusted for diet quality (mediator); indirect effect (ab), mediation effect; percentage of mediation: (indirect effect [ab]/total effect [c])×100. PRS, polygenic risk score; WC, waist circumference.
TABLE 2.
Mediating effect of food groups on the association between genetic susceptibility to obesity (PRSBMI) and BMI in the Quebec Family Study1
| a 2 | b 3 | Direct effect (c′)4 | Indirect effect (ab)5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β ± SE | 95% CI | P | β ± SE | 95% CI | P | β ± SE | 95% CI | P | β ± boot SE | Boot 95% CI | % Mediation6 | |
| Whole vegetables | –0.57 ± 0.21 | –0.99, –0.16 | 0.007 | –0.27 ± 0.11 | –0.49, –0.05 | 0.02 | 10.19 ± 0.65 | 8.92, 11.46 | <0.0001 | 0.15 ± 0.08 | 0.03, 0.32 | 1.5 |
| Fruits7 | –1.55 ± 0.36 | –2.24, –0.85 | <0.0001 | –0.24 ± 0.07 | –0.37, –0.11 | 0.0003 | 9.97 ± 0.65 | 8.70, 11.24 | <0.0001 | 0.37 ± 0.12 | 0.17, 0.64 | 3.6 |
| 100% fruit juices | –0.60 ± 0.32 | –1.23, 0.02 | 0.06 | 0.08 ± 0.07 | –0.06, 0.23 | 0.26 | 10.39 ± 0.65 | 9.13, 11.66 | <0.0001 | –0.05 ± 0.05 | –0.18, 0.04 | — |
| Dairy products | –2.15 ± 0.66 | –3.44, –0.85 | 0.001 | –0.08 ± 0.04 | –0.15, –0.01 | 0.03 | 10.17 ± 0.65 | 8.90, 11.44 | <0.0001 | 0.17 ± 0.09 | 0.02, 0.37 | 1.6 |
| Milk | –0.97 ± 0.47 | –1.89, –0.04 | 0.04 | –0.13 ± 0.05 | –0.23, –0.03 | 0.008 | 10.21 ± 0.64 | 8.95, 11.48 | <0.0001 | 0.13 ± 0.08 | 0.01, 0.30 | 1.2 |
| Yogurt | –0.47 ± 0.20 | –0.86, –0.09 | 0.02 | –0.25 ± 0.12 | –0.49, −0.02 | 0.04 | 10.22 ± 0.65 | 8.96, 11.49 | <0.0001 | 0.12 ± 0.06 | 0.02, 0.25 | 1.2 |
| Cheese | –0.71 ± 0.47 | –1.63, 0.22 | 0.13 | 0.02 ± 0.05 | –0.07, 0.12 | 0.63 | 10.36 ± 0.65 | 9.09, 11.63 | <0.0001 | –0.02 ± 0.04 | –0.10, 0.06 | — |
| Processed meats | 1.34 ± 0.43 | 0.51, 2.18 | 0.002 | 0.08 ± 0.06 | –0.03, 0.19 | 0.17 | 10.24 ± 0.65 | 8.97, 11.51 | <0.0001 | 0.10 ± 0.09 | –0.04, 0.31 | — |
| Plant-based protein foods | –0.80 ± 0.36 | –1.51, –0.08 | 0.03 | –0.09 ± 0.07 | –0.22, 0.04 | 0.17 | 10.27 ± 0.65 | 9.00, 11.54 | <0.0001 | 0.07 ± 0.06 | –0.02, 0.21 | — |
| Nuts and seeds | –0.58 ± 0.33 | –1.23, 0.06 | 0.08 | –0.10 ± 0.07 | –0.24, 0.05 | 0.19 | 10.29 ± 0.65 | 9.02, 11.55 | <0.0001 | 0.06 ± 0.05 | –0.03, 0.18 | — |
| Sugar-sweetened beverages | 1.26 ± 0.45 | 0.38, 2.14 | 0.005 | 0.20 ± 0.05 | 0.09, 0.30 | 0.0002 | 10.10 ± 0.64 | 8.84, 11.36 | <0.0001 | 0.25 ± 0.14 | 0.05, 0.60 | 2.4 |
| Sugar and sugary foods | 0.33 ± 0.97 | –1.57, 2.24 | 0.73 | –0.06 ± 0.02 | –0.10, –0.01 | 0.02 | 10.36 ± 0.64 | 9.10, 11.62 | <0.0001 | –0.02 ± 0.06 | –0.14, 0.10 | — |
| Fat and high-fat foods | 3.88 ± 0.99 | 1.94, 5.82 | 0.0001 | 0.12 ± 0.02 | 0.07, 0.16 | <0.0001 | 9.89 ± 0.64 | 8.63, 11.14 | <0.0001 | 0.46 ± 0.16 | 0.19, 0.79 | 4.4 |
n = 738. Analyses are performed on complete cases resulting in the exclusion of n = 12 participants with missing data on menopausal (n = 12), dieting (n = 1), or smoking (n = 1) status from the analyses. Mediation analyses are conducted using the Process Macro v. 3.3 for SAS that uses percentile bootstrap CIs to assess the mediating or indirect effect through which the PRSBMI influences BMI. 95% CI for indirect effect are estimated through 5000 bootstrap samples. Models are adjusted for age, sex (men, 0; women, 1), current dieting status (yes, 1; no, 0) menopausal status (yes, 1; no, 0), current smoking status (yes, 1; no, 0), misreporting of dietary intakes [(underreporting, yes, 1; no, 0) and (overreporting, yes, 1; no, 0)]. Food groups are expressed in percentage of total energy intake. Boot, bootstrap; PRS, polygenic risk score.
a, association between the PRSBMI and the mediator.
b, association between the mediator and BMI adjusted for PRSBMI.
Direct effect (c′), association between the PRSBMI and BMI adjusted for the mediator.
Indirect effect (ab), mediation effect.
% mediation calculated as (indirect effect [ab]/total effect [c])×100. Total effect (c) : β=10.34 ± 0.64; 95% CI: 9.08, 11.61, P < 0.0001 (association between the PRSBMI and BMI without adjustment for the mediator).
Excluding fruit juices.
Dietary mediators of the association between PRSBMI and WC
Similar results were observed for WC. Accordingly, a poor diet quality partly mediated the association between the PRSBMI and WC (β = 0.92 ± 0.31; 95% CI: 0.39, 1.58) (Figure 1B). Among food groups, high intakes of SSBs (β = 0.58 ± 0.31; 95% CI: 0.12, 1.29) and fat and high-fat foods (β = 1.09 ± 0.36; 95% CI: 0.48, 1.92), and low intakes of whole vegetables (β = 0.38 ± 0.18; 95% CI: 0.07, 0.77), fruits excluding fruit juices (β = 1.04 ± 0.33; 95% CI: 0.47, 1.73) and dairy products (β = 0.49 ± 0.23; 95% CI: 0.12, 0.99), particularly milk (β = 0.35 ± 0.20; 95% CI: 0.02, 0.79) and yogurt (β = 0.33 ± 0.15; 95% CI: 0.07, 0.67), were all partial mediators of the association between the PRSBMI and WC (Table 3). Again, the total effect (c) (β = 22.62 ± 1.51; 95% CI: 19.66, 25.59, P < 0.0001) was slightly reduced by these mediators, resulting in the percentage of mediation varying between 1.5 and 4.8%.
TABLE 3.
Mediating effect of food groups on the association between genetic susceptibility to obesity (PRSBMI) and WC in the Quebec Family Study1
| a 2 | b 3 | Direct effect (c′)4 | Indirect effect (ab)5 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β ± SE | 95% CI | P | β ± SE | 95% CI | P | β ± SE | 95% CI | P | β ± boot SE | Boot 95% CI | % Mediation6 | |
| Whole vegetables | –0.58 ± 0.21 | –0.99, –0.16 | 0.007 | –0.65 ± 0.26 | –1.17, –0.14 | 0.01 | 22.25 ± 1.51 | 19.28, 25.22 | <0.0001 | 0.38 ± 0.18 | 0.07, 0.77 | 1.7 |
| Fruits7 | –1.55 ± 0.36 | –2.25, –0.85 | <0.0001 | –0.67 ± 0.16 | –0.97, –0.37 | <0.0001 | 21.59 ± 1.51 | 18.62, 24.55 | <0.0001 | 1.04 ± 0.33 | 0.47, 1.73 | 4.6 |
| 100% fruit juices | –0.60 ± 0.32 | –1.22, 0.03 | 0.06 | 0.10 ± 0.18 | –0.24, 0.45 | 0.56 | 22.69 ± 1.51 | 19.71, 25.66 | <0.0001 | –0.06 ± 0.12 | –0.35, 0.15 | — |
| Dairy products | –2.12 ± 0.66 | –3.42, –0.83 | 0.001 | –0.23 ± 0.08 | –0.40, –0.07 | 0.006 | 22.13 ± 1.51 | 19.16, 25.10 | <0.0001 | 0.49 ± 0.23 | 0.12, 0.99 | 2.2 |
| Milk | –0.96 ± 0.47 | –1.89, –0.03 | 0.04 | –0.36 ± 0.12 | –0.60, –0.13 | 0.002 | 22.27 ± 1.51 | 19.32, 25.23 | <0.0001 | 0.35 ± 0.20 | 0.02, 0.79 | 1.5 |
| Yogurt | –0.47 ± 0.20 | –0.86, –0.08 | 0.02 | –0.71 ± 0.28 | –1.26, –0.16 | 0.01 | 22.29 ± 1.51 | 19.33, 25.25 | <0.0001 | 0.33 ± 0.15 | 0.07, 0.67 | 1.5 |
| Cheese | –0.69 ± 0.47 | –1.62, 0.23 | 0.14 | 0.04 ± 0.12 | –0.20, 0.27 | 0.76 | 22.65 ± 1.51 | 19.68, 25.62 | <0.0001 | –0.03 ± 0.09 | –0.21, 0.17 | — |
| Processed meats | 1.34 ± 0.43 | 0.51, 2.18 | 0.002 | 0.17 ± 0.13 | –0.09, 0.42 | 0.21 | 22.40 ± 1.52 | 19.42, 25.38 | <0.0001 | 0.22 ± 0.21 | –0.15, 0.70 | — |
| Plant-based protein foods | –0.79 ± 0.36 | –1.51, –0.08 | 0.03 | –0.22 ± 0.15 | –0.52, 0.08 | 0.15 | 22.45 ± 1.51 | 19.48, 25.42 | <0.0001 | 0.18 ± 0.14 | –0.04, 0.52 | — |
| Nuts and seeds | –0.58 ± 0.33 | –1.23, 0.07 | 0.08 | –0.26 ± 0.17 | –0.59, 0.07 | 0.13 | 22.47 ± 1.51 | 19.51, 25.44 | <0.0001 | 0.15 ± 0.13 | –0.05, 0.47 | — |
| Sugar sweetened beverages | 1.25 ± 0.45 | 0.37, 2.13 | 0.005 | 0.46 ± 0.12 | 0.22, 0.71 | 0.0002 | 22.04 ± 1.50 | 19.09, 25.00 | <0.0001 | 0.58 ± 0.31 | 0.12, 1.29 | 2.6 |
| Sugar and sugary foods | 0.38 ± 0.97 | –1.52, 2.28 | 0.69 | –0.09 ± 0.06 | –0.21, 0.02 | 0.11 | 22.66 ± 1.51 | 19.70, 25.62 | <0.0001 | –0.04 ± 0.10 | –0.27, 0.17 | — |
| Fat and high-fat foods | 3.85 ± 0.99 | 1.91, 5.80 | 0.0001 | 0.28 ± 0.06 | 0.18, 0.39 | <0.0001 | 21.53 ± 1.50 | 18.58, 24.47 | <0.0001 | 1.09 ± 0.36 | 0.48, 1.92 | 4.8 |
n = 737. Analyses are performed on complete cases resulting in the exclusion of n = 11 participants with missing data on menopausal status from the analyses. Mediation analyses are conducted using the Process Macro v. 3.3 for SAS that uses percentile bootstrap CIs to assess the mediating or indirect effect through which the PRSBMI influences WC. 95% CI for indirect effect are estimated through 5000 bootstrap samples. Mediation models are adjusted for age, sex (men, 0; women, 1), current dieting (yes, 1; no, 0), menopausal status (yes, 1; no, 0), current smoking status (yes, 1; no, 0), misreporting of dietary intakes [(underreporting, yes, 1; no, 0) and (overreporting, yes, 1; no, 0)]. Food groups are expressed in percentage of total energy intake. Boot, bootstrap; PRS, polygenic risk score; WC, waist circumference.
a, association between the PRSBMI and the mediator.
b, association between the mediator and WC adjusted for PRSBMI.
Direct effect (c′), association between the PRSBMI and WC adjusted for the mediator.
Indirect effect (ab), mediation effect.
% mediation: percentage of mediation calculated as (indirect effect [ab]/total effect [c])×100. Total effect (c): β=22.62 ± 1.51; 95% CI: 19.66, 25.59, P <0.0001 (association between the PRSBMI and WC without adjustment for the mediator).
Excluding fruit juices.
Genetic correlations between BMI and dietary intakes
Significant negative genetic correlations were observed between BMI and diet quality (ρg = –0.46 ± 0.13, P = 0.001), whole vegetables (ρg = –0.40 ± 0.17, P = 0.02), fruits excluding juices (ρg = –0.56 ± 0.15, P = 0.002), 100% fruit juices (ρg = –0.87 ± 0.73, P = 0.03), dairy products (ρg = –0.47 ± 0.19, P = 0.01), milk (ρg = –0.51 ± 0.17, P = 0.002), plant-based protein foods (ρg = –0.37 ± 0.14, P = 0.01), and nuts and seeds (ρg = –0.43 ± 0.15, P = 0.004) (Table 4). Positive genetic correlations were observed between BMI and SSBs (ρg = 0.79 ± 0.19, P = 0.0001), and fat and high-fat foods (ρg = 0.73 ± 0.18, P = 0.0002).
TABLE 4.
Genetic, environmental, and phenotypic correlations between dietary intakes and BMI in the Quebec Family Study1
| Dietary intakes | ρg ± SE | P | ρe ± SE | P | ρp ± SE | P |
|---|---|---|---|---|---|---|
| Diet quality | ||||||
| Nutrient-Rich Food Index 6.3 | −0.46 ± 0.13 | 0.001 | 0.01 ± 0.08 | 0.91 | −0.18 ± 0.04 | <0.0001 |
| Food groups | ||||||
| Whole vegetables | −0.40 ± 0.17 | 0.02 | 0.03 ± 0.08 | 0.71 | −0.11 ± 0.04 | 0.004 |
| Fruits2 | −0.56 ± 0.15 | 0.002 | –0.08 ± 0.08 | 0.33 | −0.23 ± 0.04 | <0.0001 |
| 100% fruit juices | −0.87 ± 0.73 | 0.03 | 0.15 ± 0.07 | 0.04 | –0.005 ± 0.04 | 0.90 |
| Dairy products | −0.47 ± 0.19 | 0.01 | 0.01 ± 0.08 | 0.94 | −0.13 ± 0.04 | 0.0004 |
| Milk | −0.51 ± 0.17 | 0.002 | 0.07 ± 0.08 | 0.36 | −0.13 ± 0.04 | 0.0007 |
| Yogurt | –0.04 ± 0.58 | 0.95 | –0.07 ± 0.07 | 0.34 | –0.05 ± 0.04 | 0.15 |
| Cheese | –0.16 ± 0.44 | 0.70 | 0.03 ± 0.08 | 0.67 | 0.002 ± 0.04 | 0.95 |
| Processed meats | –0.03 ± 0.22 | 0.89 | 0.16 ± 0.08 | 0.04 | 0.10 ± 0.04 | 0.007 |
| Plant-based protein foods | −0.37 ± 0.14 | 0.01 | 0.07 ± 0.08 | 0.38 | −0.10 ± 0.04 | 0.01 |
| Nuts and seeds | −0.43 ± 0.15 | 0.004 | 0.14 ± 0.08 | 0.09 | −0.08 ± 0.04 | 0.047 |
| Sugar-sweetened beverages | 0.79 ± 0.19 | 0.0001 | –0.01 ± 0.08 | 0.90 | 0.21 ± 0.04 | <0.0001 |
| Sugar and sugary foods | –0.09 ± 0.23 | 0.70 | –0.08 ± 0.08 | 0.32 | −0.08 ± 0.04 | 0.04 |
| Fat and high-fat foods | 0.73 ± 0.18 | 0.0002 | –0.04 ± 0.08 | 0.60 | 0.19 ± 0.04 | <0.0001 |
n families = 215, n = 738. Analyses are performed on complete cases resulting in the exclusion of n = 12 participants with missing data on menopausal (n = 12), dieting (n = 1), or smoking (n = 1) status from the analyses. Bivariate genetic correlation analyses taking into account the family structure of the Quebec Family Study were performed using SOLAR Eclipse version 8.4.1. Food groups are expressed in percentage of total energy intake. Analyses are adjusted for age, sex (men, 0; women, 1), current dieting status (yes, 1; no, 0) menopausal status (yes, 1; no, 0), current smoking status (yes, 1; no, 0), and misreporting of dietary intakes [(underreporting, yes, 1; no, 0) and (overreporting, yes, 1; no, 0)]. ρ e, environmental correlation; ρ g, genotypic correlation; ρ p, phenotypic correlation.
Excluding fruit juices.
Discussion
The mechanisms by which the genetic susceptibility to obesity influences body weight and adiposity are largely unknown. This study aimed to investigate whether diet quality and specific food groups mediate the genetic susceptibility to obesity, using a whole-genome PRS and BMI and WC as measures of obesity. To our knowledge, only 1 study has assessed the mediating effect of diet quality (17), and no studies have assessed the mediating effect of specific food groups on the genetic susceptibility to obesity. The results showed that a poor diet quality, as assessed by the NRF6.3, a high proportion of energy intake from SSBs and fat and high-fat foods, and a low proportion of energy intake from fruits excluding juices, vegetables, total dairy products, and specific dairy products including yogurt and milk, partly mediated the genetic susceptibility to obesity in analyses related to BMI and WC. Although the effect of individual mediator is small, the results suggest that diet quality and intakes of specific food groups explain a part of the genetic susceptibility to obesity. These results also suggest that interventions aimed at improving diet quality and the consumption of specific food groups may have beneficial effects on body weight and WC in individuals with a genetic predisposition to obesity.
In the context of understanding the implication of genes and diet in obesity, both moderation and mediation studies complement each other. Accordingly, moderation highlights the role of diet quality in modifying the genetic association with obesity, whereas mediation identifies pathways whereby genes influence obesity. Our results thus complement those of Wang et al. (44) who showed, using a gene-diet interaction design in 2 prospective US cohorts, that improvement in adherence to healthy dietary patterns attenuated the genetic association with weight gain, and this was particularly pronounced among individuals with high genetic susceptibility to obesity. They also complement those of other gene-diet interaction studies which showed that the association between genetic susceptibility to obesity and BMI was stronger in individuals with low diet quality as well as low intakes of fruits and high intakes of SSBs and fried foods (6–8). In their study investigating the mediating effect of eating patterns on genetic susceptibility to obesity, assessed using a PRSBMI, Masip et al. (17) observed no mediating effect of diet quality based on a 14-item FFQ, but this null finding may be due to the lower accuracy of brief instruments to assess the whole diet and the lack of consideration of systematic errors (i.e. misreporting of energy intake) in dietary assessment (36, 41, 45). However, they found a mediating effect of some eating patterns, such as snacking and infrequent and unhealthy eating, on the association between genetic susceptibility to obesity and BMI or WC (17).
The moderate to large genetic correlations between most statistically significant mediating variables and BMI suggest that dietary intakes and BMI share a common underlying genetic architecture. Several genes associated with obesity have also been associated with dietary intakes (16, 46–48), and unhealthy eating has been recognized as one of the leading causes of obesity (49). This collectively provides support to the hypothesis that dietary intakes may also act as mediators of the genetic susceptibility to obesity. However, because of the cross-sectional design of this study and the fact that individuals with obesity or high WC may modify their food intakes to either control or lose body weight, or to prevent or treat obesity-associated comorbidities, reverse causation between dietary intakes and obesity measures cannot be excluded. Yet, a recent Mendelian randomization study showed that a low proportion of energy from carbohydrates and a high proportion of energy from lipids were causally related to higher BMI and WC, suggesting evidence for a causal effect between dietary intakes and measures of BMI and WC (50). This is also supported by studies showing that intakes of vegetables, fruits, and yogurt were protective for weight gain, whereas SSBs and high-fat foods, which are mostly fried and ultra-processed, and similar to foods included in the fat and high-fat food group in the present study, were associated with weight gain (34, 35). Total dairy products and milk appear mostly neutral towards weight gain (34, 35, 51), but have been associated with a lower risk of abdominal obesity (52).
The associations between the PRSBMI and dietary intakes are in line with genetic correlations. Accordingly, all dietary variables negatively associated with the PRSBMI, except yogurt, showed a negative genetic correlation with BMI, indicating that shared genetic factors influence BMI and dietary intakes in the opposite direction. Similarly, all dietary variables positively associated with the PRSBMI showed a positive genetic correlation with BMI. Negative genetic correlations between BMI and fruits and vegetables and a positive genetic correlation between soft drinks (soda), a type of SSB, have also been previously reported (53, 54). The negative association between the PRSBMI and diet quality is consistent with the results of Dashti et al. (55) who showed that a high genetic risk of obesity was associated with purchasing less healthy foods and more food items, as objectively measured over a 3-mo period at a workplace cafeteria. In addition to studies demonstrating an association between genetic susceptibility to obesity and eating behavior traits (12–15, 17, 18, 56, 57), these results collectively suggest that obesity genes may influence dietary intakes. This is also supported by the fact that obesity-related genetic variants are mainly expressed in the central nervous system, in regions involved in appetite regulation, learning, cognition, emotions, and memory, such as the hypothalamus, pituitary gland, hippocampus, and limbic system (58, 59). It is thus likely that genes affect BMI through behavioral pathways related to food intake (57).
This study has several strengths and limitations. The main strength includes using a whole-genome PRS of BMI which explains a high percentage of variance in BMI and WC (i.e. 25.7% and 19.8%, respectively) compared with genetic risk scores incorporating only genome-wide significant genetic variants (4, 59). Another strength of this study is the use of a 3-d food record with consideration of misreporting (systematic error) (36, 41). This method allows a more comprehensive assessment of dietary intakes compared with a screener or a short FFQ (45), which has been previously used to assess the mediating effect of diet quality on the genetic susceptibility to obesity. However, food records are subjected to day-to-day variation (within-person random error) which may have attenuated estimates of regression models (60). The main limitation of this study is the cross-sectional design that precludes causal inference and that cannot exclude reverse causation between diet and obesity. As such, replication of these results within other cohorts and using longitudinal data is needed. Another limitation is our inability to dissociate single foods from mixed meals that have been entered as such into the food database, which contributed to a mean of 6.3 ± 7.8% of TEI, resulting mainly in the exclusion of these foods from the different food groups. Another limitation of this study is the use of the NRF6.3 to assess diet quality. Despite being a good indicator of the quality of foods in the diet (28, 29), this index focuses on nutrients rather than food patterns and does not reflect adherence to current Canadian dietary guidelines (61). However, the assessment of different food groups as mediators of the genetic susceptibility to obesity overcomes this limit and allows the identification of specific foods that could be targeted in obesity prevention and treatment. Future studies should assess if other food groups, such as whole grains and refined grains, are mediators of the genetic susceptibility to obesity.
In conclusion, this study shows that poor diet quality and intakes of specific food groups, including high intakes of high-fat foods and SSBs and low intakes of vegetables, fruits, milk, and yogurt, partly mediate the association between genetic susceptibility to obesity and both BMI and WC. To our knowledge, this study is the first to assess the role of specific food groups in mediating the genetic susceptibility to obesity. These results suggest that improvement in diet quality and in the consumption of specific food groups may reduce obesity risk among individuals with high genetic susceptibility. They also emphasize the relevance of intervening on diet quality to reduce obesity among these individuals.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—LP, VD, CL, M-EL, and RJ: designed the research; CC and RJ: analyzed data; RJ: wrote the first draft of the manuscript; LP had primary responsibility for the final content; and all authors: read, edited, and approved the final manuscript.
Notes
This study was supported by the Cardiometabolic Health, Diabetes and Obesity (CMDO) Research Network of the Fonds de recherche du Québec – Santé (FRQS) and by the Institute of Nutrition and Functional Foods (INAF). The Quebec Family Study was supported over the years by multiple grants from the Medical Research Council of Canada and the Canadian Institutes of Health Research (PG-11811, MT-13960, and GR-15187) and other agencies. RJ is the recipient of PhD scholarships from the FRQS and the Canadian Institutes of Health Research (CIHR, fellowship number: 430872). AT is the holder of the Canada Research Chair in Environment and Energy Balance. C Bouchard is partially funded by the John W Barton, Sr Chair in Genetics and Nutrition and by the National Institutes of Health (NIH) funded grant (NIH 8 P30GM118430-01). The CMDO Research Network and INAF were not involved in designing and conducting the study, analyzing and interpreting the data, or preparing and reviewing the manuscript before submission.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: DV, daily values; HEI, Healthy Eating Index; NRF6.3, Nutrient Rich Food Index 6.3; pER, predicted energy requirement; PRS, polygenic risk score; QFS, Quebec Family Study; rEI, reported energy intake; SNP, single nucleotide polymorphism; SSB, sugar-sweetened beverage; TEI, total energy intake; WC, waist circumference.
Contributor Information
Raphaëlle Jacob, Centre Nutrition, santé et société (NUTRISS), Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada; Quebec Heart and Lung Institute Research Center, Université Laval, Quebec, Canada.
Catherine Bertrand, Centre Nutrition, santé et société (NUTRISS), Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Canada; Department of Kinesiology, Faculty of Medicine, Université Laval, Quebec, Canada.
Clare Llewellyn, Department of Behavioural Science and Health, University College London, London, United Kingdom.
Christian Couture, Quebec Heart and Lung Institute Research Center, Université Laval, Quebec, Canada; Department of Kinesiology, Faculty of Medicine, Université Laval, Quebec, Canada.
Marie-Ève Labonté, Centre Nutrition, santé et société (NUTRISS), Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Canada; School of Nutrition, Université Laval, Quebec, Canada.
Angelo Tremblay, Centre Nutrition, santé et société (NUTRISS), Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Canada; Quebec Heart and Lung Institute Research Center, Université Laval, Quebec, Canada; Department of Kinesiology, Faculty of Medicine, Université Laval, Quebec, Canada.
Claude Bouchard, Pennington Biomedical Research Center, Baton Rouge, LA, USA.
Vicky Drapeau, Centre Nutrition, santé et société (NUTRISS), Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Canada; Quebec Heart and Lung Institute Research Center, Université Laval, Quebec, Canada; Department of Physical Education, Faculty of Education, Université Laval, Quebec, Canada.
Louis Pérusse, Centre Nutrition, santé et société (NUTRISS), Institute of Nutrition and Functional Foods (INAF), Université Laval, Quebec, Canada; Department of Kinesiology, Faculty of Medicine, Université Laval, Quebec, Canada.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the authors as well as the funding bodies.
References
- 1. Pérusse L, Rice TK, Bouchard C. Evidence of a genetic component to obesity from genetic epidemiology. In: Bray G, Bouchard Ceditors. Handbook of obesity—epidemiology, etiology, and physiopathology. Boca Raton (FL): CRC Press; 2013. 91–104. [Google Scholar]
- 2. Elks CE, den Hoed M, Zhao JH, Sharp SJ, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Frontiers in Endocrinology. 2012;3:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silventoinen K, Jelenkovic A, Sund R, Yokoyama Y, Hur YM, Cozen W, Hwang AE, Mack TM, Honda C, Inui F et al. Differences in genetic and environmental variation in adult BMI by sex, age, time period, and region: an individual-based pooled analysis of 40 twin cohorts. Am J Clin Nutr. 2017;106(2):457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yengo L, Sidorenko J, Kemper KE, Zheng Z, Wood AR, Weedon MN, Frayling TM, Hirschhorn J, Yang J, Visscher PM. Meta-analysis of genome-wide association studies for height and body mass index in approximately 700000 individuals of European ancestry. Hum Mol Genet. 2018;27(20):3641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corraini P, Olsen M, Pedersen L, Dekkers OM, Vandenbroucke JP. Effect modification, interaction and mediation: an overview of theoretical insights for clinical investigators. Clinical Epidemiology. 2017;9:331–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ding M, Ellervik C, Huang T, Jensen MK, Curhan GC, Pasquale LR, Kang JH, Wiggs JL, Hunter DJ, Willett WC et al. Diet quality and genetic association with body mass index: results from 3 observational studies. Am J Clin Nutr. 2018;108(6):1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Qi Q, Chu AY, Kang JH, Huang J, Rose LM, Jensen MK, Liang L, Curhan GC, Pasquale LR, Wiggs JL et al. Fried food consumption, genetic risk, and body mass index: gene-diet interaction analysis in three US cohort studies. BMJ. 2014;348(1):g1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Qi Q, Chu AY, Kang JH, Jensen MK, Curhan GC, Pasquale LR, Ridker PM, Hunter DJ, Willett WC, Rimm EB et al. Sugar-sweetened beverages and genetic risk of obesity. N Engl J Med. 2012;367(15):1387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brunkwall L, Chen Y, Hindy G, Rukh G, Ericson U, Barroso I, Johansson I, Franks PW, Orho-Melander M, Renström F. Sugar-sweetened beverage consumption and genetic predisposition to obesity in 2 Swedish cohorts. Am J Clin Nutr. 2016;104(3):809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Casas-Agustench P, Arnett DK, Smith CE, Lai CQ, Parnell LD, Borecki IB, Frazier-Wood AC, Allison M, Chen YD, Taylor KD et al. Saturated fat intake modulates the association between an obesity genetic risk score and body mass index in two US populations. Journal of the Academy of Nutrition and Dietetics. 2014;114(12):1954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Celis-Morales CA, Lyall DM, Gray SR, Steell L, Anderson J, Iliodromiti S, Welsh P, Guo Y, Petermann F, Mackay DF et al. Dietary fat and total energy intake modifies the association of genetic profile risk score on obesity: evidence from 48 170 UK Biobank participants. Int J Obes. 2017;41(12):1761–8. [DOI] [PubMed] [Google Scholar]
- 12. Jacob R, Drapeau V, Tremblay A, Provencher V, Bouchard C, Perusse L. The role of eating behavior traits in mediating genetic susceptibility to obesity. Am J Clin Nutr. 2018;108(3):445–52. [DOI] [PubMed] [Google Scholar]
- 13. Brunner EJ, Maruyama K, Shipley M, Cable N, Iso H, Hiyoshi A, Stallone D, Kumari M, Tabak A, Singh-Manoux A et al. Appetite disinhibition rather than hunger explains genetic effects on adult BMI trajectory. Int J Obes. 2021;45(4):758–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Lauzon-Guillain B, Clifton EA, Day FR, Clement K, Brage S, Forouhi NG, Griffin SJ, Koudou YA, Pelloux V, Wareham NJ et al. Mediation and modification of genetic susceptibility to obesity by eating behaviors. Am J Clin Nutr. 2017;106(4):996–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Konttinen H, Llewellyn C, Wardle J, Silventoinen K, Joensuu A, Mannisto S, Salomaa V, Jousilahti P, Kaprio J, Perola M et al. Appetitive traits as behavioural pathways in genetic susceptibility to obesity: a population-based cross-sectional study. Sci Rep. 2015;5(1):14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oyeyemi BF, Ologunde CA, Olaoye AB, Alamukii NA. FTO gene associates and interacts with obesity risk, physical activity, energy intake, and time spent sitting: pilot study in a Nigerian population. Journal of Obesity. 2017;2017:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Masip G, Silventoinen K, Keski-Rahkonen A, Palviainen T, Sipilä PN, Kaprio J, Bogl LH. The genetic architecture of the association between eating behaviors and obesity: combining genetic twin modeling and polygenic risk scores. Am J Clin Nutr. 2020;112(4):956–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Llewellyn CH, Trzaskowski M, van Jaarsveld CHM, Plomin R, Wardle J. Satiety mechanisms in genetic risk of obesity. JAMA Pediatrics. 2014;168(4):338–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wardle J, Carnell S, Haworth CM, Farooqi IS, O'Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. The Journal of Clinical Endocrinology & Metabolism. 2008;93(9):3640–3. [DOI] [PubMed] [Google Scholar]
- 20. Khera AV, Chaffin M, Wade KH, Zahid S, Brancale J, Xia R, Distefano M, Senol-Cosar O, Haas ME, Bick A et al. Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell. 2019;177(3):587–96.e9.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chaput JP, Perusse L, Despres JP, Tremblay A, Bouchard C. Findings from the Quebec Family Study on the etiology of obesity: genetics and environmental highlights. Current Obesity Reports. 2014;3(1):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bouchard C. Genetic epidemiology, association, and sib-pair linkage: results from the Québec Family Study. In: Bray G, Ryan Deditors. Molecular and genetic aspects of obesity. Baton Rouge (LA): State University Press; 1996. 470–81. [Google Scholar]
- 23. The Airlie (VA) Consensus Conference. Standardization of anthropometric measurements. Champaign (IL): Human Kinetics; 1988. [Google Scholar]
- 24. Sung YJ, Perusse L, Sarzynski MA, Fornage M, Sidney S, Sternfeld B, Rice T, Terry JG, Jacobs DR Jr, Katzmarzyk P et al. Genome-wide association studies suggest sex-specific loci associated with abdominal and visceral fat. Int J Obes. 2016;40(4):662–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Privé F, Arbel J, Vilhjálmsson BJ. LDpred2: better, faster, stronger. Bioinformatics. 2020;; 36(22–23):5424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tremblay A, Sévigny J, Leblanc C, Bouchard C. The reproducibility of a three-day dietary record. Nutr Res. 1983;3(6):819–30. [Google Scholar]
- 27. Health Canada. The Canadian nutrient file. Ottawa (Canada): 2010. [Google Scholar]
- 28. Fulgoni VL 3rd, Keast DR, Drewnowski A. Development and validation of the nutrient-rich foods index: a tool to measure nutritional quality of foods. J Nutr. 2009;139(8):1549–54. [DOI] [PubMed] [Google Scholar]
- 29. Drewnowski A. Defining nutrient density: development and validation of the nutrient rich foods index. J Am Coll Nutr. 2009;28(4):421S–6S. [DOI] [PubMed] [Google Scholar]
- 30. Garriguet D. Diet quality in Canada. Health Rep. 2009;20(3):41–52. [PubMed] [Google Scholar]
- 31. Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, Wilson MM, Reedy J. Update of the Healthy Eating Index: HEI-2015. Journal of the Academy of Nutrition and Dietetics. 2018;118(9):1591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Health Canada. Nutrition labeling—table of daily values. Ottawa (Canada): 2016. [Google Scholar]
- 33. Drapeau V, Despres JP, Bouchard C, Allard L, Fournier G, Leblanc C, Tremblay A. Modifications in food-group consumption are related to long-term body-weight changes. Am J Clin Nutr. 2004;80(1):29–37. [DOI] [PubMed] [Google Scholar]
- 34. Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133(2):187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang TT, Roberts SB, Howarth NC, McCrory MA. Effect of screening out implausible energy intake reports on relationships between diet and BMI. Obes Res. 2005;13(7):1205–17. [DOI] [PubMed] [Google Scholar]
- 37. Black AE, Cole TJ. Within- and between-subject variation in energy expenditure measured by the doubly-labelled water technique: implications for validating reported dietary energy intake. Eur J Clin Nutr. 2000;54(5):386–94. [DOI] [PubMed] [Google Scholar]
- 38. Institute of Medicine. Panel on Macronutrients. Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 39. Garriguet D. Accounting for misreporting when comparing energy intake across time in Canada. Health Rep. 2018;29(5):3–12. [PubMed] [Google Scholar]
- 40. Garriguet D. Impact of identifying plausible respondents on the under-reporting of energy intake in the Canadian community health survey. Health Rep. 2008;19:47–55. [PubMed] [Google Scholar]
- 41. Jessri M, Lou WY, L'Abbé MR. Evaluation of different methods to handle misreporting in obesity research: evidence from the Canadian national nutrition survey. Br J Nutr. 2016;115(1):147–59. [DOI] [PubMed] [Google Scholar]
- 42. Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach, Second edition. New York (NY): Guildford Press; 2017. [Google Scholar]
- 43. Blangero J, Williams JT, Almasy L. Variance component methods for detecting complex trait loci. Adv Genet. 2001;42:151–81. [DOI] [PubMed] [Google Scholar]
- 44. Wang T, Heianza Y, Sun D, Huang T, Ma W, Rimm EB, Manson JE, Hu FB, Willett WC, Qi L. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ. 2018;360:j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thompson FE, Kirkpatrick SI, Subar AF, Reedy J, Schap TE, Wilson MM, Krebs-Smith SM. The National Cancer Institute's dietary assessment primer: a resource for diet research. Journal of the Academy of Nutrition and Dietetics. 2015;115(12):1986–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bauer F, Elbers CC, Adan RA, Loos RJ, Onland-Moret NC, Grobbee DE, van Vliet-Ostaptchouk JV, Wijmenga C, van der Schouw YT. Obesity genes identified in genome-wide association studies are associated with adiposity measures and potentially with nutrient-specific food preference. Am J Clin Nutr. 2009;90(4):951–9. [DOI] [PubMed] [Google Scholar]
- 47. Livingstone KM, Celis-Morales C, Lara J, Ashor AW, Lovegrove JA, Martinez JA, Saris WH, Gibney M, Manios Y, Traczyk I et al. Associations between FTO genotype and total energy and macronutrient intake in adults: a systematic review and meta-analysis. Obes Rev. 2015;16(8):666–78. [DOI] [PubMed] [Google Scholar]
- 48. McCaffery JM, Papandonatos GD, Peter I, Huggins GS, Raynor HA, Delahanty LM, Cheskin LJ, Balasubramanyam A, Wagenknecht LE, Wing RR. Obesity susceptibility loci and dietary intake in the look AHEAD trial. Am J Clin Nutr. 2012;95(6):1477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malik VS, Willett WC, Hu FB. Global obesity: trends, risk factors and policy implications. Nat Rev Endocrinol. 2013;9(1):13–27. [DOI] [PubMed] [Google Scholar]
- 50. Freuer D, Meisinger C, Linseisen J. Causal relationship between dietary macronutrient composition and anthropometric measures: a bidirectional two-sample Mendelian randomization analysis. Clin Nutr. 2021;40(6):4120–31. [DOI] [PubMed] [Google Scholar]
- 51. Willett WC, Ludwig DS. Milk and health. N Engl J Med. 2020;382(7):644–54. [DOI] [PubMed] [Google Scholar]
- 52. Lee M, Lee H, Kim J. Dairy food consumption is associated with a lower risk of the metabolic syndrome and its components: a systematic review and meta-analysis. Br J Nutr. 2018;120(4):373–84. [DOI] [PubMed] [Google Scholar]
- 53. Martin LJ, Lee SY, Couch SC, Morrison J, Woo JG. Shared genetic contributions of fruit and vegetable consumption with BMI in families 20 y after sharing a household. Am J Clin Nutr. 2011;94(4):1138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Eney AE, Tsang S, Delaney JA, Turkheimer E, Duncan GE. Cross-sectional association between soda consumption and body mass index in a community-based sample of twins. Nutrition Journal. 2017;16(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dashti HS, Hivert MF, Levy DE, McCurley JL, Saxena R, Thorndike AN. Polygenic risk score for obesity and the quality, quantity, and timing of workplace food purchases: a secondary analysis from the Choosewell 365 randomized trial. PLoS Med. 2020;17(7):e1003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cornelis MC, Rimm EB, Curhan GC, Kraft P, Hunter DJ, Hu FB, van Dam RM. Obesity susceptibility loci and uncontrolled eating, emotional eating and cognitive restraint behaviors in men and women. Obesity. 2014;22(5):E135–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Silventoinen K, Konttinen H. Obesity and eating behavior from the perspective of twin and genetic research. Neuroscience & Biobehavioral Reviews. 2020;109:150–65. [DOI] [PubMed] [Google Scholar]
- 58. Loos RJ. The genetics of adiposity. Curr Opin Genet Dev. 2018;50:86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J et al. Genetic studies of body mass index yield new insights for obesity biology. Nature. 2015;518(7538):197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. National Institutes of Health, National Cancer Institute. Dietary assessment primer, effect of measurement error. 2021. [Internet]. [Accessed 2021 Jun 30]. Available from: https://dietassessmentprimer.cancer.gov/concepts/error/error-effects.html. [Google Scholar]
- 61. Health Canada. Canada's food guide. Ottawa (Canada): 2019. [Internet]. [Accessed 2021 Jun 30]. Available from: https://food-guide.canada.ca/en/. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending approval from the authors as well as the funding bodies.