Abstract
Vasculitis is one of the complications of COVID-19. We conducted a systematic review analysing the association of COVID-19 with vasculitis. We searched Google Scholar and PubMed from December 1, 2019, to October 11, 2021. The review included 8 studies (7 case reports and 1 case series) reporting 9 cases of vasculitis secondary to COVID-19. The mean age was 29.17 ± 28.2 years, ranging from 6 months to 83 years. The male to female ratio was 4:5. Maculopapular, violaceous, papular and erythematous rash were common. Heparin(n = 2), corticosteroids (n = 6) (methylprednisolone) and intravenous immunoglobulin (n = 4) were prescribed in these patients. Significant clinical improvement was observed in 8 out of 9 patients. One person died during treatment. Our study discusses vasculitis as one of the complications of COVID-19. Furthermore, the pathophysiology, clinical presentation, and management of COVID-19 associated vasculitis is discussed.
Keywords: IgA, Immunoglubulin A; SARS-C0V, Severe Acute Respiratory Syndrome Coronavirus 2; COVID-19, Coronavirus disease of 2019
Keywords: COVID-19, Vasculitis, SARS-CoV-2, Kawasaki disease, Leukocytoclastic vasculitis, IgA vasculitis
Highlights
-
•
This is a systematic review of all published cases of vasculitis secondary to COVID-19 from the start of COVID-19 outbreak to date.
-
•
Most common forms of vasculitis reported were Kawasaki disease Vasculitis, IgA vasculitis and Leucocytoclastic Vasculitis with symptoms being predominantly skin limited.
-
•
Steroids, LMWH and IVIG were the most common line of treatment.
-
•
An good overall prognosis was seen following treatment.
1. Introduction
With more than 241 million cases and 4.91 million deaths worldwide, coronavirus-2019 (COVID-19) has been a significant global economic and healthcare burden [1]. Several complications have been noted in patients of COVID-19. Vasculitis is the inflammation of blood vessels. It is triggered by autoimmune disorders, infections, and trauma [2]. There are different types of vasculitis, but leucocytoclastic (LCV), IgA, and Kawasaki disease like vasculitis are more commonly associated with COVID-19 patients.
IgA vasculitis (IgAV) is a systemic, immune complex-mediated, small-vessel vasculitis characterized by nonthrombocytopenic palpable purpura, arthritis, and abdominal pain. It typically occurs in children, although adults can also be a target of it. IgA vasculitis usually resolves spontaneously. Thus, only supportive treatment is advised [3]. Leukocytoclastic vasculitis, much like IgAV, is a small vessel vasculitis marked by immune complex-mediated damage. It appears as erythematous macules with palpable purpura appearing bilaterally on lower extremities and buttocks. However, unlike IgAV, it typically affects adults, although other groups are also at risk [4].
On the other hand, Kawasaki disease is a predominantly medium-vessel vasculitis that mainly affects children aged five years or younger. Patients typically present with fever, rash, and swollen hands and feet. If left untreated, it may cause heart complications and even death. Although reportedly globally, it has the highest incidence in Japan [5].
Medications commonly used to treat vasculitis include corticosteroids, immunosuppressive drugs, monoclonal antibodies, anticoagulants, anti-platelet agents, and immunoglobulin therapy. The choice of medications mainly depends on the type of vasculitis and the organ that is affected. As there is a rise in cases of COVID-19-associated vasculitis and vasculopathy, an effective treatment plan is yet to be discovered.
For over two years, varying complications of COVID-19 have been reported, and comprehensive systematic reviews of cases have come forth as a result [6,7]. However, the topic of vasculitis secondary to COVID-19 has been relatively untouched. To the best of our knowledge, this is the first systematic review of case reports and case series on the occurrence of vasculitis in COVID-19 patients. This article reviewed the relationship between COVID-19 and vasculitis, collated evidence, and summarized available literature.
2. Methodology
2.1. Literature review
Our work was conducted in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses(PRISMA) checklist (Supplementary file S1) [8]. We searched PubMed and Google scholar from December 1, 2019, to October 11, 2021, for studies published in English. Search terms were combined using appropriate Boolean operators. It included subject heading terms/keywords relevant to COVID-19 (e.g., SARS-CoV-2 OR Coronavirus Disease 2019 OR COVID-19 OR severe acute respiratory syndrome coronavirus 2 OR coronavirus infection) and vasculitis (e.g., Vasculitis OR Vasculitides OR Angiitis). The references of selected studies were also checked to ensure completeness of the search. Please see Supplementary File S1 for a sample search strategy. Furthermore, our study was registered in International prospective register of systematic reviews (PROSPERO) and holds the unique identifying number (UIN); CRD42021296389 [9].
2.2. Inclusion and exclusion criteria
Only case reports, case series, correspondence articles, and editorials that reported individual-level data on the emergence of vasculitis among COVID-19 positive patients were considered for this review. Studies were excluded if they did not present original empirical data on the clinical manifestation of the condition or reported aggregate-level data. The title, abstract, and full-text screening were completed in duplicate and independently by two reviewers (MUFAS and MK). Disagreements regarding the inclusion of studies for data extraction were resolved by the senior author (IU).
2.3. Data extraction
Duplicate studies were removed, whereafter data was extracted in case abstraction form on excel sheets. We collected data on age, gender, physical findings, type of vasculitis, treatment regimen, and treatment outcomes.
2.4. Quality assessment
We used the Joanna Briggs institute's critical appraisal tools to assess the quality of included papers [10,11]. Selected studies were examined for inclusion criteria, sample size, description of study participants and setting. Two reviewers independently assessed the methodological quality of each paper. Quality assessments were done with different tools based on different study designs. Each tool was modified to provide a numeric score. Tools had eight items for case reports and ten for case series. Included case reports (n = 7) had a mean score of 6.71 ± 0.45 with scores ranging from 6 to 7 [[12], [13], [14], [15], [16], [17], [18]]. Meanwhile, the case series by Akca et al. scored seven on a scale of 1–10 [19]. The detailed results of the quality assessment are provided in Supplementary file S1. The quality of our systematic review was assessed using AMSTAR 2 criteria [20]. The level of compliance with AMSTAR 2 came out to be “low”. As only case reports were included in the analyses and the number of studies were less, we couldn't conduct a meta-analysis.
3. Statistical analysis
We reported descriptive data of COVID-19 positive patients presenting with vasculitis. The data was presented in a table describing the symptoms, treatment, and subsequent prognosis among nine individuals who suffered from the condition(Table 1). The median and interquartile range (IQR) of the continuous variable (age) was calculated.
Table 1.
Patient information gathered from literature review of articles on individuals with vasculitis secondary to COVID-19. (y/o = years old, m/o = months old, IV = intravenous, LMWH = Low molecular weight heparin, COVID-19 = Coronavirus disease 2019, VV-ECMO = veno-venous extracorporeal membrane oxygenation, IVIG = Intravenous immunoglobulin).
| Author | Age and sex | Physical findings | Treatment | Diagnosis | Prognosis |
|---|---|---|---|---|---|
| Allez et al. [12] | 24 y/o male | Skin rash, intense asymmetric arthralgia, periarticular swelling, and abdominal pain | LMWH, IV methylprednisolone (0.8 mg. kg) | COVID-19 associated IgA vasculitis | Discharged on day 7 under oral steroids and enoxaparin |
| Jones et al. [13] | 6 m/o female | Erythematous, nonpruritic blotchy rash | Single-dose of 2 g/kg IVIG and high dose acetylsalicylic acid (20 mg/kg 4 times daily) | COVID-19 and Kawasaki Disease | Discharged on low-dose acetylsalicylic acid (3mg/kg daily) |
| Akca et al. [19] | 7 y/o male | Diffuse erythematous maculopapular rash, erosive hyperemia of the oral mucosa | IVIG, azithromycin, hydroxychloroquine, ritonavir and lopinavir, tocilizumab, and mesenchymal stem cell treatments | Kawasaki-like disease and COVID-19 | Died from severe hypoxia on the 17th day of VV-ECMO |
| Akca et al. [19] | 10 y/o female | one-sided submandibular lymphadenopathy size of 2×1.5 cm, maculopapular erythema around the neck | IVIG, anakinra, corticosteroid therapies (20 mg/kg) | Kawasaki-like disease and COVID-19 | Discharged on day 7 |
| Sokolovsky et al. [14] | 36 y/o female | Diffuse rash and arthralgias | Aspirin 650 mg, IVIG 2 g/kg, methylprednisolone 2 mg/kg | Kawasaki-like disease and COVID-19 | Discharged home |
| Gómez et al. [15] | 29 y/o male | Purple palpable papules | Corticosteroids (0.5 mg/kg/day) | Leucocytoclastic vasculitis and COVID-19 | Skin lesions disappeared entirely after 15 days, and the patient remained asymptomatic after 3 weeks of follow-up |
| Hoskins et al. [16] | 2 y/o male | Nonblanching, violaceous rash | LMWH, steroid | COVID-19 and IgA Vasculitis | Discharged home on a 4-week steroid taper, low-dose aspirin |
| Mayor- Ibarguren et al. [17] | 83 y/o female | Purple palpable papules and sero-haematic blisters | Prednisone 30mg/day | Leukocytoclastic vasculitis secondary to COVID-19 infection | Clinical improvement after 10 days |
| Dominguez- Santas et al. [18] | 71 y/o female | Pruritic macules and papules | Betamethasone dipropionate 0.05% cream twice daily. | Leukocytoclastic vasculitis secondary to COVID-19 infection | Lesions healed in the third week of follow up |
4. Results
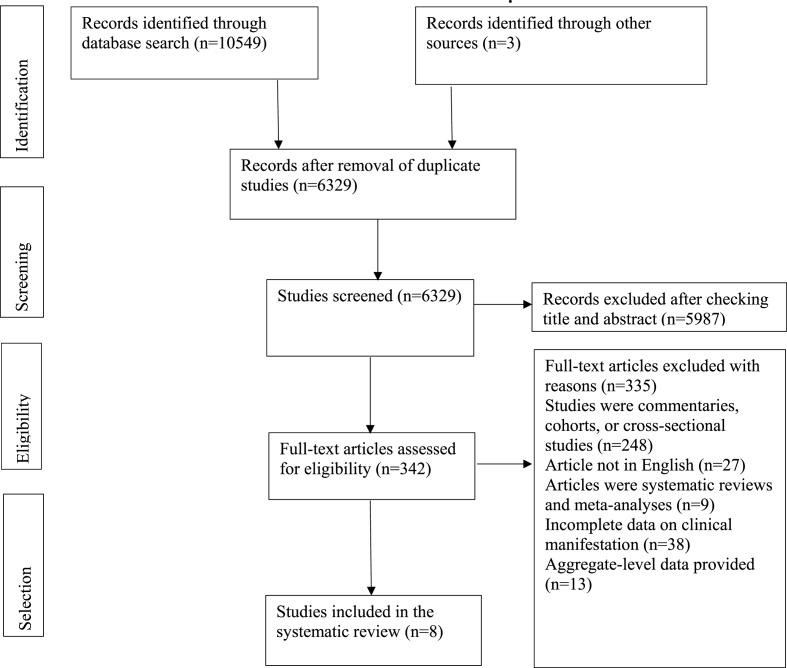
Our initial search yielded 10,552 results (Fig. 1). After removing duplicate studies (4223), titles and abstracts of the remaining studies were checked (6329). After excluding articles through title and abstract search (5987), full-text versions of the remaining articles were read (342). Studies were excluded if they were not in English (n = 27), reported aggregate-level data (n = 13), reported insufficient information on clinical events (n = 38), or were not of the desired study type (e.g., case report and case series) (n = 257). Finally, a total of 8 articles, comprising of 9 patients, met our inclusion criteria and were added to our qualitative analysis (Table 1).
Fig. 1.
Flow chart of study selected for systematic review.
Table 1 shows the information of 9 patients who developed vasculitis secondary to COVID-19. The median age of patients was 24 (49) years, ranging from 6 months to 83 years. The male to female ratio of patients was 4:5. Maculopapular, violaceous, papular, and erythematous rash were common presenting complaints. Other minor findings include arthralgia (n = 2), periarticular swelling (n = 1), lymphadenopathy (n = 1) and abdominal pain (n = 1). Although children did not complain of arthralgia, two adults presented with it. Common treatment regimen for patients with vasculitis secondary to COVID-19 included heparin(n = 2), corticosteroid therapy (n = 6) (methylprednisolone) and intravenous immunoglobulin (n = 4). Although a 7-year-old died of hypoxia, an overall good prognosis was observed (88.89%). Significant clinical improvement was observed in 8 out of 9 patients. 2 patients each were prescribed steroids and aspirin post-discharge.
5. Discussion
5.1. Pathophysiology
Endothelial inflammation, apoptosis, and dysfunction occur in patients with COVID-19 [21]. Endothelial cells are triggered by infection, oxidative stress, hypoxia, and environmental toxins. The external signals and intracellular mediators involved in this inflammatory property include anti-inflammatory cytokines, Transforming Growth Factor Beta (TGFβ), Interleukin 10 (IL-10), Interleukin 1 (IL-1) receptor agonist, and High Density Lipoprotein Cholesterol (HDL-C) [22]. Moreover, the widescale expression of Angiotensin Converting Enzyme 2 (ACE2) receptors within endothelial cells raises a question of its vulnerability to Severe Acute Respiratory Syndrome Coronavirus Disease 2 (SARS-CoV-2) binding, membrane fusion, and viral entry, causing infection, vascular injury, and dysfunction [21]. Accumulation of inflammatory cells and viral inclusions were identified within the endothelium. In autopsy and surgical tissue specimens, diffuse lymphocytic endotheliitis and apoptotic bodies were observed [23]. Thus, endotheliitis and endothelial cell injury can result in vasculitis amongst COVID-19 patients.
Kawasaki disease (KD) is an acute inflammatory disease characterized by medium-sized vasculitis with a predilection for coronary arteries, predominantly affecting children <5 years of age [24]. The leading theory for the pathogenesis of KD is that an unknown infectious agent leads to activation of the immune system in a genetically susceptible child, which can be supported by the apparent seasonality of KD [25]. It begins with neutrophil infiltration of the arterial wall, destroying the vascular wall and causing aneurysms. This phase is followed by lymphocytic infiltration with CD8+T cells, plasma cells, and monocytes, releasing pro-inflammatory cytokines Interleukin 1 Beta (IL-1β) and Tumor nNcrosis Factor Alpha (TNFα), which may continue for months to years in a few patients (chronic arteritis) [26,27]. As SARS-CoV-2 infection can accumulate inflammatory cells within the endothelium, endothelial inflammation, and dysfunction, it can trigger the development of KD. The systemic inflammatory response to pneumonia may potentiate the inflammatory response within coronary lesions, rendering endothelial dysfunction [23], resulting in the development of KD.
IgAV, formerly known as Henoch–Schönlein purpura (HSP), is a type of small-vessel vasculitis that is mediated by immune-complexes deposits containing IgA and is the most common form of systemic vasculitis in children [28]. The current etiopathogenetic hypothesis for IgAV assumes an abnormal immune response to various antigens in genetically susceptible individuals [29]. However, the exact etiology remains unknown. IgAV is usually preceded by upper respiratory tract infections, medications, vaccinations, or malignancies [30]. Thus, an upper respiratory tract infection by SARS-CoV-2 could be a triggering factor in the emergence of IgA vasculitis. It has been hypothesized that mucosal infections lead to upregulation of IL-6, which could lead to the development of Galactose Deficient IgA1 (Gd-IgA1) by altering glycosylation [31]. This variant of IgA1 antigenically recognizes structures of some microorganisms, anti-glycan IgA1 or Immunoglobulin G (IgG) antibodies, and forms circulating complexes, the deposition of these complexes can result in IgA vasculitis and nephritis. The cytokine storm and drugs are given during SARS-COV-2 infection could also be associated with COVID-19, resulting in IgA vasculitis.
Leukocytoclastic vasculitis (LCV) is a small vessel vasculitis characterized by immune complex-mediated inflammation of dermal capillaries and venules [4]. Although LCV is idiopathic in 50% of cases, it can also occur secondary to systemic autoimmune diseases, malignancies, drugs, and chronic infections. These factors induce immune complex deposition and complement-mediated damage within small vessels. Neutrophil chemotaxis followed by secondary exudation of fibrin, erythrocytes, and serum, injuring the vessel wall and causing fibrinoid necrosis. Elevated levels of serum IL-1, IL6, and IL-8 and tumor necrosis factors can also be detected [4]. Additionally, COVID-19 causes a cytokine storm linked with a rise in IL6 levels [16]. This rise in IL-6 levels and immune complex-mediated damage can result in inflammation of small vessels causing LSV.
5.2. Management
The treatment for COVID-19 induced vasculitis should be curated according to the severity of vasculitis, type of vasculitis, and patient demographics. The disease self resolves in most cases, however, based on the severity of skin-limited IgA vasculitis (sparing GI and renal involvement) a symptomatic treatment plan should be devised. Analgesics like acetaminophen should be prescribed for joint pain and muscle pain. Compression therapy and antihistamines should be prescribed for cutaneous variant of IgA vasculitis. This could reduce the formation of new lesions by inhibiting vascular dilatation [32]. Antihistamines work by inhibiting vasodilation, thereby, reducing blood supply. This prevents entry of immunoglobin to the affected area. Steroids should be given if there is a chance of incipient necrosis, which otherwise would slow down the healing process [32].
In KD, treatment comprises of high dose IVIG and aspirin [5]. Combinational therapy of IVIG, TNF inhibitors, steroids, calcineurin inhibitors, or anakinra, might be helpful to treat patients with IVIG-resistant disease [5].
LCV is mild and resolves with supportive measures like leg elevation, rest, compression stockings, and antihistamines. A 4–6 week tapering dose of corticosteroids can be used in more chronic or resistant cases. Rarely, immunosuppressive steroid-sparing agents such as methotrexate, azathioprine, mycophenolate mofetil, dapsone, cyclophosphamide, and intravenous immunoglobulin may be needed [4].
6. Limitations
This article has some limitations. For example, not all case reports included the patient's past medical history and risk factors for COVID-19 associated vasculitis, which may contribute to the new onset of rash. Some of the vasculitis cases are in asymptomatic COVID-19 patients, which raises the question about the correlation between COVID-19 and vasculitis. More cases were reported in children and adolescents, so more investigations are needed in diverse populations.
7. Conclusion
COVID-19 associated vasculitis is rare and lacks a clear treatment protocol. Steroid have shown clinical benefits in case reports. More studies are needed to establish a definitive treatment protocol for COVID-19 associated vasculitis.
Ethical approval
Since this is a review article no ethical approval was needed.
Sources of funding
The authors have no funding source to declare.
Author contribution
Kalai Wong: study concept, study design, data collection, data analysis, data interpretation, writing the paper. Mir Umer Farooq Alam Shah: study concept, data collection, data analysis, writing the paper. Maman Khurshid: study concept, data collection, data analysis, writing the paper. Irfan Ullah: study concept, data analysis, writing the paper. Muhammad Junaid Tahir: study concept, data collection, data analysis, writing. Zohaib Yousaf: study concept, data analysis, writing.
Consent
Not applicable since this is a review article.
Registration of research studies
-
1.
Name of the registry: PROSPERO
-
2.
Unique Identifying number or registration ID: CRD42021296389
-
3.
Hyperlink to your specific registration (must be publicly accessible and will be checked): https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021296389
Guarantor
Kalai Wong, Maman Khurshid, Irfan Ullah.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors have no conflict of interests to declare.
Acknowledgements
The authors have no acknowledgements to disclose.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103249.
Contributor Information
Kalai Wong, Email: kalaika@live.unc.edu.
Mir Umer Farooq Alam Shah, Email: umermir137@gmail.com.
Maman Khurshid, Email: mamankhurshid00@gmail.com.
Irfan Ullah, Email: irfanullahecp2@gmail.com.
Muhammad Junaid Tahir, Email: junaid262626@gmail.com.
Zohaib Yousaf, Email: yousaf@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.WHO Coronavirus (COVID-19) Dashboard. World Health Organization; 2021. https://covid19.who.int/ [Internet] [cited 2021 Oct 21]. Available from: [Google Scholar]
- 2.Vasculitis. National heart, lung, and blood institute. October 21,2019. https://www.nhlbi.nih.gov/health-topics/vasculitis Available from.
- 3.Reamy B.V., Servey J.T., Williams P.M. Henoch-Schönlein purpura (IgA vasculitis): rapid evidence review. Am. Fam. Physician. 2020 August 15;102(4):229–233. [PubMed] [Google Scholar]
- 4.Baigrie D., Bansal P., Goyal A., Crane J.S. Vol. 35. StatPearls Publishing; Treasure Island: 2021. https://www.ncbi.nlm.nih.gov/books/NBK482159/ (Leukocytoclastic Vasculitis). [Internet] [cited 2021 Oct 22]. 251–267 pp. Available from: [Google Scholar]
- 5.Kawasaki Disease. Centers for disease control and prevention. June 4, 2020. https://www.cdc.gov/kawasaki/index.html Available from:
- 6.Ullah I., Sohail A., Shah M.U.F.A., Khurshid M., Diwan M.N., Qadir A., et al. Central Retinal Vein Occlusion in patients with COVID-19 infection: a systematic review. Ann. Med. Surg. 2021 Oct doi: 10.1016/j.amsu.2021.102898. [Internet] [cited 2021 Oct 22];102898. Available from:/pmc/articles/PMC8500694/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dilek A., Ozaras R., Ozkaya S., Sunbul M., Sen E.I., Leblebicioglu H. COVID-19-associated mucormycosis: case report and systematic review. Travel. Med. Infect Dis. 2021 Aug 26 doi: 10.1016/j.tmaid.2021.102148. [Internet] [cited 2021 Oct 22];44:102148. Available from:/pmc/articles/PMC8387131/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int. J. Surg. 2021;88:105906. doi: 10.1016/j.ijsu.2021.105906. [DOI] [PubMed] [Google Scholar]
- 9.Prospero. https://www.crd.york.ac.uk/prospero/#myprospero [Internet] [cited 2021 Dec 31]. Available from:
- 10.Moola S., Munn Z., Tufanaru C., Aromataris E., Sears K., Sfetcu R., et al. In: JBI Manual for Evidence Synthesis. Aromataris E., Munn Z., editors. JBI; 2020. Chapter 7: systematic reviews of etiology and risk.https://synthesismanual.jbi.global Available from. [Google Scholar]
- 11.Munn Z, Barker T, Moola S, Tufanaru C, Stern C, McArthur A, et al. Methodological quality of case series studies, JBI Evidence Synthesis, doi: 10.11124/JBISRIR-D-19-00099. [DOI] [PubMed]
- 12.Allez M., Denis B., Bouaziz J., Battistella M., Zagdanski A., Bayart J., et al. Arthritis Rheumatol; Hoboken, N.j: 2020 Nov 1. Covid‐19 Related IgA Vasculitis.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7361577/ [Internet] [cited 2021 Oct 22];72(11):1952–3. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones V.G., Mills M., Suarez D., Hogan C.A., Yeh D., Segal J.B., et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp. Pediatr. 2020 Jun 1 doi: 10.1542/hpeds.2020-0123. https://hosppeds.aappublications.org/content/10/6/537 [Internet] [cited 2021 Oct 22];10(6):537–40. Available from: [DOI] [PubMed] [Google Scholar]
- 14.Sokolovsky S., Soni P., Hoffman T., Kahn P., Scheers-Masters J. COVID-19 associated Kawasaki-like multisystem inflammatory disease in an adult. Am. J. Emerg. Med. 2021 Jan 1 doi: 10.1016/j.ajem.2020.06.053. [Internet] [cited 2021 Oct 22];39:253.e1-253.e2. Available from:/pmc/articles/PMC7315983/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gómez M.C., González-Cruz C., Ferrer B., Barberá M.J. Leucocytoclastic vasculitis in a patient with COVID-19 with positive SARS-CoV-2 PCR in skin biopsy. BMJ Case Rep. 2020 Oct 29 doi: 10.1136/bcr-2020-238039. [Internet] [cited 2021 Oct 22];13(10):e238039. Available from:/pmc/articles/PMC7597471/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoskins B., Keeven N., Dang M., Keller E., Nagpal R. A child with covid-19 and immunoglobulin a vasculitis. Pediatr. Ann. 2020;50(1):e44–e48. doi: 10.3928/19382359-20201211-01. [DOI] [PubMed] [Google Scholar]
- 17.Mayor‐Ibarguren A., Feito‐Rodriguez M., Castanedo L.Q., Ruiz‐Bravo E., Vega D.M., Herranz‐Pinto P. Cutaneous small-vessel vasculitis secondary to COVID‐19 infection: a case report. J. Eur. Acad. Dermatol. Venereol. 2020 Jul 3 doi: 10.1111/jdv.16670. [Internet] [cited 2021 Oct 22];34(10):e541–2. Available from:/pmc/articles/PMC7280661/?report=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominguez‐Santas M., Diaz‐Guimaraens B., Abellas P.G., Real CM del, Burgos‐Blasco P., Suarez‐Valle A. Cutaneous small‐vessel vasculitis associated with novel 2019 coronavirus SARS‐CoV‐2 infection (COVID‐19) J. Eur. Acad. Dermatol. Venereol. 2020 Oct doi: 10.1111/jdv.16663. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7283648/ [Internet] [cited 2021 Oct 22];34(10):e536–7. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akca U.K., Kesici S., Ozsurekci Y., Aykan H.H., Batu E.D., Atalay E., et al. Kawasaki-like disease in children with COVID-19. Rheumatol. Int. 2020 Dec doi: 10.1007/s00296-020-04701-6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7492688/ [Internet] [cited 2021 Oct 22]; 40(12):1–11. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., Moher D., Tugwell P., Welch V., Kristjansson E., Henry D.A. Amstar 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017 Sep 21;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker R.C. COVID-19 update: covid-19-associated coagulopathy. J. Thromb. Thrombolysis. 2020 Jul;50(1):54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Becker R.C. COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis. 2020;50(3):499–511. doi: 10.1007/s11239-020-02230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Lancet; London, England): 2020 May 2. Endothelial Cell Infection and Endotheliitis in COVID-19.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7172722/ [Internet] [cited 2021 Oct 22];395(10234):1417–8. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gkoutzourelas A., Bogdanos D.P., Sakkas L.I. Kawasaki disease and COVID-19. Mediterr J. Rheumatol. 2020 doi: 10.31138/mjr.31.3.268. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7656130/ [Internet] [cited 2021 October 22];31(Suppl 2):268–74. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rife E., Gedalia A. Kawasaki disease: an update. Curr. Rheumatol. Rep. 2020 Sep 13 doi: 10.1007/s11926-020-00941-4. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7487199/ [Internet] [cited 2021 Oct 22];22(10):75. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rivas M.N., Arditi M. Kawasaki disease: pathophysiology and insights from mouse models. Nat. Rev. Rheumatol. 2020 May 26 doi: 10.1038/s41584-020-0426-0. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7250272/ [Internet] [cited 2021 Oct 22];16(7):391–405. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Orenstein J.M., Shulman S.T., Fox L.M., Baker S.C., Takahashi M., Bhatti T.R., et al. Three linked vasculopathic processes characterize Kawasaki disease: a light and transmission electron microscopic study. PLoS One. 2012 Jun 18 doi: 10.1371/journal.pone.0038998. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3377625/ [Internet] [cited 2021 Oct 22];7(6):e38998. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardner-Medwin J.M., Dolezalova P., Cummins C., Southwood T.R. Incidence of Henoch-Schonlein purpura, Kawasaki disease, and rare vasculitides in children of different ethnic origins. Lancet. 2002 Oct 19 doi: 10.1016/S0140-6736(02)11279-7. http://www.thelancet.com/article/S0140673602112797/fulltext [Internet] [cited 2021 Oct 22];360(9341):1197–202. Available from: [DOI] [PubMed] [Google Scholar]
- 29.Piram M., Maldini C., Biscardi S., De Suremain N., Orzechowski C., George E., et al. Incidence of IgA vasculitis in children estimated by four-source capture-recapture analysis: a population-based study. Rheumatology. 2017 Aug doi: 10.1093/rheumatology/kex158. https://academic.oup.com/rheumatology/article/56/8/1358/3749645 [Internet] [cited 2021 October 22];56(8):1358–66. Available from: [DOI] [PubMed] [Google Scholar]
- 30.Hetland L.E., Susrud K.S., Lindahl K.H., Bygum A. Henoch-schönlein purpura: a literature review. Acta Derm. Venereol. 2017 Nov 15 doi: 10.2340/00015555-2733. https://www.medicaljournals.se/acta/content/abstract/10.2340/00015555-2733 [Internet] [cited 2021 Oct 22];97(10):1160–6. Available from: [DOI] [PubMed] [Google Scholar]
- 31.Heineke M.H., Ballering A.V., Jamin A., Ben Mkaddem S., Monteiro R.C., Van Egmond M. New insights in the pathogenesis of immunoglobulin A vasculitis (Henoch-Schönlein purpura) Autoimmun. Rev. 2017 Dec doi: 10.1016/j.autrev.2017.10.009. [Internet] [cited 2021 Oct 22];16(12):1246–53. Available from: [DOI] [PubMed] [Google Scholar]
- 32.Pillebout E., Sunderkötter C. IgA vasculitis. Semin immunopathol. 2021 Jun 25. [Internet] [cited 2021 Oct 22]; Available from. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.