ABSTRACT
Background
Diet is one of the modifiable risk factors for cognitive decline. However, studies on dietary protein intake and cognitive decline have remained limited and inconclusive.
Objectives
In this study, we aimed to investigate the associations between long-term dietary protein intake and subsequent subjective cognitive decline (SCD).
Methods
We included 49,493 women from the Nurses’ Health Study (NHS) (1984–2006) and 27,842 men from the Health Professionals Follow-up Study (HPFS) (1986–2002). For the NHS, average dietary intake was calculated from 7 repeated semi-quantitative FFQs (SFFQs), and SCD was assessed in 2012 and 2014. For the HPFS, average dietary intake was calculated from 5 repeated SFFQs, and SCD was assessed in 2008 and 2012. Poisson regression was used to examine the associations between dietary protein, amino acids, and various protein food sources with subsequent SCD.
Results
Higher protein intake compared with total carbohydrates was associated with lower odds of SCD. When substituting 5% energy from protein for the equivalent percentage of energy from total carbohydrates, the pooled multivariable-adjusted ORs (95% CIs) were 0.89 (0.85, 0.94) for total protein, 0.89 (0.84, 0.94) for animal protein, and 0.74 (0.62, 0.88) for plant protein. When substituting 5% of energy from animal protein with plant protein, the OR was 0.84 (95% CI: 0.72, 0.97). For protein food sources, higher intakes of beans/legumes, fish, and lean poultry were significantly associated with lower odds of SCD, but higher intake of hotdogs was associated with higher odds of SCD.
Conclusions
Higher protein intake was associated with lower odds of SCD when compared isocalorically with carbohydrate. Plant protein sources were also associated with lower odds when compared with animal protein sources. Our findings suggest that adequate protein intake, and choices of protein sources could play a role in the maintenance of cognition and should be studied further.
Keywords: dietary protein, cognitive function, subjective cognitive decline, plant protein, amino acid, protein food, cohort study
Introduction
In our world of rapid aging, the global prevalence of age-related cognitive decline and dementia is projected to rise exponentially (1). Disability related to cognitive decline and the substantial lifetime costs (2) not only affect patients but also pose great burdens on their families and the whole society (1). Due to the lack of effective treatments for dementia, disease prevention is of great importance. The clinical course of dementia is generally viewed as a continuum of decline from normal cognitive function, through a preclinical phase, then objective cognitive impairment, and finally dementia (3). Subjective cognitive decline (SCD) is a state of self-perceived cognitive decline without detectable objective cognitive impairments and can precede clinically apparent mild cognitive impairment and dementia (3). SCD has been strongly associated with both concurrent objective cognitive function (4, 5) and subsequent cognitive decline (5), especially for people with higher education (6). Dementia-associated brain pathologies may develop years before SCD (7), making the long preclinical phase of dementia a critical period for prevention (8). Substantial literature has indicated that diet is one of the modifiable risk factors for cognitive decline and may play an important role in cognitive function (9).
Proteins and amino acids are important nutrients for normal functioning of the human body, are the building blocks for muscles and organs, and are essential for tissue/cell repair and production of neurotransmitters (10). In some animal studies, low-calorie and low-protein diets were associated with longer life span and better aging-related outcomes (11, 12), and higher dietary protein intake was associated with increased cardiovascular risk (13). However, inadequate protein intake in the older population could increase risk of sarcopenia and frailty (14), closely linked to cognitive impairment (15). To date, epidemiologic studies on dietary protein intake and cognitive decline have been inconclusive (16, 17). In addition, evidence on the impact of specific protein sources on cognitive function has been mixed (16, 18–20). Therefore, the current study aimed to investigate the relations between long-term dietary protein intake and subsequent SCD with repeated dietary assessments from >20 y of follow-up in 2 large prospective cohorts of men and women.
Methods
Study design
A total of 121,701 female registered nurses aged 30–55 y in the United States were enrolled in the Nurses’ Health Study (NHS) in 1976. Participants were followed up biennially via questionnaires that included potential risk factors and newly diagnosed diseases (21). Dietary information has been collected using a semiquantitative FFQ (SFFQ) that has been validated in multiple studies (22), and these data were collected in 1980, 1984, 1986, and then every 4 y thereafter.
Starting in 1986, 51,529 male health professionals aged 40–75 y, residing in the United States, were enrolled in the Health Professionals Follow-up Study (HPFS). Detailed questionnaires regarding information on lifestyle risk factors and medical history have been sent to participants every 2 y (23). Dietary assessments with the SFFQ began in 1986 and have continued every 4 y.
The study was approved by the Human Subjects Committees of the Harvard T. H. Chan School of Public Health and Brigham and Women's Hospital.
Assessment of dietary intake
The SFFQ (available at www.nurseshealthstudy.org and sites.sph.harvard.edu/hpfs/hpfs-questionnaires) was used for dietary assessments. Nutrient values were mainly based on the USDA database. Follow-up for this analysis began in 1984 for the NHS, when the first expanded SFFQ with 131 items was administered. Repeated dietary assessments were done in 1986 and then every 4 y. Cumulative average intakes of the percentage of energy from protein, amino acids, protein foods, other nutrients/foods, and total energy were calculated from these repeated SFFQs (from 1984 until 2006). This approach best represents a long-term diet and can reduce within-subject variation (24). Similarly, in the HPFS, average intakes were calculated from 5 repeated dietary assessments obtained every 4 y since 1986 until 2002. Energy-adjusted total protein intake (i.e., the sum of plant and animal protein intake) from the SFFQs correlated well with multiple dietary records and with protein biomarkers (urine nitrogen adjusted for energy intake using doubly labeled water) (25, 26). The estimated correlation with true protein intake using the combined assessments with dietary records and biomarkers was 0.65 (95% CI: 0.59, 0.72) (25).
Assessment of SCD
The primary outcome of SCD was assessed twice by mailed or online questionnaires (2012 and 2014 for the NHS, 2008 and 2012 for the HPFS; available at www.nurseshealthstudy.org and sites.sph.harvard.edu/hpfs/hpfs-questionnaires) (5). The descriptive term subjective cognitive function was used in our previous publications (27), but we have updated our terminology to SCD in keeping with changes in the field (28). SCD was assessed by 6 yes/no questions for the HPFS and 7 questions for the NHS. For each question, every “yes” was assigned the number 1 and every “no” the number 0. Of the participants in the NHS and HPFS, 86% and 72.4%, respectively, completed both SCD questionnaires; 11.4% and 19% completed only the first questionnaire; and 2.6% and 8.7% completed only the second questionnaire (Table 1). To minimize random errors, the average of the 2 SCD scores was used, except for participants who completed only 1 of the 2 SCD questionnaires. Dietary data were updated until 6 y prior to SCD assessment to minimize the potential impact of altered cognitive function on diet.
TABLE 1.
Characteristics of NHS and HPFS participants by quintiles of total protein intake1
| Quintile of protein intake | |||||
|---|---|---|---|---|---|
| Characteristic | Q1 | Q2 | Q3 | Q4 | Q5 |
| NHS (49,493 women) | (n = 9898) | (n = 9899) | (n = 9899) | (n = 9899) | (n = 9898) |
| Protein intake, % energy, mean ± SD | 14.9 ± 1.1 | 16.8 ± 0.4 | 17.9 ± 0.3 | 19.1 ± 0.4 | 21.4 ± 1.4 |
| Age at study baseline, y, mean ± SD | 49.1 ± 6.8 | 48.5 ± 6.6 | 48.2 ± 6.6 | 48.0 ± 6.5 | 47.9 ± 6.4 |
| BMI, kg/m2, mean ± SD | 25.0 ± 4.3 | 25.5 ± 4.4 | 26.0 ± 4.6 | 26.6 ± 4.8 | 27.4 ± 4.9 |
| Total energy intake, kcal/d, mean ± SD | 1842 ± 447 | 1808 ± 421 | 1751 ± 406 | 1694 ± 388 | 1583 ± 377 |
| Total carbohydrate, % energy, mean ± SD | 52.7 ± 6.6 | 51.3 ± 5.8 | 50.5 ± 5.7 | 49.8 ± 5.6 | 48.5 ± 5.9 |
| Total fat, % energy, mean ± SD | 31.7 ± 4.7 | 31.6 ± 4.5 | 31.5 ± 4.4 | 31.2 ± 4.6 | 30.6 ± 4.8 |
| Alcohol, g/d, mean ± SD | 7.8 ± 11.3 | 6.5 ± 8.8 | 5.8 ± 7.8 | 4.9 ± 6.7 | 3.7 ± 5.5 |
| Physical activity, MET-h/wk, mean ± SD | 17.5 ± 16.4 | 18.3 ± 15.4 | 18.5 ± 16.0 | 18.9 ± 16.0 | 19.6 ± 16.8 |
| Smoking pack-years, n (%) | |||||
| Never smoked | 4646 (46.9) | 4692 (47.4) | 4643 (46.9) | 4586 (46.3) | 4471 (45.2) |
| ≤4 pack-years | 993 (10.0) | 1050 (10.6) | 1081 (10.9) | 1107 (11.2) | 1176 (11.9) |
| 5–24 pack-years | 2089 (21.1) | 2213 (22.4) | 2269 (22.9) | 2307 (23.3) | 2341 (23.6) |
| ≥25 pack-years | 2008 (20.3) | 1792 (18.1) | 1744 (17.6) | 1735 (17.5) | 1740 (17.6) |
| Missing | 162 (1.6) | 152 (1.5) | 162 (1.6) | 164 (1.7) | 170 (1.7) |
| Cancer, n (%) | 1724 (17.4) | 1859 (18.8) | 1855 (18.7) | 1855 (18.7) | 1859 (18.8) |
| Depression, n (%) | 1895 (19.1) | 1837 (18.6) | 1876 (18.9) | 2006 (20.3) | 2014 (20.3) |
| Number of dietary assessments, n (%) | |||||
| 1 | 40 (0.4) | 14 (0.1) | 16 (0.2) | 15 (0.1) | 25 (0.3) |
| 2 | 93 (0.9) | 67 (0.7) | 65 (0.7) | 57 (0.6) | 91 (0.9) |
| 3 | 162 (1.6) | 141 (1.4) | 153 (1.5) | 135 (1.4) | 168 (1.7) |
| 4 | 357 (3.6) | 313 (3.2) | 274 (2.8) | 284 (2.9) | 380 (3.8) |
| 5 | 812 (8.2) | 691 (7.0) | 670 (6.8) | 686 (6.9) | 703 (7.1) |
| 6 | 1993 (20.1) | 1893 (19.1) | 1816 (18.3) | 1905 (19.2) | 1883 (19.0) |
| 7 | 6441 (65.1) | 6780 (68.5) | 6905 (69.8) | 6817 (68.9) | 6648 (67.2) |
| Missing year of SCD assessment, n (%) | |||||
| None | 8449 (85.4) | 8569 (86.6) | 8495 (85.8) | 8530 (86.2) | 8498 (85.9) |
| 2012 | 282 (2.8) | 229 (2.3) | 254 (2.6) | 269 (2.7) | 258 (2.6) |
| 2014 | 1167 (11.8) | 1101 (11.1) | 1150 (11.6) | 1100 (11.1) | 1142 (11.5) |
| Postmenopause and ever use hormone, n (%) | 7022 (70.9) | 7332 (74.0) | 7389 (74.6) | 7410 (74.9) | 7440 (75.2) |
| Parity, n (%) | |||||
| Nulliparous | 548 (5.5) | 530 (5.4) | 511 (5.2) | 490 (5.0) | 497 (5.0) |
| 1–2 | 738 (7.5) | 608 (6.1) | 645 (6.5) | 608 (6.1) | 690 (7.0) |
| 3+ | 8440 (85.3) | 8572 (86.6) | 8589 (86.8) | 8633 (87.2) | 8498 (85.9) |
| Missing | 172 (1.7) | 189 (1.9) | 154 (1.5) | 168 (1.7) | 213 (2.1) |
| Dietary intake, servings/d, mean ± SD | |||||
| Vegetable intake | 3.2 ± 1.6 | 3.4 ± 1.5 | 3.5 ± 1.5 | 3.7 ± 1.6 | 3.9 ± 1.7 |
| Fruit intake | 1.5 ± 0.9 | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.6 ± 0.8 | 1.6 ± 0.8 |
| Fruit juice intake | 0.8 ± 0.6 | 0.8 ± 0.5 | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.5 ± 0.4 |
| Sweets/desserts intake | 1.8 ± 1.1 | 1.4 ± 0.9 | 1.2 ± 0.7 | 1.0 ± 0.6 | 0.7 ± 0.5 |
| SSB intake | 0.5 ± 0.6 | 0.3 ± 0.4 | 0.2 ± 0.3 | 0.2 ± 0.2 | 0.1 ± 0.2 |
| HPFS (27,842 men) | (n = 5568) | (n = 5569) | (n = 5568) | (n = 5569) | (n = 5568) |
| Protein intake, % energy, mean ± SD | 14.7 ± 1.1 | 16.6 ± 0.4 | 17.8 ± 0.3 | 19.1 ± 0.4 | 21.5 ± 1.6 |
| Age at study baseline, y, mean ± SD | 51.4 ± 8.4 | 51.1 ± 8.3 | 50.9 ± 8.2 | 51.0 ± 8.1 | 50.8 ± 7.9 |
| BMI, kg/m2, mean ± SD | 25.2 ± 2.9 | 25.7 ± 3.1 | 25.8 ± 3.2 | 26.2 ± 3.3 | 26.6 ± 3.6 |
| Total energy intake, kcal/d, mean ± SD | 2124 ± 541 | 2078 ± 514 | 2034 ± 507 | 1943 ± 485 | 1785 ± 460 |
| Total carbohydrate, % energy, mean ± SD | 51.9 ± 7.7 | 50.3 ± 6.8 | 49.7 ± 6.6 | 49.0 ± 6.5 | 47.3 ± 6.9 |
| Total fat, % energy, mean ± SD | 30.3 ± 5.2 | 30.8 ± 5.1 | 30.8 ± 5.1 | 30.6 ± 5.2 | 30.3 ± 5.6 |
| Alcohol, g/d, mean ± SD | 15.9 ± 17.4 | 13.0 ± 13.5 | 11.0 ± 11.5 | 9.3 ± 10.0 | 7.1 ± 8.0 |
| Physical activity, MET-h/wk, mean ± SD | 28.4 ± 22.4 | 29.0 ± 21.3 | 29.0 ± 21.0 | 28.4 ± 20.4 | 27.6 ± 20.3 |
| Smoking pack-years, n (%) | |||||
| Never smoked | 2703 (48.6) | 2748 (49.3) | 2786 (50.0) | 2770 (49.7) | 2711 (48.7) |
| ≤24 pack-years | 1600 (28.7) | 1622 (29.1) | 1548 (27.8) | 1638 (29.4) | 1635 (29.4) |
| 25–44 pack-years | 657 (11.8) | 622 (11.2) | 623 (11.2) | 611 (11.0) | 654 (11.7) |
| ≥45 pack-years | 334 (6.0) | 292 (5.3) | 305 (5.5) | 256 (4.6) | 235 (4.2) |
| Missing | 274 (4.9) | 285 (5.1) | 306 (5.5) | 294 (5.3) | 333 (6.0) |
| Cancer, n (%) | 840 (15.1) | 898 (16.1) | 891 (16.0) | 855 (15.4) | 860 (15.4) |
| Depression, n (%) | 336 (6.0) | 314 (5.6) | 314 (5.6) | 293 (5.3) | 300 (5.4) |
| Number of dietary assessments, n (%) | |||||
| 1 | 123 (2.2) | 82 (1.5) | 90 (1.6) | 110 (2.0) | 223 (4.0) |
| 2 | 253 (4.5) | 232 (4.2) | 220 (4.0) | 229 (4.1) | 349 (6.3) |
| 3 | 434 (7.8) | 449 (8.1) | 461 (8.3) | 460 (8.3) | 568 (10.2) |
| 4 | 1010 (18.1) | 941 (16.9) | 983 (17.7) | 1040 (18.7) | 1128 (20.2) |
| 5 | 3748 (67.3) | 3865 (69.4) | 3814 (68.5) | 3730 (67.0) | 3300 (59.3) |
| Missing year of SCD assessment, n (%) | |||||
| None | 4131 (74.2) | 4122 (74.0) | 4050 (72.7) | 4002 (71.9) | 3846 (69.1) |
| 2008 | 423 (7.6) | 418 (7.5) | 463 (8.3) | 508 (9.1) | 599 (10.8) |
| 2012 | 1014 (18.2) | 1029 (18.5) | 1055 (18.9) | 1059 (19.0) | 1123 (20.2) |
| Profession, n (%) | |||||
| Dentist | 2874 (51.6) | 3013 (54.1) | 3207 (57.6) | 3336 (59.9) | 3535 (63.5) |
| Pharmacist | 653 (11.7) | 523 (9.4) | 467 (8.4) | 376 (6.7) | 328 (5.9) |
| Optometrist | 390 (7.0) | 381 (6.8) | 383 (6.9) | 375 (6.7) | 360 (6.5) |
| Osteopath | 212 (3.8) | 217 (3.9) | 187 (3.4) | 256 (4.6) | 259 (4.7) |
| Podiatrist | 122 (2.2) | 107 (1.9) | 131 (2.3) | 140 (2.5) | 197 (3.5) |
| Veterinarian | 1317 (23.7) | 1328 (23.8) | 1193 (21.4) | 1086 (19.5) | 889 (16.0) |
| Dietary intake, servings/d, mean ± SD | |||||
| Vegetable | 3.2 ± 1.7 | 3.5 ± 1.7 | 3.6 ± 1.7 | 3.7 ± 1.7 | 3.8 ± 1.9 |
| Fruit | 1.7 ± 1.2 | 1.7 ± 1.1 | 1.7 ± 1.0 | 1.7 ± 1.0 | 1.6 ± 1.0 |
| Fruit juice | 0.9 ± 0.8 | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.8 ± 0.6 | 0.6 ± 0.5 |
| Sweets/desserts | 2.0 ± 1.4 | 1.6 ± 1.1 | 1.4 ± 0.9 | 1.2 ± 0.8 | 0.8 ± 0.6 |
| SSB | 0.6 ± 0.7 | 0.4 ± 0.5 | 0.3 ± 0.4 | 0.2 ± 0.3 | 0.2 ± 0.2 |
Except for age at baseline, values of means or percentages are standardized to the age distribution of the study population. All values are averages over the follow-up period except for age at baseline. HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent; Q, quintile; NHS, Nurses’ Health Study; SCD, subjective cognitive decline; SSB, sugar-sweetened beverage.
The strong associations between the homozygous APOEℇ4 genotype and poor SCD scores in both the NHS and HPFS support the validity of this score (27). Among men, the age-standardized prevalence of the homozygous APOE ℇ4 genotype was 1.0% among participants with a good SCD score (0 points) and 4.6% among those with a poor SCD score (≥3 points; P-trend < 0.001) (27); among women, the prevalence was 1.3% for those with a good SCD score and 2.4% for those with a poor SCD score (29). Also, numerous known risk factors for dementia, such as high blood pressure, high blood cholesterol, depression, cardiovascular disease, type 2 diabetes, and heavy smoking, were all associated with poor subsequent SCD scores in our studies (27).
Covariates
Starting from baseline and in follow-up questionnaires, information on covariates was collected prospectively in the NHS and HPFS. These covariates include age, race, BMI (in kg/m2), physical activity, multivitamin use, smoking status, alcohol consumption, history of cancer, history of cardiovascular disease (CVD), high blood pressure, elevated cholesterol, diabetes, family history of dementia, and depression. For the NHS, additional information on education, husband's education, census tract income, menopausal status and the use of hormone replacement therapy, and parity was obtained. For the HPFS, we also used information on specific health professions.
Population for analysis
For both NHS and HPFS, we excluded participants with extreme energy intakes (<600 or >3500 kcal/d for women and <800 or >4200 kcal/d for men), those with >70 food items blank, and individuals who developed Parkinson disease (PD) prior to SCD assessments because patients with PD may also present with cognitive impairment. The final analysis included 49,493 women with a mean age of 48 y at baseline in 1984 and 27,842 men with a mean age of 51 y at enrollment in 1986 (Supplementary Figure 1). Compared with participants who were excluded, participants included in the analysis were younger (Supplementary Table 1).
Statistical analysis
Intake of protein was expressed as the percentage of total energy and was classified into quintiles in each cohort. Age-standardized characteristics of participants were calculated according to quintiles of total protein intake. Average SCD score was calculated from the 2 SCD assessments and was used as our primary outcome. Due to the distribution and nature of the SCD scores, Poisson regression was used to estimate the associations between protein, amino acids, and protein food intakes with SCD. ORs (95% CIs) for a 3-unit increment in SCD were calculated because 3 or more positive SCD questions have been used to indicate poor cognitive function (4, 5). Covariate information from the same time frame as dietary assessments was used (1984–2006 for the NHS and 1986–2002 for the HPFS). Both a quadratic term and linear term for age were included in all models because the relation between age and SCD was nonlinear. In multivariate analyses, the average age at the 2 SCD assessments, total energy intake, race, smoking history, depression, physical activity level, BMI, alcohol intake, family history of dementia, an indicator for having only 1 of the 2 SCD assessments, number of dietary assessments during the follow-up period, and use of multivitamin were included as covariates. For the NHS, parity, postmenopausal status and hormone replacement therapy use, census tract income, education, and husband's education were also included in the analyses, whereas specific profession was included in the HPFS. Potential mediators on the causal pathway, including hypertension, diabetes, elevated cholesterol, and CVD, were not adjusted in our primary analysis, although results remained similar when these variables were included in the models.
For protein substitution analysis, isocaloric substitution models were built, which simultaneously included total energy intake, percentage of energy intake from protein, percentage of energy from trans fat, saturated fat, MUFA, and PUFA. The coefficients from the substitution models can be interpreted as the associations when replacing the percentage of energy intake from protein for the same percentage of energy from carbohydrates. For substitution of animal protein with plant protein, the difference between the β-coefficients was exponentiated, and the variances and covariance of the 2 types of proteins were used to estimate the 95% CI (30). To examine whether the associations were independent of other dietary factors in our protein analyses, we further adjusted for intakes of carotenoids, anthocyanins, and vitamins C, D, and E. The same dietary factors were controlled in analyses of amino acid intakes. Sensitivity analyses were done by only including participants with both SCD assessments and also adjusting for flavonoid subclasses, which had significant inverse associations with SCD in our cohorts (31).
For food-based analyses, all aforementioned nondietary factors and intakes of total vegetables, fruit, fruit juice, sugar-sweetened beverages, and sweets/desserts were adjusted in the final model. Linear trends were tested by assigning median values within each quintile and modeling these variables continuously.
We further investigated whether the associations between protein and protein-containing food intakes with SCD differed by baseline age (<50 y, ≥50 y), smoking status (never smokers, past smokers, and current smokers), disease status (self-reported depression, CVD, and type 2 diabetes), and APOE ℇ4 allele carrier status (yes/no) in a subgroup of participants who had their APOE ℇ4 measured or imputed from a genome-wide association analysis.
Temporal relations between specific sources of protein intake and SCD were evaluated. We estimated the associations between dietary intake at each of the individual years with SCD. Also, both recent (the average intake from 2002–2006 in the NHS and average intake from 1998–2002 for the HPFS) and remote (the average intake from 1984–1990 in the NHS and average intake from 1986–1990 for the HPFS) intakes were mutually included in the same model to examine whether these associations were independent of each other. Covariates closest in time to the dietary assessments were used in these analyses.
Analyses were performed separately for the NHS and HPFS, and an inverse variance–weighted, meta-analysis was used to combine the results across cohorts. Because our analyses included multiple comparisons, we interpreted our findings using the conservative Bonferroni correction. All analyses were performed using SAS software, version 9.2 (SAS Institute). Figures were generated by Prism, version 8.0.0 (GraphPad Software).
Results
Population characteristics
The characteristics of study participants are shown in Table 1. For both the NHS and HPFS, participants with higher total protein intake (as a percentage of energy) had higher BMI, less alcohol consumption, and a higher vegetable intake but lower intake of sweets/desserts.
Protein analysis
Higher intakes of total protein, animal protein, and plant protein were significantly associated with lower odds of SCD in both the NHS and HPFS (Table 2). Adjusting for total energy intake (which was positively associated with SCD) and major nondietary factors attenuated the magnitude of these associations. Comparing the highest with the lowest quintiles of intakes, the pooled multivariate ORs (95% CIs) of a 3-unit increment in SCD were 0.84 (0.76, 0.91), P-trend < 0.0001 for total protein; 0.86 (0.79, 0.94), P-trend < 0.0001 for animal protein; and 0.84 (0.76, 0.91), P-trend = 0.0007 for plant protein.
TABLE 2.
OR (95% CI) for the associations between specific sources of protein intakes with subjective cognitive decline in the NHS and HPFS1
| Quintile of protein intake | |||||||
|---|---|---|---|---|---|---|---|
| Characteristic | Q1 | Q2 | Q3 | Q4 | Q5 | P-trend | Continuous2 |
| Total protein | 5% energy | ||||||
| NHS | |||||||
| Median intake, % of energy | 15.17 | 16.77 | 17.92 | 19.12 | 20.99 | ||
| Age-adjusted model | Reference | 0.96 (0.88, 1.03) | 0.94 (0.87, 1.02) | 0.85 (0.78, 0.92) | 0.77 (0.71, 0.84) | <0.0001 | 0.84 (0.79, 0.89) |
| Age- and calorie-adjusted model | Reference | 0.96 (0.89, 1.04) | 0.97 (0.90, 1.05) | 0.89 (0.82, 0.97) | 0.84 (0.77, 0.92) | 0.0002 | 0.90 (0.85, 0.95) |
| Above + nondietary factors adjusted (MV1) | Reference | 0.98 (0.91, 1.06) | 0.98 (0.90, 1.06) | 0.89 (0.82, 0.97) | 0.85 (0.77, 0.92) | 0.0002 | 0.90 (0.84, 0.95) |
| Above + dietary factors adjusted (MV2) | Reference | 1.00 (0.92, 1.08) | 1.00 (0.92, 1.09) | 0.91 (0.83, 0.99) | 0.84 (0.77, 0.93) | 0.0003 | 0.90 (0.84, 0.96) |
| HPFS | |||||||
| Median intake, % of energy | 14.93 | 16.61 | 17.79 | 19.05 | 21.06 | ||
| Age-adjusted model | Reference | 1.02 (0.90, 1.16) | 0.84 (0.74, 0.96) | 0.86 (0.75, 0.98) | 0.74 (0.64, 0.85) | <0.0001 | 0.82 (0.75, 0.89) |
| Age- and calorie-adjusted model | Reference | 1.04 (0.92, 1.18) | 0.87 (0.77, 1.00) | 0.92 (0.81, 1.05) | 0.84 (0.73, 0.96) | 0.0062 | 0.90 (0.82, 0.98) |
| Above + nondietary factors adjusted (MV1) | Reference | 1.05 (0.92, 1.19) | 0.87 (0.76, 0.99) | 0.92 (0.81, 1.06) | 0.84 (0.73, 0.97) | 0.008 | 0.90 (0.82, 0.99) |
| Above + dietary factors adjusted (MV2) | Reference | 1.06 (0.93, 1.20) | 0.87 (0.76, 1.00) | 0.93 (0.81, 1.07) | 0.82 (0.71, 0.96) | 0.0055 | 0.88 (0.80, 0.98) |
| Meta-analyzed results (MV2) | Reference | 1.00 (0.94, 1.09) | 0.97 (0.89, 1.03) | 0.91 (0.86, 0.97) | 0.84 (0.76, 0.91) | <0.0001 | 0.89 (0.85, 0.94) |
| Animal protein | 5% energy | ||||||
| NHS | |||||||
| Median intake, % of energy | 9.71 | 11.35 | 12.49 | 13.72 | 15.61 | ||
| Age-adjusted model | Reference | 1.07 (0.99, 1.15) | 0.98 (0.91, 1.06) | 0.92 (0.85, 1.00) | 0.80 (0.73, 0.87) | <0.0001 | 0.84 (0.79, 0.89) |
| Age- and calorie-adjusted model | Reference | 1.07 (0.99, 1.16) | 1.01 (0.93, 1.09) | 0.97 (0.89, 1.05) | 0.88 (0.80, 0.96) | 0.0015 | 0.90 (0.85, 0.96) |
| Above + nondietary factors adjusted (MV1) | Reference | 1.07 (0.99, 1.16) | 1.01 (0.93, 1.10) | 0.94 (0.86, 1.02) | 0.87 (0.79, 0.95) | 0.0007 | 0.89 (0.84, 0.95) |
| Above + dietary factors adjusted (MV2) | Reference | 1.08 (1.00, 1.17) | 1.02 (0.94, 1.11) | 0.95 (0.87, 1.03) | 0.86 (0.79, 0.95) | 0.0009 | 0.89 (0.84, 0.96) |
| HPFS | |||||||
| Median intake, % of energy | 9.35 | 11.11 | 12.36 | 13.65 | 15.78 | ||
| Age-adjusted model | Reference | 1.11 (0.97, 1.26) | 0.87 (0.77, 1.00) | 0.90 (0.78, 1.03) | 0.78 (0.68, 0.90) | <0.0001 | 0.82 (0.75, 0.89) |
| Age- and calorie-adjusted model | Reference | 1.12 (0.98, 1.27) | 0.91 (0.80, 1.04) | 0.96 (0.83, 1.09) | 0.89 (0.77, 1.02) | 0.0344 | 0.90 (0.82, 0.98) |
| Above + nondietary factors adjusted (MV1) | Reference | 1.11 (0.97, 1.26) | 0.89 (0.78, 1.02) | 0.94 (0.82, 1.08) | 0.86 (0.74, 0.99) | 0.0145 | 0.89 (0.81, 0.97) |
| Above + dietary factors adjusted (MV2) | Reference | 1.11 (0.98, 1.27) | 0.90 (0.78, 1.03) | 0.94 (0.82, 1.09) | 0.84 (0.72, 0.98) | 0.0094 | 0.88 (0.79, 0.97) |
| Meta-analyzed results (MV2) | Reference | 1.09 (1.03, 1.16) | 1.00 (0.91, 1.06) | 0.94 (0.89, 1.03) | 0.86 (0.79, 0.94) | <0.0001 | 0.89 (0.84, 0.94) |
| Plant protein | 5% energy | ||||||
| NHS | |||||||
| Median intake, % of energy | 4.43 | 4.97 | 5.36 | 5.77 | 6.47 | ||
| Age-adjusted model | Reference | 0.80 (0.73, 0.86) | 0.77 (0.71, 0.84) | 0.76 (0.70, 0.83) | 0.68 (0.62, 0.75) | <0.0001 | 0.50 (0.41, 0.60) |
| Age and calorie-adjusted model | Reference | 0.80 (0.74, 0.87) | 0.78 (0.72, 0.85) | 0.77 (0.71, 0.84) | 0.71 (0.65, 0.79) | <0.0001 | 0.55 (0.45, 0.67) |
| Above + nondietary factors adjusted (MV1) | Reference | 0.83 (0.77, 0.90) | 0.83 (0.76, 0.91) | 0.82 (0.75, 0.90) | 0.76 (0.69, 0.84) | <0.0001 | 0.64 (0.52, 0.79) |
| Above + dietary factors adjusted (MV2) | Reference | 0.89 (0.81, 0.96) | 0.90 (0.83, 0.98) | 0.91 (0.83, 1.00) | 0.85 (0.76, 0.94) | 0.0069 | 0.78 (0.63, 0.97) |
| HPFS | |||||||
| Median intake, % of energy | 4.24 | 4.87 | 5.35 | 5.87 | 6.78 | ||
| Age-adjusted model | Reference | 0.84 (0.74, 0.96) | 0.81 (0.71, 0.93) | 0.77 (0.66, 0.89) | 0.61 (0.52, 0.73) | <0.0001 | 0.44 (0.33, 0.58) |
| Age- and calorie-adjusted model | Reference | 0.85 (0.75, 0.97) | 0.82 (0.72, 0.95) | 0.79 (0.68, 0.91) | 0.64 (0.54, 0.76) | <0.0001 | 0.48 (0.36, 0.64) |
| Above + nondietary factors adjusted (MV1) | Reference | 0.88 (0.77, 1.01) | 0.85 (0.74, 0.98) | 0.83 (0.71, 0.97) | 0.71 (0.59, 0.85) | 0.0004 | 0.54 (0.40, 0.74) |
| Above + dietary factors adjusted (MV2) | Reference | 0.94 (0.82, 1.08) | 0.94 (0.82, 1.09) | 0.94 (0.80, 1.10) | 0.82 (0.68, 0.98) | 0.0422 | 0.66 (0.48, 0.91) |
| Meta-analyzed results (MV2) | Reference | 0.91 (0.84, 0.97) | 0.91 (0.84, 0.97) | 0.91 (0.84, 1.00) | 0.84 (0.76, 0.91) | 0.0007 | 0.74 (0.62, 0.88) |
Age-adjusted model: adjusted for age [at subjective cognitive decline (SCD) measurement, continuous, with a linear and a quadratic term, years]. Age- and calorie-adjusted model: adjusted for age and total calorie intake (kcal, continuous). Multivariate model 1 (nondietary factors): NHS: further adjusted for census tract income ($50,000, $50,000–$69,999, or $70,000/y), education (registered nursing degrees, bachelor's degree, master's or doctorate degree), husband's education (high school or lower education, college, graduate school), race (white, black, other), smoking history (never, ≤4 pack-years, 5–24 pack-years, ≥25 pack-years), depression, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–25, 25–30, >30 kg/m2) from 1984 to 2006, intakes of alcohol (g/d), postmenopausal status and hormone replacement therapy use, family history of dementia, missing indicator for SCD measurement at 2012 or 2014, number of dietary assessments during 1984–2006, multivitamin use (yes/no), parity (nulliparous, 1–2, >2). HPFS: further adjusted for smoking history (never, 1–24 pack-years, 25–44 pack-years, ≥45 pack-years), cancer (yes/no), depression, family history of dementia, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–24.9, 25–29.9, ≥30 kg/m2) from 1986 to 2002, multivitamin use (yes/no), intake of alcohol (g/d), profession (dentist, pharmacist, optometrist, osteopath, podiatrist, veterinarian), missing indicator for SCD measurement at 2008 or 2012, and number of dietary assessments during 1986–2002. Multivariate model 2 (dietary factors): in addition to variables adjusted in MV1, further adjusted for carotenoids (quintiles), anthocyanins (quintiles), and vitamins C, D, and E (quintiles). HPFS, Health Professionals Follow-up Study; MET, metabolic equivalent; Q, quintile; MV1, multivariate model 1; MV2, multivariate model 2; NHS, Nurses’ Health Study.
Indicates OR of 3-unit increments in SCD when replacing each 5% of energy intake from specific protein with the same amount of energy from total carbohydrates. All models for protein adjusted for percentage of energy intake from trans fat, saturated fat, MUFA, and PUFA. Percentages of energy from animal and plant protein were mutually adjusted. Poisson regression was used for data analysis. Pearson χ2 for Poisson regression = 74,073.8, degrees of freedom = 49,310; pseudo-R2 = 0.3655 for NHS animal and plant protein multivariate model 2. Pearson χ2 for Poisson regression = 74,080.4, degrees of freedom = 49,314; pseudo-R2 = 0.3655 for NHS total protein multivariate model 2. Pearson χ2 for Poisson regression = 40,524.2, degrees of freedom = 27,753; pseudo-R2 = 0.2475 for HPFS animal and plant protein multivariate model 2. Pearson χ2 for Poisson regression = 40,543.2, degrees of freedom = 27,757; pseudo-R2 = 0.2475 for HPFS animal and plant protein multivariate model 2.
When substituting each 5% of energy intake from protein for the equivalent percentage of energy from total carbohydrates, the pooled multivariate ORs (95% CIs) were 0.89 (0.85, 0.94) for total protein, 0.89 (0.84, 0.94) for animal protein, and 0.74 (0.62, 0.88) for plant protein. When substituting every 5% of energy from animal protein with plant protein, the pooled multivariable-adjusted OR (95% CI) was 0.84 (0.72, 0.97) (data not shown). Similar results were observed in the sensitivity analysis, which only included participants with both SCD assessments and when also adjusted for flavonoid subclasses. Results were similar across strata of baseline age, smoking status, disease status, and APOE ℇ4 allele carrier status.
Top food contributors to total protein and animal protein in our cohorts during the follow-up period were chicken without skin, fish, low-fat milk, and beef. Nuts, beans/legumes, dark bread, and cold breakfast cereal were major contributors to plant protein (Supplementary Table 2).
Amino acids
After adjusting for total energy intake, the positive associations between amino acid intakes and SCD became inverse or null. In both the NHS and HPFS, higher intake of proline was significantly associated with lower odds of SCD in the fully adjusted model (Supplementary Table 3). In the pooled results, amino acid intakes were associated with 11–26% lower odds of SCD.
Protein food and food group analysis
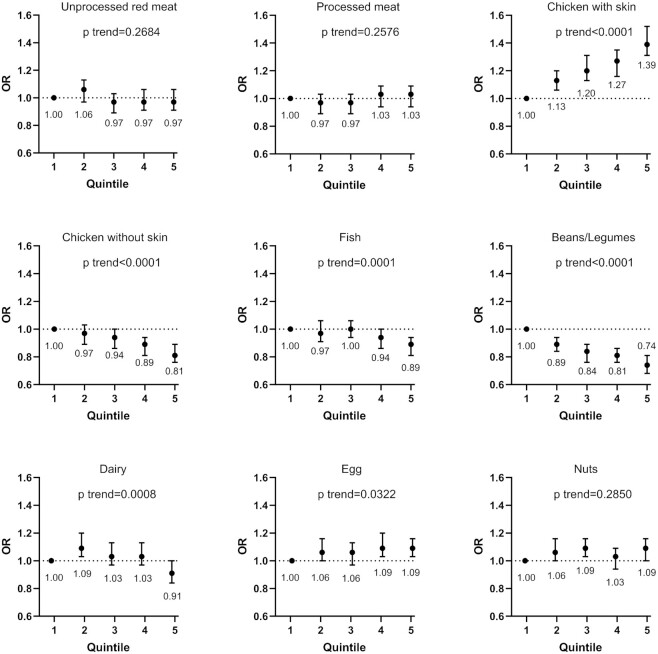
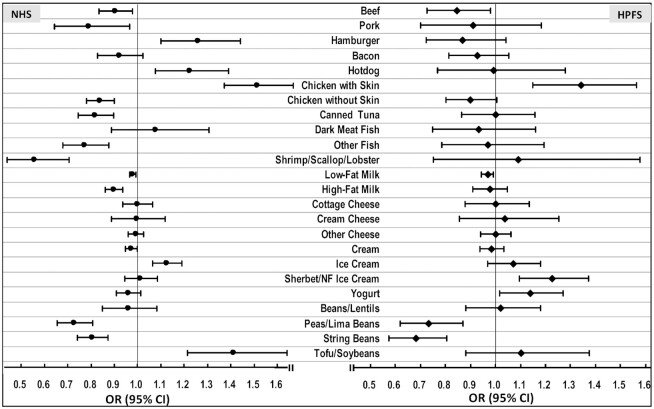
In a fully adjusted model, higher intakes of chicken without skin, fish, and beans/legumes were significantly associated with lower odds of SCD in the pooled results [multivariable-adjusted ORs (95% CIs) for each 3-serving/wk increase in intake were 0.86 (0.81, 0.91) for chicken without skin, 0.93 (0.89, 0.97) for fish, and 0.62 (0.54, 0.70) for beans/legumes; Figure 1, Supplementary Table 4]. Associations between individual protein foods and SCD are shown in Figure 2. Among foods selected as independent prospective predictors of SCD using stepwise regression, shrimp/scallop/lobster [the pooled multivariable-adjusted OR (95% CI) for each 3-serving/wk increase in intake = 0.68 (0.55, 0.84)], peas/lima beans (0.72 [0.66, 0.79]), and string beans (0.79 [0.72, 0.84]) had the strongest inverse associations with SCD. Hotdogs (1.16 [1.06, 1.31]) had a significant positive association with SCD.
FIGURE 1.
ORs of a 3-unit increment in subjective cognitive decline (SCD), associated with protein food groups in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). Multivariate model: NHS: adjusted for age (at SCD assessment, continuous, with a linear and a quadratic term, years), total energy intake (kcal, continuous), census tract income ($50,000, $50,000–$69,999, or $70,000/y), education (registered nursing degrees, bachelor's degree, master's or doctorate degree), husband's education (high school or lower education, college, graduate school), race (white, black, other), smoking history (never, ≤4 pack-years, 5–24 pack-years, ≥25 pack-years), depression, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–25, 25–30, >30 kg/m2) from 1984 to 2006, intakes of alcohol (g/d), postmenopausal status and hormone replacement therapy use, family history of dementia, missing indicator for SCD measurement at 2012 or 2014, number of dietary assessments during 1984–2006, multivitamin use (yes/no), and parity (nulliparous, 1–2, >2). HPFS: adjusted for age, total energy intake, smoking history (never, 1–24 pack-years, 25–44 pack-years, ≥45 pack-years), cancer (yes/no), depression, family history of dementia, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–24.9, 25–29.9, ≥30 kg/m2) from 1986 to 2002, multivitamin use (yes/no), intake of alcohol (g/d), profession (dentist, pharmacist, optometrist, osteopath, podiatrist, veterinarian), missing indicator for SCD measurement at 2008 or 2012, and number of dietary assessments during 1986–2002. Both cohorts also adjusted for dietary intakes of total vegetables, fruit, fruit juice, sugar-sweetened beverages, and sweets/desserts. Poisson regression was used for data analysis.
FIGURE 2.
ORs (95% CIs) of a 3-unit increment in subjective cognitive decline (SCD), associated with individual protein food sources in the Nurses’ Health Study (NHS) and Health Professionals Follow-up Study (HPFS). For each 3-serving/wk of protein foods as continuous variables. Multivariate model: NHS: adjusted for age (at SCD assessment, continuous, with a linear and a quadratic term, years), total energy intake (kcal, continuous), census tract income ($50,000, $50,000–$69,999, or $70,000/y), education (registered nursing degrees, bachelor's degree, master's or doctorate degree), husband's education (high school or lower education, college, graduate school), race (white, black, other), smoking history (never, ≤4 pack-years, 5–24 pack-years, ≥25 pack-years), depression, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–25, 25-30, >30 kg/m2) from 1984 to 2006, intakes of alcohol (g/d), postmenopausal status and hormone replacement therapy use, family history of dementia, missing indicator for SCD measurement at 2012 or 2014, number of dietary assessments during 1984–2006, multivitamin use (yes/no), and parity (nulliparous, 1–2, >2). HPFS: adjusted for age, total energy intake, smoking history (never, 1–24 pack-years, 25–44 pack-years, ≥45 pack-years), cancer (yes/no), depression, family history of dementia, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–24.9, 25–29.9, ≥30 kg/m2) from 1986 to 2002, multivitamin use (yes/no), intake of alcohol (g/d), profession (dentist, pharmacist, optometrist, osteopath, podiatrist, veterinarian), missing indicator for SCD measurement at 2008 or 2012, and number of dietary assessments during 1986–2002. Both cohorts also adjusted for dietary intakes of total vegetables, fruit, fruit juice, sugar-sweetened beverages, and sweets/desserts. Poisson regression was used for data analysis.
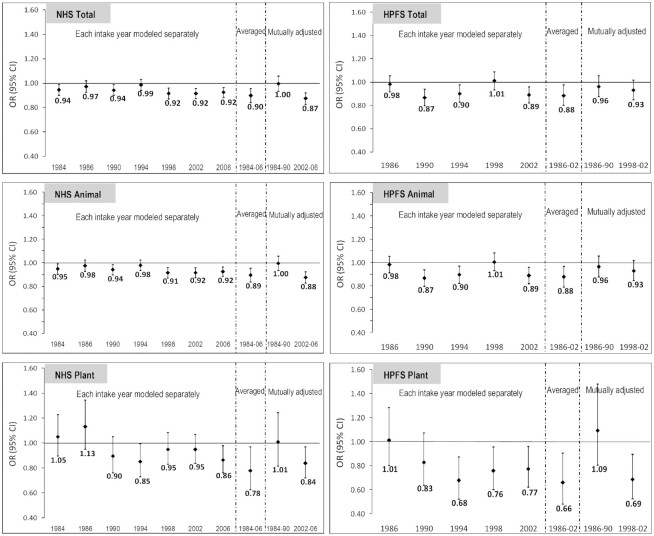
Temporal relations
Intakes of total, animal, and plant protein in recent years were associated with lower odds of SCD in both the NHS and HPFS (Figure 3). The average intakes had the strongest inverse associations. Dietary intake of protein before and after SCD assessments was compared, and no major dietary change was found.
FIGURE 3.
Intakes of specific sources of protein at each year of dietary assessment and OR of a 3-unit increment in subjective cognitive decline (SCD). Substituting every 5% of energy intake from each specific protein for the same amount of energy from total carbohydrates. Multivariate model: Nurses’ Health Study (NHS): adjusted for age (at SCD assessment, continuous, with a linear and a quadratic term, years), total energy intake (kcal, continuous), census tract income ($50,000, $50,000–$69,999, or $70,000/y), education (registered nursing degrees, bachelor's degree, master's or doctorate degree), husband's education (high school or lower education, college, graduate school), race (white, black, other), smoking history (never, ≤4 pack-years, 5–24 pack-years, ≥24 pack-years), depression, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–25, 25–30, >30 kg/m2) from 1984 to 2006, intakes of alcohol (g/d), postmenopausal status and hormone replacement therapy use, family history of dementia, missing indicator for SCD measurement at 2012 or 2014, number of dietary assessments during 1984–2006, multivitamin use (yes/no), parity (nulliparous, 1–2, >2). Health Professionals Follow-up Study (HPFS): adjusted for age, total energy intake, smoking history (never, 1–24 pack-years, 25–44 pack-years, ≥45 pack-years), cancer (yes/no), depression, family history of dementia, physical activity level (metabolic equivalent–h/wk, quintiles), BMI (<23, 23–24.9, 25–29.9, ≥30 kg/m2) from 1986 to 2002, multivitamin use (yes/no), intake of alcohol (g/d), profession (dentist, pharmacist, optometrist, osteopath, podiatrist, veterinarian), missing indicator for SCD measurement at 2008 or 2012, and the number of dietary assessments during 1986–2002. Both cohorts also adjusted for intakes carotenoids (quintiles), anthocyanins (quintiles), and vitamins C, D, and E (quintiles). All models adjusted for the percentage of energy intake from trans fat, saturated fat, MUFA, and PUFA. The percentages of energy from animal and plant protein were mutually adjusted. Poisson regression was used for data analysis.
Discussion
Higher total, animal, and plant protein intakes compared with total carbohydrates were associated with lower odds of SCD; substituting animal protein for plant protein was also associated with lower odds. These findings are highly unlikely to be due to chance because of the extreme degree of statistical significance and consistency across the 2 cohorts. For major food sources of protein, higher intakes of beans/legumes, fish, and lean poultry were significantly associated with better late-life subjective cognitive function.
Current literature on the associations between protein intake and cognitive decline showed mixed results. Some cohort studies suggested that higher protein intake was associated with less cognitive decline (17, 32), whereas other studies found null results (16, 33). In a Chinese cross-sectional study, higher protein intake was associated with an increased risk of mild cognitive impairment (34). We found that substituting each 5% of energy from total protein for the same percentage of energy from total carbohydrates was associated with 11% lower odds of SCD. Plant protein had the strongest inverse association, with a 26% lower odds of SCD compared with total carbohydrates. In addition, for every 5% of energy intake from animal protein replaced with the equivalent amount of energy from plant protein, there was a 16% lower odds of SCD after adjusting for major nondietary factors (including socioeconomic factors) and other dietary factors (including trans fat, saturated fat, MUFA, PUFA, carotenoids, flavonoids, and vitamins C, D, and E). Especially in the older population, low protein intake can be associated with a higher risk of sarcopenia (35, 36) and frailty (14), which are closely linked to the development of cognitive impairment (37). Our results supported the hypothesis that plant-based protein may be a superior source of protein. We also found many of the amino acids were inversely associated with SCD. Proline was significantly associated with lower odds of SCD in both of our cohorts. Although a detailed mechanism is not yet known, a possible protective role has been suggested for a proline-rich polypeptide in preventing dementia progression (38). Two large neutral amino acids, tyrosine and tryptophan, were suggested to be potentially beneficial because they act as precursors of serotonin and catecholamine neurotransmitters (dopamine, norepinephrine, and epinephrine), but dietary supplementation trials with either amino acid were mostly short term and limited to only healthy young adults (39). In our analyses, inverse associations of these 2 amino acids with SCD were observed in the NHS but not the HPFS.
Results from other studies on the associations between various protein food sources and cognitive function have been mixed. For legume consumption, suggestions of better cognitive function were reported (19, 40), but no association was also observed (16), and poorer subsequent cognitive function was seen in other studies (20, 41). For fish intake, beneficial associations with cognitive function were reported (16, 42), but in the PAQUID (Personnes Agées QUID) study (42), the association became null after adjusting for education, and null results for fish consumption were also seen in other studies (18, 40) [an inverse association between fish consumption and dementia was seen only among APOE ℇ4 noncarriers in the Three-City cohort study (18)]. For other protein food sources, current evidence also remained inconclusive (16, 20, 41, 42). The discrepancies among these study results may be due to the difference in lengths of study follow-up, ages of the study participants, and dietary patterns in different study populations. Our results support previous studies that showed beneficial associations between beans/legumes, fish, and lean poultry with cognitive function and those that found harmful associations for processed meat. Plant-based protein foods had the lowest amounts of advanced glycation end products (AGEs), followed by poultry and fish, with processed meat containing the highest concentrations of AGEs among major protein sources (43). A low-AGE diet was found to be associated with significantly lower brain amyloid protein accumulation (44). In our findings, beans/legumes had the strongest inverse association with SCD, followed by lean poultry and fish, with processed meat having the least favorable association, consistent with these insights (43, 44) on AGEs. Different cooking methods may also affect AGE formation: cooking with high and dry heat such as frying or roasting resulted in significantly higher concentrations of AGE (43), which may have been reflected in the positive association between chicken with skin and SCD seen in our cohorts.
Over 20 y of follow-up is a major strength of the present study, which allows the capture of potentially important exposure windows and reduces the impact of reverse causation. Large sample sizes in both cohorts provided great power for detailed analyses. Dietary intake averaged from multiple dietary assessments over time reduced the effects of random error and within-person variations. Updating dietary data ceased 6 y prior to SCD assessments minimized the impact of altered cognitive function on diet. We also included comprehensive information on many possible confounders, and we adjusted for these variables to minimize residual confounding. There are some limitations in the current study. First, baseline cognitive function was not assessed in our cohorts. However, we can assume generally high baseline cognitive function in these participants during their early adulthood due to multiple admissions and board examinations that were required for practicing health professions. These highly educated participants may also have relatively good insights in reporting subtle cognitive changes (45). Second, objective cognitive assessment was not included in our study. However, SCD has been repeatedly validated and has been found to be strongly associated with both concurrent objective cognitive function (4, 5) and subsequent cognitive decline (5). Moreover, SCD can be more advantageous in detecting subtle cognitive changes (46), especially in those with higher education (6). Third, participants who completed the first but not the second SCD assessment might have more severe cognitive impairment. However, this scenario would bias the results toward the null, and in our sensitivity analysis including only participants with both SCD assessments, the results remained similar. Finally, limited generalizability could be an issue, because the study populations were mainly Caucasian and health care professionals, who may have better health awareness and relatively high cognitive function required for their occupations. However, this relatively uniform early life cognitive function in our study participants may reduce residual confounding.
In conclusion, adequate protein intake may be important for maintaining cognitive function, with plant-based protein being generally a superior source. Choice of protein foods could also be important; in particular, higher intake of beans/legumes, fish, and lean poultry may be beneficial for cognition maintenance. However, processed meat products, such as hotdogs, may be related to poor subsequent cognitive function. These findings could have important public health implications, and future studies are warranted to verify our results.
Supplementary Material
Acknowledgments
The authors’ responsibilities were as follows—TSY: designed and conducted the analysis, interpreted the data, and wrote the manuscript; CY: contributed to data analysis and completed the technical review of the results; AA, BAR, and DB: contributed to the interpretation of the results, provided critical feedback, and revised the manuscript for important intellectual content; WCW: designed the analysis, interpreted the results, revised the manuscript for important intellectual content, and supervised the project; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the NIH (UM1 CA186107, U01 167552).
Supplemental Figure 1 and Supplemental Tables 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AGE, advanced glycation end product; CVD, cardiovascular disease; HPFS, Health Professionals Follow-up Study; NHS, Nurses’ Health Study; PD, Parkinson disease; SCD, subjective cognitive decline; SFFQ, semiquantitative FFQ.
Contributor Information
Tian-Shin Yeh, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Changzheng Yuan, Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Department of Big Data and Health Science, School of Public Health, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.
Alberto Ascherio, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Bernard A Rosner, Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Biostatistics, Harvard T. H. Chan School of Public Health, Boston, MA, USA; Department of Medicine, Harvard Medical School, Boston, MA, USA.
Deborah Blacker, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, USA; Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Walter C Willett, Department of Epidemiology, Harvard T. H. Chan School of Public Health, Harvard University, Boston, MA, USA; Channing Division of Network Medicine, Brigham and Women's Hospital and Harvard Medical School, Boston, MA, USA; Department of Nutrition, Harvard T. H. Chan School of Public Health, Boston, MA, USA.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request.
References
- 1. Ahmadi-Abhari S, Guzman-Castillo M, Bandosz P, Shipley MJ, Muniz-Terrera G, Singh-Manoux A, Kivimäki M, Steptoe A, Capewell S, O'Flaherty M et al. Temporal trend in dementia incidence since 2002 and projections for prevalence in England and Wales to 2040: modelling study. BMJ. 2017;358:j2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yeh TS, Wang JD, Ku LE. Estimating life expectancy and lifetime healthcare costs for Alzheimer's disease in Taiwan: does the age of disease onset matter?. J Alzheimers Dis. 2020;73(1):307–15. [DOI] [PubMed] [Google Scholar]
- 3. Rabin LA, Smart CM, Amariglio RE. Subjective cognitive decline in preclinical Alzheimer's disease. Annu Rev Clin Psychol. 2017;13(1):369–96. [DOI] [PubMed] [Google Scholar]
- 4. Amariglio RE, Townsend MK, Grodstein F, Sperling RA, Rentz DM. Specific subjective memory complaints in older persons may indicate poor cognitive function. J Am Geriatr Soc. 2011;59(9):1612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Samieri C, Proust-Lima C, MG M, Okereke OI, Amariglio RE, Sperling RA, Rentz DM, Grodstein F. Subjective cognitive concerns, episodic memory, and the APOE epsilon4 allele. Alzheimers Dementia. 2014;10(6):752–759.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM. Subjective memory complaints, education, and risk of Alzheimer's disease. Alzheimers Dementia. 2007;3(2):92–7. [DOI] [PubMed] [Google Scholar]
- 7. Galluzzi S, Frisoni GB. Imaging, subjective complaints, and MCI: 30 years before. J Nutr Health Aging. 2008;12(Suppl 1):S80–3. [DOI] [PubMed] [Google Scholar]
- 8. Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, Iwatsubo T, Jack CR Jr, Kaye J, Montine TJ et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dementia. 2011;7(3):280–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tucker KL. Nutrient intake, nutritional status, and cognitive function with aging. Ann N Y Acad Sci. 2016;1367(1):38–49. [DOI] [PubMed] [Google Scholar]
- 10. Wu G. Dietary protein intake and human health. Food Function. 2016;7(3):1251–65. [DOI] [PubMed] [Google Scholar]
- 11. Levine ME, Suarez JA, Brandhorst S, Balasubramanian P, Cheng CW, Madia F, Fontana L, Mirisola MG, Guevara-Aguirre J, Wan J et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014;19(3):407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakagawa S, Lagisz M, Hector KL, Spencer HG. Comparative and meta-analytic insights into life extension via dietary restriction. Aging Cell. 2012;11(3):401–9. [DOI] [PubMed] [Google Scholar]
- 13. Lagiou P, Sandin S, Lof M, Trichopoulos D, Adami HO, Weiderpass E. Low carbohydrate-high protein diet and incidence of cardiovascular diseases in Swedish women: prospective cohort study. BMJ. 2012;344:e4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Morais JA, Chevalier S, Gougeon R. Protein turnover and requirements in the healthy and frail elderly. J Nutr Health Aging. 2006;10(4):272–83. [PubMed] [Google Scholar]
- 15. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—a review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840–51. [DOI] [PubMed] [Google Scholar]
- 16. Vercambre MN, Boutron-Ruault MC, Ritchie K, Clavel-Chapelon F, Berr C. Long-term association of food and nutrient intakes with cognitive and functional decline: a 13-year follow-up study of elderly French women. Br J Nutr. 2009;102(3):419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts RO, Roberts LA, Geda YE, Cha RH, Pankratz VS, O'Connor HM, Knopman DS, Petersen RC. Relative intake of macronutrients impacts risk of mild cognitive impairment or dementia. J Alzheimers Dis. 2012;32(2):329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barberger-Gateau P, Raffaitin C, Letenneur L, Berr C, Tzourio C, Dartigues JF, Alperovitch A. Dietary patterns and risk of dementia: the Three-City cohort study. Neurology. 2007;69(20):1921–30. [DOI] [PubMed] [Google Scholar]
- 19. Mazza E, Fava A, Ferro Y, Moraca M, Rotundo S, Colica C, Provenzano F, Terracciano R, Greco M, Foti D et al. Impact of legumes and plant proteins consumption on cognitive performances in the elderly. J Transl Med. 2017;15(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ozawa M, Shipley M, Kivimaki M, Singh-Manoux A, Brunner EJ. Dietary pattern, inflammation and cognitive decline: the Whitehall II prospective cohort study. Clin Nutr. 2017;36(2):506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Colditz GA, Manson JE, Hankinson SE. The Nurses' Health Study: 20-year contribution to the understanding of health among women. J Womens Health. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 22. Klipstein-Grobusch K, den Breeijen JH, Goldbohm RA, Geleijnse JM, Hofman A, Grobbee DE, Witteman JC. Dietary assessment in the elderly: validation of a semiquantitative food frequency questionnaire. Eur J Clin Nutr. 1998;52(8):588–96. [DOI] [PubMed] [Google Scholar]
- 23. Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet North Am Ed. 1991;338(8765):464–8. [DOI] [PubMed] [Google Scholar]
- 24. Bernstein AM, Rosner BA, Willett WC. Cereal fiber and coronary heart disease: a comparison of modeling approaches for repeated dietary measurements, intermediate outcomes, and long follow-up. Eur J Epidemiol. 2011;26(11):877–86. [DOI] [PubMed] [Google Scholar]
- 25. Al-Shaar L, Yuan C, Rosner B, Dean SB, Ivey KL, Clowry CM, Sampson LA, Barnett JB, Rood J, Harnack LJ et al. Reproducibility and validity of a semi-quantitative food frequency questionnaire in men assessed by multiple methods. Am J Epidemiol. 2021;190(6):1122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan C, Spiegelman D, Rimm EB, Rosner BA, Stampfer MJ, Barnett JB, Chavarro JE, Rood JC, Harnack LJ, Sampson LK et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yuan C, Fondell E, Bhushan A, Ascherio A, Okereke OI, Grodstein F, Willett WC. Long-term intake of vegetables and fruits and subjective cognitive function in US men. Neurology. 2019;92(1):e63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Molinuevo JL, Rabin LA, Amariglio R, Buckley R, Dubois B, Ellis KA, Ewers M, Hampel H, Kloppel S, Rami L et al. Implementation of subjective cognitive decline criteria in research studies. Alzheimers Dementia. 2017;13(3):296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan C, Fondell E, Ascherio A, Okereke OI, Grodstein F, Hofman A, Willett WC. Long-term intake of dietary carotenoids is positively associated with late-life subjective cognitive function in a prospective study in US women. J Nutr. 2020;150(7):1871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Würtz AML, Jakobsen MU, Bertoia ML, Hou T, Schmidt EB, Willett WC, Overvad K, Sun Q, Manson JE, Hu FB et al. Replacing the consumption of red meat with other major dietary protein sources and risk of type 2 diabetes mellitus: a prospective cohort study. Am J Clin Nutr. 2021;113(3):612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yeh TS, Yuan C, Ascherio A, Rosner BA, Willett WC, Blacker D. Long-Term Dietary Flavonoid Intake and Subjective Cognitive Decline in US Men and Women. Neurology. 2021;97(10):1–16.. doi:10.1212/WNL.0000000000012454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shang X, Hill E, Li Y, He M. Energy and macronutrient intakes at breakfast and cognitive declines in community-dwelling older adults: a 9-year follow-up cohort study. Am J Clin Nutr. 2021;113(5):1093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deschamps V, Astier X, Ferry M, Rainfray M, Emeriau JP, Barberger-Gateau P. Nutritional status of healthy elderly persons living in Dordogne, France, and relation with mortality and cognitive or functional decline. Eur J Clin Nutr. 2002;56(4):305–12. [DOI] [PubMed] [Google Scholar]
- 34. Ding B, Xiao R, Ma W, Zhao L, Bi Y, Zhang Y. The association between macronutrient intake and cognition in individuals aged under 65 in China: a cross-sectional study. BMJ Open. 2018;8(1):e018573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bopp MJ, Houston DK, Lenchik L, Easter L, Kritchevsky SB, Nicklas BJ. Lean mass loss is associated with low protein intake during dietary-induced weight loss in postmenopausal women. J Am Diet Assoc. 2008;108(7):1216–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, Lee JS, Sahyoun NR, Visser M, Kritchevsky SB. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150–5. [DOI] [PubMed] [Google Scholar]
- 37. Chang KV, Hsu TH, Wu WT, Huang KC, Han DS. Association between sarcopenia and cognitive impairment: a systematic review and meta-analysis. J Am Med Dir Assoc. 2016;17(12):1164.e7–1164.e15. [DOI] [PubMed] [Google Scholar]
- 38. Bilikiewicz A, Gaus W. Colostrinin (a naturally occurring, proline-rich, polypeptide mixture) in the treatment of Alzheimer's disease. J Alzheimers Dis. 2004;6(1):17–26. [DOI] [PubMed] [Google Scholar]
- 39. van de Rest O, van der Zwaluw NL, de Groot LC. Literature review on the role of dietary protein and amino acids in cognitive functioning and cognitive decline. Amino Acids. 2013;45(5):1035–45. [DOI] [PubMed] [Google Scholar]
- 40. Chen X, Huang Y, Cheng HG. Lower intake of vegetables and legumes associated with cognitive decline among illiterate elderly Chinese: a 3-year cohort study. J Nutr Health Aging. 2012;16(6):549–52. [DOI] [PubMed] [Google Scholar]
- 41. Xu X, Parker D, Shi Z, Byles J, Hall J, Hickman L. Dietary pattern, hypertension and cognitive function in an older population: 10-Year longitudinal survey. Front Public Health. 2018;6:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S. Fish, meat, and risk of dementia: cohort study. BMJ. 2002;325(7370):932–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Uribarri J, Woodruff S, Goodman S, Cai W, Chen X, Pyzik R, Yong A, Striker GE, Vlassara H. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110(6):911–916.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cai W, Uribarri J, Zhu L, Chen X, Swamy S, Zhao Z, Grosjean F, Simonaro C, Kuchel GA, Schnaider-Beeri M et al. Oral glycotoxins are a modifiable cause of dementia and the metabolic syndrome in mice and humans. Proc Natl Acad Sci U S A. 2014;111(13):4940–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li Y, Schoufour J, Wang DD, Dhana K, Pan A, Liu X, Song M, Liu G, Shin HJ, Sun Q et al. Healthy lifestyle and life expectancy free of cancer, cardiovascular disease, and type 2 diabetes: prospective cohort study. BMJ. 2020;368:l6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jessen F, Wiese B, Bachmann C, Eifflaender-Gorfer S, Haller F, Kölsch H, Luck T, Mösch E, van den Bussche H, Wagner M et al. Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch Gen Psychiatry. 2010;67(4):414–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.