Abstract
The etiologic agent of a large 1998 outbreak of poststreptococcal acute glomerulonephritis (PSGN) in Nova Serrana, Brazil, was found likely to be a specific strain of Streptococcus equi subsp. zooepidemicus from contaminated cheese (S. Balter et al., Lancet 355:1776–1780, 2000). In the present study, we used a serologic screen for a known surface-exposed virulence factor to confirm the epidemiologic findings. Using primers flanking a previously characterized M-like protein gene (J. F. Timoney et al., Infect. Immun. 63:1440–1445, 1995), we amplified and sequenced the M-like protein (designated Szp5058) gene and found it to be identical among four independent acute-phase PSGN patient isolates. Convalescent-phase sera from 33 of 44 patients in the PSGN outbreak were found to contain antibodies highly reactive to a purified Szp5058 fusion protein, compared with 1 of 17 control sera (P < 0.0001), suggesting that Szp5058 was expressed during infection and further implicating this strain as the cause of the PSGN outbreak. The predicted signal sequence and cell wall association motif of Szp5058 were highly conserved with the corresponding sequence from S. equi subsp. zooepidemicus SzpW60, while the predicted surface-exposed portions differed markedly between these two proteins. The 5′ end of the szp5058 gene, including its variable region, was identical to the szp gene from another strain associated with a previous PSGN outbreak in England (M. Barham et al., Lancet i:945–948, 1983), and the corresponding szp sequence found from the Lancefield group C type strain isolated from a guinea pig. In addition, the hypervariable (HV) portion of szp5058 was identical to a previously published HV sequence from a horse isolate (J. A. Walker and J. F. Timoney, Am. J. Vet. Res. 59:1129–1133, 1998). Three other strains of S. equi subsp. zooepidemicus, including another strain previously associated with a PSGN outbreak, were each found to contain a distinct szp gene. Two of these szp genes had HV regions identical to szp regions from isolates recovered from different host species.
Streptococcus equi subsp. zooepidemicus causes disease in several animal species and is a frequently isolated pathogen in horses, where it exists as normal flora (10). This organism has been known to cause a variety of serious infections in humans, including meningitis (13), pneumonia (20), septic arthritis (5), endocarditis (15), and poststreptococcal acute glomerulonephritis (PSGN) (1, 2, 4, 7). Transmission to humans has been associated with equine contact (14, 20) or dairy product consumption (2, 4, 7).
In 1998, a large outbreak of PSGN was linked to a specific strain of S. equi subsp. zooepidemicus on the basis of throat culture identification from patients (1). Patients were more likely than matched controls to have consumed a locally produced cheese product, and throat cultures of individuals who prepared the cheese were also positive for this specific S. equi subsp. zooepidemicus strain. Illness was severe; of 133 confirmed cases, 3 persons died, 7 required dialysis, and 96 were hospitalized. Because of a limited number of culture confirmations, it was important to solidify the link between the bacterial isolates and the outbreak using a serologic approach.
Similar to Streptococcus pyogenes, acid protein extracts from different strains of S. equi subsp. zooepidemicus contain a protein that elicits protective opsonic activity and exhibits extensive antigenic variability between strains (16, 21, 22). The gene encoding the S. equi subsp. zooepidemicus protein, designated szpW60, was cloned from one S. equi subsp. zooepidemicus strain and sequenced (22). SzpW60, other than similarities in membrane export and wall attachment motif, did not share high sequence homology with other known surface proteins of gram-positive bacteria; however, certain structural and opsonogenic features of SzpW60 were found to be analogous to the antiphagocytic M proteins of S. pyogenes. Unlike M proteins, which are extremely variable within their N-terminal 40 to 50 residues, SzpW60 and other Szp proteins were found to have their most variable region situated approximately 70 residues from the mature N terminus (24). While the N termini of M proteins contain type-specific and opsonic epitopes, the relationship of the Szp hypervariable (HV) segments to Szp serologic types or opsonic antibody elicitation has not yet been established (24). All of the Szp types that have been associated with normal equine tonsil isolates have been represented by equine pneumonia isolates, suggesting that in horses, S. equi subsp. zooepidemicus is an endogenous opportunist (23).
The aims of this study were twofold. We wished to strengthen the circumstantial data linking the S. equi subsp. zooepidemicus strain as the etiologic agent of the 1998 PSGN outbreak in Brazil by demonstrating reactivity between convalescent-phase sera and the M-like protein (Szp5058) of this strain. We also wanted to compare the deduced sequence of Szp5058 to the sequences of Szp proteins from other known PSGN outbreak isolates and animal isolates of S. equi subsp. zooepidemicus. We show that szp5058 variable-region sequences are shared between two different S. equi subsp. zooepidemicus PSGN outbreak strains and guinea pig and horse strains. One other example of identical szp sequences shared between isolates from different host species is presented, indicating that at least some szp sequences are not unique to specific host species.
MATERIALS AND METHODS
Strains.
Streptococcus equi subsp. zooepidemicus isolates 5058, 5059, 5060, and 5064 were recovered from the throats of acute glomerulonephritis patients in Nova Serrana, Brazil, during the 1998 outbreak (1).
PCR and sequence analysis.
PCR and DNA sequencing were performed as previously described (3) with PCR and sequencing primers cf1 (gataattaggagacatcatgtctagata), cf2 (ggctagcttcagtatcggcagccttgt), cr1 (aagctttaccactggggtat), and cr2 (gcaagagctgccgcggtgaa gaatggat) derived from the sequence with accession no. U04620 (21; bases 181 to 208, 274 to 300, 1362 to 1383, and 1276 to 1303, respectively).
Purification of His-Emz1 fusion protein.
The szp-specific amplicon from strain 5057 was digested with PstI, and the 1,018-bp szp5058 PstI fragment encoding the putative surface-exposed region was cloned in the proper orientation into the PstI site of pQE-30 using methods described in the instructions for the Qiaexpress system (QIAgen). Transformants were screened for the correct orientation of the coding fragment by PvuII restriction analysis of plasmid minipreps. The six-histidine (His6)-tagged fusion protein was overexpressed and purified by Ni-nitrilotriacetic acid affinity chromatography as described in the Qiaexpress system.
Human sera.
During the outbreak investigation (1), 44 human serum specimens were obtained from adults who met the case definition. Cases were defined as residents of the state where the outbreak occurred (Minas Gerais) with onset of illness between December 1997 and August 1998 and at least two of the following symptoms of disease: (i) systolic blood pressure of >140 mm Hg or diastolic blood pressure of >90 mm Hg for adults and >95th percentile of the age-specific normal limit for children, (ii) edema, or (iii) hematuria or proteinuria. All of the sera used for this study were collected 7 or more days after initial complaints of illness occurred. Pharyngeal cultures were obtained from 6 of the 44 patients who provided the study serum samples. Two of these six cultures were positive for the S. equi subsp. zooepidemicus strain that was determined to be the cause of the outbreak (1). One of these two isolates was 5060-98, which was one of the four isolates from patients used for this study. Control sera were obtained from 17 randomly selected adult blood donors at a hospital in a nearby city.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotting procedures were performed with 10% polyacrylamide gels as described previously (5). Samples containing 80 μg of the Szp5058 fusion protein were loaded onto a single-well gel and electrophoresed. Kaleidoscope prestained standards (Bio-Rad Laboratories) were used as molecular weight markers. After transfer, the nitrocellulose membranes with bound Szp5058 were incubated at room temperature with Brazilian serum samples diluted 1:500 in casein-thimerosal buffer (CTB) for 1 h (11). After three washes (5 min each) with CTB, the membranes were exposed to goat anti-human immunoglobulin G horseradish peroxidase conjugate (Bio-Rad Laboratories) for 1 h at room temperature. The wash step with CTB was repeated, and the membranes were developed with 3,3′-diaminobenzidine tetrahydrochloride. The development was stopped after 5 min by rinsing with deionized water.
Statistical analysis.
Intensities of reactive bands observed on Western blots were quantified with a fluor-chem digital imager (Alpha Innotech, San Leandro, Calif.) to obtain an integrated density value (IDV) for each of the 61 Brazilian serum samples. Positive reactivity was defined as any log10 IDV greater than or equal to the logarithmic mean of the normal controls plus 1 standard deviation, which was 3.62. The Wilcoxon rank sum test was used to compare distributions of the serology results from cases and controls.
Nucleotide sequence accession numbers.
The GenBank accession numbers for the sequences obtained in this work, including the 1,151-bp szp5058 structural gene and putative ribosome binding sequence, as well as the ribosome binding sequence and 70 to 80% of szp1028, szp1345, and szp1216, are listed in Table 1. Signal sequence predictions were made at the web site http://www.cbs.dtu.dk /services/SignalP/ (17).
TABLE 1.
Strain sources, relationships to GenBank matches, and HV motifs of szp sequences obtained in this study
| Strain or isolates/szp GenBank accession no. | Disease/country/yr of isolation | GenBank match (description)/source/yr isolated | HV motifa |
|---|---|---|---|
| 5058, 5059, 5060, 5064/AF150748 (szp5058) | AGNb/Brazil/1998 | AF021915 (100% identity over 183-base HV region)/horse nasal specimen/1969 | HV5 |
| SS1215/none | AGN/England/1982 | AF021915 (100% identity over 183-base HV region)/horse nasal specimen/1969; AF150748 (100% identity over 730-base overlap)/Brazil AGN associated/1998 | HV5 |
| SS189/none | None (from guinea pig)/unknown (Lancefield type strain)/prior to 1948 | AF021915 (100% identity over 183-base HV region)/horse nasal specimen/1969; AF150748 (100% identity over 730-base overlap)/Brazil AGN associated/1998 | HV5 |
| SS1028/AF244521 (szp1028) | AGN/Romania/1968 | None (unique) | HV3 |
| SS1345 (ATCC 43079)/AF244522 (szp1345) | Bovine mastitis/England/unknown | AF021907 (100% over 183-base overlap)/horse pleuritic fluid/1968 | HV2 |
| SS1216/AF244523 (szp1216) | Food-borne bacteremia/New Mexico/1983 | None (unique) | HV4 |
Motifs HV1 to -5 are described in reference 24.
AGN, acute glomerulonephritis.
RESULTS AND DISCUSSION
Crude chromosomal templates from four independent isolates (isolates 5058, 5059, 5060, and 5064) previously associated with the Brazil PSGN outbreak (1) were used to amplify the szp gene with two different primer sets (cf1 plus cr1 and cf2 plus cr2). As expected, the four PCR products shared sequence identity over their entire lengths (1,128-bp structural gene plus 19 bp of upstream sequence). The full-length deduced protein, designated Szp5058, had 86.5% overall sequence identity with the single, complete S. equi subsp. zooepidemicus M-like protein in the GenBank database (designated SzpW60; GenBank accession no. U04620) which was previously obtained from a horse nasal discharge isolate (22). The 32-residue signal sequence and 124 C-terminal residues (residues 254 to 377), including 20 repeats of PEPK upstream of its LPSTGE wall attachment motif (9), were identical between Szp5058 and SzpW60. Most of the variation between these two proteins was over the first 143 mature N-terminal residues, with only 69% sequence identity within this region. As with SzpW60, Szp5058 appears to be alpha helical over most of its N-terminal two-thirds and lacks the region A, B, and C repeat regions characteristic of M proteins (8).
We subsequently analyzed 5′ partial sequences predicted to encompass about 70 to 80% of the szp structural genes from five Centers for Disease Control and Prevention culture collection S. equi subsp. zooepidemicus strains from diverse sources (Table 1). It was interesting that the szp gene from strain SS1215, which originated from a PSGN outbreak in England during 1982 (2), shared sequence identity with szp5058 over its entire overlap (Table 1). We also found that the szp gene from SS189, the Lancefield type C reference strain of guinea pig origin isolated prior to 1933 (strain K64 in reference 10), shared identity over this region with szp5058. According to previous data (24), still another S. equi subsp. zooepidemicus strain of horse origin shared sequence identity over at least Szp5058 residues 107 to 167 (Fig. 1; Table 1; see GenBank accession no. AF021915). Strains SS1028, SS1345, and SS1216 were each found to have distinct szp gene sequences (Fig. 1; Table 1). It is notable that residues 107 to 167 of Szp5058 represent the most variable region among these four proteins (Fig. 1), which is in agreement with previous findings (24). In this previous work, five subgroups based upon similarities in this 60-residue HV region were found among 15 different S. equi subsp. zooepidemicus strains and were designated HV1 to 5. As Table 1 indicates, four distinct szp sequences were found among the six strains analyzed in this study, and these sequences were found to belong to one of three different HV types based upon the 60-residue HV region (Table 1). At least 15 antigenic types of S. equi subsp. zooepidemicus acid extracts are known to exist (16); however, the relationship of the HV region to the Szp antigenic type is unknown since certain distinct serotypes have been shown to share sequence identity in the HV region (24). The relationship of the HV region to protective opsonic epitopes is also unknown, since all known S. equi subsp. zooepidemicus antigenic types are opsonized by antisera to the SzpW60 protein (24).
FIG. 1.
Sequence comparison of the Szp5058 N terminus with corresponding regions of Szp proteins from three S. equi subsp. zooepidemicus strains. The inverted arrow indicates the predicted signal sequence cleavage site. Shaded segments represent shared identity among at least three of the four proteins. The entire deduced Szp5058 sequence shown was identical to the deduced Szp sequence from strains SS1215 (England PSGN outbreak strain) and SS189 (guinea pig isolate) (Table 1). The bold segment of Szp5058 (Brazil PSGN outbreak strain) indicates a region identical to that deduced from a partial szp gene sequence obtained from a horse pleuritic fluid isolate (GenBank accession no. AF021907). The bold segment of Szp1345 (bovine mastitis isolate) indicates a region identical to that deduced from a partial szp gene from a horse nasal aspirate (GenBank accession no. AF021907).
These results show that szp genes may show significant variation among different human PSGN outbreak strains. Although szp5058 was found to be shared between the Brazil PSGN outbreak strain and the England PSGN outbreak strain (Table 1 and references 4 and 5), the sequence of szp5058 varied considerably from that of szp1028 from the Romania PSGN outbreak strain (Table 1 and reference 11). Additionally, these three PSGN outbreak strains appeared to be genomically unrelated since they had unrelated SmaI-digested chromosomal DNA pulsed-field gel electrophoresis profiles (1). szp5058 was also found to share sequence identity with the szp gene from a guinea pig isolate recovered prior to 1933 (Table 1). Again, pulsed-field gel electrophoresis results revealed no relatedness between these strains (1). To examine the genetic relatedness of these strains in more detail, it may prove useful to compare them by multilocus sequence typing. It is possible that allelic identities would show closer relatedness between the outbreak strain and other szp5058-containing strains compared to those containing szp alleles other than szp5058.
From other sequencing results, it is shown here that besides isolates from two human sources and one guinea pig source, an szp5058-specific sequence was also found in a horse nasal aspirate isolate (GenBank accession no. AF021915). An szp1345-specific sequence from both bovine and equine sources was also found (Table 1). This work shows conclusively that specific szp genes are shared among isolates recovered from different mammalian hosts and clinical specimens.
Sequencing of the szp5058 structural gene revealed two fortuitously situated PstI sites (one PstI site overlapping the sequence encoding the signal peptide cleavage site and the other situated about 60 bp downstream of the region encoding the wall attachment motif LPSTGE [see GenBank accession no. AF150748 and U04620 for the exact locations of these conserved PstI sites in these two szp genes]) that expedited the construction of a histidine-tagged fusion product. The 1,018-bp PstI fragment was cloned in the proper orientation into the His6 fusion vector for purification of the resultant fusion protein. The predicted molecular mass of the His6-Szp1 fusion protein was approximately 38 kDa. SDS–10% PAGE analysis revealed that the His6-Szp5058 fusion protein was approximately 54 kDa, which is consistent with a previous report that these proteins migrate on SDS-PAGE at a much slower mobility than that predicted by their actual molecular weight (22).
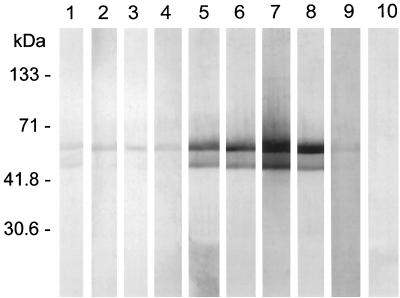
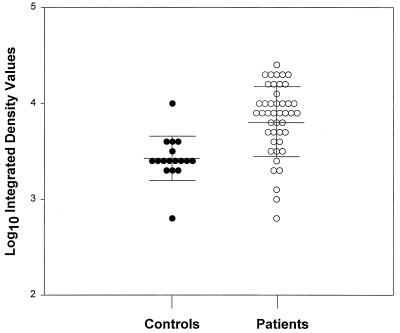
To evaluate the immune response and to determine if patients clinically diagnosed with PSGN respond serologically to the Szp5058 fusion protein, 61 serum samples (representing 44 cases and 17 case-matched controls) were analyzed by Western blotting. Representative examples of positive and negative seroreactivity to the Szp5058 fusion protein are shown in Fig. 2. The major reactive band migrated at a calculated molecular mass of ∼54 kDa. An additional positive reactive band observed at ∼52 kDa was possibly a degradation product of the 54-kDa fusion protein. Logarithmic IDVs were normally distributed in both the case and control groups; the mean logarithmic IDV of case patients was significantly higher than that of control persons (P < 0.001). Thirty-three of 44 case persons and 1 of 17 control persons were seropositive for the Szp5058 fusion protein (Fig. 3). All six of the case patients who had pharyngeal cultures were seropositive (data not shown). Compared to the clinically and epidemiologically defined criteria (1), the test specificity was 94%, the sensitivity was 71%, and the positive predictive value was 97%.
FIG. 2.
Immunoblot analysis of patient and control sera with purified His6-Szp5058 fusion protein. Lanes: 1 to 4, controls; 5 to 8, nephritis patient sera; 9, nonreactive group C rabbit polyclonal antibody to S. equisimilis control; 10, CTB control strip with no added antibody. The blot shows an upper band of 54 kDa and a lower band of 52 kDa.
FIG. 3.
Logarithmic IDVs reflecting immunoblot reactivity of nephritis patient and control sera against purified His6-Szp5058 fusion protein. The solid lines represent the mean and the standard deviation for the control and patient groups.
In summary, the data indicate that antibodies to the Szp5058 protein were generated in the human host as a result of infection with the Brazil PSGN outbreak S. equi subsp. zooepidemicus strain and provide additional important evidence that this strain was the etiologic agent. A standardized M-like-protein-based approach (e.g., enzyme-linked immunosorbent assay) of screening convalescent-phase sera could potentially be used to determine likely etiologic causes of PSGN. It is possible that representative streptococcal M or M-like proteins from group A and G and other group C species could also be used in this manner, although differentiation of protein type specificity (e.g., Szp5058 versus Szp1028 versus Szp1345 versus Szp1216) would probably require the use of specific HV segment peptides rather than full-length proteins which share extensive sequence identity.
The pathogenic mechanism of PSGN is still unknown. PSGN in mice following infection by group A streptococci requires streptokinase (18, 19); however, other streptococcal factors may be additionally required. It is possible that Szp proteins have an antiphagocytic role analogous to that of group A streptococcal M proteins that is essential in establishing human infection. Both M and Szp proteins stimulate opsonic antibodies, are protective in animal models, and share key structural similarities (22–24). The apparent strong antibody response evoked by Szp5058 during human infection (Fig. 2 and 3) and the variability of the N termini of Szp proteins are features that are consistent with surface virulence proteins under immunologic selection.
ACKNOWLEDGMENTS
M.L.N. was supported by an American Society for Microbiology-National Centers for Infectious Diseases postdoctoral fellowship. L.F. was supported by an Emerging Infectious Diseases Advanced Laboratory Training Fellowship sponsored through the Centers for Disease Control and Prevention and the Association of Public Health Laboratories.
REFERENCES
- 1.Balter S, Benin A, Pinto S W L, Teixeira L M, Alvim G G, Luna E, Jackson D, LaClaire L, Elliott J, Facklam R, Schuchat A. Epidemic nephritis in Nova Serrana, Brazil, 1998. Lancet. 2000;355:1776–1780. doi: 10.1016/s0140-6736(00)02265-0. [DOI] [PubMed] [Google Scholar]
- 2.Barham M, Thorton T J, Lange K. Nephritis caused by Streptococcus zooepidemicus (Lancefield group C) Lancet. 1983;i:945–948. doi: 10.1016/s0140-6736(83)92078-0. [DOI] [PubMed] [Google Scholar]
- 3.Beall B, Facklam R, Thompson T. Sequencing emm-specific PCR products for routine and accurate typing of group A streptococci. J Clin Microbiol. 1996;34:953–958. doi: 10.1128/jcm.34.4.953-958.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Centers for Disease Control. Group C streptococcal infections associated with eating homemade cheese, New Mexico. Morbid Mortal Weekly Rep. 1983;32:510–516. [PubMed] [Google Scholar]
- 5.Collazos J, Echevarria M J, Ayarza R, Miguel J. Streptococcus zooepidemicus septic arthritis: case report and review of group C streptococcal arthritis. Clin Infect Dis. 1992;15:744–746. doi: 10.1093/clind/15.4.744-a. [DOI] [PubMed] [Google Scholar]
- 6.Crook J, Tharpe J A, Johnson S E, Williams D B, Stinson A R, Facklam R R, Ades E W, Carlone G M, Sampson J S. Immunoreactivity of five monoclonal antibodies against the 37kDa common cell wall protein of Streptococcus pneumoniae. Clin Diag Lab Immunol. 1998;5:205–210. doi: 10.1128/cdli.5.2.205-210.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duca E, Teodorovici G, Radu C, et al. A new nephritogenic streptococcus. J Hyg. 1969;67:691–698. doi: 10.1017/s0022172400042145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischetti V A, Pancholi V, Schneewind O. Conservation of a hexapeptide sequence in the anchor region of surface proteins from gram-positive cocci. Mol Microbiol. 1990;4:1603–1605. doi: 10.1111/j.1365-2958.1990.tb02072.x. [DOI] [PubMed] [Google Scholar]
- 10.Kasai K, Nobata R, Rya E. On the incidence of Streptococcus hemolyticus in the normal tonsils of horses and the typing of equine tonsillar streptococci. Jpn J Vet Sci. 1944;6:116–123. [Google Scholar]
- 11.Kenna J G, Major G N, Williams R S. Methods for reducing non-specific antibody binding in enzyme-linked immunosorbent assays. J Immunol Methods. 1985;85:409–419. doi: 10.1016/0022-1759(85)90150-4. [DOI] [PubMed] [Google Scholar]
- 12.Lancefield R C. A serological differentiation of human and other groups of hemolytic streptococci. J Exp Med. 1933;57:571–595. doi: 10.1084/jem.57.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Latorre M, Alvarez M, Fernandez J M, Berdonces P, Llanos P, Cisterna R. A case of meningitis due to Streptococcus zooepidemicus. Clin Infect Dis. 1993;17:932–933. doi: 10.1093/clinids/17.5.932. [DOI] [PubMed] [Google Scholar]
- 14.Low D E, Young M R, Harding G K M. Group C streptococcal meningitis in an adult: probably acquisition from a horse. Arch Intern Med. 1980;140:977–978. [PubMed] [Google Scholar]
- 15.Martinez-Luengas F, Inclan G M, Pastor A, et al. Endocarditis due to Streptococcus zooepidemicus. Can Med Assoc J. 1982;127:13. [PMC free article] [PubMed] [Google Scholar]
- 16.Moore B O, Bryans J T. Antigenic classification of group C animal streptococci. J Am Vet Med Assoc. 1969;155:416–420. [PubMed] [Google Scholar]
- 17.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 18.Nordstrand A, Norgren M, Ferretti J J, Holm S E. Streptokinase as a mediator of acute poststreptococcal glomerulonephritis in an experimental mouse model. Infect Immun. 1998;66:315–321. doi: 10.1128/iai.66.1.315-321.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nordstrand A, Norgren M, Holm S E. Pathogenic mechanism of acute post-streptococcal glomerulonephritis. Scand J Infect Dis. 1999;31:523–537. doi: 10.1080/00365549950164382. [DOI] [PubMed] [Google Scholar]
- 20.Rose H D, Allen J R, Witte G. Streptococcus zooepidemicus (group C) pneumonia in a human. J Clin Microbiol. 1980;11:76–78. doi: 10.1128/jcm.11.1.76-78.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Timoney J F, Mukhtar M. Variability in the M proteins of equine strains of Streptococcus equi subsp. zooepidemicus. In: Plowright W, Rossdale P D, Wade J F, editors. Equine infectious diseases VI. Newmarket, England: R&W Publications; 1992. pp. 15–20. [Google Scholar]
- 22.Timoney J F, Walker J, Zhou M, Ding J. Cloning and sequence analysis of a protective M-like gene from Streptococcus equi subsp. zooepidemicus. Infect Immun. 1995;63:1440–1445. doi: 10.1128/iai.63.4.1440-1445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Timoney J F, Anzai T, Blair M. Clonal invasion of the equine respiratory tract by Streptococcus zooepidemicus. Adv Exp Med Biol. 1997;418:611–613. doi: 10.1007/978-1-4899-1825-3_142. [DOI] [PubMed] [Google Scholar]
- 24.Walker J A, Timoney J F. Molecular basis of variation in protective Szp proteins of Streptococcus zooepidemicus. Am J Vet Res. 1998;59:1129–1133. [PubMed] [Google Scholar]