Abstract
Aims
Cardiac dysfunction in coronavirus disease-19 (COVID-19) has been reported during acute phase but serial changes have not been well studied. To determine serial changes in type and severity of echocardiographic left and right heart functions we performed a prospective study.
Methods and results
Successive COVID-19 patients at discharge from the hospital from June to December 2020 were enrolled. Clinical details were obtained and echocardiography was performed using Philips IE33X-Matrix. Follow-up evaluation was performed after 3 months. In total, 1789 COVID-19 patients were evaluated. Baseline echocardiography was performed in 1000 eligible patients (men 611, women 389). Mean age was 50.2 ± 15 years, hypertension was in 44.0%, diabetes in 49.4%, and coronary disease in 10.8%. COVID-19 was mild in 47.0%, moderate in 39.5%, and severe in 13.5%. Baseline cardiac parameters were more impaired in severe vs. moderate or mild COVID-19. At 3 months, in 632 patients where baseline and follow-up data were available, decline was observed in select left [left ventricular internal diameter in diastole +0.9 ± 0.2 mm, left atrial volume +7.6 ± 0.1 mL/m2, mitral E/e′ +4.8 ± 0.1, and left ventricular ejection fraction (LVEF) −3.7 ± 0.2%] and right [right ventricular internal diameter in diastole +2.1 ± 0.1 mm, right atrial internal dimension +1.6 ± 0.1 mm, tricuspid Vmax +1.0 ± 0.1 cm, and tricuspid annulus plane systolic excursion (TAPSE) −2.7 ± 0.2 mm] heart variables (P < 0.001). Compared to mild COVID-19, decline was significantly greater in moderate/severe disease, LVEF −1.1 ± 0.3 vs. −3.8 ± 0.3%; mitral E/e′ +3.2 ± 0.1 vs. +4.8 ± 0.1, tricuspid Vmax +0.3 ± 0.1 vs. +1.0 ± 0.1 cm, and TAPSE −0.7 ± 0.2 vs. −2.7 ± 0.2 mm (P < 0.001).
Conclusion
This study shows impaired cardiac functions in severe and moderate COVID-19 compared to mild at hospital discharge and progressive decline in left and right heart functions at 3 months. Impairment is significantly greater in patients with moderate to severe disease.
Keywords: SARS-CoV-2, cardiovascular disease, cardiac function, diastolic dysfunction, left heart function, right heart function, echocardiography
Graphical Abstract
Introduction
Cardiovascular complications are important causes of morbidity and mortality in coronavirus disease-19 (COVID-19).1 Acute COVID-19 leads to myocarditis, myocardial infarction, small vessel vasculitis, cardiac arrhythmias, along with complications secondary to hypoxia, shock, pulmonary, and systemic disease.2,3 Cardiac manifestations have also been reported in post-acute COVID-19 syndrome (PACS, long COVID) due to long-term sequelae of acute pathology and lead to increased cardiometabolic demand, myocardial fibrosis and scarring, arrhythmias, tachycardia, and autonomic dysfunction.4,5
Echocardiography has emerged as an important diagnostic tool for the assessment of cardiovascular disease in COVID-19.6,7 This technique is cost-effective, widely available and provides information that is clinically useful and can influence patient management.8 A number of studies have evaluated cardiac function using echocardiography in acute and post-acute COVID-19.7–12 Earlier studies that evaluated cardiac injury in COVID-19 relied mainly on biomarkers, e.g. troponins and N-terminal pro B-type natriuretic peptide.7,13–16 Small studies that evaluated left and right ventricular function using echocardiography are available and reported a predominantly left ventricular dysfunction due to acute coronary syndrome, small vessel vasculitis, and myocarditis.5,6 Studies have also reported the presence of right ventricular dysfunction due to pulmonary vascular thrombosis or venous pulmonary thromboembolism.9–11 A cardiac magnetic resonance imaging (MRI) study reported evidence of myocardial damage,17 although many subsequent studies have been inconclusive.5 Fayol et al.,18 reported persistent left ventricular functional abnormalities at 6-month follow-up using echocardiographic parameters. No follow-up studies exist from India where the burden of COVID-19 is among the highest in the world.19 Therefore, to identify immediate and 3 months changes in left and right heart function we performed a prospective study among patients at discharge from the hospital after recovery from acute COVID-19.
Methods
Participants
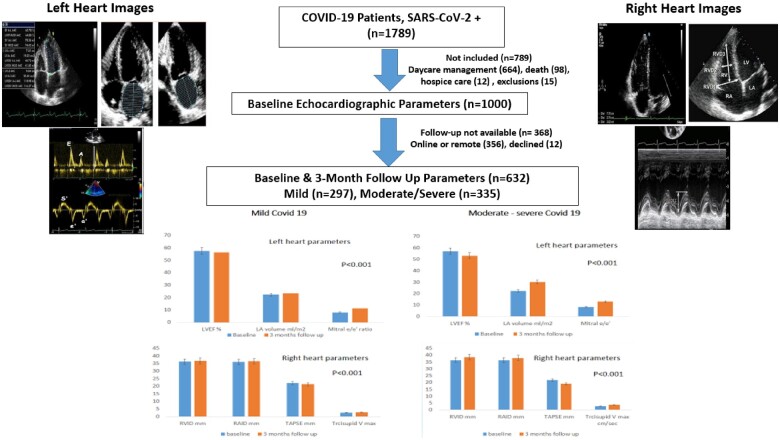
The study was approved by the hospital institutional ethics committee (CDSCO Registration No. ECR/615/Inst/RJ/2014/RR-20) and is part of the prospective COVID-19 registry at this hospital.20 All patients (n = 1789) with virologically confirmed diagnosis of COVID-19 (severe acute respiratory syndrome coronavirus 2 genes identified using reverse transcriptase-polymerase chain reaction, RT-PCR) and admitted to the hospital from June to December 2020 were included.20 Of these patients, 789 were excluded due to various reasons (Graphical Abstract). Patients younger than 18 years of age, patients with a previous history of chronic obstructive pulmonary disease, atrial fibrillation, valvular heart disease, pulmonary hypertension, chronic renal failure, and pulmonary embolism were also excluded from the study (n = 15). Therefore, 1000 patients who consented for the echocardiographic evaluation at discharge were included. At 3-month follow-up, 632 patients were available for re-evaluation. Details of recruitment and follow-up are shown in the Graphical Abstract.
Graphical Abstract.
Data collection and echocardiography
Details regarding patient demographics were obtained from hospital electronic medical records. Data on clinical characteristics were also obtained and patients were classified as having mild, moderate, or severe disease according to WHO guidelines.21 Baseline echocardiographic evaluation was performed at the time of discharge from the hospital. All patients who underwent echocardiography were requested for a follow-up evaluation at 3 months following the initial evaluation. Total 632 patients were available at follow-up at 3 months post-discharge and data of these patients were compared to baseline evaluation.
In all patients, a transthoracic echocardiography was performed using Philips IE33X Matrix equipment (Philips Healthcare Inc., Andover, MA, USA) using an X5 transducer. Echocardiographic evaluation was performed in left lateral decubitus position. Standard views were obtained including short-axis and long-axis parasternal, apical, and subcostal views. Detailed two-dimensional and M-mode recordings were made and Doppler velocities across various valves determined. Chamber volumes and other functions were calculated according to guidelines recommended by the American Society of Echocardiography and European Association of Cardiovascular Imaging.22 In parasternal view, left ventricular internal diameter in diastole (LVIDd) and left ventricular internal diameter in systole were measured in mm. Left ventricular ejection fraction (LVEF) was measured in apical four-chamber and two-chamber views using Simpson’s volume method. Left atrial (LA) volume was measured in apical four-chamber and two-chamber views. Right ventricular (RV) and right atrial (RA) basal internal diameter were measured in apical four-chamber view. Tricuspid annulus plane systolic excursion (TAPSE) was measured at lateral tricuspid annulus in apical four-chamber view in M-mode image. Doppler velocity across mitral valve E value was measured by putting pulse wave across the mitral leaflets tips and average e′ was measured by taking average of septal and lateral mitral annulus tissue Doppler velocity. Pericardial effusion was assessed in parasternal as well as in apical views.
All the aseptic precautions were followed before, during, and after the examination according to the recommendations by the Cardiological Society of India.23 At the end of each day two independent and experienced cardiologists who were blinded to the clinical data of patients, analysed all the recorded echocardiographic images. Intra- and interobserver reliability for LVEF, LA volume, RA, and RV diameter values were assessed in 30 randomly selected patients and intraclass correlation coefficients (ICCs) were calculated. ICCs for intraobserver and interobserver reliability for LVEF were 0.90 (95% confidence intervals, CIs 0.85–0.95) and 0.87 (95% CI 0.82–0.95), respectively; for LA volume, they were 0.91 (95% CI 0.87–0.96) and 0.88 (95% CI 0.83–0.94), and for RA and RV diameter, they were 0.91 (95% CI 0.83–0.95) and 0.88 (95% CI 0.84–0.94), respectively.
Statistical analysis
All the statistical calculations were performed using a freely available commercial software (https://www.medcalc.org/). Continuous data are expressed as mean ± standard deviations, while categorical data are expressed as percentages. Significance of intergroup comparisons of continuous variables have been assessed using paired t-test and categorical data were assessed using χ2 test. P-values <0.05 are considered significant.
Results
During the study period from June to December 2020, 1789 RT-PCR confirmed COVID-19 patients were admitted to the hospital. Six hundred and sixty-four patients were admitted in day-care facility, 15 met the exclusion criteria vide supra, and 110 either died in hospital (n = 98) or transferred to hospice care (n = 12). We enrolled remaining 1000 patients who were discharged alive from the hospital [men 611 (61.1%), women 389 (38.9%)] (Graphical Abstract) and echocardiographic examination was performed just before discharge.
Baseline characteristics
Mean age of the patients was 50.2 ± 15 years and important comorbidities were hypertension in 44.0%, diabetes in 49.4%, and coronary heart disease in 10.8% (Table 1). The severity of COVID-19 was classified according to WHO criteria21 and was mild in 470 (47.0%), moderate in 395 (39.5%), and severe in 135 (13.5%). Patients with moderate and severe COVID-19, compared to those with mild disease, were significantly older, had more cardiovascular risk factors and comorbidities and duration of hospitalization was significantly greater (Table 1). Baseline echocardiographic measurements in this cohort (n = 1000) showed normal left and right heart functions. The left ventricular systolic and diastolic parameters were: LVIDd 48.3 ± 4.8 mm, LVEF 57.0 ± 5.4%, LA volume 22.4 ± 1.6 mL/m2, and mitral E/e′ 8.1 ± 1.4. LVEF <55% was in 7.7%, LVIDd >55 mm in 5.0%, LA volume >26 mL/m2 in 1.7%, and mitral E/e′ >13 was in 1.1%. The right heart parameters were also within normal range and were: basal right ventricular internal diameter in diastole (RVIDd) 36.4 ± 2.6 mm, basal right atrial internal dimension (RAID) 36.1 ± 2.5 mm, TAPSE 21.7 ± 3.7 mm, and tricuspid Vmax 2.6 ± 1.2 cm/s. Low prevalence of right heart function abnormalities were observed: RVIDd >42 mm in 1.3%, RAID >44 mm in 1.0%, and TAPSE <16 mm in 0.9%. Pericardial effusion was observed in 6 patients (0.6%). In patients with moderate and severe COVID-19, the LA volume was significantly higher (one-way analysis of variance, P < 0.05). There was greater prevalence of reduced LVEF, increased LA volume and mitral E/e′ also, although the numbers are low (Table 1).
Table 1.
Baseline characteristics among mild, moderate, and severe COVID-19 patients
| Total (n = 1000) | Mild COVID-19 (n = 470) | Moderate COVID-19 (n = 395) | Severe COVID-19 (n = 135) | P-value (ANOVA or χ2 test) | |
|---|---|---|---|---|---|
| Age (years) | 51.1 ± 13.8 | 48.4 ± 13.3 | 52.9 ± 14.8 | 51.3 ± 12.9 | <0.0001 |
| Age groups | |||||
| <40 | 296 (29.6) | 163 (34.6) | 112 (33.4) | 21 (15.5) | 0.0117 |
| 40–59 | 583 (58.3) | 236 (50.2) | 249 (74.3) | 98 (72.5) | 0.0662 |
| 60+ | 121 (12.1) | 45 (9.5) | 28 (7.0) | 48 (35.5) | <0.0001 |
| Comorbidities | |||||
| Hypertension | 440 (44.0) | 159 (33.8) | 201 (50.8) | 95 (70.3) | 0.0001 |
| Diabetes | 494 (49.4) | 200 (42.5) | 193 (48.9) | 101 (74.8) | 0.0041 |
| Coronary heart disease | 108 (10.8) | 42 (8.9) | 41 (10.3) | 25 (18.5) | 0.0519 |
| Congestive heart failure | 77 (7.7) | 29 (6.1) | 33 (8.3) | 15 (11.1) | 0.3360 |
| Obesity (BMI >30 kg/m2) | 43 (4.3) | 16 (3.4) | 16 (4.0) | 11 (8.1) | 0.1576 |
| Duration of illness (days) | 11.7 ± 4.9 | 08.4 ± 4.7 | 12.4 ± 3.5 | 15.9 ± 4.2 | <0.0001 |
| Echo parameters left heart | |||||
| LVIDd, mm | 48.3 ± 4.8 | 48.3 ± 4.5 | 48.4 ± 4.3 | 49.1 ± 4.2 | 0.2886 |
| LVEF, % | 57.0 ± 5.4 | 57.4 ± 5.2 | 57.3 ± 4.2 | 56.9 ± 3.9 | 0.4374 |
| Left atrial volume, mL/m2 | 22.4 ± 1.6 | 22.2 ± 1.6 | 22.3 ± 1.6 | 22.8 ± 1.5 | 0.0011 |
| Mitral E/e′ ratio | 8.1 ± 1.4 | 8.0 ± 1.4 | 8.0 ± 1.5 | 8.3 ± 1.2 | 0.1029 |
| Echo parameters right heart | |||||
| RVID basal, mm | 36.4 ± 2.6 | 36.2 ± 2.5 | 36.3 ± 2.3 | 36.6 ± 2.2 | 0.3067 |
| RAID basal, mm | 36.1 ± 2.5 | 36.0 ± 2.4 | 36.1 ± 2.5 | 36.4 ± 2.3 | 0.4283 |
| TAPSE, mm | 21.7 ± 3.7 | 21.9 ± 3.8 | 21.7 ± 3.4 | 21.5 ± 3.3 | 0.6468 |
| Tricuspid Vmax, cm/s | 2.6 ± 1.2 | 2.5 ± 1.2 | 2.5 ± 1.1 | 2.7 ± 1.3 | 0.1448 |
| Categorical | |||||
| LVEF <55% | 49 (4.9) | 5 (1.1) | 28 (7.1) | 16 (11.9) | <0.0001 |
| LA volume >26 mL/m2 | 11 (1.1) | 2 (0.4) | 3 (0.7) | 6 (4.4) | 0.0024 |
| Mitral E/e′ ratio > 13 | 7 (0.7) | 1 (0.2) | 2 (0.5) | 4 (2.9) | 0.0166 |
| RVID >42 mm | 10 (1.0) | 3 (0.6) | 3 (0.7) | 4 (2.9) | 0.1372 |
| RAID >44 mm | 8 (0.8) | 3 (0.6) | 2 (0.5) | 3 (2.2) | 0.2948 |
| TAPSE <16 mm | 8 (0.8) | 2 (0.4) | 3 (0.7) | 3 (2.2) | 0.2631 |
| Pericardial effusion | 5 (0.5) | 1 (0.2) | 2 (0.5) | 2 (1.5) | 0.3724 |
Numbers ± are 1 SD; numbers in parentheses are percent; P-values have been calculated using one-way ANOVA for continuous variables and χ2 test for categorical variables.
LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension diastole; RAID, right atrial internal dimension; RVIDd, right ventricular internal dimension diastole; TAPSE, tricuspid annular plane systolic excursion.
Follow-up evaluation
Six hundred and thirty-two (63.2%) of the baseline cohort of 1000 COVID-19 patients were available for echocardiographic re-evaluation at 3 months. Details of follow-up are in the Graphical Abstract. We compared echocardiographic details of left and right heart parameters in these 632 COVID-19 patients who were available at both baseline and 3-month follow-up. Various measurements are shown in Table 2. At 3-month follow-up, statistically significant decline was observed in left heart function with increased mean LVIDd (+0.9 ± 0.2 mm), LA volume (+7.6 ± 0.1 mL/m2), and mitral E/e′ +4.8 ± 0.1 and reduced LVEF −3.7 ± 0.2% (P < 0.001). The right heart parameters also deteriorated significantly with increased mean RVIDd (+2.1 ± 0.1 mm), RAID (+1.6 ± 0.1 mm), and tricuspid Vmax (+1.0 ± 0.1 cm/s) and reduced TAPSE (−2.7 ± 0.2 mm) (P < 0.001). Categorical variables for left and right heart functions are also in Table 2 and show significant increase in prevalence of reduced left ventricular systolic (LVEF) and diastolic (LA volume and mitral E/e′) functions (P < 0.01). The right ventricular categorical parameters did not change significantly.
Table 2.
Echocardiographic parameters at baseline and 3-month follow-up in 632 patients
| Baseline (n = 632) | 3 months (n = 632) | Difference (95% CI) | P-value | |
|---|---|---|---|---|
| Left heart measurements | ||||
| LVIDd, mm | 48.6 ± 4.7 | 49.2 ± 4.2 | +0.6 ± 0.5 (+0.10, 1.1) | 0.0169 |
| LVEF, % | 57.2 ± 5.2 | 53.3 ± 4.6 | −3.9 ± 0.6 (−3.3, −4.4) | <0.0001 |
| Left atrial volume, mL/m2 | 22.2 ± 1.4 | 30.0 ± 2.8 | +7.8 ± 1.4 (+7.5, 8.0) | <0.0001 |
| Mitral E/e′ ratio | 8.6 ± 1.5 | 12.9 ± 1.6 | +4.3 ± 0.1 (+4.1, 4.4) | <0.0001 |
| Right heart measurements | ||||
| RVIDd basal, mm | 36.6 ± 2.5 | 38.5 ± 2.1 | +1.9 ± 0.4 (+1.6, 2.1) | <0.0001 |
| RAID basal, mm | 36.3 ± 2.7 | 37.7 ± 2.3 | +1.4 ± 0.4 (+1.1, 1.7) | <0.0001 |
| TAPSE, mm | 21.9 ± 3.6 | 19.0 ± 3.3 | −2.9 ± 0.3 (−3.2, −2.5) | <0.0001 |
| Tricuspid Vmax, cm | 2.5 ± 1.1 | 3.6 ± 1.5 | +1.1 ± 0.4 (+0.9, +1.2) | <0.0001 |
| Categorical measurements | % Change (χ2 statistic) | |||
| LVEF <55% | 49 (7.7) | 122 (19.3) | +11.6 (26.672) | <0.0001 |
| Mitral E/e′ ratio >13 | 7 (1.1) | 89 (14.0) | +12.9 (63.6317) | <0.0001 |
| LA volume >26 mL/m2 | 11 (1.7) | 114 (18.0) | +16.3 (76.0129) | <0.0001 |
| RVIDd >42 mm | 10 (1.6) | 21 (3.3) | +1.7 (3.1334) | 0.0767 |
| RAID >44 mm | 8 (1.3) | 12 (1.9) | +0.6 (0.4383) | 0.5079 |
| TAPSE <16 mm | 8 (1.3) | 20 (3.2) | +1.9 (4.2111) | 0.0401 |
| Pericardial effusion | 5 (0.8) | 13 (2.1) | +1.3 (2.6732) | 0.1020 |
Numbers ± are 1 SD; numbers in parentheses are percent; P-values have been calculated using t-test for continuous variables and χ2 test with Yates’ correction for categorical variables.
LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension diastole; RAID, right atrial internal dimension; RVIDd, right ventricular internal dimension diastole; TAPSE, tricuspid annular plane systolic excursion.
Echocardiography in mild vs. moderate-severe COVID-19
Changes in the echocardiographic parameters in mild vs. moderate to severe COVID-19 patients are shown in Table 3. This shows that compared to mild COVID-19, the anatomic and functional decline was significantly greater in patients with moderate and severe disease. In mild vs. moderate to severe COVID-19, a significantly greater change in left heart parameters—LVIDd (+0.6 ± 0.3 vs. 1.0 ± 0.3 mm), LA volume (+1.3 ± 0.1 vs. +7.7 ± 0.1 mL/m2), LVEF (−1.1 ± 0.3 vs. −3.8 ± 0.3%), and mitral E/e′ (+3.2 ± 0.1 vs. +4.8 ± 0.1) was observed (P < 0.01). Compared to mild COVID-19, the changes in right heart parameters were also more in moderate–severe COVID-19 patients: basal RVIDd (+0.4 ± 0.1 vs. +2.2 ± 0.1 mm), RAID basal (0.4 ± 0.1 vs. +1.6 ± 0.1 mm), TAPSE (−0.7 ± 0.2 vs. −2.7 ± 0.2 mm), and tricuspid Vmax (+0.3 ± 0.1 vs. +1.0 ± 0.1 cm/s) (P < 0.01).
Table 3.
Changes in left and right heart-sided echocardiographic parameters at baseline and 3-month follow-up in mild vs. moderate to severe disease
| Mild COVID-19 |
Moderate and severe COVID-19 |
|||||||
|---|---|---|---|---|---|---|---|---|
| Baseline (n = 297) | 3 months (n = 297) | Difference (95% CI) | P-value | Baseline (n = 335) | 3 months (n = 335) | Difference (95% CI) | P-value | |
| Left heart measurements | ||||||||
| LVIDd, mm | 48.3 ± 4.5 | 48.9 ± 4.1 | +0.6 ± 0.3 | 0.089 | 48.2 ± 4.7 | 49.2 ± 4.2 | +1.0 ± 0.3 | 0.003 |
| (0.09–1.29) | (0.32–1.67) | |||||||
| LVEF, % | 57.4 ± 5.2 | 56.3 ± 4.7 | −1.1 ± 0.3 | 0.007 | 57.0 ± 4.8 | 53.2 ± 4.6 | −3.8 ± 0.3 | <0.001 |
| (1.89–0.30) | (4.51–3.08) | |||||||
| LA volume, mL/m3 | 22.2 ± 1.6 | 23.5 ± 2.2 | +1.3 ± 0.1 | <0.001 | 22.4 ± 1.6 | 30.1 ± 2.8 | +7.7 ± 0.1 | <0.001 |
| (0.99–1.61) | (7.35–8.04) | |||||||
| Mitral E/e′ ratio | 8.0 ± 1.4 | 11.2 ± 1.3 | +3.2 ± 0.1 | <0.001 | 8.1 ± 1.5 | 12.9 ± 1.6 | +4.8 ± 0.1 | <0.001 |
| (2.98–3.40) | (4.56–5.03) | |||||||
| Right heart measurements | ||||||||
| RVIDd basal, mm | 36.2 ± 2.5 | 36.7 ± 2.2 | +0.4 ± 0.1 | 0.009 | 36.4 ± 2.6 | 38.6 ± 2.1 | +2.2 ± 0.1 | <0.001 |
| (0.12–0.87) | (1.84–2.55) | |||||||
| RAID basal, mm | 36.0 ± 2.4 | 36.4 ± 2.0 | +0.4 ± 0.1 | 0.027 | 36.3 ± 2.6 | 37.9 ± 2.3 | +1.6 ± 0.1 | <0.001 |
| (1.22–1.97) | ||||||||
| (0.04–0.75) | ||||||||
| TAPSE, mm | 21.9 ± 3.8 | 21.2 ± 3.2 | −0.7 ± 0.2 | 0.015 | 21.7 ± 3.6 | 19.0 ± 3.3 | −2.7 ± 0.2 | <0.001 |
| (1.26–0.13) | (3.22–2.17) | |||||||
| Tricuspid Vmax, cm | 2.5 ± 1.2 | 2.8 ± 1.4 | +0.3 ± 0.1 | 0.005 | 2.6 ± 1.2 | 3.6 ± 1.5 | 1.0 ± 0.1 | <0.001 |
| (0.09–0.51) | (0.79–1.20) | |||||||
| Categorical measurements | Percent change | Percent change | ||||||
| RVIDd >42 mm | 3 (1.0) | 4 (1.3) | +0.3 | 0.996 | 10 (2.9) | 17 (5.1) | +2.2 | 0.260 |
| RAID >44 mm | 3 (1.0) | 4 (1.3) | +0.3 | 0.996 | 7 (2.1) | 8 (2.4) | +0.3 | 0.995 |
| TAPSE <16 mm | 2 (0.7) | 4 (1.3) | +0.6 | 0.687 | 7 (2.1) | 16 (4.8) | +2.7 | 0.102 |
| Pericardial effusion | 1 (0.3) | 2 (0.7) | +0.4 | 0.997 | 5 (1.5) | 11 (3.3) | +1.8 | 0.218 |
Numbers ± are 1 SD; numbers in parentheses are percent; P-values have been calculated using t-test for continuous variables and χ2 test with Yates’ correction for categorical variables.
LA, left atrium; LVEF, left ventricular ejection fraction; LVIDd, left ventricular internal dimension diastole; RAID, right atrial internal dimension; RVIDd, right ventricular internal dimension diastole; TAPSE, tricuspid annular plane systolic excursion.
Discussion
This study shows that echocardiographic left heart systolic and diastolic functional parameters are slightly impaired following hospital discharge in COVID-19 patients, more in moderate and severe disease. Significant systolic and diastolic left and right heart dysfunction emerges at 3-month follow-up and these changes are more in moderate-to-severe COVID-19 patients compared to those with mild disease.
Previous echocardiographic studies in COVID-19 survivors have reported impaired left ventricular systolic function (increased LVIDd and reduced LVEF).7–9,13 Some studies have also reported left ventricular diastolic dysfunction with increased LA volume, increased peak tricuspid regurgitation velocity (Vmax), and changes in mitral E/e′.7,10–12,24 Our study shows similar findings. Other left heart functional abnormalities that have been reported include impaired diastolic function and subtle abnormalities that are identified by speckle-tracking myocardial imaging on echocardiography.25,26 Cardiac MRI may be a better tool to delineate left ventricular and LA myocardial abnormalities6 but is expensive and difficult in acute disease due to logistical reasons. The impaired left ventricular functions could be consequence of subtle or silent acute coronary syndrome leading to minor left ventricular functional abnormalities.27 We do not have data on cardiac injury biomarkers (troponins, etc.) and hence cannot comment on the degree of myocardial damage, but more impaired cardiac functions (reduced LVEF, increased LA volume, and mitral E/e′) at baseline among patients with severe disease is suggestive of greater myocardial damage in these patients.
Right heart functional abnormalities have also been reported during the acute phase of illness and are attributed to pulmonary pathologies such as endothelialitis, small vessel vasculitis, subclinical pulmonary thromboembolism, and pulmonary necrosis in COVID-19 apart from right ventricular myocarditis.2,3,7,11,28 Right ventricular dysfunction is specific to COVID-19 and may be due to increased afterload and decreased contractility caused by various factors such as acute respiratory distress syndrome, pulmonary thrombosis, direct viral injury, hypoxia, inflammatory response, and autoimmune injury and is an important prognostic marker.29,30 Studies have reported right heart systolic and diastolic functional abnormalities in COVID-19.7,11 Our study shows that some of the right heart functional abnormalities emerge only at follow-up, these findings are similar to a previous study.18 Greater increase in right heart abnormalities suggest ongoing pulmonary vascular disease and right-sided myocardial damage.29,30 It has been suggested that right ventricular dysfunction is prevalent in severe COVID-19 patients and more focused studies that involves cardiac MRI for better assessment of right heart anatomy and function are needed.6 Greater functional abnormalities in moderate to severe COVID-19 patients in our cohort suggests that all these factors could be important. There is need for larger and more focused studies.
Limitations
The study has a few limitations. An important limitation is lack of cardiac biomarker data (not routinely performed at our hospital), which are important prognostic markers of myocardial damage in COVID-19.5,15 There is a strong correlation of cardiac biomarkers with clinical severity of COVID-19.27 In our cohort too, disease severity, as judged by clinical parameters, is associated with significantly greater cardiac functional abnormality (Table 1). Secondly, we could perform echocardiography in only about two-thirds of the patients who presented to the hospital and excluded patients who opted for day-care and home-based care and also could not evaluate patients who died or opted for hospice care. This is important study limitation as greater cardiac dysfunction has been reported in fatal COVID-19.2,5 Previous studies have also reported minimal cardiac involvement among day-care patients or those with mild COVID-19.7,24 Thirdly, although serial echocardiograms reveal a subtle deterioration of left and right heart function, clinical significance of these minor changes is difficult to assess.31 Only long-term prospective follow-up studies shall be able to provide guidance in this regard.
Conclusions
This study demonstrates mild, albeit significant, impairment of left and right heart echocardiographic parameters following COVID-19 with increase in cardiac dysfunction, including both left and right heart functions, especially diastolic dysfunction at 3 months of discharge from the hospital. These changes may be secondary to progression of left and right heart myocarditis27–30 or due to COVID-19-induced progressive pulmonary disease.4 Pulmonary disease, including fibrosis have been well described as part of long-COVID-19 or PACS.4 Whether these changes resolve spontaneously or need pharmacological therapy via anti-fibrotic drugs can be answered by clinical trials. Routine evaluation of left and right heart function following COVID-19, especially severe infections, may be useful for identification of subclinical cardiac functional abnormalities and prognostication. Meanwhile, primary prevention of COVID-19 infection, utilizing public health measures including mass vaccination,32 remains the key to prevent the secondary cardiovascular and other complications of the disease.
Conflict of interest: none declared.
Data availability
The data is available upon reasonable request from the corresponding author.
References
- 1. Giustino G, Pinney SP, Lala A, Reddy VY, Johnston-Cox HA, Mechanick JI et al. Coronavirus and cardiovascular disease, myocardial injury and arrhythmia: JACC focus seminar. J Am Coll Cardiol 2020;76:2011–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chung MK, Zidar DA, Bristow MR, Cameron SJ, Chan T, Harding CV et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res 2021;128:1214–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bavishi C, Bonow RO, Trivedi V, Abbott JD, Messerli FH, Bhatt DL. Acute myocardial injury in patients hospitalized with COVID-19 infection: a review. Prog Cardiovasc Dis 2020;63:682–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS et al. Post-acute COVID-19 syndrome. Nat Med 2021;27:601–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abbasi J. Researchers investigate what COVID-19 does to the heart. JAMA 2021;325:808–11. [DOI] [PubMed] [Google Scholar]
- 6. Rudski L, Januzzi JL, Rigolin VH, Bohula EA, Blankstein R, Patel VH et al. ; Expert Panel From the ACC Cardiovascular Imaging Leadership Council. Multimodality imaging in evaluation of cardiovascular complications in patients with COVID-19. J Am Coll Cardiol 2020;76:1345–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Carrizales-Sepúlveda EF, Vera-Pineda R, Flores-Ramírez R, Hernández-Guajardo DA, Pérez-Contreras E, Lozano-Ibarra MM et al. Echocardiographic manifestations in COVID-19: a review. Heart Lung Circ 2021;30:1117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yau OY, Gin K, Luong C, Jue J, Abolmaesumi P, Tsang M et al. Point-of-care ultrasound in the COVID-19 era: a scoping review. Echocardiography 2021;38:329–42. [DOI] [PubMed] [Google Scholar]
- 9. Szekely Y, Lichter Y, Taieb P, Banai A, Hochstadt A, Merdler I et al. Spectrum of cardiac manifestations in COVID-19: a systematic echocardiographic study. Circulation 2020;142:342–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pishgahi M, Toudeshki KK, Safari S, Yousefifard M. Echocardiographic abnormalities as independent prognostic factor of in-hospital mortality among COVID-19 patients. Arch Acad Emerg Med 2021;9:e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bieber S, Kraechan A, Hellmuth JC, Muenchhoff M, Scherer C, Schroeder I et al. Left and right ventricular dysfunction in patients with COVID-19 associated myocardial injury. Infection 2021;49:491–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hadzibegovic S, Lena A, Churchill TW, Ho JE, Potthoff S, Denecke C et al. Heart failure with preserved ejection fraction according to HFA-PEFF score in COVID-19 patients: clinical correlates and echocardiographic findings. Eur J Heart Fail 2021;23:1891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lala A, Johnson KW, Januzzi JL, Russak AJ, Paranjpe I, Richter F et al. Prevalence and impact of myocardial injury in patients hospitalized with COVID-19 infection. J Am Coll Cardiol 2020;76:533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giustino G, Croft LB, Stefanini GG, Bragato R, Silbiger JJ, Vicenzi M et al. Characterization of myocardial injury in patients with COVID-19. J Am Coll Cardiol 2020;76:2043–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019; results of a multicenter study. JAMA Cardiol 2020;5:1274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao YD, Ding M, Dong X, Zhang J-J, Azkur AK, Azkur D et al. Risk factors for severe and critically ill COVID-19 patients; a review. Allergy 2021;76:428–55. [DOI] [PubMed] [Google Scholar]
- 17. Puntmann VO, Carerj ML, Wieters I, Fahi M, Arendt C, Hoffman J et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fayol A, Livrozet M, Boutouyrie P, Khettab H, Betton M, Tea V et al. ; French COVID cohort study group. Cardiac performance in patients hospitalized with COVID-19: a 6 month follow-up study. ESC Heart Fail 2021;8:2232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ritchie H, Mathieu E, Rodes-Guirao L, Appel C, Ortiz-Ospina E, Hasell J et al. India: Coronavirus Pandemic Country Profile. https://ourworldindata.org/coronavirus/country/india (24 October 2021, date last accessed).
- 20. Khedar RS, Mittal K, Ambaliya HC, Mathur A, Gupta JB, Sharma KK et al. Greater COVID-19 severity and mortality in hospitalized patients in the second (delta) wave compared to the first wave of epidemic: single centre prospective study in India. medRxiv preprints 2021; doi: 10.1011/2021.09.03.21263091. [DOI] [Google Scholar]
- 21. World Health Organization. Coronavirus Disease (COVID-19) Technical Guidance: Patient Management. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/patientmanagement (14 May 2021, date last accessed).
- 22. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrng A, Ernande L et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from American Society of Echocardiography and European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 23. Gupta R, Das MK, Mohanan PP, Deb PK, Parashar SK, Chopra HK et al. Cardiological Society of India document on safety measure during echo evaluation of cardiovascular disease in the time of COVID-19. Indian Heart J 2020;72:145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joy G, Artico J, Kurdi H, Seraphim A, Lau C, Thornton GD et al. Prospective case-control study of cardiovascular abnormalities 6 months following mild COVID-19 in healthcare workers. JACC Cardiovasc Imaging 2021;14:2155–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu H, Cirillo C, Nabeebaccus AA, Sun Z, Fang L, Xie Y et al. First-phase ejection fraction, a measure of preclinical heart failure is strongly associated with increased mortality in patients with COVID-19. Hypertension 2021;77:2014–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ozer S, Candan L, Ozyildiz AG, Turan OE. Evaluation of left ventricular global functions with speckle tracking echocardiography in patients recovered from COVID-19. Int J Cardiovasc Imaging 2021;37:2227–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shorikova DV, Shorikov EL. COVID-19 and acute coronary syndrome: emphasis on ACS without atherothrombosis. E-J Cardiovasc Pract 2021;21:e5. [Google Scholar]
- 28. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F et al. Pulmonary vascular endothelialitis, thrombosis and angiogenesis in COVID-19. N Engl J Med 2020;383:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lan Y, Liu W, Zhou Y. Right ventricular damage in COVID-19: association between myocardial injury and COVID-19. Front Cardiovasc Med 2021;8:606318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation 2021;143:1031–42. [DOI] [PubMed] [Google Scholar]
- 31. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 32. Goldfarb JL, Kreps S, Brownstein JS, Kriner DL. Beyond the first dose-COVID-19 vaccine follow-through and continued protective measures. N Engl J Med 2021;385:101–103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data is available upon reasonable request from the corresponding author.