Abstract
Deep brain stimulation (DBS) is a well-established treatment for drug-resistant involuntary movements. However, the conventional quadripole cylindrical lead creates electrical fields in all directions, and the resulting spread to adjacent eloquent structures may induce unintended effects. Novel directional leads have therefore been designed to allow directional stimulation (DS). Directional leads have the advantage of widening the therapeutic window (TW), compensating for slight misplacement of the lead and requiring less electrical power to provide the same effect as a cylindrical lead. Conversely, the increase in the number of contacts from four to eight and the addition of directional elements has made stimulation programming more complex. For these reasons, new treatment strategies are required to allow effective directional DBS. During lead implantation, the directional segment should be placed in a “sweet spot,” and the orientation of the directional segment is important for programming. Trial-and-error testing of a large number of contacts is unnecessary, and efficient and systematic execution of the programmed procedure is desirable. Recent improvements in imaging technologies have enabled image-guided programming. In the future, optimal stimulations are expected to be programmed by directional recording of local field potentials.
Keywords: directional lead, deep brain stimulation, Parkinson’s disease, tremor, dystonia
Introduction
Since the 1998 report by Benabid et al.,1) deep brain stimulation (DBS) has become well established as a treatment for drug-resistant involuntary movements such as advanced Parkinson’s disease (PD), essential tremor (ET), and dystonia. This is because of the effectiveness, minimal invasiveness, and regulatory properties of this method. DBS systems made by Medtronic (Minneapolis, MN, USA) were approved by the United States Food and Drug Administration (FDA) in 1998. In Japan, Medtronic systems gained insurance coverage for the treatment of ET and PD in 2000. Initially, two types of DBS lead with four cylindrical contacts (Medtronic 3387: four contacts of 1.5 mm length separated by interspaces of 1.5 mm; Medtronic 3389: same contact lengths, interspaces of 0.5 mm) and an implantable pulse generator (IPG) (Soletra, Medtronic) were available. After more than 10 years, the IPG was upgraded to the Activa SC (Medtronic), which provided a choice between a constant voltage mode and a constant current mode, and added interleaving stimulation that was able to stimulate alternately by two different settings. A rechargeable IPG (Activa RC, Medtronic) that can stimulate eight contacts in two leads also became available. That same year, St. Jude Medical (St. Paul, MN, USA) released a rechargeable constant current IPG (Brio), but that lead only provided four contacts at that stage.
A cylindrical contact creates a spherical electrical field around the active contact, so expansion of the electrical field to adjacent eloquent structures with an increase in stimulus power can result in side effects such as muscle contractions or numbness. Further increases in stimulus power then become difficult, even if the therapeutic effect at that point is insufficient. The position of leads in the target nucleus is important,2,3) and deviation from the optimum position increases the risk of side effects. However, since lead malpositioning due to human and mechanical errors is difficult to completely avoid, current steering by bipolar stimulation or interleaving stimulation has been performed to avoid side effects, and replacement of DBS leads is sometimes required.3,4) In 2014, Boston Scientific (Valencia, CA, USA) released the Vercise Twisted lead with eight contacts (interspace: 0.5 mm) and the rechargeable Vercise IPG equipped with a multiple independent current control system (MICC), enabling precise current steering along the lead axis direction. However, the electrode was very long for the usual DBS treatment of PD, and the MICC also created a spherical stimulation field, so the problem of side effects was only slightly ameliorated. In July 2017, Boston Scientific released the Vercise Cartesia directional lead and Vercise PC non-rechargeable IPG, followed by the Vercise Gevia rechargeable IPG in September 2017. In December 2017, Abbott-St. Jude Medical (Little Canada, MN, USA) released the Infinity DBS systems in Japan. Directional DBS (d-DBS) has since become possible with the advent of these directional leads (D-leads).
Development of d-DBS systems
D-lead
Stimulation and in vitro experiments reported by Wei et al. in 2005 found that a segmented cylindrical electrode produced an edge effect that increased mean current density over the electrode surface, and required less current to create the same volume of tissue activated (VTA) as a cylindrical electrode.5) A simulation study by Butson et al. in 2006 confirmed that VTA size and shape correlated with the size and shape of the stimulating electrode contact, and reducing the number of contacts or changing contact shape could thus potentially maximize desired effects while minimizing the side effects of DBS.6) Another computational model by Teplitzky et al. suggested that single monopolar stimulation through segmented contacts could shift the center of the VTA as much as 1.0–1.3 mm.7) Keane et al. expected that stimulation with directionally segmented electrodes could improve the therapeutic effect, based on computational simulations using information on the anatomical structures and electrode positions of patients undergoing DBS to the thalamic ventral intermediate nucleus (Vim) for ET.8)
Two initial clinical trials of D-leads were performed intraoperatively only in subthalamic nucleus (STN)-DBS for PD patients with prototype leads. Pollo et al. used the Direct STN Acute directional lead (Fig. 1A), in which the distal two of four cylindrical contacts were divided into three directions, and compared the therapeutic window (TW) between directional stimulation (DS) and omnidirectional stimulation (OS). DS facing the optimal direction increased the TW by 41.3% and decreased therapeutic current strength (TCS) by 43%.9) Contarino et al. also reported that DS increased the side effect threshold and TW using a Medtronic Sapience with 40 circular contacts (diameter, 0.8 mm each) (Fig. 1B).10) In both reports, conventional cylindrical leads were used for the actual implantation for chronic stimulation.
Fig. 1.
Review of clinical directional leads. (A) The Direct STN Acute directional lead has two distal contacts that are divided into three smaller 1.0-mm2 contacts (numbers of contacts are 3-3-1-1 from distal to proximal). This lead was used only for intraoperative stimulation in Reference 9. (B) The Medtronic-Sapiens directional lead has 40 oval-shaped contacts around the lead and was used only for intraoperative stimulation in Reference 10. (C, D) The Infinity directional lead (Abbott-St. Jude Medical) has two middle contacts that are divided into three smaller, 1.15-mm-wide contacts (1-3-3-1). Each contact has a length of 1.5 mm, and the spacing between contacts is 0.5 mm (C) or 1.5 mm (D). (E) The Vercise Cartesia directional lead (Boston Scientific) has two middle contacts that are divided into three smaller, 1.0-mm-wide contacts (1-3-3-1), and the entire distal end of the lead is covered with metal to the tip. Each contact has a length of 1.5 mm, and the spacing between contacts is 0.5 mm.
In 2015, two types of D-lead from Boston Scientific and Abbott-St. Jude Medical obtained the CE mark, and chronic implantation became feasible. FDA approval was obtained by Abbott in 2016 and Boston Scientific in 2017, and both devices were launched in Japan in 2017.
The Vercise Cartesia DBS lead (Boston Scientific) and Infinity DBS lead (Abbott-St. Jude) show almost the same structure, with only slight differences. The middle two contacts of the conventional four cylindrical contacts are divided into three directions with each direction of 120°, providing a total of eight contacts (1-3-3-1). The biggest difference between the two products is seen in the most distal contact. The Infinity D-lead has a conventional cylindrical shape with a non-metallic tip of 1 mm length (Fig. 1C and 1D), while the tip of the Vercise Cartesia lead is fully metallic (Fig. 1E). This difference affects the electrical field propagation when the distal contact is used for stimulation, but exerts little difference on DS and thus is not described in detail in this article. The Vercise Cartesia lead has spacing of only 0.5 mm, whereas the Infinity provides two types of the lead, with spacing of 0.5 or 1.5 mm (Fig. 1C and 1D). Furthermore, the numbering systems used to indicate contact positions for programming also differ. The four cylindrical contacts for Medtronic are numbered 0-1-2-3 and 8-9-10-11 from the distal end, while those for Infinity leads are numbered 1-2-3-4 and 9-10-11-12 from the distal end, with A, B, and C describing directional segments (e.g., Fig. 2A–C) clockwise from the position of the radiopaque marker situated proximal to the contacts (Fig. 2B and 2C). On the other hand, with the Vercise Cartesia, the eight contacts including the directional segment are numbered serially 1–8 and 9–16, and the directional segments are numbered counterclockwise from the position of the marker (Fig. 2). When the position of a contact needs to be marked, in principle we follow the naming conventions used by Abbott, which make the position in this document easy to visualize.
Fig. 2.
Naming of the contacts by the three vendors. (A) The contacts of the Medtronic 3387 and 3397 leads are numbered 0, 1, 2, and 3 from the distal end. In the Activa PC/RC, the second lead can be connected and the resulting contacts are numbered 8, 9, 10, and 11. B: The contacts of the Infinity directional lead (Abbott-St. Jude Medical) are numbered 1–4 and 9–12 from the distal end. Directional segments of 2/3 and 10/11 are named by adding A, B, and C, such as 2A, 2B, and 2C clockwise from the direction of the radiopaque marker situated proximal to the contacts. (C) The contacts of the Vercise Cartesia directional lead (Boston Scientific) are numbered 1–8 and 9–16 from the distal end, including directional segments labeled counterclockwise from the direction of the radiopaque marker situated proximal to the contacts.
IPG
Boston Scientific has already released the Vercise equipped with a MICC and the Twist lead with eight cylindrical contacts. The D-lead (Vercise Cartesia) was released as the non-rechargeable Vercise PC and, slightly later, as the rechargeable Vercise Gevia. Both can stimulate two D-leads and have a MICC. Since MICCs have an independent power supply for each of the 16 contacts, the amplitude of each contact is freely adjustable regardless of each impedance (pulse width and frequency are restricted), and the degree of freedom for current steering is thus extremely high. The effectiveness of MICCs in STN-DBS for PD using the Twist lead was clarified in the INTREPID study11) and VANTAGE study,12) and this system is also expected to prove effective for d-DBS. On the other hand, the increase in the number of contacts from four to eight and the addition of directional elements resulted in much more complicated programming for stimulation with d-DBS.13) MICCs offer a high degree of freedom in adjusting parameters, and programing protocols have not been standardized, making adjustment more difficult. In Japan, the Vercise Genus (non-rechargeable Genus P16 and rechargeable Genus R16) was released in 2020. Vercise PC and Vercise Gevia both require a wand with USB connection for communication between the IPG and the programming device used by the doctor (Microsoft Surface; Microsoft, Redmond, WA, USA), and the communication distance is limited to less than 45 cm. However, the Vercise Genus System uses Bluetooth for communication, allowing communication up to several meters (theoretically 10 m), but resulting in no marked changes in DS function.
Infinity systems are only non-rechargeable, but two different sizes are available. The Infinity 5 is similar in size to Medtronic’s Activa SC and the Infinity 7 is similar to the Activa PC and Vercise PC. Another type of Infinity that can connect to Medtronic extensions is also available; this was intended to replace the Activa. Infinity systems are thus provided in a total of four types. Although Infinity systems are functionally similar to Activa, they can use short-pulse stimulations up to 20 microseconds (μs) and can stimulate multiple contacts as one contact, so unlike the current mode of Activa (only one cathode and one anode allowed), multiple cathodes and anodes can be set (coactivation). However, since the Infinity offers a single current source, current distribution depends on the impedance of each contact and is not always even.14) Infinity systems can use the MultiStim set (MSS), which is the same as the interleaving stimulation of the Activa. Programming devices for doctors are iPadOS (Apple, Cupertino, CA, USA) devices (especially the iPad mini; Apple), while patients use an iPod touch via Bluetooth communication. Programming is easily adjusted while the patient is walking. Patients accustomed to smartphones can also self-adjust the amplitude within a range set by the doctor. The features of the Infinity include easy use with Apple systems and the possibility of adding functions by upgrading the program. The MSS could not be used at the time the Infinity was released, but was supported in a version upgrade. This platform is expected to support telemedicine in the future.
Directional DBS
Even in post-marketing studies of chronic implantations, Steigerwald et al. reported that in seven PD patients with STN-DBS, the DS positioned in the optimal direction at the optimal level widened the TW by increasing the threshold for side effects compared to OS.15) However, even DS positioned in the optimal direction but at a suboptimal level still widened the TW by not only increasing the side effect threshold, but also decreasing the efficacy threshold. From these results, Steigerwald et al. pointed out that even an electrode deviating from the optimal position may compensate within a certain range. Dembek et al. also observed an increase in the TW due to the increase in the side effect threshold in a prospective, double-blinded trial of STN-DBS in 10 PD patients.16) Furthermore, Rebelo et al. showed that DS of the single segment increased the TW by 91% and decreased TCS by 31% and power consumption by 6% in Vim-DBS for eight drug-resistant tremors.17) Such results suggest that DS may extend the battery life of IPGs.
These three single-center trials showed that DS widened the TW in STN-DBS, and those results have been verified in several subsequent small studies.18–20) However, the observation periods for those trials were short, and a long-term, large-scale, double-blinded randomized controlled trial has been required. In May 2021, Schnitzler et al. reported results from the PROGRESS study using Abbott systems. The PROGRESS study was a randomized, double-blinded, multicenter trial in which 37 institutions mainly in Europe and the United States performed STN-DBS for PD.21) The aim of the study was to determine whether a wider TW could be achieved with DS. Follow-up for up to 36 months was scheduled for 234 patients, and the report by Schnitzler et al. included interim results up to the 6-month follow-up of registrants. In the blinded period, 90.6% of patients showed a wider TW with DS than with OS, and 62.2% had a wider TW with single-segment DS than with double-segment DS. The TW was increased by 41% in DS compared to OS. Using the contact with the lowest TCS, DS was able to reduce the current required to achieve symptom relief by 39% compared to OS, while no significant difference in clinical effect was evident between DS and OS. At the end of the blinded period, 52.8% of patients and 58.5% of physicians reported that DS was preferred, with OS preferred by 25.9% and 21.2%, respectively.
Another randomized, double-blinded trial showed that for best DS, the TW was wider and therapeutic threshold was lower than OS also in Vim/posterior subthalamic area (PSA)-DBS, while side effect threshold and clinical efficacy did not differ from those with OS.22)
From these studies, a key advantage of DS is the widening of the TW, the possibility of compensating for slight (about 1 mm) misplacement of the lead, and the possibility of reduced power consumption and prolonged battery life. However, disadvantages are also seen in that stimulation programing becomes much more complicated. To make use of these advantages, new treatment strategies are necessary, including for D-lead placement and simplification of stimulation programming.
Placement of D-lead
Depth
Even if the D-lead can compensate for misplacement to some degree, the range is about 1 mm at most, and attention to accurate stereotactic placement of the lead cannot be reduced. Since DS is possible only with the middle two contacts of the D-lead, for example, when a conventional cylindrical quadripole lead (Medtronic 3389) was placed into STN, the optimal stimulation site (the so-called “sweet spot”) should be within a range of 7.5 mm, as the total electrode length. The permissible range can be said to be narrowed to 3.5 mm in D-lead placement. The D-lead requires more accurate lead placement.23,24)
The optimal stimulation site in STN-DBS remains controversial, and the dorsolateral inside STN, upper border of the STN, and structures above the STN, such as the zona incerta (ZI) and Forel H2 field, have all been mentioned.25–27) On the other hand, the medial part of the STN is less effective and tends to cause side effects such as mental symptoms (effects on the limbic STN) and disorders of eye movements (effects on the oculomotor nerve). Spread to the substantia nigra under the STN may cause akinesia, while spread to the internal capsule on the anterior to lateral sides of the STN can cause muscle contractions. The spread of stimulation to the medial and posterior medial side (medial lemniscus) can cause sensory symptoms.26,28) With a conventional cylindrical quadripole lead, 1 is placed at the bottom of the STN, 1–3 are in the STN, and 4 is above the STN (Fig. 3A).26,29) With a D-lead, some authors proposed that directional segments (2, 3) should be placed in sweet spots,23,30) as decided from microelectrode recording (MER) and test stimulation, but no specific indicators were shown. In our hospital, 4 was often the optimal stimulation site in the conventional arrangement in STN-DBS and Vim-DBS, so we have changed the arrangement so that 2 and 3 in the part where the kinesthetic response and tremor rhythm are recorded by MER. However, Asahi et al. placed the midpoint between the two directional segments at the upper border of the STN (1 and 2 in the STN, 3 and 4 above the STN) as detected by MER.31) Side effects were evaluated with electrical stimulation using the directional part of the lead after surgery, and the direction of final stimulation was investigated. As a result, the most frequently used contacts were located above the STN (63%), followed by the upper part of the STN (32%). This electrode placement (Fig. 3B) is shallower by about one contact than that of a conventional quadripole lead, but is considered rather appropriate, because the optimal stimulation site in STN-DBS may be located dorsolateral to the boundary of the STN or above. Structures above the STN, particularly the caudal ZI (cZI), are part of PSA, which has been considered a new target for treating tremor in recent years.32) Furthermore, simultaneous stimulation of STN and cZI may provide better clinical outcomes than conventional STN-DBS in selected patients.33–37) Further studies are needed to clarify the effects and side effects of d-DBS to this site.
Fig. 3.

Sagittal brain section depicting the STN 12 mm from midline with DBS leads in position. (A) When the cylindrical quadripole lead (Medtronic 3389) is ordinarily placed into the STN, the distal contact (0 according to the Medtronic numbering system) is placed at the bottom of the STN, the 3 distal contacts (0, 1, and 2 according to the Medtronic numbering system) are placed within the STN, and the most proximal contact (3 according to the Medtronic numbering system) is placed above the STN (References 26, 29). (B) The midpoint between the two directional contacts is placed at the upper border of the STN (as in Reference 31). Contacts 1 and 2 are placed in the STN, 3 is above the STN, and 4 is in the thalamus (Voa). DBS: deep brain stimulation, PC: posterior commissure, RAPRL: prelemniscal radiations, S.N.: substantia nigra, STN: subthalamic nucleus, Vim: ventral intermedius nucleus, Vop: ventral oralis posterior, Voa: ventral oralis anterior, Z.I.: zona incerta.
Direction
The orientation of directional segments is important for predicting the effects and side effects of stimulation for DBS programming. Both Vercise Cartesia and Infinity leads have a radiopaque marker proximal to the contacts, and the orientation of the directional segments is determined from the position of this marker. The optimal direction in the STN varied from patient to patient,9) so the direction of the marker differs depending on the report, but facing forward (A facing anterior, B right posterior, and C left posterior) has been common. We ordinarily place the marker facing forward in the STN-DBS and globus pallidus internus-DBS, but often facing backwards in Vim-DBS. This is because the ventral caudal nucleus (Vc) behind the Vim tends to cause side effects of sensory symptoms, and the internal capsule (IC), which tends to cause motor symptoms, lies anterolaterally. When the marker is placed backward, A is in the direction of the Vc, and B on the left and C on the right are in the direction of the IC. This placement allows easy understanding of the directions from which side effects are likely to occur.
Since the marker disappears with insertion of the lead into the brain, alignment of the direction of the screw of the stopper with the direction of the marker is common. However, lead direction cannot be determined using only this method, so confirmation on X-ray or fluoroscopy is necessary during the operation. As a method of confirming the marker direction intraoperatively, the concept of the “iron sight” sign on X-ray or fluoroscopy from a lateral view is useful (Fig. 4). If the directional segment faces straightforward, the iron sight sign can be seen through the overlapping gaps between A and B and between A and C in the lateral view.38,39) Other studies have examined new algorithms and methods to detect the rotational angle of the D-lead.40–43) However, even with placement in such a strict way, the D-lead may rotate after surgery.44–46) Dembek et al. reported D-lead rotation >30° occurred in 42% of patients and rotation angle was within ±90°.44) Lange et al. observed D-lead rotation in about 50% of cases. Such rotation occurred within 24 h after surgery45) and was stable thereafter.46) Confirmation of marker direction from X-ray or CT was thus necessary >24 h after surgery. On the other hand, Krüger et al. stated that rotational angle was small when the iron sight sign was observed intraoperatively, so the rotation of the D-lead was suggested to occur when torque was applied to the lead during lead fixation or with different surgical techniques.47) The need for some form of locking mechanisms was suggested.47)
Fig. 4.
Explanation of “iron sight sign.” If the markers are correctly placed facing the front, the spaces between the front contact (A) and the two rear contacts (B and C) will overlap, allowing X-rays to pass through in the lateral view and revealing the “iron sight” line described by Reinacher et al. in Reference 29. X-rays of “iron sight” signs (arrow) from the Vercise Cartesia directional lead (upper) and Infinity directional lead (lower). The Infinity shows slightly narrower spacing of directional segments, so the iron sight sign is hard to see unless the direction matches more strictly than that with the Vercise Cartesia.
MER and anesthesia
The conventional method for determining lead placement is MER under awake conditions and test stimulation. During the surgery, the patient has to endure lying on a bed with their head fixed for several hours. Some recent reports have described MER under general anesthesia for placement of the DBS lead. Senemmar et al. reported that asleep STN-DBS surgery led to a significantly wider TW than awake surgery for both DS and OS, with no difference in clinical effects between methods.48) On the other hand, many reports have described DBS lead placement using only a neuroimaging guide, finding no difference in clinical effects from placement with MER guidance.49–52) Although D-lead placement with only a neuroimaging guide has been reported21) and the asleep STN-DBS surgery with D-lead is expected to increase in the future, no reports have examined indicators for lead placement or compared clinical effects. As mentioned earlier, the D-lead requires more accurate placement, so further studies are therefore expected.
Even with Vim-DBS, the optimal site is not definitive, and lead placement with MER and test stimulation is common. Recently, segmentation of the thalamic nuclei53) and detection of optimal site using imaging methods such as drawing the dentato- rubro-thalamic tract54,55) and connectivity analysis56) have become possible thanks to advances in imaging technology. On the other hand, Gravbrot, et al. reported that about 12 patients received asleep D-lead implantation to Vim for ET.57) They used atlas-based targeting and interventional magnetic resonance imaging without MER and achieved favorable tremor control with surgical complications and stimulation-related adverse effects comparable to awake surgery. They reported not only clinical effects, complications, and adverse effect, but also details for their procedure of setting the D-lead, providing crucial information for further studies.
Programming
No validated, well-established programming protocol has been defined for DBS.58) The first step in programming is determination of a contact for stimulation. In programing for DBS with a cylindrical lead, it is common to perform “monopolar review.” Many authors have described protocols for monopolar review, often by measuring the TW with gradually increasing amplitude at all contacts, then selecting the contact with the widest TW for chronic stimulation.58–60) In STN-DBS, tremor and rigidity are typically used because these symptoms respond to stimulation adjustment within seconds, whereas bradykinesia and axial symptoms often require several minutes to several hours to respond. For this reason, most monopolar reviews that measure the TW are conducted for tremor and rigidity.28) The number of contacts of the D-lead has increased from four to eight, and eight combinations of horizontal contacts (six pairs and two ring modes) are possible. Performing all tests would take much longer than when using the quadripole lead, and such longer testing would lead to patient fatigue and inaccurate responses. Steigerwald et al. and Fricke et al. therefore noted that the measurement of the TW with OS should be tried as a first step, focusing the monopolar review on the DS at the level of the best TW on OS.23,29,30) The Informity tool for monopolar review is available from Abbott. Informity works with a user-friendly interface on an iPad (Apple), and users can easily switch between each contact alone, combinations of two contacts, and OS using all three contacts, gradually increasing the stimulus, and recording amplitude and symptoms with effects and side effects (Fig. 5A). Since the results are displayed in a chart (Fig. 5B), visualization of the TW can help determine optimal contact in programming.
Fig. 5.
Screenshot of Informity in demonstration mode. (A) The user interface can be used to easily change the amplitude, pulse width, frequency, and cathode contacts as well as record the efficacy and side effects. (B) The report chart created by Informity (fictious value).
The next step is adjustment of the parameters. In OS, a pulse width of 60 μs and a frequency of 130 Hz are commonly used, and the effects are adjusted by changing the amplitude. When side effects occur as amplitude increases, changes in pulse width and frequency, bipolar stimulation, and interleaving stimulation have been tried. Recently, a short pulse of less than 60 μs has been used in newer devices to raise the side effect threshold and widen the TW.61–63) The combination of DS and a short pulse decrease the side effect threshold, so most cases of STN-DBS can be adjusted by monopolar stimulation. On the other hand, in Vim-DBS, some reports have described side effects occurring even with DS, and directional bipolar stimulation has been required.64,65)
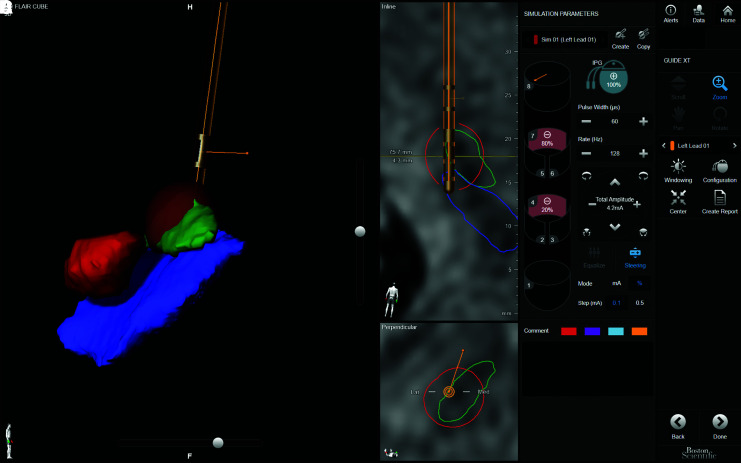
The programming of d-DBS is strongly dependent on the experience and skill of the physician, and simpler, better organized programming methods are required. Algorithms combining VTA visualization by computer simulation and image guidance have been reported,66–68) but require advanced engineering expertise and are thus available in only limited facilities. Boston Scientific recently provided “Guide XT” as a useful tool. Guide XT is based on Brainlabo (Munich, Germany) technology, segmenting, and displaying nuclei in three dimensions using preoperative MRI, and superimposing the lead position from postoperative CT and the programmed VTA (Fig. 6). Research into where the effects and side effects occur using D-lead is progressing,19,55) and visual programming is expected to become possible in the near future.
Fig. 6.
Screenshot of Guide XT. A 69-year-old man underwent bilateral DBS for STN at the age of 65 with the Vercise Cartesia directional lead and Vercise PC. The image shows the Guide XT screen of the left STN-DBS with the stimulus settings at the last visit. (A) 3D image of the lead; red nucleus (RN, red), STN (green), substantia nigra (SN, blue), and VTA (brown). (B) MRI-FLAIR image and electrode position on the lead plane; STN (green line), SN (blue line), and VTA (red line). (C) MRI-FLAIR image and lead position in a cross-section perpendicular to the lead. The lead was located on the lateral boundary of the STN (green line), and a larger VTA was formed toward the STN by DS. (D) Stimulation condition settings. Values of 4 and 7 were assigned to the cathode for monopolar stimulation. Stimuli with amplitude of 4.2 mA, pulse width of 60 μs, and frequency of 128 Hz were allocated to 4 at 20% (0.84 mA) and 7 at 80% (3.36 mA) using the multiple independent current control system. DBS: deep brain stimulation, DS: directional stimulation, STN: subthalamic nucleus, VTA: volume of tissue activated.
Another indicator of optimal direction is local field potential (LFP). For quite some time, enhanced beta band power of subthalamic local field potential activity (13–30 Hz) has been identified as an electrophysiological marker in PD. Recently, many studies have recorded LFP intraoperatively from D-leads.69–72) Bour et al. performed intraoperative recording of LFP in STN-DBS surgery using a prototype D-lead with 32 oval-shaped contacts. They reported that LFP could be used to more accurately detect the STN border, and DS toward the location of highest LFP power in the beta band produced the best clinical effects.73) Tinkhauser et al. demonstrated that the two segmented contacts of the Vercise Cartesia lead with maximal STN beta activity were highly likely to include the contact that turned out to offer the best efficacy with a wide TW.74) Telkes et al. reported that LFP data collected intraoperatively via the Abbott Infinity lead showed that the normalized beta band power observed in the STN was higher in the anterior direction and tended to be positively associated with bradykinesia/rigidity in the dorsoanterior direction and with axial scores in the dorsomedial direction.75) Nguyen et al. noted that spectral power in the total beta band in STN was the best predictor for ranking the three directions for the TW with the Direct STN Acute lead (Fig. 1A), and directional LFPs recorded with segmented leads supported the hypothesis that spectral power might be indicative of the best stimulation direction.76) Noor et al. used a computational model of human STN neural activity to simulate LFP recordings, and stated that STN LFP recordings from an 8-contact directional lead (28 possible bipolar pairs) could provide more information than 4-contact cylindrical leads (6 possible bipolar pairs), but also introduced additional complexity to signal analysis.77) Such reports suggest that the LFP from directional contact was effective for simplifying complicated programming for d-DBS. However, development of new hardware and software is necessary for efficient programming.
At the end of 2020, Medtronic released Percept PC equipped with Brain Sense, which records LFP from the leads and adaptive DBS to allow automatic adjustment of stimulation with abnormal β activity as an index. In particular, the first approval in the world for adaptive DBS was made in Japan, and although the efficacy of the method is still unknown, expectations are high. The Percept PC uses a traditional lead with four cylindrical contacts; in other words, the risk of side effects is as before. The Medtronic SenSight received the CE mark of approval in March 2021 and was approved by the FDA in June. SenSight is a directional DBS lead system capable of LFP recording, and will be launched in Japan by the end of 2021. This is the first system in the world to integrate LFP recording and the directional lead into the same system, but unfortunately LFP recording is currently possible only in ring mode, not directional LFP. Directional LFP recording is expected to become possible in the future.
Conclusion
The d-DBS offers many advantages to compensate for the weaknesses of conventional DBS, and D-leads can also be used in the same way as conventional leads, so implantation of a D-lead is recommended as standard for new DBS surgery. Programming of the d-DBS is complicated and time-consuming, but programming with image guidance is now becoming possible, and these problems are expected to be resolved by integration with Adaptive DBS in the future.
Footnotes
Conflicts of Interest Disclosure
No disclosures to declare.
References
- 1).Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J: Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol 50: 344–346, 1987 [DOI] [PubMed] [Google Scholar]
- 2).Rezai AR, Kopell BH, Gross RE, et al. : Deep brain stimulation for Parkinson’s disease: surgical issues. Mov Disord 21 Suppl 14: S197–218, 2006 [DOI] [PubMed] [Google Scholar]
- 3).Okun MS, Tagliati M, Pourfar M, et al. : Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Arch Neurol 62: 1250–1255, 2005 [DOI] [PubMed] [Google Scholar]
- 4).Falowski SM, Bakay RA: Revision surgery of deep brain stimulation leads. Neuromodulation 19: 443–450, 2016 [DOI] [PubMed] [Google Scholar]
- 5).Wei XF, Grill WM: Current density distributions, field distributions and impedance analysis of segmented deep brain stimulation electrodes. J Neural Eng 2: 139–147, 2005 [DOI] [PubMed] [Google Scholar]
- 6).Butson CR, McIntyre CC: Role of electrode design on the volume of tissue activated during deep brain stimulation. J Neural Eng 3: 1–8, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Teplitzky BA, Zitella LM, Xiao Y, Johnson MD: Model-based comparison of deep brain stimulation array functionality with varying number of radial electrodes and machine learning feature sets. Front Comput Neurosci 10: 58, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Keane M, Deyo S, Abosch A, Bauwa JA, Jonson MD: Improved spatial targeting with directionally segmented deep brain stimulation leads for treating essential tremor. J Neural Eng 9: 046005, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Pollo C, Kaelin-Lang A, Oertel MF, et al. : Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain 137: 2015–2026, 2014 [DOI] [PubMed] [Google Scholar]
- 10).Contarino MF, Bour LJ, Verhagen R, et al. : Directional steering: a novel approach to deep brain stimulation. Neurology 83: 1163–1169, 2014 [DOI] [PubMed] [Google Scholar]
- 11).Vitek JL, Jain R, Chen L, et al. : Subthalamic nucleus deep brain stimulation with a multiple independent constant current-controlled device in Parkinson’s disease (INTREPID): a multicentre, double-blind, randomised, sham-controlled study. Lancet Neurol 19: 491–501, 2020 [DOI] [PubMed] [Google Scholar]
- 12).Timmermann L, Jain R, Chen L, et al. : Multiple-source current steering in subthalamic nucleus deep brain stimulation for Parkinson’s disease (the VANTAGE study): a non-randomised, prospective, multicentre, open-label study. Lancet Neurol 14: 693–701, 2015 [DOI] [PubMed] [Google Scholar]
- 13).Ten Brinke TR, Odekerken VJJ, Dijk JM, van den Munckhof P, Schuurman PR, de Bie RMA: Directional deep brain stimulation: first experiences in centers across the globe. Brain Stimul 11: 949–950, 2018 [DOI] [PubMed] [Google Scholar]
- 14).Schüpbach WMM, Chabardes S, Matthies C, et al. : Directional leads for deep brain stimulation: Opportunities and challenges. Mov Disord 32: 1371–1375, 2017 [DOI] [PubMed] [Google Scholar]
- 15).Steigerwald F, Müller L, Johannes S, Matthies C, Volkmann J: Directional deep brain stimulation of the subthalamic nucleus: a pilot study using a novel neurostimulation device. Mov Disord 31: 1240–1243, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Dembek TA, Reker P, Visser-Vandewalle V, et al. : Directional DBS increases side-effect thresholds-A prospective, double-blind trial. Mov Disord 32: 1380–1388, 2017 [DOI] [PubMed] [Google Scholar]
- 17).Rebelo P, Green AL, Aziz TZ, et al. : Thalamic directional deep brain stimulation for tremor: spend less, get more. Brain Stimul 11: 600–606, 2018 [DOI] [PubMed] [Google Scholar]
- 18).Nguyen TAK, Djilas M, Nowacki A, et al. : Analysis of patient-specific stimulation with segmented leads in the subthalamic nucleus. PLoS One 14: e0217985, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19).Nguyen TAK, Nowacki A, Debove I, et al. : Directional stimulation of subthalamic nucleus sweet spot predicts clinical efficacy: proof of concept. Brain Stimul 12: 1127–1134, 2019 [DOI] [PubMed] [Google Scholar]
- 20).Shao MM, Liss A, Park YL, et al. : Early experience with new generation deep brain stimulation leads in Parkinson’s disease and essential tremor patients. Neuromodulation 23: 537–542, 2020 [DOI] [PubMed] [Google Scholar]
- 21).Schnitzler A, Mir P, Brodsk MA, et al. : Directional deep brain stimulation for Parkinson’s disease: results of an international crossover study with randomized, double-blind primary endpoint. Neuromodulation 2021, Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 22).Bruno S, Nikolov P, Hartmann CJ, et al. : Directional deep brain stimulation of the thalamic ventral intermediate area for essential tremor increases therapeutic window. Neuromodulation 24: 343–352, 2021 [DOI] [PubMed] [Google Scholar]
- 23).Steigerwald F, Matthies C, Volkmann J: Directional deep brain stimulation. Neurotherapeutics 16: 100–104, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24).Dayal V, De Roquemaurel A, Grover T, et al. : Novel programming features help alleviate subthalamic nucleus stimulation-induced side effects. Mov Disord 35: 2261–2269, 2020 [DOI] [PubMed] [Google Scholar]
- 25).Caire F, Ranoux D, Guehl D, Burbaud P, Cuny E: A systematic review of studies on anatomical position of electrode contacts used for chronic subthalamic stimulation in Parkinson’s disease. Acta Neurochir (Wien) 155: 1647–1654; discussion 1654, 2013 [DOI] [PubMed] [Google Scholar]
- 26).Bari AA, Fasano A, Muchoz RP, Lozano AM: Improving outcomes of subthalamic nucleus deep brain stimulation in Parkinson’s disease. Experv Rev Neurother 15: 1151–1160, 2015 [DOI] [PubMed] [Google Scholar]
- 27).Bot M, Schuurman PR, Odekerken VJJ, et al. : Deep brain stimulation for Parkinson’s disease: defining the optimal location within the subthalamic nucleus. J Neurol Neurosurg Psychiatry 89: 493–498, 2018 [DOI] [PubMed] [Google Scholar]
- 28).Koeglsperger T, Palleis C, Hell F, Mehrkens JH, Bötzel K: Deep brain stimulation programming for movement disorders: current concepts and evidence-based strategies. Front Neurol 10: 410, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29).van den Munckhof P, Bot M, Schuurman PR: Targeting of the subthalamic nucleus in patients with Parkinson’s disease undergoing deep brain stimulation surgery. Neurol Ther 10: 61–73, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30).Fricke P, Nickl R, Breun M, et al. : Directional leads for deep brain stimulation: technical notes and experiences. Stereotact Funct Neurosurg 19: 305–312, 2021 [DOI] [PubMed] [Google Scholar]
- 31).Asahi T, Ikeda K, Yamamoto J, Tsubono H, Sato S: Pilot study for considering subthalamic nucleus anatomy during stimulation using directional leads. J Mov Disord 12: 97–102, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).Blomstedt P, Stenmark Persson R, Hariz GM, et al. : Deep brain stimulation in the caudal zona incerta versus best medical treatment in patients with Parkinson’s disease: a randomised blinded evaluation. J Neurol Neurosurg Psychiatry 89: 710–716, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33).Mostofi A, Evans JM, Partington-Smith L, Yu K, Chen C, Silverdale MA: Outcomes from deep brain stimulation targeting subthalamic nucleus and caudal zona incerta for Parkinson’s disease. NPJ Park Dis 5: 17, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34).Falconer RA, Rogers SL, Shenai M: Using directional deep brain stimulation to co-activate the subthalamic nucleus and zona incerta for overlapping essential tremor/Parkinson’s disease symptoms. Front Neurol 9: 544, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35).De Marco R, Bhargava D, Macerollo A, Osman-Farah J: Could ZI have a role in DBS for Parkinson’s disease? an observational study to optimize DBS target localization. J Clin Neurosci 77: 89–93, 2020 [DOI] [PubMed] [Google Scholar]
- 36).Ossowska K: Zona incerta as a therapeutic target in Parkinson’s disease. J Neurol 267: 591–606, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37).Sasagawa A, Enatsu R, Kitagawa M, et al. : Target selection of directional lead in patients with Parkinson’s disease. Neurol Med Chir 60: 622–628, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38).Reinacher PC, Krüger MT, Coenen VA, et al. : Determining the orientation of directional deep brain stimulation electrodes using 3D rotational fluoroscopy. AJNR Am J Neuroradiol 38: 1111–1116, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Sitz A, Hoevels M, Hellerbach A, et al. : Determining the orientation angle of directional leads for deep brain stimulation using computed tomography and digital X-ray imaging: a phantom study. Med Phys 44: 4463–4473, 2017 [DOI] [PubMed] [Google Scholar]
- 40).Egger K, Rau A, Urbach H, Reisert M, Reinacher PC: 3D X-ray based visualization of directional deep brain stimulation lead orientation. J Neuroradiol S0150–9861 (21) 00107–3, 2021, ahead of print [DOI] [PubMed] [Google Scholar]
- 41).Hunsche S, Neudorfer C, Majdoub FE, Maarouf M, Sauner D: Determining the rotational orientation of directional deep brain stimulation leads employing flat-panel computed tomography. Oper Neurosurg (Hagerstown) 16: 465–470, 2019 [DOI] [PubMed] [Google Scholar]
- 42).Hellerbach A, Dembek TA, Hoevels M, et al. : DiODe: Directional orientation detection of segmented deep brain stimulation leads: a sequential algorithm based on CT imaging. Stereotact Funct Neurosurg 96: 335–341, 2018 [DOI] [PubMed] [Google Scholar]
- 43).Sedrak M, Sabelman E, Pezeshkian P, et al. : Biplanar X-ray methods for stereotactic intraoperative localization in deep brain stimulation surgery. Oper Neurosurg (Hagerstown) 19: 302–312, 2020 [DOI] [PubMed] [Google Scholar]
- 44).Dembek TA, Hoevels M, Hellerbach A, et al. : Directional DBS leads show large deviations from their intended implantation orientation. Parkinsonism Relat Disord 67: 117–121, 2019 [DOI] [PubMed] [Google Scholar]
- 45).Lange F, Steigerwald F, Engel D, et al. : Longitudinal assessment of rotation angles after implantation of directional deep brain stimulation leads. Stereotact Funct Neurosurg 99: 150–158, 2021 [DOI] [PubMed] [Google Scholar]
- 46).Dembek TA, Asendorf AL, Wirths J, Barbe MT, Visser-Vandewalle V, Treuer H: Temporal stability of lead orientation in directional deep brain stimulation. Stereotact Funct Neurosurg 99: 167–170, 2021 [DOI] [PubMed] [Google Scholar]
- 47).Krüger MT, Naseri Y, Cavalloni F, et al. : Do directional deep brain stimulation leads rotate after implantation? Acta Neurochir (Wien) 163: 197–203, 2021 [DOI] [PubMed] [Google Scholar]
- 48).Senemmar F, Hartmann CJ, Slotty PJ, Vesper J, Schnitzler A, Groiss SJ: Asleep surgery may improve the therapeutic window for deep brain stimulation of the subthalamic nucleus. Neuromodulation 24: 279–285, 2021 [DOI] [PubMed] [Google Scholar]
- 49).Chen T, Mirzadeh Z, Ponce FA: “Asleep” deep brain stimulation surgery: a critical review of the literature. World Neurosurg 105: 191–198, 2017 [DOI] [PubMed] [Google Scholar]
- 50).Kochanski RB, Sani S: Awake versus asleep deep brain stimulation surgery: technical considerations and critical review of the literature. Brain Sci 8: 17, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Liu Z, He S, Li L: General anesthesia versus local anesthesia for deep brain stimulation in Parkinson’s disease: a meta-analysis. Stereotact Funct Neurosurg 97: 381–390, 2019 [DOI] [PubMed] [Google Scholar]
- 52).Wang J, Ponce FA, Tao J, et al. : Comparison of awake and asleep deep brain stimulation for Parkinson’s disease: a detailed analysis through literature review. Neuromodulation 23: 444–450, 2020 [DOI] [PubMed] [Google Scholar]
- 53).Krüger MT, Kurtev-Rittstieg R, Kägi G, Naseri Y, Hägele-Link S, Brugger F: Evaluation of automatic segmentation of thalamic nuclei through clinical effects using directional deep brain stimulation leads: a technical note. Brain Sci 10: 642, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Petry-Schmelzer JN, Dembek TA, Steffen JK, et al. : Selecting the most effective DBS contact in essential tremor patients based on individual tractography. Brain Sci 10: 1015, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55).Nordin T, Zsigmond P, Pujol S, Westin CF, Wårdell K: White matter tracing combined with electric field simulation - a patient-specific approach for deep brain stimulation. Neuroimage Clin 24: 102026, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Akram H, Dayal V, Mahlknecht P, et al. : Connectivity derived thalamic segmentation in deep brain stimulation for tremor. Neuroimage Clin 18: 130–142, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Gravbrot N, Burket A, Saranathan M, Kasoff WS: Asleep deep brain stimulation of the nucleus ventralis intermedius for essential tremor using indirect targeting and interventional magnetic resonance imaging: single-institution case series. Mov Disord Clin Pract 7: 521–530, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58).Picillo M, Lozano AM, Kou N, Puppi Munhoz R, Fasano A: Programming deep brain stimulation for Parkinson’s disease: The Toronto Western Hospital algorithms. Brain Stimul 9: 425–437, 2016 [DOI] [PubMed] [Google Scholar]
- 59).Wagle Shukla A, Zeilman P, Fernandez H, Bajwa JA, Mehanna R: DBS programming: an evolving approach for patients with Parkinson’s disease. Park Dis 2017: 8492619, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60).Merola A, Romagnolo A, Krishna V, et al. : Current directions in deep brain stimulation for Parkinson’s disease-directing current to maximize clinical benefit. Neurol Ther 9: 25–41, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61).Steigerwald F, Timmermann L, Kühn A, et al. : Pulse duration settings in subthalamic stimulation for Parkinson’s disease. Mov Disord 33: 165–169, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Bouthour W, Wegrzyk J, Momjian S, et al. : Short pulse width in subthalamic stimulation in Parkinson’s disease: a randomized, double-blind study. Mov Disord 33: 169–173, 2018 [DOI] [PubMed] [Google Scholar]
- 63).Choe CU, Hidding U, Schaper M, et al. : Thalamic short pulse stimulation diminishes adverse effects in essential tremor patients. Neurology 91: e704–e713, 2018 [DOI] [PubMed] [Google Scholar]
- 64).Reker P, Dembek TA, Becker J, Visser-Vandewalle V, Timmermann L: Directional deep brain stimulation: a case of avoiding dysarthria with bipolar directional current steering. Parkinsonism Relat Disord 31: 156–158, 2016 [DOI] [PubMed] [Google Scholar]
- 65).Steffen JK, Reker P, Mennicken FK, et al. : Bipolar directional deep brain stimulation in essential and Parkinsonian tremor. Neuromodulation 23: 543–549, 2020 [DOI] [PubMed] [Google Scholar]
- 66).Anderson DN, Osting B, Vorwerk J, Dorval AD, Butson CR: Optimized programming algorithm for cylindrical and directional deep brain stimulation electrodes. J Neural Eng 15: 026005, 2018 [DOI] [PubMed] [Google Scholar]
- 67).Carl B, Bopp M, Saß B, Waldthaler J, Timmermann L, Nimsky C: Visualization of volume of tissue activated modeling in a clinical planning system for deep brain stimulation. J Neurosurg Sci S0390-5616.19.04827-6, 2020, ahead of print. [DOI] [PubMed] [Google Scholar]
- 68).Lévy JP, Nguyen TAK, Lachenmayer L, et al. : Structure-function relationship of the posterior subthalamic area with directional deep brain stimulation for essential tremor. Neuroimage Clin 28: 102486, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69).Chen CC, Pogosyan A, Zrinzo LU, et al. : Intra- operative recordings of local field potentials can help localize the subthalamic nucleus in Parkinson’s disease surgery. Exp Neurol 198: 214–221, 2006 [DOI] [PubMed] [Google Scholar]
- 70).Hammond C, Bergman H, Brown P: Pathological synchronization in Parkinson’s disease: networks, models and treatments. Trends Neurosci 30: 357–364, 2007 [DOI] [PubMed] [Google Scholar]
- 71).Zaidel A, Spivak A, Grieb B, Bergman H, Israel Z: Subthalamic span of beta oscillations predicts deep brain stimulation efficacy for patients with Parkinson’s disease. Brain 133: 2007–2021, 2010 [DOI] [PubMed] [Google Scholar]
- 72).Horn A, Neumann WJ, Degen K, Schneider GH, Kühn AA: Toward an electrophysiological “sweet spot” for deep brain stimulation in the subthalamic nucleus. Hum Brain Map 38: 3377–3790, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73).Bour LJ, Lourens MA, Verhagen R, et al. : Directional recording of subthalamic spectral power densities in Parkinson’s disease and the effect of steering deep brain stimulation. Brain Stimul 8: 730–741, 2015 [DOI] [PubMed] [Google Scholar]
- 74).Tinkhauser G, Pogosyan A, Debove I, Nowacki A, et al. : Directional local field potentials: a tool to optimize deep brain stimulation. Mov Disord 33: 159–164, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75).Telkes I, Sabourin S, Durphy J, et al. : Functional use of directional local field potentials in the subthalamic nucleus deep brain stimulation. Front Hum Neurosci 14: 145, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Nguyen TAK, Schüpbach M, Mercanzini A, Dransart A, Pollo C: Directional local field potentials in the subthalamic nucleus during deep brain implantation of Parkinson’s disease patients. Front Hum Neurosci 14: 521282, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Noor MS, McIntyre CC: Biophysical characterization of local field potential recordings from directional deep brain stimulation electrodes. Clin Neurophysiol 132: 1321–1329, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]