Abstract
A simulation model was developed to better understand the mechanisms of brain injuries in sports. A three-dimensional model comprising approximately 1.22 million elements was constructed from cranial computed tomography images of adult male volunteers by the voxel method. To simulate contact sports that permit actions such as tackling, a sinusoidal wave with duration of 10 ms and maximum acceleration of 2000 m/s2 was applied to the lowest point of the model to apply rotational acceleration to the head from different directions. The von Mises stress was then observed at five points in the coronal plane of the brain: cingulate gyrus (CG), corpus callosum (CC), brain stem (BS), lateral temporal lobe (LT), and medial temporal lobe (MT). LS-DYNA universal finite element analysis software with explicit time integration was used for the analysis. Concentrations of stress started to appear in the CC and BS at 10 ms post-impact, after which they also became evident in the CG and MT. The maximum changes in stress at each location occurred 10–15 ms post-impact. The von Mises stress was 9–14 kPa in the CG, 8–24 kPa in the CC, 12–24 kPa in the BS, 7–12 kPa in the LT, and 12–18 kPa in the MT. The highest stress in every part of the brain occurred after lateral impact, followed by oblique impact and sagittal impact. Such simulations may help elucidate the mechanisms of brain injuries in sports and help develop measures to prevent chronic traumatic encephalopathy.
Keywords: sports head injury, head injury simulation, concussion
Introduction
In many different sports, repeated concussions or other minor traumatic brain injuries (mTBIs) may lead to the development of dementia and other forms of chronic traumatic encephalopathy (CTE), and this has become a social issue.1–4) Sporting authorities are now taking measures such as rule revisions to prevent repeated mTBIs, but the underlying mechanism of traumatic brain injury due to soft impact is unclear, and from this perspective, CTE prevention measures are making little headway.
The following are among the reasons why it is so difficult to elucidate the mechanism of head injury in these contact sports. First, the complex structure of the brain is covered by structures with widely varying physical values, such as the cranial bones, dura mater, and cerebrospinal fluid. Second, in practice, injuries are sustained in many different ways, making it difficult to accurately assess factors such as the energy at the time of injury. Third, although the pathological effects of injury are becoming known through autopsy studies, and since impacts causing injury and evaluation of their consequences differ much in time, the correspondence between individual findings and the mechanism of injury remains unclear. Finally, animal experiments involving cranial injury are restricted to rats and other small animals, and in addition to the anatomical–physiological differences, it is difficult to say that their biological response is applicable to humans.
Because animal experiments are difficult to conduct in today’s social milieu, the mechanism of TBI is being analyzed using simulations. Such simulations may improve our understanding of the mechanisms of traumatic brain injuries in sports and contribute to improving protective gear and formulating rules in the sporting world to avoid the tragic outcome of CTE. The authors have previously observed changes in intracerebral stress as a result of translational acceleration and rotational types of acceleration,5,6) but those studies paid particular attention to changes in intracerebral stress during lateral impact (LI) by modelling it only on rotational acceleration. In the present study, however, the same simulation model was improved to reproduce the structures of the brain in detail, as well as building on Gennarelli et al.’s experiments to reproduce impacts from three directions (lateral, sagittal, and oblique),7) and observe over time changes of intracerebral stress in the coronal plane in five locations in particular: the cingulate gyrus (CG), corpus callosum (CC), brain stem (BS), lateral temporal lobe (LT), and medial temporal lobe (MT).
Use of this simulation is intended to improve our understanding of the mechanisms of traumatic brain injuries in sports and contribute to improving protective gear and formulating rules in the sporting world to avoid the tragic outcome of CTE.
Methods
Simulation model
The cranial simulation model used was the same as that reported in our previous study,6) a three-dimensional model comprising approximately 1.22 million elements constructed from cranial computed tomography images of adult male volunteers by the voxel method developed by Hollister and Kikuchi.8)
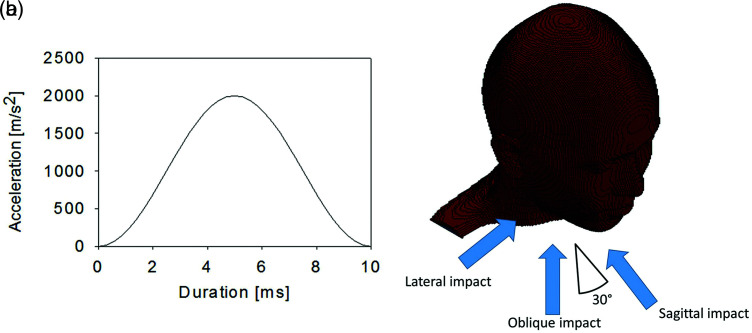
To simulate contact sports that permit actions such as tackling, a sinusoidal wave with duration of 10 ms and maximum acceleration of 2000 m/s2, as shown in Fig. 1a, was applied to the lowest point of the model to induce rotational acceleration to the head. This acceleration profile corresponds to head injury criterion (HIC) = 1000. The HIC is the index used in the safety evaluation of automobile accidents to assess whether brain injury might have occurred, and HIC = 1000 is the level at which brain injury occurs in 50% of cases.5) The directions of impact included sagittal impact (SI), LI, and oblique impact (OI) at an angle of 30° to the SI used in Gennarelli et al.’s experiments (Fig. 1b).7)
Fig. 1.
(a) Acceleration profile used for impact. The impact duration is 10 ms, and the profile has a peak translational acceleration of 2000 m/s2 in the middle. (b) Impact directions. The SI is simulated as striking the jaw, the LI as striking the temple, and the OI as striking the cheek at a 30° angle to the SI. LI: lateral impact, OI: oblique impact, SI: sagittal impact.
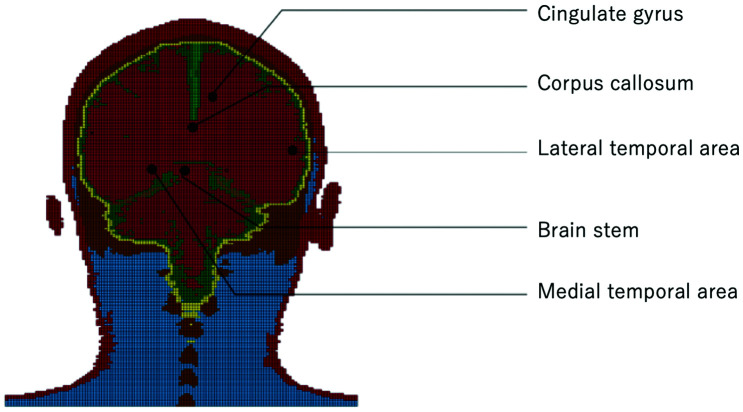
The von Mises stress was observed at five points in the coronal plane of the brain: CG, CC, BS, LT, and MT (Fig. 2).
Fig. 2.
Intracerebral stress measurement sites.
Analysis
LS-DYNA universal finite element analysis software with explicit time integration was used for the analysis. The physical properties of intracranial structures were assigned as shown in Table 1, with the skin, dura mater, falx cerebri, and tentorium cerebelli treated as elastic bodies, bone as an elasto-plastic body, and the brain, soft tissues, and eyes as viscoelastic bodies. Cerebrospinal fluid and the cerebral ventricles were not considered to behave as fluids during impact, but they were treated as viscoelastic bodies with a low modulus of shearing elasticity.
Table 1. Material properties of tissues in the head model.
| Tissue | Young’s modulus E (Pa) | Poisson’s ratio ν | Density ρ (kg/m3) | Yield stress σy (Pa) | Tangent modulus Et (Pa) |
|---|---|---|---|---|---|
| (a) Elastic and elasto-plastic bodies | |||||
| Skin | 1.67 × 107 | 0.420 | 1300 | – | – |
| Falx cerebri | 3.15 × 107 | 0.450 | 1130 | – | – |
| Dura mater | 3.15 × 107 | 0.450 | 1130 | – | – |
| Tentorium cerebelli | 3.15 × 107 | 0.450 | 1130 | – | – |
| Skull | 8.75 × 109 | 0.261 | 1456 | 4.18 × 107 | 4.62 × 109 |
| Tissue | Bulk modulus K (Pa) | Short-term shear modulus G0 (Pa) | Long-term shear modulus G∞ (Pa) | Density ρ (kg/m3) | Decay factor β [s-1] |
|---|---|---|---|---|---|
| (b) Viscoelastic bodies | |||||
| Brain | 2.19 × 109 | 1.25 × 104 | 2.5 × 103 | 1040 | 80 |
| Soft tissue | 2.19 × 109 | 1.25 × 104 | 2.5 × 103 | 1040 | 80 |
| Eyes | 2.19 × 109 | 1.25 × 104 | 2.5 × 103 | 1040 | 80 |
| CSF | 2.19 × 109 | 5.0 × 102 | – | 1040 | 5.0 × 105 |
| Ventricle | 2.19 × 109 | 5.0 × 102 | – | 1040 | 5.0 × 105 |
Results
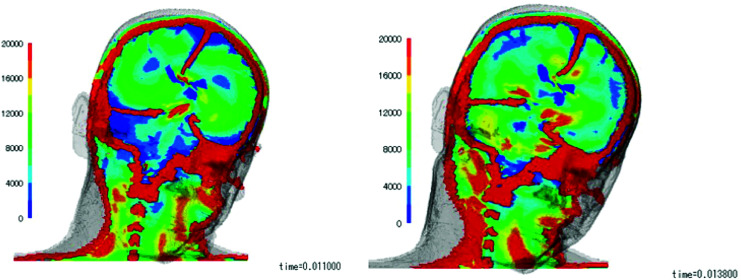
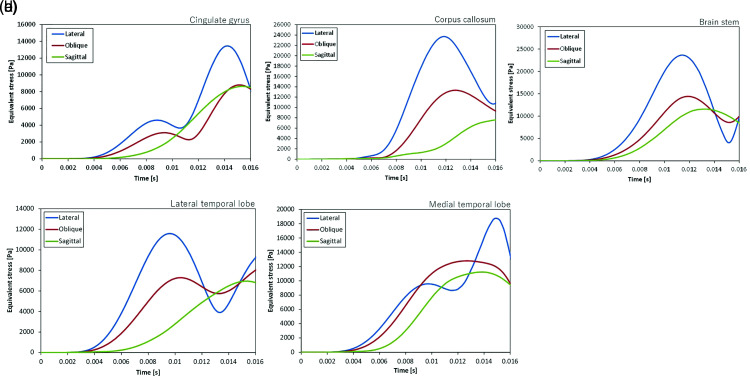
Observations of the changes in intracerebral stress over time following impacts from each direction, SI, LI, and OI (Fig. 3), showed that concentrations of stress started to appear in the CC and BS at 10 ms post-impact, after which they also became evident in the CG and MT. The maximum changes in stress at each location occurred at 10–15 ms post-impact. Measured as von Mises stress, the intensity of the stress was 9–14 kPa in the CG, 8–24 kPa in the CC, 12–24 kPa in the BS, 7–12 kPa in the LT, and 12–18 kPa in the MT, with the highest stress concentrations evident in the BS and CC, followed by the MT (Fig. 4).
Fig. 3.
Changes over time in intracerebral stress. Left: At 11 ms, concentrations of stress start to appear in the CC and BS. Right: At 13 ms, concentrations of stress also appear in the CG and the medial temporal area. BS: brain stem, CC: corpus callosum, CG: cingulate gyrus.
Fig. 4.
Von Mises stress at various sites. (a) Von Mises stress in the CG. Stress peaks at around 14 ms, with stress of 14 kPa evident after LI and stress of 8 kPa after oblique or SI. (b) Von Mises stress in the CC. For LI, stress peaks at 24 kPa at around 12 ms, whereas for OI, the peak is approximately 13 kPa and occurs at around 12 ms, and for SI, the peak is later and approximately 8 kPa. (c) Von Mises stress in the BS. Stress peaks at around 12 ms, with a high level of stress of 24 kPa evident after LI, and high levels of stress of 14 kPa after OI and 12 kPa after SI are also evident. (d) Von Mises stress in the LT. After LI, stress peaks somewhat early at 10 ms at approximately 12 kPa. After OI, stress peaks at 7 kPa at the same time as for LI, and after SI, it peaks at around 14 ms at approximately 7 kPa. (e) Von Mises stress in the MT. After LI, a high level of stress of 19 kPa is evident at around 15 ms, and stresses of 12 kPa are also evident at a somewhat early stage after both OI and SI. BS: brain stem, CC: corpus callosum, CG: cingulate gyrus, LI: lateral impact, LT: lateral temporal lobe, MT: medial temporal lobe, OI: oblique impact, SI: sagittal impact.
A comparison of the directions of impact showed that the highest stress in every part of the brain occurred after LI, followed by OI and SI. The intensity was approximately 1.5 to 2 times greater after LI than after OI and SI in every part of the brain.
Discussion
The brain’s behavior at the moment of injury had been unknown until Pudenz and Shelden recorded the movement of the surface of the brain in macaques in 1946, and that study only observed the surface of the cerebral hemispheres.9) For the deeper parts of the brain, Gennarelli et al.’s experiments on primates were a milestone in understanding cranial trauma, because they made clear the association between the behavior (symptoms) of the brain under an external force and pathological findings.7) Subsequently, Tagge et al. continued to verify these findings pathologically in small animals,10) but it is still currently impossible to investigate changes in the deep structures of the brain at the moment of injury and their mechanisms in humans; therefore, simulations must be used instead. The use of simulations enables us to understand the intracerebral response, and studies have been performed with the goal of enabling the proposal of methods of alleviating impact.
Immobilization of the neck in the present study made it similarly possible to reproduce rotational acceleration, which contributes to the occurrence of concussion, as indicated in a past simulation study.11) This enabled observation of physical damage to the brain as von Mises stress, which is currently considered to provide a good index for ascertaining the deformation of the brain.5) The challenge imposed by stress on brain tissue physically causes axonal or microvascular injury and subsequent changes that are believed to lead to the appearance of symptoms associated with concussion and CTE.10,12)
The distinctive feature of the present study is that, because previous studies already identified areas of the brain where concentrations of stress occur, it was possible to investigate five selected areas that are considered important in this respect (CG, CC, BS, LT, and MT) (Fig. 2). The different directions of impact (SI, LI, and OI) of Gennarelli et al.’s experiments were also examined,7) and it was possible to observe the correlation between their pathological findings and the current simulation.
Of the five locations observed, high concentrations of stress were observed in the BS, CC, and MT (Fig. 4). This suggested that stress on the brainstem may cause impaired signal transduction over a wide area, mainly in axons, resulting in the appearance of a wide variety of symptoms. In the CC, this has been implicated in changes in self-motivation and emotions, and in the MT to orientation and memory (related to amnesia). Previous simulation studies have also reported high stress in the CC.13) These sites of damage are also associated with pathological findings of diffuse axonal damage.
Concerning the direction of impact, the stress generated in LI was about 1.5 to 2 times as intense as that in SI, a result that reflects Gennarelli et al.’s findings, consistent with pathological findings and clinical symptoms from primates, and Sahoo et al.’s findings that the greatest intracerebral impact occurred in LI14); all of the above indicate the validity of the simulation in the present study. Actual sports involve the interaction of complex elements, but in addition to purely LIs when a player is taken completely by surprise, impacts received when a player is consciously trying to avoid a frontal impact are also common, and these often correspond to OI in the present study. The fact that higher stress was generated by an OI than by an SI in the present study suggests that OI may merit caution in practice.
In the present study, since only a single cross-section of the brain was observed, it is not possible to assess detailed changes in stress at other locations of interest because of the complex structure within the cranium. Furthermore, brain behavior along with whole-body movement with variable neck stiffness is another issue, though Murayama et al. have already delineated the relationship between body actions and head acceleration in judo.15,16)
Although simulations are only theoretical, digitalized model investigations, they offer the only means of exploring the mechanism of sports-related brain injury at present. In the present study, impacts were simulated with an energy corresponding to HIC = 1000, the level at which serious brain injury occurs more than half the time, but by investigating the threshold for impacts that actually causes concussion in sports,17) it may be possible to confirm the response to increasing and decreasing energy levels and identify the anatomical changes in corresponding simulations more practically.
From the perspective of prevention, the use of simulation as a tool enables the evaluation of changes in how the brain responds to impact with the use of a range of different protective devices, such as helmets and mouthpieces, which are not regarded as definite protective gear.18)
Ethical Approval Statement
This study is based on digital simulation data and does not contain any information from human subjects; therefore, ethics committee approval was not required.
Footnotes
Conflicts of Interest Disclosure
Aiphone Co., Ltd. has provided a donation to our institution not related to this project. Allm Inc. has provided research funds not related to this project. The authors declare that they have no other potential conflicts of interest relevant to this research.
References
- 1).McAllister TW, Flashman LA, Maerlender A, et al. : Cognitive effects of one season of head impacts in a cohort of collegiate contact sport athletes. Neurology 78: 1777–1784, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2).McCrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A: What is the evidence for chronic concussion-related changes in retired athletes: behavioural, pathological and clinical outcomes? Br J Sports Med 47: 327–330, 2013 [DOI] [PubMed] [Google Scholar]
- 3).Mez J, Daneshvar DH, Kiernan PT, et al. : Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318: 360–370, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Mackay DF, Russell ER, Stewart K, MacLean JA, Pell JP, Stewart W: Neurodegenerative disease mortality among former professional soccer players. N Engl J Med 381: 1801–1808, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5).Watanabe D, Yuge K, Nishimoto T, Murakami S, Takao H: Development of a human head FE model and impact simulation on the focal brain injury. J Comput Sci Tech 3: 252–263, 2009 [Google Scholar]
- 6).Watanabe D, Yuge K, Nishimoto T, Murakami S, Takao H: Simulation of a human head subject to a lateral rotational impact and study on the cause of a diffuse axonal injury. Int J Vehicle Safety 5: 333–344, 2011 [Google Scholar]
- 7).Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP: Diffuse axonal injury and traumatic coma in the primate. Ann Neurol 12: 564–574, 1982 [DOI] [PubMed] [Google Scholar]
- 8).Hollister SJ, Kikuchi N: Homogenization theory and digital imaging: a basis for studying the mechanics and design principles of bone tissue. Biotechnol Bioeng 43: 586–596, 1994 [DOI] [PubMed] [Google Scholar]
- 9).Pudenz RH, Shelden CH: The lucite calvarium; a method for direct observation of the brain; cranial trauma and brain movement. J Neurosurg 3: 487–505, 1946 [DOI] [PubMed] [Google Scholar]
- 10).Tagge CA, Fisher AM, Minaeva OV, et al. : Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141: 422–458, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Zhang J, Yoganandan N, Pintar FA, Gennarelli TA: Role of translational and rotational accelerations on brain strain in lateral head impact. Biomed Sci Instrum 42: 501–506, 2006 [PubMed] [Google Scholar]
- 12).Bain AC, Meaney DF: Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J Biomech Eng 122: 615–622, 2000 [DOI] [PubMed] [Google Scholar]
- 13).Yoganandan N, Li J, Zhang J, Pintar FA, Gennarelli TA: Influence of angular acceleration-deceleration pulse shapes on regional brain strains. J Biomech 41: 2253–2262, 2008 [DOI] [PubMed] [Google Scholar]
- 14).Sahoo D, Robbe C, Deck C, Meyer F, Papy A, Willinger R: Head injury assessment of non-lethal projectile impacts: a combined experimental/computational method. Injury 47: 2424–2441, 2016 [DOI] [PubMed] [Google Scholar]
- 15).Murayama H, Hitosugi M, Motozawa Y, Ogino M, Koyama K: Biomechanical analysis of the head movements of a person thrown by the judo technique ‘Seoi-nage’. Neurol Med Chir (Tokyo) 60: 101–106, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Murayama H, Hitosugi M, Motozawa Y, Ogino M, Koyama K: Ukemi technique prevents the elevation of head acceleration of a person thrown by the judo technique ‘Osoto-gari’. Neurol Med Chir (Tokyo) 60: 307–312, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Crisco JJ, Wilcox BJ, Machan JT, et al. : Magnitude of head impact exposures in individual collegiate football players. J Appl Biomech 28: 174–183, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Daneshvar DH, Baugh CM, Nowinski CJ, McKee AC, Stern RA, Cantu RC: Helmets and mouth guards: the role of personal equipment in preventing sport-related concussions. Clin Sports Med 30: 145–163, x, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]