Abstract
The protozoan Toxoplasma gondii is one of the most common infectious pathogenic parasites and can cause severe medical complications in infants and immunocompromised individuals. We report here the development of a real-time PCR-based assay for the detection of T. gondii. Oligonucleotide primers and a fluorescence-labeled TaqMan probe were designed to amplify the T. gondii B1 gene. After 40 PCR cycles, the cycle threshold values (CT) indicative of the quantity of the target gene were determined. Typically, a CT of 25.09 was obtained with DNA from 500 tachyzoites of the T. gondii RH strain. The intra-assay coefficients of variation (CV) were 0.4, 0.16, 0.24, and 0.79% for the four sets of quadruplicate assays, with a mean interassay CV of 0.4%. These values indicate the reproducibility of this assay. Upon optimization of assay conditions, we were able to obtain a standard curve with a linear range (correlation coefficient = 0.9988) across at least 6 logs of DNA concentration. Hence, we were able to quantitatively detect as little as 0.05 T. gondii tachyzoite in an assay. When tested with 30 paraffin-embedded fetal tissue sections, 10 sections (33%) showed a CT of <40 and were scored as positive for this test. These results were consistent with those obtained through our nested-PCR control experiments. We have developed a rapid, sensitive, and quantitative real-time PCR for detection of T. gondii. The advantages of this technique for the diagnosis of toxoplasmosis in a clinical laboratory are discussed.
The protozoan parasite Toxoplasma gondii has emerged as an important opportunistic infectious pathogen affecting organ transplant recipients, AIDS patients, and other immunocompromised patients. Toxoplasmic encephalitis and extracerebral toxoplasmosis are among the major life-threatening T. gondii infections of these patients (4, 6, 19, 29). In addition, toxoplasmic infection during pregnancy may lead to severe, if not fatal, infection of the fetus (7, 11, 25). If the fetus is infected in the first trimester, the result is spontaneous abortion, stillbirth, or severe disease. If infection occurs after the first trimester, disease manifestations include epilepsy, encephalitis, retardation, blindness, and other neurological disorders. Emphasis is placed on preventive measures and early diagnosis of the infection in order to prevent these severe complications of toxoplasmosis.
Current diagnosis of toxoplasmosis relies either on serological detection of specific anti-Toxoplasma immunoglobulin, on culture of amniotic fluid or fetal blood, or on other nonspecific indicators of infection (14, 25). Although serological testing has been one of the major diagnostic techniques for toxoplasmosis, it has many limitations. For example, it may fail to detect specific anti-Toxoplasma immunoglobulin G (IgG) or IgM during the active phase of T. gondii infection, because these antibodies may not be produced until after several weeks of parasitemia. Therefore, the high risk of congenital toxoplasmosis of a fetus may be undetected because the pregnant mother might test negative during the active phase of T. gondii infection. Furthermore, the test may fail to detect T. gondii infection in certain immunocompromised patients due to the fact that the titers of specific anti-Toxoplasma IgG or IgM may fail to rise in this type of patient (23). An alternative method of identifying T. gondii by mouse inoculation or tissue culture of the clinical specimen may confirm the infection by parasites. However, this method usually requires several days to obtain results and is labor-intensive (20). Thus, a more efficient method is needed to provide rapid and quantitative results for the diagnosis of T. gondii infection.
Several PCR-based techniques (16, 18, 24) have been developed for the diagnosis of toxoplasmosis using various clinical specimens, including amniotic fluid (3, 11), blood (1, 13, 17), cerebrospinal fluid (27), and tissue biopsy (15). Among these techniques, nested PCR followed by hybridization of PCR products has been the most sensitive method. However, the major disadvantage of these methods is that they are quite time-consuming and do not provide quantitative data. The recent advent of a real-time quantitative PCR technique has proven useful in various applications, including pathogen detection, gene expression and regulation, and allelic discrimination (5, 9, 28). Real-time PCR utilizes the 5′ nuclease activity of Taq DNA polymerase (12) to cleave a nonextendible, fluorescence-labeled hybridization probe during the extension phase of PCR. The fluorescence of the intact probe is quenched by a second fluorescent dye, usually 6-carboxy-tetramethyl-rhodamine (TAMRA). The nuclease cleavage of the hybridization probe during the PCR releases the effect of quenching resulting in an increase of fluorescence proportional to the amount of PCR product, and can be monitored by a sequence detector, such as the GenAmp 5700 Sequence Detection System (PE Applied Biosystem, Foster City, Calif.). In this study, we describe the development of a real-time quantitative PCR for the detection of T. gondii. The use of this methodology will facilitate the diagnosis of T. gondii in clinical laboratories.
MATERIALS AND METHODS
Materials.
A GenomicPrep cell and tissue DNA isolation kit was purchased from Amersham Pharmacia (Uppsala, Sweden). The TaqMan universal PCR master mix reagent kit, primers, and probe for real-time and nested PCR were purchased from PE Applied Biosystem. The T. gondii RH strain tachyzoites were kindly provided by Gan-Nan Chang, Department of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung, Taiwan, Republic of China. All the paraffin-embedded fetal tissue sections were from the Department of Pathology, Chang Gung Memorial Hospital, Tao-Yuan, Taiwan, Republic of China.
Preparation of DNA templates for PCR.
The T. gondii tachyzoites were obtained after peritoneal lavage of mice inoculated with the RH strain. Parasites collected from the mouse ascitic fluid were washed and resuspended in phosphate-buffered saline. The concentration of tachyzoites was determined by phase-contrast microscopy using the counting chamber. For preparation of positive control DNA, indicated amounts of T. gondii tachyzoites (RH strain) were incubated at 95°C for 10 min to denature the parasite and to release the DNA. The suspension was then used as a positive control in both the nested PCR and the real-time PCR.
High-molecular-weight DNA was extracted from paraffin-embedded tissue sections using a GenomicPrep cell and tissue DNA isolation kit as described by the manufacturer. Briefly, tissue sections were suspended in a cell lysis solution with proteinase K (20 μg/μl). After overnight incubation at 55°C, the lysates were heated at 95°C for 10 min to inactivate proteinase K and then were deproteinated with a protein precipitation solution. The precipitates were removed by centrifugation, and the DNA-containing supernatant was pipetted into a new 1.5-ml centrifuge tube. The DNA was then precipitated with isopropanol and resuspended in ultrapure water.
Detection of T. gondii B1 gene by real-time quantitative PCR.
The forward primer (TOXO-F), reverse primer (TOXO-R), and TaqMan probe for real-time PCR amplification were designed with the PrimerExpress software (PE Applied Biosystem) to specifically amplify the T. gondii B1 gene. The target DNA for real-time PCR amplification was the published sequence of the 35-fold repetitive B1 gene of the T. gondii RH strain (2). Briefly, template DNA was added to a reaction mixture containing 25 μl of 2× PCR universal master mix, 5 μl of the forward primer TOXO-F (5 μM, 5′-TCCCCTCTGCTGGCGAAAAGT-3′), 5 μl of the reverse primer TOXO-R (5 μM, 5′-AGCGTTCGTGGTCAACTATCGATTG-3′), and 5 μl of TaqMan probe (2 μM, 6FAM-TCTGTGCAACTTTGGTGTATTCGCAG-TAMRA) in a final volume of 50 μl. The PCRs were performed with the GenAmp 5700 Sequence Detection System (PE Applied Biosystem). After initial activation of AmpliTaq Gold DNA polymerase at 95°C for 10 min, 40 PCR cycles of 95°C for 15 s and 60°C for 1 min were performed. The cycle threshold value (CT), indicative of the quantity of target gene at which the fluorescence exceeds a preset threshold, was determined. This threshold was defined as 20 times the standard deviation of the baseline fluorescent signal, i.e., the normalized fluorescent signal of the first few PCR cycles. After reaching the threshold, the sample was considered positive.
Nested PCR for detection of T. gondii B1 gene.
Template DNA was added to a final volume of 50 μl of PCR mixture consisting of 5 μl of 10× PCR buffer (50 mM Tris-HCl [pH 9.1], 16 mM ammonium sulfate, 3.5 mM MgCl2, and 150 μg/ml of bovine serum albumin), 8 μl of 1.25 mM deoxynucleoside triphosphate, 0.5 μl of Taq DNA polymerase (5 U/μl), 1.5 μl of 20-pmol forward primer (TOXO 1; 5′-GGAACTGCATCCGTTCATGAG-3′), and 1.5 μl of 20-pmol reverse primer (TOXO 2; 5′-TCTTTAAAGCGTTCGTGGTC-3′). The mixture was denatured at 94°C for 10 min, followed by 30 PCR cycles of 94°C for 1 min, 60°C for 15 s, and 72°C for 45 s. One microliter of the resulting PCR product was reamplified under identical conditions in a reaction mixture identical in composition to that of the first-round PCR, except that the primer TOXO 1 was replaced with the primer TOXO 4 (5′-TGCATAGGTTGCAGTCACTG-3′). The second PCR product was analyzed by electrophoresis on a 2% agarose gel stained with ethidium bromide.
RESULTS
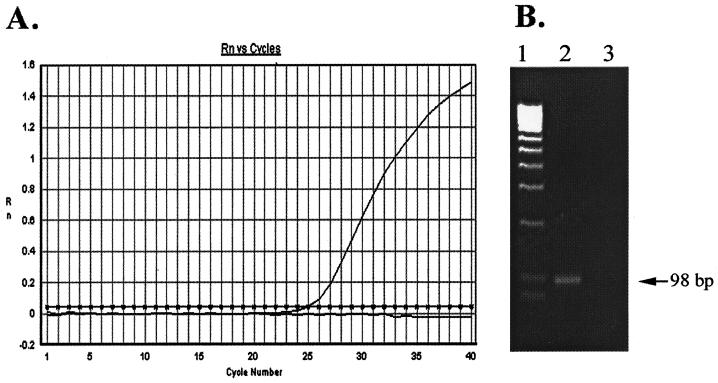
The primers used are shown in Fig. 1. DNA extracted from the T. gondii RH strain equivalent to 500 tachyzoites was used as a template for the establishment of this PCR technique. A typical amplification plot (change in fluorescent signal versus cycle numbers) with a CT of 25.09 was obtained (Fig. 2A). Electrophoretic analysis of the real-time PCR product on a 2% agarose gel showed an expected 98-bp band (Fig. 2B). DNA sequence analysis confirmed the specific amplification of the B1 gene fragment (data not shown).
FIG. 1.
Design of real-time PCR for detection of the T. gondii B1 gene. The relative positions of the primers and TaqMan probe in the B1 gene for real-time PCR and nested PCR are shown.
FIG. 2.
Real-time PCR detection of the T. gondii B1 gene. (A) Typical amplification plot with 500 tachyzoites as the initial DNA template. Rn, fluorescent signal. (B) The PCR product from panel A was fractionated on a 2% agarose gel followed by visualization with ethidium bromide staining. Lane 1, DNA molecular weight marker; lane 2, 500 tachyzoites; lane 3, no-template control.
To assess the reproducibility and reliability of our real-time PCR assay, the B1 gene real-time PCR experiment was repeated four times under identical conditions. Each experiment was performed in quadruplicate. For the four experiments, the mean CTs were 25.02, 25.08, 25.32, and 25.13 and the intra-assay coefficients of variation (CVs) within each experiment (i.e., variations among the four sets of quadruplicates) were 0.40, 0.16, 0.24, and 0.79%. Accordingly, the mean CT was 25.14 [(25.02 + 25.08 + 25.32 + 25.13)/4], and the mean interassay CV was 0.40% [(0.40% + 0.16% + 0.24% + 0.79%)/4].
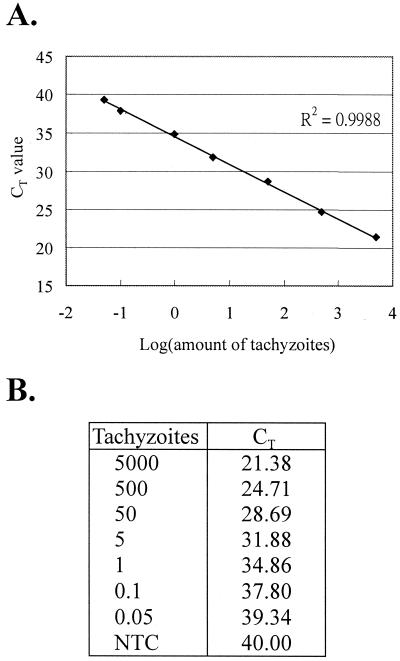
To determine the detection limit of our method and to establish a standard curve that could be used for quantification, a serial dilution of T. gondii DNA with a final concentration from 5,000 to 0.05 tachyzoites was subjected to real-time PCR analysis. We were able to detect the B1 gene at a concentration as low as 0.05 tachyzoite (CT = 39.34) in a 50-μl reaction volume (Fig. 3B). The standard curve showed a linear range across at least 6 logs of DNA concentrations with a correlation coefficient of 0.9988 (Fig. 3A).
FIG. 3.
Establishment of the standard curve for quantification of T. gondii. Serial dilutions of T. gondii DNA, ranging from 5,000 to 0.05 tachyzoites, were used as the template for real-time PCR analyses. (A) CT values were plotted against log (amount of tachyzoites). (B) CT values for all data points. Similar results were obtained in three independent experiments. NTC, no-template control.
We further assessed the ability of our real-time PCR to detect T. gondii infections in clinical specimens. Thirty paraffin-embedded fetal tissue sections were used for this study. DNA was isolated from these tissue sections using a GenomicPrep cell and tissue DNA isolation kit. An amount of 1/10 of each DNA sample isolated from each tissue section was subjected to real-time PCR analysis. In this assay, an increase of fluorescent signal above a preset threshold within 40 PCR cycles was considered positive (i.e., CT < 40). Of the 30 tissue sections we analyzed, 10 (33%) were positive, with CTs ranging from 32.03 to 39.80 (Table 1). These results were consistent with those obtained by the nested PCR (Table 1). Furthermore, the relative quantity of tachyzoites in each DNA sample was determined using the standard curve presented in Fig. 3A. The amount of tachyzoites among the positive samples varied from 0.34 (sample 1) to 28.44 (sample 21) per tissue section (Table 1).
TABLE 1.
Results of real-time and nested PCR detection of the T. gondii B1 gene in paraffin-embedded tissues
| Sample no. | Result of nested PCR | Real-time PCR values
|
|
|---|---|---|---|
| CT | T. gondii quantitya | ||
| 1 | + | 39.80 | 0.34 |
| 2 | − | 40.00 | |
| 3 | + | 37.23 | 1.78 |
| 4 | + | 38.91 | 0.60 |
| 5 | − | 40.00 | |
| 6 | − | 40.00 | |
| 7 | + | 35.00 | 7.49 |
| 8 | − | 40.00 | |
| 9 | − | 40.00 | |
| 10 | − | 40.00 | |
| 11 | − | 40.00 | |
| 12 | + | 34.17 | 12.79 |
| 13 | − | 40.00 | |
| 14 | − | 40.00 | |
| 15 | − | 40.00 | |
| 16 | − | 40.00 | |
| 17 | + | 33.67 | 17.65 |
| 18 | − | 40.00 | |
| 19 | − | 40.00 | |
| 20 | − | 40.00 | |
| 21 | + | 32.93 | 28.44 |
| 22 | + | 36.06 | 3.78 |
| 23 | − | 40.00 | |
| 24 | − | 40.00 | |
| 25 | − | 40.00 | |
| 26 | − | 40.00 | |
| 27 | − | 40.00 | |
| 28 | − | 40.00 | |
| 29 | + | 37.24 | 1.77 |
| 30 | + | 37.36 | 1.64 |
The quantities were expressed as the number of tachyzoites per tissue section.
DISCUSSION
Toxoplasmosis has emerged as a major cause of encephalitis in AIDS patients. Severe manifestations in these patients may include hemiparesis, seizures, visual impairment, confusion, and lethargy. Congenital toxoplasmosis also occurs in infants born to mothers who are infected during pregnancy. To prevent severe toxoplasmosis complications, early diagnosis and routine screening of patients early in the course of human immunodeficiency virus infection and before organ transplantation are warranted.
Currently the enzyme-linked immunosorbent assay for detecting IgM antibodies appears to be a reliable procedure for the diagnosis of acute T. gondii infections. However, this test is generally unsatisfactory for AIDS patients with latent or reactivated infections because they fail to produce an IgM response or an increasing IgG titer. Several PCR-based techniques have been developed as alternative diagnostic measurements for T. gondii infection. These techniques make use of the most conserved gene sequences among different strains of T. gondii (8), including the B1 gene repetitive sequence, the P30 (SAG1) gene, and ribosomal DNA. The use of the B1 gene for T. gondii detection originated with Burg et al. in 1989 (2), who combined PCR amplification with Southern blotting to detect a specific B1 gene product. Since then, several variations of assays have been reported that have improved sensitivity or specificity. For example, Pujol-Rique et al. designed a one-tube heminested PCR method with a sensitivity equivalent to 0.1 parasite (24). Pelloux et al. designed a new set of PCR primers for T. gondii detection in amniotic fluid (22). In the present study, we have developed a real-time PCR-based B1 gene-specific TaqMan assay for quantitative detection of T. gondii. We have demonstrated that real-time PCR of the B1 gene is extremely sensitive (0.05 parasite/reaction) and highly reproducible (mean interassay CV of 0.4%). This method has also been applied for the analysis of clinical specimens, including whole blood and amniotic fluids (data not shown). Although both nested and real-time PCR are useful in the analysis of clinical specimens (Table 1) and may achieve similar levels of assay sensitivity, the major advantages of real-time PCR are its ability to quantify the infection load of a clinical specimen and its long linear range over at least 6 logs of DNA concentrations (Fig. 2). Quantification of infection load has been used to assess disease severity and treatment outcome in human immunodeficiency virus and hepatitis C virus infections (10). To date there have not been comprehensive studies relating this application to T. gondii infection. A preliminary report suggested that quantitative PCR is useful in the diagnosis of ocular toxoplasmosis (21). The quantitative analysis may also be useful in comparing different drug regimens and in determining the prognostic value of treatment. To quantify the amount of T. gondii tachyzoites, Lee et al. had developed competitive nested PCR (18). However, this method not only is labor-intensive but also provides only semiquantitative data, with a narrow linear range of 2 to 3 logs of DNA concentrations. Secondly, the potential PCR carryover associated with conventional PCR is usually avoided in real-time PCR, since the latter is performed in a closed-tube environment. Thirdly, it is much less labor-intensive, since there is no need for post-PCR handling, such as agarose gel electrophoresis of the PCR product. In our hands, it takes a mere 2.5 h to complete the analysis of 10 specimens, as opposed to approximately 6 h for nested PCR. Finally, the adaptability of real-time PCR to a high-throughput 96-well format should significantly reduce the overall time spent per sample in a clinical laboratory.
In summary, the real-time PCR-based method described in this study provides a rapid, sensitive, and quantitative way of detecting T. gondii in clinical specimens. Thus, this method may be suitable for routine screening of T. gondii infection in the clinical laboratory in conjunction with other diagnostic techniques, such as serological tests. This technique is particularly useful in screening AIDS patients, who usually fail to generate specific IgM or increased IgG titers. Future study is warranted to further explore the clinical value of this technique.
ACKNOWLEDGMENTS
We thank Arnold Stern (New York University) and Daniel Tsun-Yee Chiu (Chang Gung University) for their critical review of the manuscript.
This work was supported by grants NSC89-2320-B-182-054 from the National Science Council, Republic of China, and CMRP850 from Chang Gung Memorial Hospital.
REFERENCES
- 1.Bergstrom T, Ricksten A, Nenonen N, Lichtenstein M, Olofsson S. Congenital Toxoplasma gondii infection diagnosed by PCR amplification of peripheral mononuclear blood cells from a child and mother. Scand J Infect Dis. 1998;30:202–204. doi: 10.1080/003655498750003681. [DOI] [PubMed] [Google Scholar]
- 2.Burg J L, Grover C M, Pouletty P, Boothroyd J C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costa J M, Dardé M-L, Assouline B, Vidaud M, Bretagne S. Microsatellite in the beta-tubulin gene of Toxoplasma gondii as a new genetic marker for use in direct screening of amniotic fluids. J Clin Microbiol. 1997;35:2542–2545. doi: 10.1128/jcm.35.10.2542-2545.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristina N, Pelloux H, Goulhot C, Brion J P, Leclercq P, Ambroise-Thomas P. Detection of Toxoplasma gondii in AIDS patients by the polymerase chain reaction. Infection. 1993;21:150–153. doi: 10.1007/BF01710533. [DOI] [PubMed] [Google Scholar]
- 5.Das H, Koizumi T, Sugimoto T, Chakraborty S, Ichimura T, Hasegawa K, Nishimura R. Quantitation of Fas and Fas ligand gene expression in human ovarian, cervical and endometrial carcinoma using real-time quantitative RT-PCR. Br J Cancer. 2000;82:1682–1688. doi: 10.1054/bjoc.2000.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derouin F, Devergie A, Auber P, Gluckman E, Beauvais B, Garin Y J, Lariviere M. Toxoplasmosis in bone marrow-transplant recipients: report of seven cases and review. Clin Infect Dis. 1992;15:267–270. doi: 10.1093/clinids/15.2.267. [DOI] [PubMed] [Google Scholar]
- 7.Desmonts G, Couvreur J. Congenital toxoplasmosis: a prospective study of 378 pregnancies. N Engl J Med. 1974;290:1110–1116. doi: 10.1056/NEJM197405162902003. [DOI] [PubMed] [Google Scholar]
- 8.Ellis J T. Polymerase chain reaction approaches for the detection of Neospora caninum and Toxoplasma gondii. Int J Parasitol. 1998;28:1053–1060. doi: 10.1016/s0020-7519(98)00096-4. [DOI] [PubMed] [Google Scholar]
- 9.Fujii K, Matsubara Y, Akanuma J, Takahashi K, Kure S, Suzuki Y, Imaizumi M, Iinuma K, Sakatsume O, Rinaldo P, Narisawa K. Mutation detection by TaqMan-allele specific amplification: application to molecular diagnosis of glycogen storage disease type Ia and medium-chain acyl-CoA dehydrogenase deficiency. Hum Mutat. 2000;15:189–196. doi: 10.1002/(SICI)1098-1004(200002)15:2<189::AID-HUMU8>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Hisada M, O'Brien T R, Rosenberg P S, Goedert J J. Viral load and risk of heterosexual transmission of human immunodeficiency virus and hepatitis C virus by men with hemophilia. The multicenter hemophilia cohort study. J Infect Dis. 2000;181:1475–1478. doi: 10.1086/315396. [DOI] [PubMed] [Google Scholar]
- 11.Hohlfeld P, Daffos F, Costa J-M, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase chain reaction test on amniotic fluid. N Engl J Med. 1994;331:695–699. doi: 10.1056/NEJM199409153311102. [DOI] [PubMed] [Google Scholar]
- 12.Holland P M, Abramson R D, Watson R, Gelfand D H. Detection of specific polymerase chain reaction product by utilizing the 5′—3′ exonuclease activity of Thermus aquaticus DNA polymerase. Proc Natl Acad Sci USA. 1991;88:7276–7280. doi: 10.1073/pnas.88.16.7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho-Yen D O, Joss A W L, Balfour A H, Smyth E T M, Baird D, Chatterton J M W. Use of the polymerase chain reaction to detect Toxoplasma gondii in human blood samples. J Clin Pathol. 1992;45:910–913. doi: 10.1136/jcp.45.10.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James G S, Sintchenko V G, Dickeson D J, Gilbert G L. Comparison of cell culture, mouse inoculation, and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. J Clin Microbiol. 1996;34:1572–1575. doi: 10.1128/jcm.34.6.1572-1575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson J D, Butcher P D, Savva D, Holliman R E. Application of the polymerase chain reaction to the diagnosis of human toxoplasmosis. J Infect. 1993;26:147–158. doi: 10.1016/0163-4453(93)92788-x. [DOI] [PubMed] [Google Scholar]
- 16.Jones C D, Okhravi N, Adamson P, Tasker S, Lightman S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T. gondii in aqueous humor. Investig Ophthalmol Vis Sci. 2000;41:634–644. [PubMed] [Google Scholar]
- 17.Joss A W L, Chatterton J M W, Evans R, Ho-Yen D O. Toxoplasma polymerase chain reaction on experimental blood samples. J Med Microbiol. 1993;38:38–43. doi: 10.1099/00222615-38-1-38. [DOI] [PubMed] [Google Scholar]
- 18.Lee P Y C, Mangan J, Holliman R E, Butcher P D. Quantitation of Toxoplasma gondii DNA in a competitive nested polymerase chain reaction. J Clin Pathol. 1999;52:61–64. doi: 10.1136/jcp.52.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luft B J, Hafner R. Toxoplasmic encephalitis. AIDS. 1990;4:593–595. doi: 10.1097/00002030-199006000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Luft B J, Remington J S. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15:211–222. doi: 10.1093/clinids/15.2.211. [DOI] [PubMed] [Google Scholar]
- 21.Norose K, Tokushima T, Yano A. Quantitative polymerase chain reaction in diagnosing ocular toxoplasmosis. Am J Ophthalmol. 1996;121:441–442. doi: 10.1016/s0002-9394(14)70443-x. [DOI] [PubMed] [Google Scholar]
- 22.Pelloux H, Weiss J, Simon J, Muet F, Fricker-Hidalgo H, Goullier-Fleuret A, Ambroise-Thomas P. A new set of primers for the detection of Toxoplasma gondii in amniotic fluid using polymerase chain reaction. FEMS Microbiol Lett. 1996;138:11–15. doi: 10.1111/j.1574-6968.1996.tb08127.x. [DOI] [PubMed] [Google Scholar]
- 23.Porter S B, Sande M A. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Engl J Med. 1992;327:1643–1648. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 24.Pujol-Rique M, Derouin F, Garcia-Quintanilla A, Valls M E, Miro J M, Jimenez de Anta M T. Design of a one-tube hemi-nested PCR for detection of Toxoplasma gondii and comparison of three DNA purification methods. J Med Microbiol. 1999;48:857–862. doi: 10.1099/00222615-48-9-857. [DOI] [PubMed] [Google Scholar]
- 25.Remington J S, McLeod R, Desmonts G. Toxoplasmosis. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. 4th ed. Philadelphia, Pa: The W. B. Saunders Co.; 1995. pp. 140–267. [Google Scholar]
- 26.Robert-Gangneux F, Gavinet M-F, Ancelle T, Raymond J, Tourte-Schaefer C, Dupouy-Camet J. Value of prenatal diagnosis and early postnatal diagnosis of congenital toxoplasmosis: retrospective study of 110 cases. J Clin Microbiol. 1999;37:2893–2898. doi: 10.1128/jcm.37.9.2893-2898.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roberts T C, Storch G A. Multiplex PCR for diagnosis of AIDS-related central nervous system lymphoma and toxoplasmosis. J Clin Microbiol. 1997;35:268–269. doi: 10.1128/jcm.35.1.268-269.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takeuchi T, Katsume A, Tanaka T, Abe A, Inoue K, Tsukiyama-Kohara K, Kawaguchi R, Tanaka S, Kohara M. Real-time detection system for quantification of hepatitis C virus genome. Gastroenterology. 1999;116:636–642. doi: 10.1016/s0016-5085(99)70185-x. [DOI] [PubMed] [Google Scholar]
- 29.Zangerle R, Allerberger F, Pohl P, Fritsch P, Dierich M P. High risk of developing toxoplasmic encephalitis in AIDS patients seropositive to Toxoplasma gondii. Med Microbiol Immunol. 1991;180:59–66. doi: 10.1007/BF00193846. [DOI] [PubMed] [Google Scholar]