Abstract
Mucosal immune response in the upper respiratory tract is crucial for initial control of viral replication, clearance of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and progression of coronavirus disease 2019 (COVID-19). We analyzed SARS-CoV-2 RNA load and expression of selected immune genes in the upper respiratory tract (nasopharynx) of 255 SARS-CoV-2–infected patients and evaluated their association with severe COVID-19. SARS-CoV-2 replication in nasopharyngeal mucosa induces expression of several innate immune genes. High SARS-CoV-2 viral load and low CCL5 expression levels were associated with intensive care unit admission or death, although CCL5 was the best predictor of COVID-19 severity.
Keywords: CCL5, COVID-19, death, gene expression, ICU, innate immunity, nasopharynx, SARS-CoV-2, viral load
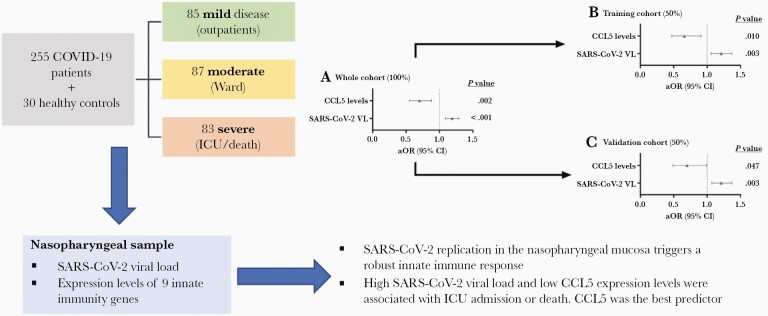
Graphical Abstract
Graphical Abstract.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection causes coronavirus disease 2019 (COVID-19), a disease with high mortality and morbidity rates worldwide. Clinical manifestations range from mild symptoms to pneumonia and severe acute respiratory syndrome. The proinflammatory response and viral replication are closely related to the severity of the disease and risk of death [1].
SARS-CoV-2 infection initiates in the upper respiratory tract, mainly in nasal epithelial cells, where the binding of the virus to the angiotensin-converting enzyme 2 (ACE2) cell receptor occurs [1]. After cellular and viral membrane fusion, the viral genome replicates inside the cell. Viral RNAs activate the innate immune system, triggering signaling pathways leading to the expression of type I and III interferons (IFN-α/β and IFN-λ), proinflammatory cytokines, and chemokines, and the recruitment of inflammatory myeloid cells. Interferon-mediated signaling, in turn, upregulates the expression of multiple antiviral interferon-stimulated genes (ISGs) [1].
Mucosal immune response in the upper respiratory tract is crucial for the initial control of viral replication, the clearance of SARS-CoV-2, and the progression of the COVID-19 [1, 2]. A characteristic of SARS-CoV-2 infection is the high viral loads in the upper airways before symptom onset, which may be related to the reduced activation of some innate immunity pathways [3, 4]. Also, dysregulated antiviral immunity in the nasal epithelium seems to predict progression to severe COVID-19 [5, 6]. Therefore, differentiating protective immune response in the nasopharynx from that leading to fatal outcomes is essential in developing preventative and therapeutic strategies against SARS-CoV-2 infection. This study aimed to analyze the viral RNA load and the expression of selected immune genes in the upper respiratory tract (nasopharynx) of SARS-CoV-2–infected patients and evaluate their association with severe COVID-19.
METHODS
Study Design and Patients
We conducted a retrospective study of 255 SARS-CoV-2–infected patients from Hospital Universitario Príncipe de Asturias between 9 November 2020 and 8 March 2021, who had a positive real-time polymerase chain reaction (RT-PCR) test at the emergency admission. Patients were initially enrolled to complete 3 groups with different severity stages: 85 outpatients that were examined at the emergency room and discharged within the first 24 hours (mild cases), 87 hospitalized in medicine wards that did not require critical care (moderate cases), and 83 critical patients that were admitted to the intensive care unit (ICU) or died within 28 days after hospital admission (severe cases). In parallel to the COVID-19 patients, we enrolled 30 healthy individuals with a negative PCR test for SARS-CoV-2 and no suspicion of any other respiratory infection. Demographic and clinical data were extracted from medical records.
This study was approved by the Hospital Universitario Príncipe de Asturias Ethics Committee (reference EXPRES-INMUNE-COVID) and the Hospital’s Institutional Review Board. Informed consent waiver was authorized by the Ethics Committee.
Laboratory Assays
Nasopharyngeal Swabs Samples
Biological samples (nasopharyngeal swabs) were obtained during the first 24 hours after the emergency admission. Samples were placed in a transport medium with guanidinium isothiocyanate (NEST Disposable Nasopharyngeal VTM Sampler kit; Wuxi NEST Biotechnology) for COVID-19 testing, and stored at −80°C. This medium served as an inactivator and preservative for SARS-CoV-2 RNA and mucosal biomarkers mRNA.
RT-PCR Assay for COVID-19 Diagnosis
Viral RNA was obtained at the hospital from clinical samples using 2 automatic extractors: MagCore HF16 (RBC Bioscience) and ELITe Ingenius (ELITechGroup). RNA amplification was performed using 2 RT-PCR platforms, randomly according to the usual laboratory workflow: Viasure SARS-CoV-2 RealTime PCR Detection Kit (Certest Biotech SL; detected genes ORF1ab and N) and GeneFinder COVID-19 Plus RealAmp Kit (Osang Healthcare Co.; detected genes E, N, and RdRP). The concordance of these 2 platforms showed 100% agreement and similar cycle threshold (Ct) values (Supplementary Table 1). Samples were considered positive when all SARS-CoV-2 genes included in each RT-PCR assay were amplified.
RT-PCR for Quantification of Mucosal Biomarkers
RNA previously extracted as above was reverse transcribed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) following the manufacturer’s instructions. The expression of the selected genes (mucosal biomarkers) was quantified in the generated cDNA by RT-PCR using TaqMan Gene Expression Assays specific for each gene (Applied Biosystems). The PCRs were done in triplicate in a Step One instrument (Applied Biosystems) following the manufacturer’s instructions. TaqMan Gene Expression Assays containing TaqMan MGB probes (FAM dye-labeled; Applied Biosystems) were used for the following cellular genes: actin-β (ACTB; Hs99999903_m1), DExD/H-box helicase 58 (DDX58; RIG-I, Hs00204833_m1), tumor necrosis factor (TNF; Hs00174128_m1), interleukin 6 (IL6; Hs00985639_m1), interleukin 8 (IL8, CXCL8; Hs00174103_m1), interferon-β1 (IFNB1; Hs01077958_s1), interferon-stimulated gene 15 (ISG15; Hs00192713_m1), interferon-induced protein with tetratricopeptide (IFIT1; Hs00174103_m1), chemokine C-X-C motif ligand 10 (CXCL10; Hs00171042_m1), and chemokine C-C motif ligand 5 (CCL5; Hs00982282_m1). We used ACTB mRNA as an endogenous control to normalize the quantitation of an mRNA target for differences in the amount of total RNA added to each reaction. We also performed a relative quantification by the comparative Ct (ΔΔCt) method (Applied Biosystems User Bulletin no. 2, http://assets.thermofisher.com/TFS-Assets/LSG/manuals/cms_040980.pdf) using as a reference sample (calibrator) a random mixture of samples from 100 SARS-CoV-2 positive individuals. Target mRNA expression was quantified relative to the calibrator for all experimental samples and expressed as n-fold.
Expression of SARS-CoV-2 nucleocapsid gene was performed according to the manufacturer’s instructions, using SYBR-Green reaction mix (Power-Up SYBR-Green Master MIX; Applied Biosystems) and the following primers: ACTB (forward, 5ʹ-CACCAACTGGGACGACAT-3ʹ; reverse, 5ʹ-ACAGCCTGGATAGCAACG-3ʹ) and N (forward, 5ʹ-GGGAGCCTTGAATACACCAAAA-3ʹ; reverse, 5ʹ-TGTAGCACGATTGCAGCATTG-3ʹ). The expression of ACTB was used as endogenous control, and relative quantification was made by the comparative Ct (ΔΔCt) method using the same calibrator as above.
Severe COVID-19 Outcomes
The primary endpoint during hospital admission was ICU admission or death within 28 days (ICU admission/death). A single episode was considered for each patient. When a patient was discharged from the emergency department and later readmitted during the study period, only the first hospital admission episode was considered for the analysis, and the corresponding respiratory sample for mucosal biomarkers.
Statistical Analysis
Statistical analysis was performed using Stata IC 15.1 (StataCorp). Figures were generated using GraphPad Prism 8.0 (GraphPad Software, Inc). All P values were 2-tailed, and the significance level was set at .05 (2-tailed).
The differences between independent groups were assessed using the Mann-Whitney U test, χ2 test, or Fisher exact test. Correlation analysis between mucosal biomarkers was performed using the Pearson coefficient (r). Logistic regression analysis was employed to determine the association of mucosal biomarkers with COVID-19 outcomes, providing the odds ratio (OR) and their 95% confidence intervals (CIs). In all cases, we performed univariate and multivariate regression analyses adjusted by significant covariables prior to SARS-CoV-2 infection (age, sex, and comorbidities) and covariables at baseline (time from COVID-19 symptoms to sample collection, international normalized ratio, C-reactive protein, ferritin, lactate dehydrogenase, alanine aminotransferase, albumin, estimated glomerular filtration rate, creatinine, glucose, thrombocytes, neutrophils, lymphocytes, and hematocrit). Covariables were selected by a stepwise forward selection method (pin < 0.05 and pout < 0.10) to avoid model overfitting. For association analysis, mucosal biomarker values were log2 transformed (base-2 logarithms). The predictive performance of severe COVID-19 was assessed by area under the receiver-operating characteristic curve (AUC), which was considered excellent (0.90–1), good (0.80–0.90), reasonable (0.70–0.80), and poor (0.60–0.70). Also, we divided the study population in half into training and validation cohorts to evaluate the predictive value of the tested genes.
RESULTS
Characteristics of COVID-19 Patients
Baseline characteristics of COVID-19 patients are shown in Supplementary Table 2. Patients were 60% men, the median age was 63.8 years, and the main comorbidities were chronic heart disease, hypertension, chronic obstructive pulmonary disease, obesity, diabetes, and dyslipidemia. After hospital admission, 19.6% entered the ICU, 16.1% had invasive mechanical ventilation, and 14.1% died within 28 days. Healthy controls were 47.7% men and the median age was 60 years.
Innate Immunity Genes Are Upregulated in COVID-19 Patients
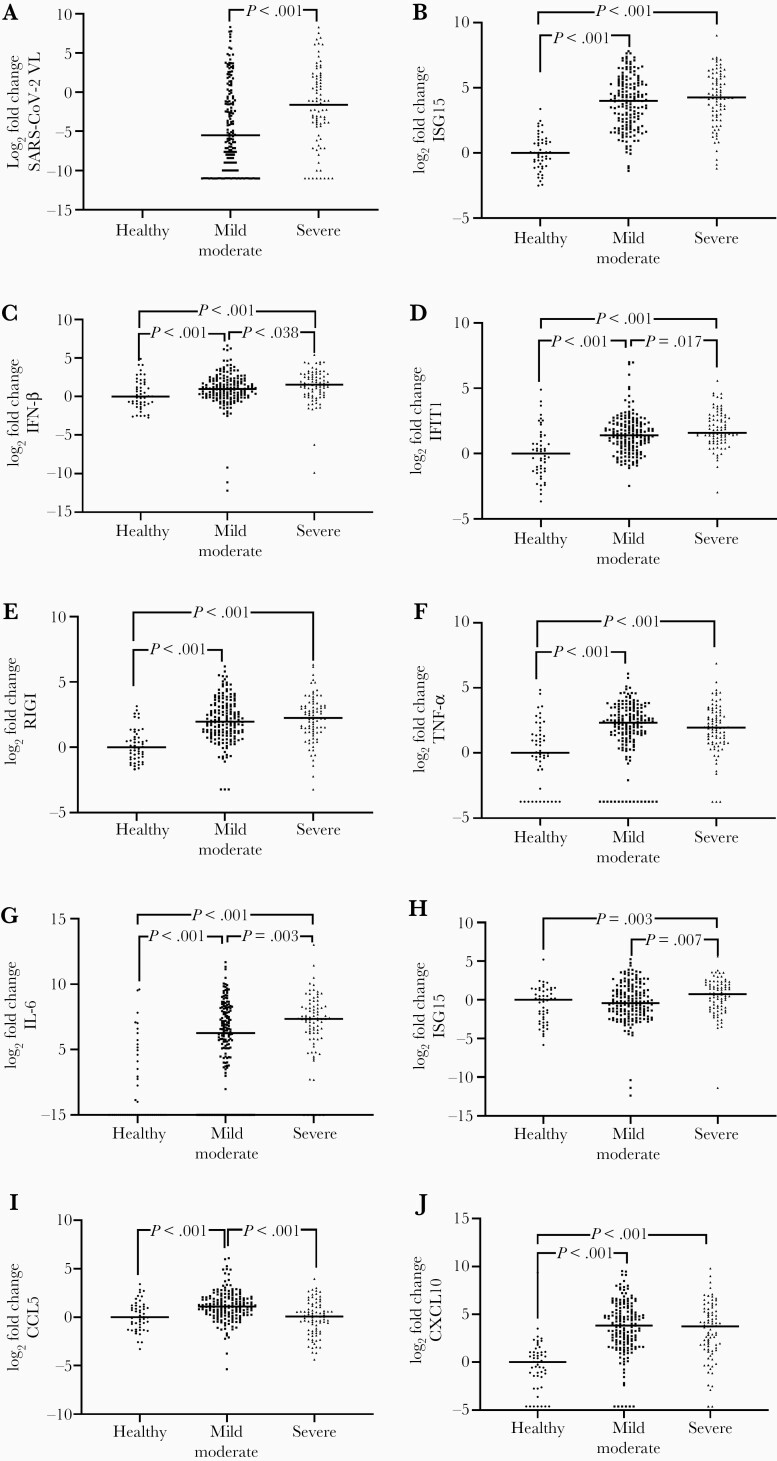
COVID-19 patients had higher gene expression levels of interferon-stimulated gene 15 (ISG15), interferon-β (IFN-β), interferon-induced protein with tetratricopeptide repeats 1 (IFIT1), retinoic acid-inducible gene I (RIGI), tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6), chemokine (C-C motif) ligand 5 (CCL5), and C-X-C motif chemokine ligand 10 (CXCL10) than healthy controls (P value < .05; Supplementary Figure 1). Patients with severe COVID-19 had significantly higher values for SARS-CoV-2 viral load, IFN-β, IFIT1, IL-6, and IL-8 than patients with mild or moderate disease, while CCL5 values were substantially lower in patients with severe COVID-19 (P value < .05; Figure 1). Moreover, we found a strong direct correlation between SARS-CoV-2 viral load and ISG15, RIGI, TNF-α, IL-6, and CXCL10 (P value < .001; Supplementary Figure 2).
Figure 1.
A–J, Relative expression of innate immunity genes and SARS-CoV-2 viral load in the upper respiratory tract of COVID-19 patients. Values are expressed as log2 (fold-changes) to the median of healthy controls to facilitate comparisons between groups. The differences between groups were assessed using the Mann-Whitney U test. Abbreviations: CCL5, chemokine (C-C motif) ligand 5; COVID-19, coronavirus disease 2019; CXCL10, C-X-C motif chemokine ligand 10; IFIT1, interferon-induced protein with tetratricopeptide repeats 1; IFN-β, interferon-β; IL-6, interleukin 6; IL-8, interleukin 8; ISG15, interferon-stimulated gene 15; RIGI, retinoic acid-inducible gene I; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; TNF-α, tumor necrosis factor-α; VL, viral load.
Mucosal Biomarkers Predict COVID-19 Outcomes
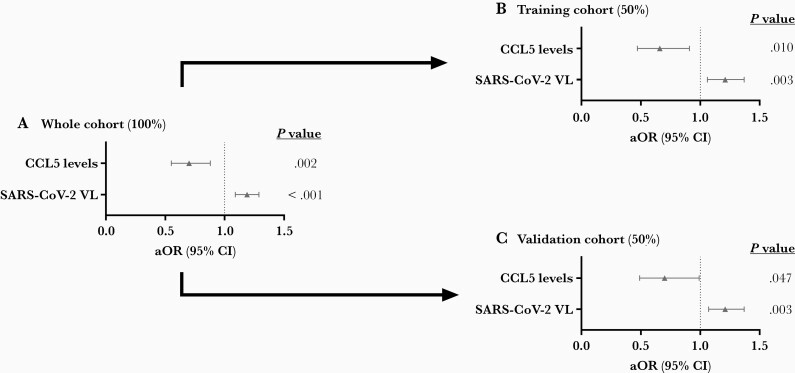
In adjusted regression models (Figure 2; full description in Supplementary Table 3), SARS-CoV-2 viral load was a risk factor (adjusted OR [aOR] = 1.19, P value < .001), and CCL5 was a protective factor for ICU admission or death during hospital admission (aOR = 0.70, P value = .002). When the whole cohort was divided in half (training and validation cohorts), we also found significant associations for SARS-CoV-2 viral load and CCL5 in both cohorts (P value < .05), supporting that the association between high levels of SARS-CoV-2 viral load and low levels of CCL5 expression with COVID-19 severity is robust.
Figure 2.
A–C, Association between mucosal biomarkers (log2 values) in the upper respiratory tract and ICU admission or death during hospital admission. The association analysis was performed by logistic regression adjusted by the most significant covariables. Abbreviations: aOR, adjusted odds ratio; CCL5, chemokine (C-C motif) ligand 5; CI, confidence interval; ICU, intensive care unit; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; VL, viral load.
Moreover, CCL5 was the only mucosal biomarker tested with an AUC > 0.70 (Supplementary Figure 3A), and the combination with other mucosal biomarkers did not significantly improve the AUC value (data not shown). CCL5 was also directly correlated with higher peripheral oxygen saturation (Spo2) values at emergency admission (r = 0.295; P < .001; Supplementary Figure 3B).
DISCUSSION
This study found that decreased expression of CCL5 and elevated SARS-CoV-2 viral load in nasopharyngeal samples were associated with poor COVID-19 outcomes. In our study, COVID-19 patients had upregulated innate immunity genes, which were directly associated with SARS-CoV-2 viral load. Characterizing the immune response triggered in the upper respiratory tract by SARS-CoV-2 is essential because the virus infection, replication, and dissemination begins in this place. An adequate immune response in the nasopharynx contributes to controlling virus replication, preventing its spread towards the lower respiratory tract, and avoiding disease complications. Conversely, an unbalanced innate immune response is closely related to COVID-19 severity and the risk of death [1].
We found that COVID-19 patients with high values of SARS-CoV-2 viral load had higher odds of ICU admission or death. In agreement with our data, some studies have found that SARS-CoV-2 viral loads on the upper respiratory tract are positively associated with disease severity [7–9]. However, others have found no correlation [4, 10, 11]. This difference may be related to different times between sample collection and SARS-CoV-2 viral load quantification.
Moreover, higher levels of CCL5 were associated with better COVID-19 outcomes, and it was also the only biomarker with acceptable predictive performance. CCL5 (also known as regulated upon activation normal T-cell expressed and secreted, RANTES) is a potent chemoattractant for several immune cells such as monocytes and natural killer (NK) cells and promotes interaction between T cells and dendritic cells, essential for virus control [12]. Thus, the CCL5 expression may help eliminate SARS-CoV-2 infection and prevent patients from developing severe COVID-19 [13]. In agreement with this, it has been described that in patients with critical COVID-19, CD8+ cytotoxic lymphocytes expressed lower levels of CCL5 [14]. In addition, COVID-19 patients with mild disease had higher nasopharyngeal CCL5 expression than those with severe pneumonia at the emergency room [15]. Thus, profiling the expression of the CCL5 gene in the nasopharyngeal mucosa with the same sample used for diagnosis could help improve the prognosis of patients at the initial phase of infection and guide potential treatments.
However, our study can be considered preliminary and has several limitations, such as it was retrospective, had a limited sample size, and only a few biomarkers were evaluated. Further studies should confirm our findings and corroborate the potential use of nasopharyngeal biomarkers to predict the clinical course of COVID-19.
CONCLUSION
SARS-CoV-2 replication in the nasopharyngeal mucosa induces the expression of several innate immune genes. High SARS-CoV-2 viral load and low CCL5 expression levels were associated with ICU admission or death, although CCL5 was the best predictor of COVID-19 severity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments . We acknowledge the patients in this study for their participation. This study would not have been possible without the collaboration of all medical and nursing staff and data managers who have taken part in the project.
Author contributions . J. F. B. M., S. R., and I. M. acquired funding. S. R. and I. M. developed the study concept and design, and supervised the work. F. P. G., L. C. G., I. H. F., V. G. V., and F. C. G. performed patient selection and clinical data acquisition. M. M. V., R. L. R. G., M. J. M. G., and E. J. V. A. carried out sample preparation, RNA isolation, and RT-PCRs. F. P. G., S. R., and I. M. performed statistical analysis and interpretation of data. F. P. G., M. M. V., S. R., and I. M. wrote the original draft. J. F. B. M. edited the manuscript.
Disclaimer . The funding sources played no role in the study’s design, collection, analysis, interpretation of the data, or manuscript writing.
Financial support . This work was supported by the Canadian Institutes of Health Research (grant number CIHR OV2-170357); Research Nova Scotia, Atlantic Genome/Genome Canada; Li-Ka Shing Foundation; Dalhousie Medical Research Foundation ( to J. F. B. M.); Instituto de Salud Carlos III (grant numbers COV20/00110 to J. F. B. M. and CIBERES 06/06/0028 to S. R.); Convocatoria extraordinaria y urgente de la Gerencia Regional de Salud de Castilla y León para la financiación de proyectos de investigación en enfermedad COVID-19 (grant number GRS COVID 53/A/20 to J. F. B. M.); and the Centro de Investigación Biomédica en Red en Enfermedades Infecciosas (grant number CB21/13/00044 to S. R. and I. M.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Availability of data . The datasets used and analyzed during the current study are available from the corresponding authors upon reasoned request.
References
- 1. Hu B, Guo H, Zhou P, Shi ZL.. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 2021; 19:141–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gallo O, Locatello LG, Mazzoni A, Novelli L, Annunziato F.. The central role of the nasal microenvironment in the transmission, modulation, and clinical progression of SARS-CoV-2 infection. Mucosal Immunol 2021; 14:305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mick E, Kamm J, Pisco AO, et al. Upper airway gene expression reveals suppressed immune responses to SARS-CoV-2 compared with other respiratory viruses. Nat Commun 2020; 11:5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ng DL, Granados AC, Santos YA, et al. A diagnostic host response biosignature for COVID-19 from RNA profiling of nasal swabs and blood. Sci Adv 2021; 7:eabe5984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ziegler CGK, Miao VN, Owings AH, et al. Impaired local intrinsic immunity to SARS-CoV-2 infection in severe COVID-19. Cell 2021; 184:4713–33.e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blanco-Melo D, Nilsson-Payant BE, Liu WC, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020; 181:1036–45.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guo X, Jie Y, Ye Y, et al. Upper respiratory tract viral ribonucleic acid load at hospital admission is associated with coronavirus disease 2019 disease severity. Open Forum Infect Dis 2020; 7:ofaa282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maltezou HC, Raftopoulos V, Vorou R, et al. Association between upper respiratory tract viral load, comorbidities, disease severity, and outcome of patients with SARS-CoV-2 infection. J Infect Dis 2021; 223:1132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanner AR, Phan H, Brendish NJ, et al. SARS-CoV-2 viral load at presentation to hospital is independently associated with the risk of death. J Infect 2021; 83:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yilmaz A, Marklund E, Andersson M, et al. Upper respiratory tract levels of severe acute respiratory syndrome coronavirus 2 RNA and duration of viral RNA shedding do not differ between patients with mild and severe/critical coronavirus disease 2019. J Infect Dis 2021; 223:15–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rodriguez C, de Pros, N, Fourati S, et al. Viral genomic, metagenomic and human transcriptomic characterization and prediction of the clinical forms of COVID-19. PLoS Pathog 2021; 17:e1009416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Griffith JW, Sokol CL, Luster AD.. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol 2014; 32:659–702. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Y, Qin L, Zhang P, et al. Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight 2020; 5:e139834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chua RL, Lukassen S, Trump S, et al. COVID-19 severity correlates with airway epithelium-immune cell interactions identified by single-cell analysis. Nat Biotechnol 2020; 38:970–9. [DOI] [PubMed] [Google Scholar]
- 15. Montalvo Villalba MC, Valdes Ramirez O, Mune Jimenez M, et al. Interferon gamma, TGF-beta1 and RANTES expression in upper airway samples from SARS-CoV-2 infected patients. Clin Immunol 2020; 220:108576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.