Abstract
In 1998, an outbreak of systemic infections caused by Bacillus cereus occurred in the Neonatal Intensive Care Unit of the University Hospital Vrije Universiteit, Amsterdam, The Netherlands. Three neonates developed sepsis with positive blood cultures. One neonate died, and the other two neonates recovered. An environmental survey, a prospective surveillance study of neonates, and a case control study were performed, in combination with molecular typing, in order to identify potential sources and transmission routes of infection. Genotypic fingerprinting by amplified-fragment length polymorphism (AFLP) showed that the three infections were caused by a single clonal type of B. cereus. The same strain was found in trachea aspirate specimens of 35 other neonates. The case control study showed mechanical ventilation with a Sensormedics ventilation machine to be a risk factor for colonization and/or infection (odds ratio, 9.8; 95% confidence interval, 1.1 to 88.2). Prospective surveillance showed that colonization with B. cereus occurred exclusively in the respiratory tract of mechanically ventilated neonates. The epidemic strain of B. cereus was found on the hands of nursing staff and in balloons used for manual ventilation. Sterilization of these balloons ended the outbreak. We conclude that B. cereus can cause outbreaks of severe opportunistic infection in neonates. Typing by AFLP proved very useful in the identification of the outbreak and in the analysis of strains recovered from the environment to trace the cause of the epidemic.
Members of the genus Bacillus are aerobic spore-forming rods which are ubiquitous in nature (18). Despite their widespread distribution, even as normal skin flora, Bacillus spp. rarely cause infections. The exception is Bacillus cereus, which is a well-known cause of food poisoning and a dreaded cause of posttraumatic endophthalmitis (18). B. cereus can also cause opportunistic infections, mainly in the immunocompromised host (5, 18). Over the past 20 years, infections with this microorganism in neonates have been reported occasionally (7, 9, 12). B. cereus, however, is also known as a cause of pseudo-outbreaks (6, 8, 11). Differentiation between an outbreak and a pseudo-outbreak and determination of the source require careful analysis of patient data and accurate typing of the outbreak strain. Over the last several years, the isolation of Bacillus spp. from trachea aspirates of mechanically ventilated neonates in the Neonatal Intensive Care Unit (NICU) of our hospital has been common but had no apparent clinical implications. Between January and August 1998, three neonates developed serious invasive infection with B. cereus, and 35 neonates were found to be colonized with this microorganism. We describe this outbreak, the identification of the outbreak strain through genetic typing by amplified-fragment length polymorphism (AFLP), the risk factors for colonization, the vector of infection, and the measures taken to control the outbreak.
MATERIALS AND METHODS
Microbiologic methods.
B. cereus was identified as large aerobic, gram-positive, catalase-positive, spore-forming rods growing as irregular hemolytic colonies on sheep blood agar. Genotypic fingerprints were obtained by AFLP. DNA was isolated as described previously (3). Purified DNA was aliquoted and stored at −20°C. All procedures relating to the preparation of AFLP templates were performed essentially as described by Koeleman et al. (10). Briefly, total cellular DNA (50 ng) was digested with the restriction enzymes EcoRI and MseI, and restriction half-site-specific double-stranded oligonucleotide adapters were ligated to all restriction fragments. Restriction and ligation were carried out simultaneously for 3 h at 37°C. Amplification of sets of restriction fragments was performed by PCR with a primer combination with one selective base (Eco-A and Mse-C). The Eco-A primer was Texas red fluorescently labeled (Isogen Bioscience BV, Maarssen, The Netherlands). Amplification was performed in a Gene Amp PCR system 9700 thermal cycler (Perkin-Elmer) for 35 cycles: denaturation (30 s at 94°C), annealing (30 s at 65 to 56°C), and DNA molecule extension (1 min at 72°C). In the first 12 cycles, the annealing temperature was lowered by 0.7°C per cycle. After denaturation (1 min at 95°C, cooling on ice), fluorescent amplified fragments were separated by polyacrylamide gel electrophoresis (Rapid Gel-XL-6; Amersham Life Science, Cleveland, Ohio) according to the manufacturer's instructions in a Vistra 725 automated DNA sequencer (Amersham Life Science). Gel images were further processed with the Gel Compar 4.0 software (Applied Maths, Kortrijk, Belgium). Levels of similarity between fingerprints were calculated with the Pearson product moment correlation (r), and grouping was obtained with the unweighted pair group method using average linkages.
Strains.
Typing of the following B. cereus isolates was performed: isolates from the three symptomatic cases as well as from 35 neonates that were asymptomatically colonized in the respiratory tract, isolates from balloons used for manual ventilation and from hands of medical staff, and other environmental isolates. In our laboratory, blood culture isolates are stored for future reference. Therefore, we were able to include control isolates obtained in previous years from blood cultures. These included one isolate from a neonate in January 1997 and 14 isolates from other patients.
Study of the incidence of colonization with B. cereus.
The incidence of colonization of newborn babies with B. cereus was determined retrospectively by reviewing laboratory work sheets from January 1997 through January 1998. Although not instructed to mention the growth of colonies typical for Bacillus spp. on the laboratory work sheet, the majority of laboratory personnel did so on a routine basis. In this period, B. cereus isolates were not stored. In February 1998, all laboratory personnel were informed about the serious infections caused by Bacillus spp. in the neonates and instructed to report any growth of B. cereus. From that moment, the incidence of colonization was assessed prospectively, and B. cereus isolates were typed by AFLP and stored.
Case control study of risk factors for colonization with B. cereus.
To identify risk factors for acquisition of B. cereus, a case control study was conducted in April 1998. Because most isolates were cultured from sputum specimens, our study focused on the respiratory tract and oral feeding. A case patient was defined as any patient admitted to the NICU between 7 January and 7 April 1998 from whom B. cereus was isolated from any site. Cases were identified by reviewing laboratory records. Risk factors for cases were recorded for the period preceding the first culture with B. cereus. Control patients, who stayed in the NICU during the same period and were negative for B. cereus in sputum, were selected randomly. Risk factors for control patients were recorded until the last negative culture.
Surveillance of neonates.
To establish the route of colonization, surveillance cultures were obtained from neonates that were present in the NICU in April and May 1998. Culture specimens were obtained two to three times per week from four sites: (i) umbilicus, (ii) armpits, (iii) anus, and; (iv) trachea aspirate (if the neonate was mechanically ventilated) or throat (if the neonate was not mechanically ventilated).
Surveillance of health care workers.
Hand carriage with B. cereus was determined by printing the fingers of the dominant hand on a blood agar plate. Prints of fingers were collected from randomly selected members of the nursing staff of the NICU. For comparison, nursing staff members of other departments in the hospital were investigated by the same method. To determine the frequency of carriers of B. cereus outside the hospital, prints of fingers of randomly selected first-year medical students were obtained.
Environmental survey.
In order to identify reservoirs of B. cereus in the NICU, environmental samples were obtained with sterile swabs premoistened with sterile saline. An initial survey was based on potential sources of B. cereus spores known from the literature (1, 4, 6, 13, 17). Based on the results of the case control study and the surveillance study, a second survey was directed at materials used in mechanical ventilation.
Observation of procedures for cleaning and disinfection.
All procedures for mechanical and manual ventilation were critically evaluated by an infection control practitioner, either by personal observation or by interviewing the nursing staff. Adherence to existing protocols for cleaning and disinfection of equipment used in intubation and mechanical ventilation was verified.
Intervention.
During the second weekend of July, all Ambu balloons and Jackson-Reese balloons of the unit were sterilized by autoclaving (4 min at 134°C in a validated steam autoclave). From the moment of the intervention, the use of a balloon was restricted to one patient and the balloon was sterilized after discharge of the patient, before use in a new patient.
Statistical analysis.
Data for cases and controls were collected on standardized forms and analyzed with the SPSS statistical package version 7.5 and EXCEL version 4.0. For categorical variables, odds ratios were calculated, and continuous variables were compared with Student's t test. Whenever cells in a cross table contained zero and thus an odds ratio could not be calculated, 0.5 was added to every cell. Odds ratios with a 95% confidence interval not containing unity and mean differences with a 95% confidence interval not containing zero were considered statistically significant.
RESULTS
Description of neonates with infections caused by B. cereus.
Salient features of cases are summarized in Table 1.
TABLE 1.
Salient features of the cluster of B. cereus infections
| Feature | Datum (-a) for patient no.a:
|
||
|---|---|---|---|
| 1 | 2 | 3 | |
| Birth date | 11 January 1998 | 11 February 1998 | 11 March 1998 |
| Place of birth | UHVU | UHVU | At home |
| Sex | Male | Female | Male |
| Gestational age (wk/day) | 28/5 | 26/4 | 37/3 |
| Birth wt (g) | 895 | 1,000 | 2,780 |
| Reason for admission to NICU | IRDS (ventilator with HFOV), PDA | IRDS (ventilator with HFOV) | Perinatal asphyxia, persistent fetal circulation (ventilation with HFOV and NO), hepatosplenomegaly |
| Period of mechanical ventilation (day) | 0–12 | 0–33 | 0–16 |
| Day of colonization of respiratory tract with B. cereus | 5 | 5 | 14 |
| Day of infection | 11 | 12; 28 | 20 |
| Clinical picture | Shock, DIC, hemorrhagic meningoencephalitis | Sepsis; arthritisb | Sepsis with diarrhea |
| Cultures positive for B. cereus | Blood, CSF, trachea aspirate, necrotic skin lesion at insertion site of arterial catheter | Blood; aspirate from left kneeb | Blood, CSF, tip of peripheral catheter |
| Treatment | Amoxicillin and cefotaxime (1 day, until death) | Meropenem and vancomycin (10 days); Meropenem (3 wk)b | Meropenem and vancomycin (21 and 7 days, respectively) |
| Outcome | Died on day 12 | Recovered; recoveredb | Recovered |
Abbreviations: UHVU, University Hospital Vrije Universiteit; IRDS, infant respiratory distress syndrome; PDA, persistent ductus arteriosus; DIC, diffuse intravasal clotting; CSF, cerebrospinal fluid; NO, nitric oxide.
Data for infections at day 12 and day 28, respectively.
Typing of strains.
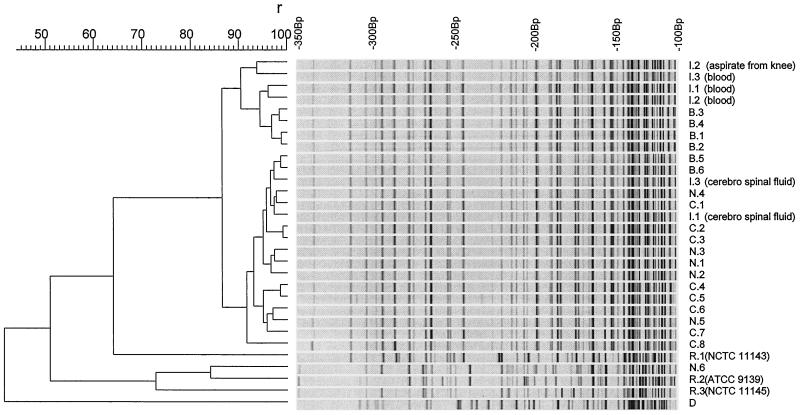
The results of genotypic fingerprinting are shown in Fig. 1. B. cereus strains isolated from the neonates who developed serious infection and from the 35 neonates who were colonized in the respiratory tract and isolates found in balloons used in manual ventilation and on the hands of some members of nursing staff of the NICU were closely related. Reference strains (NCTC 11143 and 11145 and ATCC 9139), strains isolated from other wards (N.6), and a strain isolated from a dust sample in the NICU differed markedly from the outbreak strain. It differed also from a strain isolated once before on the NICU in January 1997 and from 14 other nonrelated clinical isolates of B. cereus from other wards in the hospital (1990 to 1997, data not shown).
FIG. 1.
Genotypic fingerprints. Digitized AFLP patterns and dendrogram of a selection of B. cereus isolates. The dendrogram was constructed using Gelcompar cluster analysis by unweighted pair group method using average linkages. Percentages of similarity and molecular sizes are shown on top of the figure. The cut-off value for identical strains is 85%. Abbreviations: I, neonate infected by B. cereus; patient number corresponds with those used in Table 1; B, balloon used in mechanical ventilation; C, neonate asymptomatically colonized with B. cereus in the respiratory tract; N, nursing staff from NICU (N.1 to N.5) and another ward (N.6); culture of print of dominant hand; R, reference strain; D, dust on baseboard in the NICU.
Incidence of colonization with B. cereus.
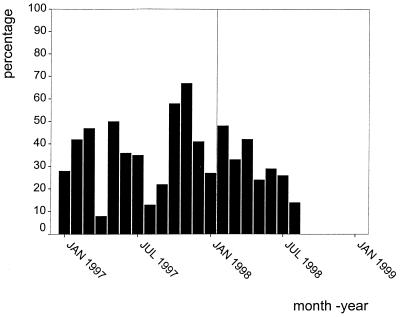
The incidence of colonization of mechanically ventilated neonates between January 1997 and January 1999 is shown in Fig. 2. Eighty-two percent of mechanically ventilated neonates became colonized during the first 5 days of life. The presumed vector of B. cereus was eliminated on the second weekend of July 1998; the incidence of colonization declined rapidly, and since September 1998 the epidemic strain has been isolated only once from a trachea aspirate.
FIG. 2.
Percentage of newly admitted mechanically ventilated neonates who became colonized with B. cereus in the respiratory tract between January 1997 and January 1999. Balloons were sterilized by autoclaving starting from 14 July 1998. For January 1997 to January 1998, numbers were determined retrospectively by review of laboratory work sheets. For February 1998 to January 1999, numbers were prospectively determined. Jan, January; Jul, July.
Case control study.
Results of the case control study are shown in Table 2. General features and enteral feeding did not differ significantly for cases and controls. All cases and controls had been mechanically ventilated. Mechanical ventilation with the Sensormedics, a machine used for high-frequency oscillatory ventilation (HFOV), was the only risk factor for colonization. There were no significant differences in antibiotic treatment (data not shown).
TABLE 2.
Risk factors for colonization and infection with B. cereus among patients in the NICU
| Risk factor | Value for group:
|
Odds ratio (95% CI)a or P value | |
|---|---|---|---|
| Cases (n = 22) | Controls (n = 22) | ||
| General features | |||
| Mean duration of study (days) | 7.1 | 8.3 | NS |
| Mean birth wt (g) | 2,018 | 2,245 | NS |
| Mean gestational age (wk/day) | 32/6 | 34/5 | NS |
| Median Apgar score at 5 min | 8 | 8 | NS |
| Way of delivery | |||
| Vaginal | 13 | 14 | NS |
| Cesarean section | 9 | 8 | |
| Place of birth | |||
| UHVUb | 12 | 9 | NS |
| Other hospital | 7 | 11 | |
| At home | 3 | 2 | |
| Enteral feeding | |||
| Feeding | |||
| Breast milk | 8 | 13 | 0.40 (0.12–1.33) |
| Nenatal | 6 | 9 | 0.54 (0.15–1.92) |
| Nutrilon Premium | 3 | 9 | 0.23 (0.05–1.01) |
| Additives | |||
| Corn oil | 0 | 1 | 0.32 (0.01–8.25)c |
| Dextrose | 0 | 0 | |
| Fantomalt | 1 | 1 | 1.00 |
| Fortifier | 4 | 6 | 0.59 (0.14–2.48) |
| Mechanical ventilation | |||
| Method | |||
| With endotracheal intubation | 21 | 22 | 0.32 (0.01–8.25) |
| Without endotracheal intubation | 4 | 10 | 0.27 (0.07–1.05) |
| Ventilation machine | |||
| Babylog | 19 | 22 | 0.12 (00.1–2.55) |
| Sensormedics | 7 | 1 | 9.80 (1.09–88.2) |
| Infant Flow | 4 | 1 | 4.67 (0.48–45.6) |
| Atomization with ventilation | |||
| Nitric oxide | 2 | 1 | 2.10 (0.18–25.0) |
| Ipratropium | 1 | 0 | 3.14 (0.12–81.4)c |
| Salbutamol | 0 | 0 | |
| Surfactant | 0 | 1 | 0.32 (0.01–8.25)c |
| Humidifiers | |||
| Fischer Paikel | 19 | 22 | 0.12 (0.01–2.55) |
| Aquamod | 4 | 0 | 11.0 (0.55–216)c |
Relative risk and 95% confidence interval (CI). NS, not significant.
UHVU, University Hospital Vrije Universiteit.
Estimation of the odds ratio (see Materials and Methods).
Surveillance of neonates.
During the surveillance period, 6 out of 32 neonates became colonized with B. cereus in the respiratory tract. These six neonates were all mechanically ventilated at that moment. Trachea aspirate was the first specimen from which B. cereus was isolated for every one of them. In one neonate, 3 days after colonization of the respiratory tract was assessed, a culture of the armpits grew B. cereus on only one occasion. Cultures of anus and umbilicus remained negative. The other mechanically ventilated neonates and neonates who were not mechanically ventilated remained negative for B. cereus.
Surveillance of nursing staff.
Results are shown in Table 3. The percentage of carriers of B. cereus was higher, although not statistically significant, among nursing staff of the NICU compared to that of other wards and to students. From the prints of fingers from the nursing staff of the NICU, both the strain of B. cereus found in neonates and nonrelated strains were isolated. For nursing staff of the other wards, only miscellaneous strains were found.
TABLE 3.
Colonization of fingers with Bacillus species (March 1998)
| Ward or group | No. of fingerprints | No. (%) positive for Bacillus spp. | OR (95% CI)a |
|---|---|---|---|
| NICU | 30 | 11b (37) | 3.29 (0.92–10.0) (NICU) |
| Surgical intensive care unit | 12 | 1 (8) | |
| Traumatology ward | 13 | 1 (8) | |
| Surgical gastroenterology ward | 9 | 0 (0) | 1.14 (0.33–3.98) (other wards) |
| Surgical oncology ward | 4 | 1 (25) | |
| Plastic surgery and urology ward | 11 | 2 (18) | |
| Students | 66 | 6 (9) | Reference group |
OR, odds ratio; CI, confidence interval.
Five of eleven were positive for the epidemic strain.
Environmental culture survey.
Results of the two culture surveys are shown in Table 4. In the initial survey, only a sample of dust on a baseboard was positive for B. cereus. This strain was not related to the epidemic strain (Fig. 1). The culture survey of materials used in mechanical ventilation identified manual ventilation balloons as the possible vector.
TABLE 4.
Environmental culture survey
| Culture specimen | Remarks | No. of cultures | No. positive for B. cereus |
|---|---|---|---|
| Initial survey | |||
| Items situated in incubators of two colonized neonates | e.g., toys, matress | 11 | 0 |
| Other items in the NICU | e.g., disposable paper napkins, towel, curtains, nurse's uniform, five ventilation grids, dust samples, two cultures of Miele washing machine | 32 | 1 (dust on baseboard) |
| Selected items from the kitchen | e.g., tap water, 24 h old and freshly made drip feed, filtration paper | 9 | 0 |
| Bottles containing 70% alcohol or soap | 7 | 0 | |
| With reference to case control study and surveillance of neonates | |||
| Two Sensormedics ventilators | 8 | 0 | |
| Sensormedics used in culture-positive neonate | Including disposable hose set | 11 | 0 |
| Materials used in intubation | e.g., McGill forceps, laryngoscopes, Silcospray | 9 | 0 |
| Three Aquamod humidifiers | One culture of water reservoir and two heating elements per humidifier | 9 | 0 |
| Randomly selected Ambu balloonsa | 16 | 2 | |
| Randomly selected Jackson-Reese balloonsa | The three positive balloons and one negative balloon also were positive for coagulase-negative staphylococci | 11 | 3 |
Specimens were obtained from the interior of the outlet of the balloon.
Observation of procedures for cleaning and disinfection.
Mechanical ventilators were cleaned and disinfected according to standard procedures. Laryngoscopes and McGill forceps were disinfected with 70% alcohol before use. Balloons for manual ventilation were regularly cleaned manually with water and soap.
Intervention.
The result of the intervention is shown in Fig. 2. In July and August, after the intervention (sterilization of balloons), five new cases of colonization with the epidemic strain of B. cereus occurred. Cultures of prints of fingers of 15 randomly selected members of nursing staff of the NICU, performed in August 1998, revealed one person who was positive for the epidemic strain of B. cereus.
DISCUSSION
In this article, we describe an outbreak caused by B. cereus in a NICU, confirmed by molecular genetic fingerprinting. Contaminated balloons, used for manual ventilation, were found to be the vector of the microorganism, and introduction of routine sterilization of these devices ended the outbreak. Because of the widespread distribution of B. cereus, including as skin flora, the hospital environment can contain spores of B. cereus (1, 4, 13, 16). Although this microorganism is known to cause invasive infections in immunocompromised hosts, it is often considered a contaminant when it is cultured from clinical specimens. Caution in the interpretation of positive culture results is also dictated by the reports on pseudo-outbreaks due to contamination of equipment or even disinfectants (8, 11). For maternity units and NICUs, dissemination of B. cereus has been described previously, with and without apparent cases of infection (2, 7, 20).
A cluster of three cases of serious systemic infection (with positive cultures of blood, cerebrospinal fluid, and synovial fluid) within a period of 3 months prompted our investigation. Colonization of the respiratory tract of neonates with B. cereus had been a common finding over the previous year. The regular isolation of B. cereus from tracheal aspirates was not viewed as a cause for concern because infections caused by B. cereus were not regularly seen; in previous years, infection had been reported only once, in January 1997. We determined that B. cereus had been isolated regularly throughout 1997 and that approximately one-third of newly admitted neonates became colonized during their stay.
The clonal nature of the strains that caused the cluster of invasive infections in 1998 and of the strains found in colonized infants was confirmed by AFLP. This recently developed technique has a high degree of reproducibility and excellent discriminatory power (15). The routine protocol used in our laboratory for various bacterial species (digestion with EcoRI and MseI and PCR with a primer combination with one selective base, Eco-A and Mse-C [10]) proved easily applicable and useful for typing of B. cereus.
We can only speculate about the cause of this outbreak and the serious infections caused by this microorganism. It may have been due to the introduction of a more virulent strain of B. cereus in the unit. Little is known, however, about virulence factors for B. cereus. We observed that the epidemic strain produced hemolysin on blood agar, a factor which is associated with virulence in other bacterial species. Hemolysin production, however, is a common feature of B. cereus, and thus it cannot explain the virulence of the epidemic strain. Most of the neonates admitted to our NICU are preterm babies and therefore immunocompromised, but no clear changes in the general characteristics of the patients admitted to the NICU occurred over the previous year.
A case control study for colonization with B. cereus identified some interesting facts. Firstly, well-known risk factors for nosocomial pathogens in neonates, such as lower birth weight, lower gestational age, and longer duration of hospitalization, did not appear associated with colonization. Secondly, the Sensormedics machine, which is used for intensive mechanical ventilation (e.g., HFOV), was identified as a significant risk factor for colonization. A prospective surveillance of newly admitted neonates also pointed to mechanical ventilation as a risk factor for colonization. Therefore, culture specimens were obtained from most materials used in mechanical ventilation. The probable cause of colonization of neonates with B. cereus was identified when the interior of the outlet of several balloons used in manual ventilation proved to be culture positive. AFLP typing showed that the strains isolated from these balloons were identical to those found in both colonized and infected babies.
After intubation, neonates are manually ventilated before being connected to the mechanical ventilator, during transport, or during cleaning of the ventilator. Most neonates became colonized within the first 5 days of life, and so it is possible that B. cereus was blown into the respiratory tract during manual ventilation before connecting the neonate to the ventilation machine. All three neonates who developed systemic infection were mechanically ventilated with the Sensormedics machine. Abrasions of the trachea caused by the oscillating tube during HFOV with this machine may have served as a porte d'entrée. Indeed, sanguinary trachea aspirates are common with this type of mechanical ventilation.
The exterior of the balloons was regularly cleaned with detergent. This method is insufficient to kill spores of B. cereus; spores can survive even in 70% alcohol (8, 14). The interior of the balloons was not reached, and so the spores were also not removed mechanically. After the sterilization of the balloons by autoclaving in July, still five new cases of colonization occurred. This was probably due to person-to-person transmission through the hands of health care workers. In September, all trachea aspirates were free of B. cereus.
In summary, we provide evidence for a true outbreak of invasive infections caused by B. cereus in a NICU. We conclude that colonization of neonates with this microorganism should be prevented.
ACKNOWLEDGMENT
W. C. van der Zwet is supported by an AGIKO grant of The Netherlands Organization for Scientific Research.
REFERENCES
- 1.Barrie D, Wilson J A, Hoffman P N, Kramer J M. Bacillus cereus meningitis in two neurosurgical patients: an investigation into the source of the organism. J Hosp Infect. 1992;25:291–297. doi: 10.1016/0163-4453(92)91579-z. [DOI] [PubMed] [Google Scholar]
- 2.Birch B R, Perera B S, Hyde W A, Ruehorn V, Ganguli L A, Kramer J M, Turnbull P C. Bacillus cereus cross-infection in a maternity-unit. J Hosp Infect. 1981;2:349–354. doi: 10.1016/0195-6701(81)90067-0. [DOI] [PubMed] [Google Scholar]
- 3.Boom R, Sol C J, Salimans M M, Jansen C L, Wertheim-Van Dillen P M, Van der Noorda J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bryce E A, Smith J A, Tweeddale M, Andruschak B J, Maxwell M R. Dissemination of Bacillus cereus in an intensive care unit. Infect Control Hosp Epidemiol. 1993;14:459–462. doi: 10.1086/646779. [DOI] [PubMed] [Google Scholar]
- 5.Drobniewski F A. Bacillus cereus and related species. Clin Microbiol Rev. 1993;6:324–338. doi: 10.1128/cmr.6.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldstein B, Abrutyn E. Pseudo-outbreak of Bacillus species: related to fibreoptic bronchoscopy. J Hosp Infect. 1985;6:194–200. [PubMed] [Google Scholar]
- 7.Gray J, George R H, Durbin G M, Ewer A K, Hocking M D, Morgan M E. An outbreak of Bacillus cereus respiratory tract infections on a neonatal intensive care unit due to contaminated ventilator circuits. J Hosp Infect. 1999;41:19–22. doi: 10.1016/s0195-6701(99)90032-4. [DOI] [PubMed] [Google Scholar]
- 8.Hsueh P, Teng L, Yang P, Pan H, Ho S, Luh K. Nosocomial pseudoepidemic caused by Bacillus cereus traced to contaminated ethyl alcohol from a liquor factory. J Clin Microbiol. 1999;37:2280–2284. doi: 10.1128/jcm.37.7.2280-2284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jevon G P, Dunne W M, Hicks M J. Bacillus cereus pneumonia in premature neonates: a report of two cases. Pediatr Infect Dis J. 1993;12:251–253. doi: 10.1097/00006454-199303000-00019. [DOI] [PubMed] [Google Scholar]
- 10.Koeleman J G, Parlevliet G A, Dijkshoorn L, Savelkoul P H, Vandenbroucke-Grauls C M. Nosocomial outbreak of multi-resistant Acinetobacter baumannii on a surgical ward: epidemiology and risk factors for acquisition. J Hosp Infect. 1997;37:113–123. doi: 10.1016/s0195-6701(97)90181-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu P Y, Ke S C, Chen S L. Use of pulsed-field gel electrophoresis to investigate a pseudo-outbreak of Bacillus cereus in a pediatric unit. J Clin Microbiol. 1997;35:1533–1535. doi: 10.1128/jcm.35.6.1533-1535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patrick C C, Langston C, Baker C J. Bacillus species infections in neonates. Rev Infect Dis. 1989;11:612–615. doi: 10.1093/clinids/11.4.612. [DOI] [PubMed] [Google Scholar]
- 13.Rowan N J, Anderson J G. Growth and enterotoxin production by diarrhoeagenic Bacillus cereus in dietary supplements prepared for hospitalized HIV patients. J Hosp Infect. 1998;38:139–146. doi: 10.1016/s0195-6701(98)90067-6. [DOI] [PubMed] [Google Scholar]
- 14.Russell A D. Bacterial spores and chemical sporicidal agents. Clin Microbiol Rev. 1990;3:99–119. doi: 10.1128/cmr.3.2.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Savelkoul P H, Aarts H J, de Haas J, Dijkshoorn L, Duim B, Otsen M, Rademaker J L, Schouls L, Lenstra J A. Amplified-fragment length polymorphism analysis: the state of an art. J Clin Microbiol. 1999;37:3083–3091. doi: 10.1128/jcm.37.10.3083-3091.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simini B. Outbreak of Bacillus cereus endophthalmitis in Rome. Lancet. 1998;351:1258. [Google Scholar]
- 17.Thuler L C, Velasco E, De Souza Martins C A, De Faria L M, Da Fonseca N P, De Castro Dias L M. An outbreak of Bacillus species in a cancer hospital. Infect Control Hosp Epidemiol. 1998;19:856–858. doi: 10.1086/647746. [DOI] [PubMed] [Google Scholar]
- 18.Tuazon C U. Other Bacillus species. In: Mandell G E, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. Edinburgh, United Kingdom: Churchill Livingstone; 1995. pp. 1890–1894. [Google Scholar]
- 19.Weisse M E, Bass J W, Jarrett R V, Vincent J M. Nonanthrax Bacillus infections of the central nervous system. Pediatr Infect Dis J. 1991;10:243–246. doi: 10.1097/00006454-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 20.Youngs E R, Roberts C, Kramer J M, Gilbert R J. Dissemination of Bacillus cereus in a maternity unit. J Infect. 1985;10:228–232. doi: 10.1016/s0163-4453(85)92538-1. [DOI] [PubMed] [Google Scholar]