ABSTRACT
Background
Numerous studies demonstrate acute anti-inflammatory properties of individual spices, but none have examined the effect of longer-term consumption of a spice blend incorporated in a meal.
Objectives
We investigated the effect of longer-term spice consumption on inflammatory cytokines and monocyte subsets [classical (CM), intermediate (IM), nonclassical (NCM)] in adults at risk of cardiometabolic disease.
Methods
A 3-period, randomized, crossover, controlled feeding trial was conducted. Participants (n = 71 recruited; n = 63 completed) randomly consumed diets differing in terms of the quantity of spices: 0.547 g (low-dose spice diet; LSD), 3.285 g (medium-dose spice diet; MSD), or 6.571 g (high-dose spice diet; HSD) · d−1 · 2100 kcal−1, for 4 wk with a ≥2-wk washout between diets. At baseline and after each diet period, proinflammatory cytokines (IL-1β, IL-6, IL-8, monocyte chemoattractant protein-1, and TNF-α) in plasma and LPS-stimulated peripheral blood mononuclear cell culture supernatants, and the phenotype and function of monocyte subsets, were measured in fasted participants. Postprandial proinflammatory cytokines also were quantified at baseline by consumption of a low-spice-dose test meal, and after each diet period by consumption of a test meal containing a spice dose corresponding to daily spice consumption during the preceding 4-wk diet period.
Results
Fasting plasma IL-6 was reduced (mean ± SEM: −118.26 ± 50.63 fg/mL; P < 0.05) after MSD compared with baseline. Postprandial plasma IL-1β, IL-8, and TNF-α were lower (mean ± SEM : −9.47 ± 2.70 fg/mL, −0.20 ± 0.05 pg/mL, and −33.28 ± 12.35 fg/mL, respectively) after MSD compared with LSD (main diet effect; P < 0.05). CM adherence was reduced (mean ± SEM: −0.86 ± 0.34; P = 0.034) after HSD compared with LSD. IM migration was reduced after MSD and HSD compared with LSD (mean ± SEM: −0.39 ± 0.09 and −0.56 ± 0.14, respectively; P < 0.05).
Conclusions
Four weeks of MSD consumption reduced fasting plasma IL-6 and postprandial plasma IL-1β, IL-8, and TNF-α as well as altering monocyte function.
This trial was registered at clinicaltrials.gov as NCT03064932.
Keywords: inflammatory cytokines, monocytes, nutritional intervention, cardiovascular disease, randomized controlled trial
Introduction
The prevalence of obesity has tripled since 1960 (1), affecting 42.4% of adults in the United States in 2018 (2). Obesity is associated with chronic low-grade inflammation (3) and increases the risk of cardiovascular disease (CVD) and atherosclerosis (4). Atherosclerosis is the dominant cause of CVD, and monocytes and macrophages play a crucial role in the initiation and progression of atherosclerosis (5). Monocytes invade the arterial wall, differentiate into macrophages, and engulf oxidized LDL, ultimately forming foam cells that contribute to atherosclerotic plaque formation (5). Monocytes also produce a myriad of inflammatory mediators that contribute to monocyte adherence and invasion into the intima of the arterial wall (6).
Three subsets of monocytes are identified in humans by differential surface expression of CD14 (LPS receptor) and CD16 (low-affinity Fc receptor III for IgG): classical (CD14++CD16−; CMs), intermediate (CD14++CD16+; IMs), and nonclassical (CD14+CD16++; NCMs) monocytes (7). Each monocyte subset exhibits distinct properties that may confer different roles during the inflammatory response (8). CMs express high levels of C-C chemokine receptor type 2 (CCR2) and present the greatest capacity for trans-endothelial migration (9, 10). IMs have robust proinflammatory responses and may be attracted to atherosclerotic lesions in a CCR5-dependent manner (11, 12). NCMs patrol the vascular endothelium and can detect damaged cells (13). Our recent meta-analysis highlighted the importance of each monocyte subset and their association with cardiometabolic disorders and CVD (14). In addition, all monocyte subsets can accumulate intracellular lipids to become foamy monocytes (14). Foamy monocytes may have enhanced capacity to infiltrate into early atherosclerotic lesions in a CD11c-dependent manner (15), suggesting that foamy monocytes are an additional indicator of atherosclerotic risk. A better understanding of the phenotype and function of monocyte subsets may have important clinical implications for the prevention and treatment of atherosclerosis.
Spices can confer numerous health benefits including a reduction in inflammatory mediators (16), which may be mediated by bioactive compounds (17). Preclinical and clinical studies have demonstrated anti-inflammatory effects of individual spices, such as cinnamon (18, 19), cumin (20), and ginger (21, 22). We previously demonstrated a postprandial anti-inflammatory effect of consuming a spice blend (6 g) delivered in a single high-saturated-fat, high-carbohydrate meal in men with overweight/obesity (n = 12) (16). However, no studies to date have examined the effect of longer-term consumption of a spice blend on inflammation. Therefore, this study was designed to 1) investigate the effect of 4 wk of spice consumption, using 3 doses of a spice blend in an average American diet, on fasting proinflammatory cytokine concentrations and the phenotype and function of monocyte subsets; and 2) evaluate the effect of 4 wk of spice consumption on postprandial inflammatory responses, in adults with overweight or obesity at risk of CVD.
Methods
Participants
We recruited nonsmoking adults (30–75 y) with overweight or obesity [BMI (in kg/m2): 25–35], elevated waist circumference (≥94 cm for men and ≥80 cm for women), and ≥1 of the following risk factors for CVD: altered lipid profile (LDL cholesterol > 130 mg/dL, triglyceride ≥ 150 mg/dL, or HDL cholesterol < 40 mg/dL for men and < 50 mg/dL for women), elevated C-reactive protein (CRP) (>1 mg/L), elevated blood pressure (systolic ≥ 130 mm Hg or diastolic ≥ 85 mm Hg), or elevated fasting glucose (≥100 mg/dL). Exclusion criteria included having diabetes (fasting glucose > 126 mg/dL) or hypertension (systolic blood pressure > 160 mm Hg or diastolic blood pressure > 100 mm Hg); taking any antihypertensive or glucose-lowering drugs; having established CVD, stroke, diabetes, liver, kidney, or autoimmune disease; using cholesterol/lipid-lowering medication or supplements (psyllium, fish oil, soy lecithin, and phytoestrogens) and botanicals; pregnancy or lactation; and having weight loss of ≥10% of body weight within 6 mo before enrolling in the study. Participants who self-reported as vegetarians were excluded because the menus reflected an average American diet, which contains meat, poultry, fish, and dairy.
Recruitment, screening, and random assignment
Participants were recruited between January 2017 and September 2019 through StudyFinder (https://studyfinder.psu.edu/) and advertisements placed in campus buildings, facilities on campus and in surrounding areas (gyms, churches, supermarkets, coffee shops, etc.), local newspapers and radio stations, and coupon packets sent by mail. Individuals who were on the university email listserv and who participated in previous studies were contacted. Potentially eligible participants were scheduled for a screening visit at the Clinical Research Center on the Pennsylvania State University, University Park campus. Participants fasted for 12 h and abstained from alcohol for 48 h before the screening visit. Height, weight, blood pressure, and waist circumference were measured. Women were asked to provide a urine sample for a pregnancy test. A blood sample was collected and sent to Quest Diagnostics (Pittsburgh, PA) for measurement of liver and kidney function, glucose, lipid/lipoprotein profile, complete blood count, and CRP. After screening, 71 eligible participants were randomly assigned before the baseline visit, using a computer-generated 6-sequence scheme to assign their diet sequence order (www.randomization.com). The randomization code was kept by the kitchen staff preparing the meals and the code was broken at the end of the study.
Study design
The study was a 3-period, randomized, crossover, controlled feeding trial (NCT03064932) that was designed to examine the effect of spice consumption for 4 wk on serum LDL cholesterol as a primary outcome (23). To detect an LDL cholesterol difference of 12.5 ± 25.9 mg/dL (mean ± within-participant SD) with 90% power (α = 0.05), 63 participants were estimated as the necessary sample size using a power calculation for crossover design studies (21, 24–28). We anticipated a dropout rate of 15%, thus we planned to recruit 74 participants. The outcomes in the current study are all secondary outcomes in the trial. In random order, participants consumed an average American diet containing 3 different doses of a spice blend for 4 wk at each dose: a low-dose spice diet (LSD), a medium-dose spice diet (MSD), or a high-dose spice diet (HSD), with a ≥2-wk washout period between each intervention period. There were a total of 4 study visits, including a baseline visit and a study visit after each dietary intervention period (Figure 1). A 7-d rotating meal plan was designed in 300-kcal increments (1800–3900 kcal/d) for weight maintenance (Supplemental Table 1). All food was prepared in the metabolic kitchen at the Pennsylvania State University. Participants were provided with meals daily Monday through Friday and all of their weekend meals were distributed on Friday. Table 1 shows the average daily nutrient profile of the background diet based on 2100 kcal/d. Energy requirements were calculated using the Harris–Benedict equation (29). Each participant's body weight was monitored daily when they picked up their food. The calorie amounts were adjusted so that the participants maintained their body weight throughout the duration of the study. Table 2 shows the mean composition of the spice blend in the LSD, MSD, and HSD over the 7-d menu based on 2100 kcal/d. Based on the different daily calorie intakes, the spice intake ranged from 0.450 to 1.025 g/d, 2.715 to 5.785 g/d, and 5.430 to 11.571 g/d for the LSD, MSD, and HSD, respectively (Supplemental Table 2). Compliance was monitored by the metabolic kitchen managers using daily checklists to determine if/how much food was uneaten. Participants were allowed to consume noncaloric beverages ad libitum; however, caffeinated beverages and alcoholic drinks were limited to 5 cups/d (<1184 mL/d) and 2 drinks/wk, respectively. The spices were chosen based on previous studies that reported benefits on CVD and inflammatory outcomes (24, 27, 30, 31), and are among the most widely consumed spices in the United States (32, 33). Thus, the blend of spices used could be feasibly consumed in the average American diet. The dosages of spices were chosen to include dosages below, near, and above the average daily spice consumption per capita in the United States in 2015 (mean: 4.5 g/d) (33, 34). The meal plan contained the same foods for all test diets and only varied in the quantity of spices provided; thus, the micronutrient and macronutrient profiles of all test diets were identical.
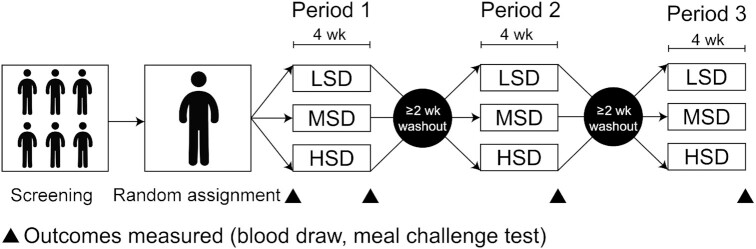
FIGURE 1.
Study design used to examine the effect of consuming an LSD, MSD, and HSD for 4 wk in adults at risk of cardiometabolic disease. HSD, high-dose spice diet; LSD, low-dose spice diet; MSD, medium-dose spice diet.
TABLE 1.
The nutrient profile of the background study diet1
| Nutrient profile | Values |
|---|---|
| Carbohydrate2 | 50 |
| Protein2 | 17 |
| Total fat2 | 33 |
| Saturated fat2 | 11 |
| Monounsaturated fat2 | 11 |
| Polyunsaturated fat2 | 8 |
| Fiber, g/d | 22 |
| Sodium, mg/d | 3023 |
Based on the 2100-kcal/d diet.
Percentage of total calories.
TABLE 2.
Mean composition of the spice blend over the 7-d menu based on 2100 kcal/d1
| Spices, g/d (% of total dose) | LSD | MSD | HSD |
|---|---|---|---|
| Cinnamon | 0.099 (18.55) | 0.595 (18.51) | 1.190 (18.51) |
| Coriander | 0.069 (12.98) | 0.417 (12.96) | 0.833 (12.96) |
| Ginger | 0.055 (10.23) | 0.328 (10.21) | 0.656 (10.21) |
| Cumin | 0.045 (8.42) | 0.270 (8.40) | 0.540 (8.40) |
| Parsley | 0.041 (7.72) | 0.238 (7.42) | 0.477 (7.42) |
| Black pepper | 0.039 (7.36) | 0.239 (7.45) | 0.479 (7.45) |
| Garlic | 0.028 (5.25) | 0.174 (5.42) | 0.348 (5.42) |
| Turmeric | 0.026 (4.88) | 0.156 (4.87) | 0.313 (4.87) |
| Onion powder | 0.026 (4.85) | 0.156 (4.85) | 0.311 (4.85) |
| Paprika | 0.020 (3.80) | 0.122 (3.79) | 0.244 (3.79) |
| Chili powder | 0.014 (2.67) | 0.086 (2.67) | 0.171 (2.67) |
| Rosemary | 0.013 (2.41) | 0.080 (2.50) | 0.161 (2.50) |
| Cilantro | 0.013 (2.38) | 0.076 (2.38) | 0.153 (2.38) |
| Oregano | 0.013 (2.35) | 0.075 (2.35) | 0.151 (2.35) |
| Basil | 0.011 (2.13) | 0.068 (2.13) | 0.137 (2.13) |
| Red pepper | 0.009 (1.59) | 0.051 (1.59) | 0.102 (1.59) |
| Thyme | 0.008 (1.50) | 0.050 (1.57) | 0.101 (1.57) |
| Bayleaf | 0.006 (1.21) | 0.040 (1.25) | 0.080 (1.25) |
| Cardamom | 0.004 (0.76) | 0.024 (0.76) | 0.049 (0.76) |
| Sesame seeds | 0.002 (0.44) | 0.014 (0.44) | 0.029 (0.44) |
| Sage | 0.002 (0.33) | 0.011 (0.33) | 0.021 (0.33) |
| Poppy seeds | 0.001 (0.22) | 0.007 (0.22) | 0.014 (0.22) |
| Dill weed | <0.001 (0.08) | 0.003 (0.08) | 0.005 (0.08) |
| Allspice | <0.001 (0.08) | 0.003 (0.08) | 0.005 (0.08) |
| Total | 0.547 | 3.285 | 6.571 |
HSD, high-dose spice diet; LSD, low-dose spice diet; MSD, medium-dose spice diet.
All the experiments in this study were performed with the approval of the Institutional Review Board of the Pennsylvania State University, University Park campus (STUDY00003603).
Meal challenge test
In men and postmenopausal women, a meal challenge test was conducted at baseline and at the end of each 4-wk dietary intervention period (Figure 1) to evaluate the effect of 4 wk of spice consumption on postprandial proinflammatory cytokine concentrations in plasma. Premenopausal women were excluded from the meal challenge test in order to exclude the possible effect of estrogen on inflammatory processes (35). The participants consumed a ∼1200-kcal standardized test meal containing ∼33% kcal from fat and 49% kcal from carbohydrate (Supplemental Table 3). The test meal contained either a low (0.613 g), a medium (3.675 g), or a high (7.350 g) dose of a spice blend (cardamom, cinnamon, coriander, cumin, garlic, ginger, paprika, red pepper, turmeric) (Supplemental Table 4). The test meal at baseline was a low-dose spice meal (0.613 g). The dose of the spice blend in the test meal after each intervention period corresponded to the dose of the spice blend in the diet that the participants consumed for the prior 4-wk intervention period (i.e., low-dose spice meal after LSD, medium-dose spice meal after MSD, high-dose spice meal after HSD). The test meal was chicken tikka masala and an apple pie yogurt parfait. At baseline and at the end of each intervention period, a premeal blood sample was collected, and participants were asked to consume the test meal within 15 min. After the meal consumption, blood samples were collected at timed intervals (30, 60, 120, 180, and 240 min).
Blood sample collection
Fasting blood samples were collected in sterile EDTA (K2)-coated blood tubes (BD Biosciences) at baseline and at the end of each intervention period to measure proinflammatory cytokines in plasma and culture supernatants from LPS-stimulated peripheral blood mononuclear cells (PBMCs), and the distribution, phenotype, adherence, and migratory capacity of monocyte subsets. In addition, blood samples were collected before and after the meal challenge test (≤4 h after meal consumption) to evaluate the effect of 4 wk of spice consumption on postprandial proinflammatory cytokine concentrations in plasma. Both fasting and postprandial plasma was dispensed into microcentrifuge tubes and frozen at −80°C until analysis.
Proinflammatory cytokine secretion assay
PBMCs were isolated from blood as previously described (16, 36). PBMCs (2 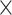 105/mL) were stimulated with 0.625 µg LPS/mL (Sigma-Aldrich) in round-bottomed 96-well plates, and supernatants were harvested after 4 h incubation and frozen at −80°C until analysis.
105/mL) were stimulated with 0.625 µg LPS/mL (Sigma-Aldrich) in round-bottomed 96-well plates, and supernatants were harvested after 4 h incubation and frozen at −80°C until analysis.
Measurement of proinflammatory cytokine concentrations
Cytokines and chemokines [IL-1β, IL-6, IL-8, TNF-α, and monocyte chemoattractant protein (MCP)-1] in plasma and culture supernatants were measured using the V-PLEX Proinflammatory Panel 1 Human Kit and V-PLEX Human MCP-1 kit (Meso Scale Diagnostics) as per manufacturers’ instructions. For the data points below the detection range, half of the lower limit of detection was used as the values in the analyses. High-sensitivity CRP was measured using a Cobas C311 chemistry analyzer (Roche Diagnostics) in the Biomarker Core Laboratory at the Pennsylvania State University.
Monocyte trans-endothelial migration assay
The monocyte trans-endothelial migration assay was performed by following a modified version of a previously described protocol (9, 10). Briefly, human umbilical vein endothelial cells (HUVECs) (ATCC) were grown in endothelial growth medium [vascular cell basal medium (ATCC) and endothelial cell growth medium plus vascular endothelial growth factor (VEGF) (ATCC)], and seeded onto fibronectin-coated transwell migration inserts (Corning Inc.) at a density of 1×105 HUVECs/cm2 membrane area. Migration medium with or without 2.5 ng/mL TNF-α (R&D systems) was added into the dish below the transwell insert. PBMCs in 500 µL of migration medium were placed inside each transwell insert. After 6 h of incubation at 37°C, supernatants containing nontransmigrated PBMCs (floating) were obtained. Transmigrated PBMCs (transmigrated) were obtained from the lower compartment. The PBMCs adhering to the HUVEC monolayer (adherent) were obtained together with the HUVECs by brief trypsinization. Cells from the 3 compartments were stained with fluorescently labeled antibodies for the identification of monocyte subsets. Monocyte adherence or transmigration was represented as an index of adherence or migration, calculated for each monocyte subset by dividing the number of adherent or transmigrated cells in the TNF-α-containing well by the number of adherent or transmigrated cells in the control well. The monocyte trans-endothelial migration was measured in 6 participants (all were women; mean age: 38.5 y; mean BMI: 31.5).
Flow cytometric analysis
PBMCs and whole blood were stained with fluorescence-labeled antibodies as previously described (36, 37). Antibodies included CD3, CD11b, CD11c, CD14, CD16, CD18, CD19, CD45, CD49d, CD56, CD62L, CD66, CD162, CD192, CD195, and human leukocyte antigen-DR isotype (HLA-DR). Antibody isotype controls included mouse IgG1 and mouse IgG2a. Whole blood was stained with Nile red using a Lipid Droplets Fluorescence Assay Kit (Cayman Chemical Company) to measure foamy monocytes, i.e., monocytes containing intracellular lipid droplets. A total of 300,000 events were acquired using a BD LSR-Fortessa (BD Biosciences). Data were analyzed and plotted using FlowJo 10 (FlowJo, LLC). The gating strategies to identity monocyte subsets in whole blood and PBMCs are presented in Supplemental Figures 1 and 2, respectively. The distribution of monocyte subsets and the expression of adhesion molecules and chemokine receptors on monocytes were measured in 23 participants (48% women; mean age: 42.5 y; mean BMI: 28.7). Foamy monocytes and CD11c expression were measured in 12 participants (82% women; mean age: 41.9 y; mean BMI: 31.4).
Statistical analyses
Statistical analyses were performed using SAS version 9.4 (SAS Institute). Q-Q plot assessment was used to confirm the normality of the data. Residual-versus-predicted value plot assessment was used to confirm the equal variance of the data. Data points that were >3SDs from the mean were regarded as outliers and removed. A mixed-models procedure for repeated measures was used to test the effect of treatment (diet) on each outcome. For the outcomes assessed in fasted participants (cytokine concentrations in plasma and PBMC culture supernatants; percentages of CMs, IMs, and NCMs; percentage of foamy monocytes; surface expression of adhesion molecules and chemokine receptors on monocytes; and adherence and migration indexes), change from baseline was calculated by subtracting measurements taken at baseline from post-intervention period values. The baseline values (single measurements at the beginning of the trial) were included in the model as a fixed effect. We evaluated both between- and within-diet differences using change from baseline values. For postprandial cytokine concentrations in plasma, change from the premeal value was calculated by subtracting measurements taken before the ingestion of the test meal from the postprandial values. We evaluated the effects of time, diet, and time-by-diet interaction on the change from the premeal value. For outcomes measured in a fasted and a postprandial state, participant was included as a random effect and sex was included as a fixed effect. To detect a carryover effect, the diet-by-period interaction was included as a fixed effect. When no diet-by-period interaction was detected, diet-by-period was removed from the model. Selection of model covariance structures was based on optimizing fit statistics, which was evaluated as the lowest Bayesian Information Criterion. In cases where a significant between-diet effect was detected, a Tukey–Kramer post hoc test was performed to correct for multiple comparisons. Spearman correlation was used to evaluate the association between the percentage of foamy monocytes and CD11c expression for each monocyte subset. In SAS, the effect of diet on each outcome was assessed using PROC MIXED and the aforementioned correlations were assessed using PROC CORR. Statistical significance was accepted at P < 0.05 for all outcomes. Graphs were plotted using Prism version 7 (GraphPad). Descriptive data are reported as mean ± SD. Values in the figures are reported as mean ± SEM.
Results
Participants and baseline characteristics
Supplemental Figure 3 provides a diagram of the flow of participants through the study. Briefly, 223 participants were assessed for eligibility via phone screening; 113 were screened for eligibility based on biochemical parameters; 71 were randomly assigned; and 63 completed the trial. The dropout rate after random assignment of eligible participants was 11%.
Participants self-reported that they consumed all of the provided foods on 94% of the study days. Diet compliance was comparable across the 3 study periods (93%, period 1; 94%, period 2; 95%, period 3) and on each of the diets (94% for the LSD, MSD, and HSD).
Table 3 shows the baseline characteristics of the study participants who completed the trial. The participants included 28 men and 35 women (age: 45.3 ± 11.2 y) with overweight or obesity (BMI: 29.6 ± 3.0) and increased waist circumference (102.2 ± 7.1 cm). There was a wide range in blood pressure and biochemical measures among participants because only 1 additional risk factor for CVD was required as per the inclusion criteria. Supplemental Table 5 presents the baseline characteristics of the study participants that were included in the meal challenge test.
TABLE 3.
Baseline characteristics of participants who completed the trial1
| Characteristic | Values |
|---|---|
| Age, y | 45.3 ± 11.2 (30–67) |
| Male n / female n | 28/35 |
| BMI, kg/m2 | 29.6 ± 3.0 (25.0–35.7) |
| Waist circumference, cm | 102.2 ± 7.1 (88–117) |
| Blood pressure, mm Hg | |
| Systolic | 130.1 ± 12.8 (106–162) |
| Diastolic | 81.4 ± 10.3 (61–102) |
| Glucose, mg/dL | 99.6 ± 7.3 (80.5–123.5) |
| Insulin, µU/mL | 11.7 ± 6.4 (3.9–35.4) |
| Total cholesterol, mg/dL | 192.9 ± 33.2 (131.5–271.5) |
| HDL cholesterol, mg/dL | 49.4 ± 11.8 (31.3–77.6) |
| LDL cholesterol, mg/dL | 126.2 ± 27.8 (78.4–188.6) |
| Triglyceride, mg/dL | 112.0 ± 50.7 (31.0–284.5) |
| High-sensitivity C-reactive protein, mg/L | 3.0 ± 2.8 (0.3–13.2) |
n = 63. Values are mean ± SD (range).
Fasting proinflammatory cytokine concentrations in plasma and culture supernatants
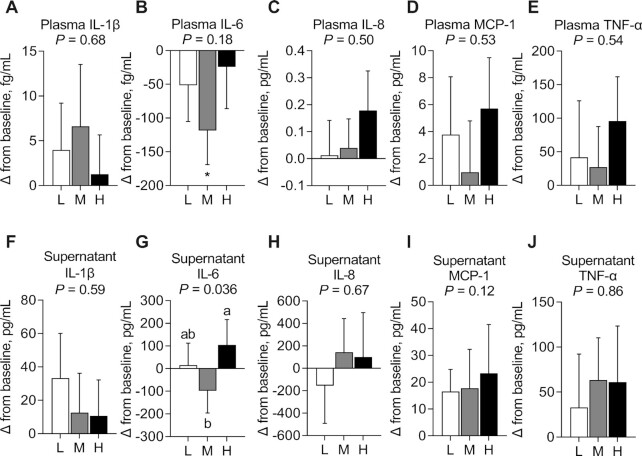
No evidence of a carryover effect was observed in cytokine concentrations in plasma and culture supernatants. No significant between-diet differences were observed in any plasma cytokine (Figure 2A–E, Supplemental Table 6) and no significant within-diet differences were observed in plasma IL-1β, IL-8, MCP-1, and TNF-α (Figure 2A, C–E) in fasted participants. However, there was a significant reduction from baseline in plasma IL-6 after MSD consumption (−118.26 ± 50.63 fg/mL; P < 0.05) (Figure 2B, Supplemental Table 6).
FIGURE 2.
Change in plasma IL-1β (A), IL-6 (B), IL-8 (C), MCP-1 (D), and TNF-α (E) concentrations and change in IL-1β (F), IL-6 (G), IL-8 (H), MCP-1 (I), and TNF-α (J) secretion from LPS-stimulated peripheral blood mononuclear cells after consuming an L, M, and H for 4 wk in fasted adults at risk of cardiometabolic disease. Labeled means without a common letter differ, P < 0.05. *Significant change from baseline, P < 0.05. Data are mean ± SEM. n = 63. H, high-dose spice diet; L, low-dose spice diet; M, medium-dose spice diet; MCP, monocyte chemoattractant protein.
There were no significant between-diet differences in IL-1β, IL-8, MCP-1, and TNF-α secretion from LPS-stimulated PBMCs collected from fasted participants (Figure 2F, H–J, Supplemental Table 6). However, there was a significant main effect of diet on IL-6 secretion (Figure 2G, Supplemental Table 6) (P = 0.036). IL-6 secretion was significantly lower (−202.06 ± 86.04 pg/mL; P = 0.041) after MSD consumption than after HSD consumption, but not compared with LSD in fasted participants. No change from baseline was observed for any cytokine after consuming any of the diets (Figure 2F–J, Supplemental Table 6).
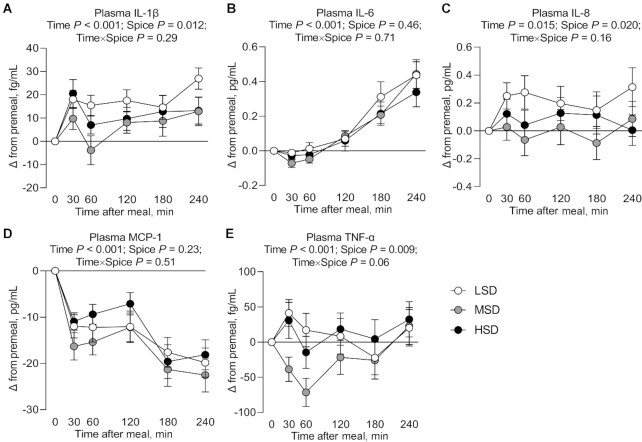
Postprandial proinflammatory cytokine concentrations in plasma
A carryover effect was observed in postprandial plasma IL-1β and TNF-α concentrations. There was a significant main effect of time on postprandial plasma IL-1β (P < 0.001), IL-6 (P < 0.001), IL-8 (P = 0.015), MCP-1 (P < 0.001), and TNF-α (P < 0.001) (Figure 3A–E, Supplemental Table 7). There was a significant main effect of diet on postprandial plasma IL-1β (P = 0.012), IL-8 (P = 0.020), and TNF-α (P = 0.009) (Figure 3A, C, E, Supplemental Table 7), but not on plasma IL-6 and MCP-1 (Figure 3B, D, Supplemental Table 7). Mean postprandial plasma IL-1β, IL-8, and TNF-α concentrations were significantly lower (−9.47 ± 2.70 fg/mL, −0.20 ± 0.05 pg/mL, and −33.28 ± 12.35 fg/mL, respectively; P = 0.008, P = 0.015, and P = 0.013, respectively) after consuming a medium-dose spice test meal containing 3.675 g of spices after 4 wk on the MSD intervention than after consuming a low-dose spice test meal containing 0.613 g of spices after 4 wk on the LSD intervention.
FIGURE 3.
Change in postprandial plasma IL-1β (A), IL-6 (B), IL-8 (C), MCP-1 (D), and TNF-α (E) concentrations after test meal ingestion after LSD, MSD, and HSD consumption for 4 wk in adults at risk of cardiometabolic disease. Data are mean ± SEM. n = 43. HSD, high-dose spice diet; LSD, low-dose spice diet; MCP, monocyte chemoattractant protein; MSD, medium-dose spice diet.
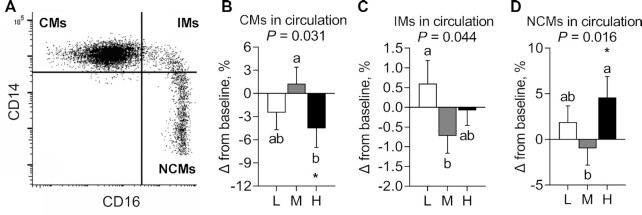
Distribution of monocyte subsets
A representative dot plot shows the CD14-versus-CD16 staining and gating strategy used to identify CMs, IMs, and NCMs in fasted participants (Figure 4A). No evidence of a carryover effect was observed in percentage of circulating monocyte subsets. Using between-diet analysis, a significant main effect of diet on the percentage of CMs (Figure 4B) (P = 0.031), IMs (Figure 4C) (P = 0.044), and NCMs (Figure 4D) (P = 0.016) was observed (Supplemental Table 6). The percentage of CMs was significantly reduced (−5.81% ± 2.06%; P = 0.028) after consuming HSD compared with MSD, but not compared with LSD. The percentage of IMs was significantly reduced (−1.33% ± 0.60%; P = 0.034) after consuming MSD, but not HSD, compared with LSD. The percentage of NCMs was significantly increased (5.58% ± 1.92%; P = 0.012) after consuming HSD compared with MSD, but not compared with LSD. Using within-diet analysis, after consuming the HSD, there was a reduction from baseline in the percentage of CMs (−4.53% ± 2.45%; P < 0.05) and an increase from baseline in the percentage of NCMs (4.61% ± 2.29%; P < 0.05) (Figure 4B, D, Supplemental Table 6).
FIGURE 4.
Change in the percentage of monocyte subsets after consuming L, M, and H for 4 wk in fasted adults at risk of cardiometabolic disease. (A) Representative dot plot of CD14 and CD16 expression and the gate of monocyte subsets. (B–D) Change in the percentages of (B) CMs, (C) IMs, and (D) NCMs after spice consumption. Labeled means without a common letter differ, P < 0.05. *Significant change from baseline, P < 0.05. Data are mean ± SEM. n = 23. CM, classical monocyte; H, high-dose spice diet; IM, intermediate monocyte; L, low-dose spice diet; M, medium-dose spice diet; NCM, nonclassical monocyte.
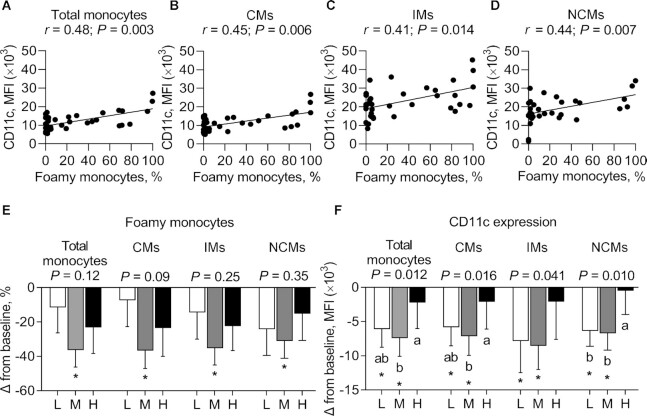
Foamy monocytes and the expression of CD11c on monocyte subsets
No evidence of a carryover effect was observed in the percentage of foamy monocytes and their surface expression of CD11c. There was a positive correlation between the percentage of foamy monocytes and the mean fluorescence intensity (MFI) of CD11c among the total monocyte population (r = 0.48; P = 0.003), CMs (r = 0.45; P = 0.006), IMs (r = 0.41; P = 0.014), and NCMs (r = 0.44; P = 0.007) in fasted participants (data pooled from participants after 4 wk on all 3 diets) (Figure 5A–D).
FIGURE 5.
Correlation between the percentage of foamy monocytes and the expression of CD11c on total monocytes (A), CMs (B), IMs (C), and NCMs (D) and change in the percentage of foamy monocytes in monocyte subsets (E) and their surface expression of CD11c (F) after consuming L, M, and H for 4 wk in fasted adults at risk of cardiometabolic disease. Labeled means without a common letter differ, P < 0.05. *Significant change from baseline, P < 0.05. Data are mean ± SEM. n = 12. CM, classical monocyte; H, high-dose spice diet; IM, intermediate monocyte; L, low-dose spice diet; M, medium-dose spice diet; MFI, mean fluorescence intensity; NCM, nonclassical monocyte.
Using between-diet analysis, no significant effect of diet was observed on the percentage of foamy monocytes among total monocytes or within each monocyte subset (Figure 5E, Supplemental Table 6). However, using within-diet analysis, after consuming the MSD, there was a reduction from baseline in the percentage of foamy monocytes in the total monocyte population (−36.43 ± 9.82 MFI; P < 0.05); in CMs (−36.66 ± 10.42 MFI; P < 0.05); in IMs (−35.21 ± 9.88 MFI; P < 0.05); and in NCMs (−31.06 ± 10.13 MFI; P < 0.05) (Figure 5E, Supplemental Table 6).
Between-diet analysis showed a significant main effect of diet on CD11c expression on total monocytes (P = 0.012), CMs (P = 0.016), IMs (P = 0.041), and NCMs (P = 0.010) (Figure 5F, Supplemental Table 6). There was a greater reduction in CD11c expression on total monocytes after consuming MSD than after consuming HSD (−5.18 ± 1.69 MFI; P = 0.010), but not compared with LSD; on CMs after consuming MSD than after consuming HSD (−4.99 ± 1.65 MFI; P = 0.012), but not compared with LSD; and on NCMs after consuming LSD and MSD than after consuming HSD (−6.20 ± 2.64 and −5.87 ± 2.03 MFI, respectively; P = 0.016 and P = 0.040, respectively) (Figure 5F, Supplemental Table 6). Using within-diet analysis, there was a significant reduction from baseline in CD11c expression on total monocytes after consuming LSD and MSD (−6.12 ± 2.62 and −7.40 ± 2.68 MFI, respectively; P < 0.05), on CMs after consuming LSD and MSD (−5.83 ± 2.72 and −7.13 ± 2.80 MFI, respectively; P < 0.05), on IMs after consuming LSD and MSD (−7.84 ± 4.61 and −8.54 ± 3.46 MFI, respectively; P < 0.05), and on NCMs after consuming LSD and MSD (−6.37 ± 2.24 and −6.70 ± 2.48 MFI, respectively; P < 0.05) (Figure 5F, Supplemental Table 6).
The expression of adhesion molecules and chemokine receptors on monocyte subsets
Evidence of a carryover effect was detected in CCR2 expression on NCMs. Between-diet analysis showed no main effect of diet on CCR2 expression in CMs (Figure 6A, Supplemental Table 6), but a significant main effect of diet on CCR2 expression in IMs (P = 0.037) and in NCMs (P = 0.002) (Figure 6B, C, Supplemental Table 6). There was a greater expression of CCR2 in IMs after consuming HSD, but not MSD, than after consuming LSD (12.05 ± 4.97 × 103 MFI; P = 0.031); and in NCMs after consuming MSD, but not HSD, than after consuming LSD (1.04 ± 0.36 × 103 MFI; P = 0.002). Within-diet analysis showed that there was a significant increase from baseline in CCR2 expression in IMs after consuming HSD (10.52 ± 6.36 × 103 MFI; P < 0.05) and in NCMs after consuming MSD (0.89 ± 0.34 × 103 MFI; P < 0.05) (Figure 6B, C, Supplemental Table 6).
FIGURE 6.
Change in the expression of CCR2 on CMs (A), IMs (B), and NCMs (C), in the adherence of monocyte subsets to HUVECs (D), and in the trans-endothelial migration of monocyte subsets through HUVECs (E) after consuming L, M, and H for 4 wk in fasted adults at risk of cardiometabolic disease. Labeled means without a common letter differ, P < 0.05. *Significant change from baseline, P < 0.05. Data are mean ± SEM. CCR2, n = 23. Adherence and migration indexes, n = 6. CCR2, C-C chemokine receptor type 2; CM, classical monocyte; H, high-dose spice diet; HUVEC, human umbilical vein endothelial cell; IM, intermediate monocyte; L, low-dose spice diet; M, medium-dose spice diet; MFI, mean fluorescence intensity; NCM, nonclassical monocyte.
No other significant between- and within-diet differences were observed on the expression of CCR5, CD11b, CD49d, L-selectin, and P-selectin glycoprotein ligand-1 (PSGL-1) in each monocyte subset (Supplemental Figure 4, Supplemental Table 6).
Adherence and migration indexes of monocyte subsets
No evidence of a carryover effect was observed in the adherence and migration indexes of monocyte subsets. Between-diet analysis showed a significant main effect of diet on the adherence index of CMs (P = 0.040), but not on the adherence indexes of IMs and NCMs (Figure 6D, Supplemental Table 6). There was a lower adherence index of CMs after consuming HSD, but not MSD, than after consuming LSD (−0.86 ± 0.34; P = 0.034). Within-diet analysis showed a reduction from baseline in the adherence index of IMs after consuming MSD (−0.54 ± 0.08; P < 0.05) (Figure 6D, Supplemental Table 6).
Between-diet analysis showed a significant main effect of diet on the migration index of IMs (P = 0.005) and NCMs (P = 0.013), but not in CMs (Figure 6E, Supplemental Table 6). The migration index of IMs was lower after consuming MSD and HSD than after consuming LSD (−0.39 ± 0.09 and −0.56 ± 0.14, respectively; P = 0.041 and 0.004, respectively); the migration index of NCMs was lower after consuming HSD than after consuming MSD (−0.55 ± 0.15; P = 0.012), but not compared with LSD. Within-diet analysis showed that there was a decrease from baseline in the migration index of CMs after consuming LSD, MSD, and HSD (−0.34 ± 0.10, −0.32 ± 0.10, and −0.51 ± 0.23, respectively; P < 0.05); an increase from baseline in the migration index of IMs after consuming LSD (0.43 ± 0.12; P < 0.05); and an increase from baseline in the migration index of NCMs after consuming MSD (0.46 ± 0.22; P < 0.05) (Figure 6E, Supplemental Table 6).
Discussion
This is the first controlled feeding study to evaluate the effect of 4 wk of spice consumption at different doses in an average American diet on inflammatory mediators related to cardiometabolic risk. We observed a reduction in plasma IL-6 and IL-6 secretion from LPS-stimulated PBMCs after 4 wk of MSD consumption, but no other changes in circulating or PBMC-derived cytokines when participants were fasted. Moreover, we observed a significant postprandial reduction in plasma IL-1β, IL-8, and TNF-α concentrations after ingestion of a test meal containing a medium dose (3.675 g) of spices after 4 wk of MSD consumption compared with ingesting a test meal containing a low dose (0.613 g) of spices after 4 wk of LSD consumption. In fasted participants, HSD and MSD had differential effects on the distribution of, and CCR expression in, monocyte subsets. Furthermore, consumption of the HSD reduced adherence of CMs (compared with the LSD) and migration of IMs (compared with the LSD) and NCMs (compared with the MSD). Collectively, 4 wk of MSD consumption may reduce circulating IL-6 and blunt meal-induced proinflammatory cytokines in circulation, and modulate the phenotype and function of monocytes.
We previously demonstrated no effect of acute spice consumption (6 g) on postprandial plasma cytokine concentrations, but a robust effect of acute spice consumption (6 g) on IL-1β, IL-8, and TNF-α secretion from LPS-stimulated PBMCs when the spice blend was delivered in a high-saturated-fat, high-carbohydrate meal (16). Findings from the current study and our previous work (16) suggest 4 wk of MSD consumption diminishes postprandial plasma cytokine responses but the timing at which cytokines are measured in plasma in relation to meal ingestion may be a critical factor to consider. Plasma polyphenol concentrations reach their peak 3 h after consuming a polyphenol-rich beverage and return to fasting concentrations 6 h after meal consumption (38). Our data support these findings and suggest circulating bioactive compounds in the MSD may mediate a beneficial effect during the postprandial period.
Monocytes invade the arterial wall during atherogenesis and drive disease progression (6). Each monocyte subset exhibits distinct phenotypic and functional properties that contribute to inflammatory responses (8, 39–43) and may play a role in atherogenesis. Based on the proatherogenic function of CMs observed in mice (44) and humans (45), CMs are hypothesized to be involved in the pathogenesis of CVD (44). A higher percentage of IMs is observed in patients with atherosclerosis than in healthy controls, suggesting IMs may also be involved in the process of CVD development (14, 46). We demonstrated that HSD consumption reduced the percentage of CMs compared with MSD, and MSD consumption reduced the percentage of IMs compared with LSD, suggesting that an MSD and HSD may influence the distribution of monocyte subsets with inflammatory potential. In contrast, we observed an increase in the percentage of NCMs after consuming an HSD compared with an MSD and baseline. Several studies report a proatherogenic role of NCMs in humans (47, 48), whereas other studies report an atheroprotective role of NCMs in preclinical models (49, 50). Additional studies are needed to determine if consuming an HSD reduces atherosclerotic risk via modulating the percentage of NCMs.
We observed that MSD consumption reduced the percentage of total foamy monocytes compared with baseline, and reduced CD11c expression on total monocytes, CMs, and NCMs compared with an HSD. Previous studies report an inhibitory effect of spice-derived bioactives, such as curcumin (51), capsaicin (52), and piperine (53), on intracellular lipid accumulation in THP-1 human monocytes and RAW 264.7 murine macrophages. However, the dose of spice needed to achieve any potential atheroprotective effect is unclear, because we observed differential effects of the MSD and HSD on the percentage of foamy monocytes, CD11c expression, and adhesion/migration of monocytes.
In a chronic low-grade inflammatory state, TNF-α activates vascular endothelial cells to produce MCP-1 (54, 55). Monocytes are recruited to the site of vascular inflammation through CCR2, an MCP-1 receptor (56, 57). We observed an increased CCR2 expression on IMs after HSD consumption and on NCMs after MSD consumption. However, increased CCR2 expression on monocytes did not correspond to elevated adherence and migration into the subendothelial space, a crucial first step for atherogenesis (58). We demonstrated HSD consumption reduced the adherence of CMs to HUVECs and trans-endothelial migration of IMs and NCMs. Previous in vitro studies reported that spice-derived bioactives, such as gingerenone A (59, 60) and curcumin (61, 62), attenuated adherence and/or migration of monocytes through vascular endothelial cells. The reduction in migration of CMs compared with baseline after consuming all 3 diets may be due to differences in the habitual diets of the participants compared with the meal plan developed for this study. Many adults in the United States do not meet the dietary recommendations for fruits and vegetables (63); thus, our experimental diets likely included more fruits and vegetables than those the participants typically consume, which may have had a beneficial effect on monocyte migration.
Although a dose–response relation between phytochemical consumption and chronic disease risk is reported (64), we observed differences between the MSD and HSD on proinflammatory cytokine concentrations, with MSD consumption conferring greater reduction than HSD consumption. There may be a nonlinear relation (e.g., U-shaped dose response) between spice consumption and inflammatory cytokine modulation. The HSD may have a high phenolic content with pro-oxidant activity that may induce oxidative stress either by generating reactive oxygen species or by inhibiting the antioxidant defense system (52). Thus, it is plausible there is a dosage of bioactive compounds that is optimal in the MSD range (2.715–5.785 g/d), and the HSD may have higher concentrations of bioactives with counteractive effects. Additional studies are needed to test these hypotheses.
A major strength of our study is the controlled-feeding design that eliminated the influence of dietary factors other than spices on the endpoints measured. Moreover, this is the first study that we know of to examine the effect of multiple doses of longer-term (4 wk) spice consumption on inflammatory cytokines and the phenotype/function of monocyte subsets. Previous studies highlighted an association between monocyte subset distribution and the development of atherosclerosis and CVD (65), and that dietary factors may modulate this relation (66). Thus, studying the effect of dietary factors on each monocyte subset may clarify their role in the inflammatory process and atherosclerotic risk. A limitation of our study is that our spice blend included 24 different spices, thus the role of any individual spice on inflammatory outcomes cannot be determined. We also did not measure polyphenolic content in plasma, which may underlie the differences observed between the MSD and HSD on inflammatory outcomes and monocyte function. Furthermore, we only had 1 baseline visit after randomization, which limited our ability to definitively evaluate any carryover effect of the diets. Lastly, analysis of multiple outcomes may increase the likelihood of a type 1 error. Thus, these results need to be interpreted with caution and evaluated in subsequent studies.
In conclusion, incorporation of a medium (2.715–5.785 g/d) dosage of spices into an average American diet for 4 wk may reduce inflammatory cytokines and alter monocyte function, which may confer an atheroprotective effect. However, additional studies are needed to explore the mechanisms underlying the differences in inflammatory outcomes after consumption of an MSD compared with an HSD, cytokine response kinetics in a fed compared with a fasted state, and how spice consumption alters monocyte phenotype and function.
Supplementary Material
Acknowledgments
We thank the staff at the Penn State Clinical Research Center for their assistance with data collection and the Penn State Flow Cytometry Facility, University Park, PA for technical support.
The authors’ responsibilities were as follows—ESO, KSP, PMK-E, and CJR: designed the research; ESO and KSP: conducted the research and collected the data; ESO and CJR: participated in data analysis and interpretation and wrote the paper; CJR: had primary responsibility for the final content; and all authors read and approved the final manuscript. CJR and PMK-E received funding from McCormick Science Institute for the research reported in this article. ESO and KSP report no conflicts of interest.
Notes
Supported by McCormick Science Institute grant MSI-10001 (to PMK-E and CJR). Supported in addition by NIH TL1 training program TL1TR002016 (to ESO) and National Center for Advancing Translational Sciences at the NIH grant 1UL1TR002014-01. The McCormick Science Institute and the NIH had no role in study design, data collection, data analysis, data interpretation, or writing the manuscript. Chefs from McCormick and Company, Inc. designed the menus based on the nutrient criteria of the experimental diets that were defined by the investigators. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication. The content is solely the responsibility of the authors and does not represent the official views of the NIH.
Supplemental Tables 1–7 and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CCR2, C-C chemokine receptor type 2; CM, classical monocyte; CRP, C-reactive protein; CVD, cardiovascular disease; HSD, high-dose spice diet; HUVEC, human umbilical vein endothelial cell; IM, intermediate monocyte; LSD, low-dose spice diet; MCP, monocyte chemoattractant protein; MFI, mean fluorescence intensity; MSD, medium-dose spice diet; NCM, nonclassical monocyte; PBMC, peripheral blood mononuclear cell.
Contributor Information
Ester S Oh, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
Kristina S Petersen, Department of Nutritional Sciences, Texas Tech University, Lubbock, TX, USA.
Penny M Kris-Etherton, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA.
Connie J Rogers, Department of Nutritional Sciences, The Pennsylvania State University, University Park, PA, USA; Center for Molecular Immunology and Infectious Disease, Huck Institutes for the Life Sciences, The Pennsylvania State University, University Park, PA, USA.
Data Availability
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.
References
- 1. Fryar CD, Carroll MD, Ogden CL. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2015–2016. Hyattsville, MD: National Center for Health Statistics; 2018. [Google Scholar]
- 2. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief, No. 360. Hyattsville, MD: National Center for Health Statistics; 2020. [Google Scholar]
- 3. Manna P, Jain SK. Obesity, oxidative stress, adipose tissue dysfunction, and the associated health risks: causes and therapeutic strategies. Metab Syndr Relat Disord. 2015;13(10):423–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ndumele CE, Matsushita K, Lazo M, Bello N, Blumenthal RS, Gerstenblith G, Nambi V, Ballantyne CM, Solomon SD, Selvin E et al. Obesity and subtypes of incident cardiovascular disease. J Am Heart Assoc. 2016;5(8):e003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moroni F, Ammirati E, Norata GD, Magnoni M, Camici PG. The role of monocytes and macrophages in human atherosclerosis, plaque neoangiogenesis, and atherothrombosis. Mediators Inflamm. 2019:7434376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Randolph G. The fate of monocytes in atherosclerosis. J Thromb Haemost. 2009;7:28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJM, Liu Y-J, MacPherson G, Randolph GJ et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116(16):e74–e80. [DOI] [PubMed] [Google Scholar]
- 8. Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, Chalasani G, Taboas JM, Lakkis FG, Metes DM. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12(4):e0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kränkel N, Kuschnerus K, Madeddu P, Lüscher TF, Landmesser U. A novel flow cytometry-based assay to study leukocyte–endothelial cell interactions in vitro. Cytom A. 2011;79(4):256–62. [DOI] [PubMed] [Google Scholar]
- 10. Connaughton EP, Naicker S, Hanley SA, Slevin SM, Eykelenboom JK, Lowndes NF, O'Brien T, Ceredig R, Griffin MD, Dennedy MC. Phenotypic and functional heterogeneity of human intermediate monocytes based on HLA-DR expression. Immunol Cell Biol. 2018;96(7):742–58. [DOI] [PubMed] [Google Scholar]
- 11. Zawada AM, Rogacev KS, Rotter B, Winter P, Marell R-R, Fliser D, Heine GH. SuperSAGE evidence for CD14++CD16+ monocytes as a third monocyte subset. Blood. 2011;118(12):e50–e61. [DOI] [PubMed] [Google Scholar]
- 12. Rogacev KS, Seiler S, Zawada AM, Reichart B, Herath E, Roth D, Ulrich C, Fliser D, Heine GH. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur Heart J. 2011;32(1):84–92. [DOI] [PubMed] [Google Scholar]
- 13. Vlacil A-K, Schuett J, Schieffer B, Grote K. Variety matters: diverse functions of monocyte subtypes in vascular inflammation and atherogenesis. Vasc Pharmacol. 2019;113:9–19. [DOI] [PubMed] [Google Scholar]
- 14. Oh ES, Na M, Rogers CJ. The association between monocyte subsets and cardiometabolic disorders/cardiovascular disease: a systematic review and meta-analysis. Front Cardiovasc Med. 2021;8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu H, Ballantyne CM. PCSK9 inhibitors and foamy monocytes in familial hypercholesterolaemia. Nat Rev Cardiol. 2017;14(7):385–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oh ES, Petersen KS, Kris-Etherton PM, Rogers CJ. Spices in a high-saturated-fat, high-carbohydrate meal reduce postprandial proinflammatory cytokine secretion in men with overweight or obesity: a 3-period, crossover, randomized controlled trial. J Nutr. 2020;150(6):1600–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kunnumakkara AB, Sailo BL, Banik K, Harsha C, Prasad S, Gupta SC, Bharti AC, Aggarwal BB. Chronic diseases, inflammation, and spices: how are they linked?. J Transl Med. 2018;16(1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong J-W, Yang G-E, Kim YB, Eom SH, Lew J-H, Kang H. Anti-inflammatory activity of cinnamon water extract in vivo and in vitro LPS-induced models. BMC Complement Altern Med. 2012;12(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Joshi K, Awte S, Bhatnagar P, Walunj S, Gupta R, Joshi S, Sabharwal S, Bani S, Padalkar A. Cinnamomum zeylanicum extract inhibits proinflammatory cytokine TNF∝: in vitro and in vivo studies. Res Pharm Biotech. 2010;2(2):14–21. [Google Scholar]
- 20. Jafari S, Sattari R, Ghavamzadeh S. Evaluation the effect of 50 and 100 mg doses of Cuminum cyminum essential oil on glycemic indices, insulin resistance and serum inflammatory factors on patients with diabetes type II: a double-blind randomized placebo-controlled clinical trial. J Tradit Complement Med. 2017;7(3):332–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arablou T, Aryaeian N, Valizadeh M, Sharifi F, Hosseini A, Djalali M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int J Food Sci Nutr. 2014;65(4):515–20. [DOI] [PubMed] [Google Scholar]
- 22. Atashak S, Peeri M, Azarbayjani MA, Stannard SR, Haghighi MM. Obesity-related cardiovascular risk factors after long-term resistance training and ginger supplementation. J Sports Sci Med. 2011;10(4):685–91. [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen KS, Davis KM, Rogers CJ, Proctor DN, West SG, Kris-Etherton PM. Herbs and spices at a relatively high culinary dosage improves 24-hour ambulatory blood pressure in adults at risk of cardiometabolic diseases: a randomized, crossover, controlled-feeding study. Am J Clin Nutr. 2021; Sep 12 (Epub ahead of print; doi:10.1093/ajcn/nqab291). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zare R, Heshmati F, Fallahzadeh H, Nadjarzadeh A. Effect of cumin powder on body composition and lipid profile in overweight and obese women. Complement Ther Clin Pract. 2014;20(4):297–301. [DOI] [PubMed] [Google Scholar]
- 25. Chuengsamarn S, Rattanamongkolgul S, Phonrat B, Tungtrongchitr R, Jirawatnotai S. Reduction of atherogenic risk in patients with type 2 diabetes by curcuminoid extract: a randomized controlled trial. J Nutr Biochem. 2014;25(2):144–50. [DOI] [PubMed] [Google Scholar]
- 26. Mahluji S, Attari VE, Mobasseri M, Payahoo L, Ostadrahimi A, Golzari SE. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int J Food Sci Nutr. 2013;64(6):682–6. [DOI] [PubMed] [Google Scholar]
- 27. Khan A, Zaman G, Anderson RA. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J Clin Biochem Nutr. 2009;44(1):52–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakayama H, Tsuge N, Sawada H, Masamura N, Yamada S, Satomi S, Higashi Y. A single consumption of curry improved postprandial endothelial function in healthy male subjects: a randomized, controlled crossover trial. Nutr J. 2014;13(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bendavid I, Lobo DN, Barazzoni R, Cederholm T, Coëffier M, de van der Schueren M, Fontaine E, Hiesmayr M, Laviano A, Pichard C et al. The centenary of the Harris–Benedict equations: how to assess energy requirements best? Recommendations from the ESPEN expert group. Clin Nutr. 2021;40(3):690–701. [DOI] [PubMed] [Google Scholar]
- 30. Srinivasan K. Dietary spices as beneficial modulators of lipid profile in conditions of metabolic disorders and diseases. Food Funct. 2013;4(4):503–21. [DOI] [PubMed] [Google Scholar]
- 31. Lal AAS, Kumar T, Murthy PB, Pillai KS. Hypolipidemic effect of Coriandrum sativum L. in triton-induced hyperlipidemic rats. Indian J Exp Biol. 2004;42(9):909–12. [PubMed] [Google Scholar]
- 32. Isbill J, Kandiah J, Khubchandani J. Use of ethnic spices by adults in the United States: an exploratory study. Health Promot Perspect. 2018;8(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. USDA ERS . Summary data on annual food imports, values and volume by food category and source country, 1999-2017. Version current 1 May 2018. [Internet]. [accessed 2021 May 2]. Available from: https://www.ers.usda.gov/data-products/us-food-imports/us-food-imports/#All%20tables%20in%20one%20file. [Google Scholar]
- 34. Nguyen L, Duong LT, Mentreddy RS. The US import demand for spices and herbs by differentiated sources. J Appl Res Med Aromat Plants. 2019;12:13–20. [Google Scholar]
- 35. Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm. 2014:615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Meng H, Ba Z, Lee Y, Peng J, Lin J, Fleming JA, Furumoto EJ, Roberts RF, Kris-Etherton PM, Rogers CJ. Consumption of Bifidobacterium animalis subsp. lactis BB-12 in yogurt reduced expression of TLR-2 on peripheral blood-derived monocytes and pro-inflammatory cytokine secretion in young adults. Eur J Nutr. 2017;56(2):649–61. [DOI] [PubMed] [Google Scholar]
- 37. Krychtiuk KA, Kastl SP, Pfaffenberger S, Lenz M, Hofbauer SL, Wonnerth A, Koller L, Katsaros KM, Pongratz T, Goliasch G et al. Association of small dense LDL serum levels and circulating monocyte subsets in stable coronary artery disease. PLoS One. 2015;10(4):e0123367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Banaszewski K, Park E, Edirisinghe I, Cappozzo JC, Burton-Freeman BM. A pilot study to investigate bioavailability of strawberry anthocyanins and characterize postprandial plasma polyphenols absorption patterns by Q-TOF LC/MS in humans. J Berry Res. 2013;3(2):113–26. [Google Scholar]
- 39. Wong KL, Tai JJ-Y, Wong W-C, Han H, Sem X, Yeap W-H, Kourilsky P, Wong S-C. Gene expression profiling reveals the defining features of the classical, intermediate, and nonclassical human monocyte subsets. Blood. 2011;118(5):e16–e31. [DOI] [PubMed] [Google Scholar]
- 40. Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol. 2015;35(6):1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–70. [DOI] [PubMed] [Google Scholar]
- 42. Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, Hedrick CC, Cook HT, Diebold S, Geissmann F. Nr4a1-dependent Ly6Clow monocytes monitor endothelial cells and orchestrate their disposal. Cell. 2013;153(2):362–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cros J, Cagnard N, Woollard K, Patey N, Zhang S-Y, Senechal B, Puel A, Biswas SK, Moshous D, Picard C et al. Human CD14dim monocytes patrol and sense nucleic acids and viruses via TLR7 and TLR8 receptors. Immunity. 2010;33(3):375–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Heine G, Ulrich C, Seibert E, Seiler S, Marell J, Reichart B, Krause M, Schlitt A, Köhler H, Girndt M. CD14++CD16+ monocytes but not total monocyte numbers predict cardiovascular events in dialysis patients. Kidney Int. 2008;73(5):622–9. [DOI] [PubMed] [Google Scholar]
- 46. Williams H, Cassorla G, Pertsoulis N, Patel V, Vicaretti M, Marmash N, Hitos K, Fletcher JP, Medbury HJ. Human classical monocytes display unbalanced M1/M2 phenotype with increased atherosclerotic risk and presence of disease. Int Angiol. 2016;36(2):145–55. [DOI] [PubMed] [Google Scholar]
- 47. Ong S-M, Hadadi E, Dang T-M, Yeap W-H, Tan CT-Y, Ng T-P, Larbi A, Wong S-C. The pro-inflammatory phenotype of the human non-classical monocyte subset is attributed to senescence. Cell Death Dis. 2018;9(3):266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Devêvre EF, Renovato-Martins M, Clément K, Sautès-Fridman C, Cremer I, Poitou C. Profiling of the three circulating monocyte subpopulations in human obesity. J Immunol. 2015;194(8):3917–23. [DOI] [PubMed] [Google Scholar]
- 49. Hanna RN, Shaked I, Hubbeling HG, Punt JA, Wu R, Herrley E, Zaugg C, Pei H, Geissmann F, Ley K et al. NR4A1 (Nur77) deletion polarizes macrophages toward an inflammatory phenotype and increases atherosclerosis. Circ Res. 2012;110(3):416–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hamers AAJ, Vos M, Rassam F, Marinković G, Kurakula K, van Gorp PJ, de Winther MPJ, Gijbels MJJ, de Waard V, de Vries CJM. Bone marrow–specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ Res. 2012;110(3):428–38. [DOI] [PubMed] [Google Scholar]
- 51. Soltani B, Bodaghabadi N, Ghaemi N, Sadeghizadeh M. Radiation-induced surge of macrophage foam cell formation, oxidative damage, and cytokine release is attenuated by a nanoformulation of curcumin. Int J Radiat Biol. 2017;93(3):303–14. [DOI] [PubMed] [Google Scholar]
- 52. Eghbaliferiz S, Iranshahi M. Prooxidant activity of polyphenols, flavonoids, anthocyanins and carotenoids: updated review of mechanisms and catalyzing metals. Phytother Res. 2016;30(9):1379–91. [DOI] [PubMed] [Google Scholar]
- 53. Matsuda D, Ohte S, Ohshiro T, Jiang W, Rudel L, Hong B, Si S, Tomoda H. Molecular target of piperine in the inhibition of lipid droplet accumulation in macrophages. Biol Pharm Bull. 2008;31(6):1063–6. [DOI] [PubMed] [Google Scholar]
- 54. Fatkhullina A, Peshkova I, Koltsova E. The role of cytokines in the development of atherosclerosis. Biochemistry. 2016;81(11):1358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. McKellar GE, McCarey DW, Sattar N, McInnes IB. Role for TNF in atherosclerosis? Lessons from autoimmune disease. Nat Rev Cardiol. 2009;6(6):410–17. [DOI] [PubMed] [Google Scholar]
- 56. Charo IF, Taubman MB. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95(9):858–66. [DOI] [PubMed] [Google Scholar]
- 57. Weber C, Schober A, Zernecke A. Chemokines: key regulators of mononuclear cell recruitment in atherosclerotic vascular disease. Arterioscler Thromb Vasc Biol. 2004;24(11):1997–2008. [DOI] [PubMed] [Google Scholar]
- 58. Čejková S, Králová-Lesná I, Poledne R. Monocyte adhesion to the endothelium is an initial stage of atherosclerosis development. Cor Vasa. 2016;58(4):e419–25. [Google Scholar]
- 59. Suk S, Kwon GT, Lee E, Jang WJ, Yang H, Kim JH, Thimmegowda N, Chung MY, Kwon JY, Yang S et al. Gingerenone A, a polyphenol present in ginger, suppresses obesity and adipose tissue inflammation in high-fat diet-fed mice. Mol Nutr Food Res. 2017;61(10):1700139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim HJ, Son JE, Kim JH, Lee CC, Yang H, Yaghmoor SS, Ahmed Y, Yousef JM, Abualnaja KO, Al-Malki AL et al. Gingerenone A attenuates monocyte-endothelial adhesion via suppression of I Kappa B kinase phosphorylation. J Cell Biochem. 2018;119(1):260–8. [DOI] [PubMed] [Google Scholar]
- 61. Zhang L, Wang X, Zhang L, Virgous C, Si H. Combination of curcumin and luteolin synergistically inhibits TNF-α-induced vascular inflammation in human vascular cells and mice. J Nutr Biochem. 2019;73:108222. [DOI] [PubMed] [Google Scholar]
- 62. Monfoulet L-E, Mercier S, Bayle D, Tamaian R, Barber-Chamoux N, Morand C, Milenkovic D. Curcumin modulates endothelial permeability and monocyte transendothelial migration by affecting endothelial cell dynamics. Free Radic Biol Med. 2017;112:109–20. [DOI] [PubMed] [Google Scholar]
- 63. Micha R, Peñalvo JL, Cudhea F, Imamura F, Rehm CD, Mozaffarian D. Association between dietary factors and mortality from heart disease, stroke, and type 2 diabetes in the United States. JAMA. 2017;317(9):912–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Holst B, Williamson G. Nutrients and phytochemicals: from bioavailability to bioefficacy beyond antioxidants. Curr Opin Biotechnol. 2008;19(2):73–82. [DOI] [PubMed] [Google Scholar]
- 65. Kapellos TS, Bonaguro L, Gemünd I, Reusch N, Saglam A, Hinkley ER, Schultze JL. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dias JA, Wirfält E, Drake I, Gullberg B, Hedblad B, Persson M, Engström G, Nilsson J, Schiopu A, Fredrikson GN et al. A high quality diet is associated with reduced systemic inflammation in middle-aged individuals. Atherosclerosis. 2015;238(1):38–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the article, code book, and analytic code will be made available upon request pending application and approval.