Abstract
Objectives
To summarize the epidemiologic, clinical, and laboratory characteristics of autoimmune hemolytic anemia (AIHA) secondary to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection or vaccination.
Methods
We conducted a systematic review using standardized keyword search to identify all reports of SARS-CoV-2 infection or vaccination and AIHA across PubMed, Web of Science, Scopus, and Google Scholar through September 24, 2021.
Results
Fifty patients (mean [SD] age, 50.8 [21.6] years) diagnosed with coronavirus disease 2019 (COVID-19) and AIHA were identified. AIHA subtypes and number of patients were as follows: cold AIHA (n = 18), warm AIHA (n = 14), mixed-type AIHA (n = 3), direct antiglobulin test (DAT)–negative AIHA (n = 1), DAT-negative Evans syndrome (n = 1), Evans syndrome (n = 3), and subtype not reported (n = 10). Mean (SD) hemoglobin at AIHA diagnosis was 6.5 [2.8] g/dL (95% confidence interval, 5.7-7.3 g/dL). Median time from COVID-19 symptom onset to AIHA diagnosis was 7 days. In total, 19% (8/42) of patients with COVID-19–associated AIHA with reported outcomes were deceased. Four patients (mean [SD] age, 73.5 [16.9] years) developed AIHA following SARS-CoV-2 vaccination: Pfizer-BioNTech BNT162b2 vaccine (n = 2); Moderna mRNA-1273 vaccine (n = 1); undisclosed mRNA vaccine (n = 1). AIHA occurred after 1 dose in 3 patients (median, 5 days).
Conclusions
SARS-CoV-2 infection and vaccination are associated with multiple AIHA subtypes, beginning approximately 7 days after infectious symptoms and 5 days after vaccination.
Keywords: COVID-19, SARS-CoV-2, SARS-CoV-2 vaccine, Autoimmune hemolytic anemia, Hemolysis, Cold agglutinin disease
KEY POINTS.
The epidemiologic, clinical, and laboratory characteristics of patients with coronavirus disease 2019 (COVID-19) and autoimmune hemolytic anemia (AIHA) are unknown.
COVID-19 is associated with warm and cold AIHA, with a mean hemoglobin of 6.5 g/dL at AIHA diagnosis, which occurs approximately 7 days after COVID-19 symptom onset.
COVID-19 is a risk factor for the development of AIHA, and patients should be monitored for this rare but potentially fatal outcome.
Introduction
The manifestations of symptomatic coronavirus disease 2019 (COVID-19) are heterogenous, with a broad spectrum of systemic complications described. Evidence is mounting that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the viral agent responsible for COVID-19, can induce a multitude of hematologic abnormalities, with an accumulation of case reports and small case series suggesting that autoimmune hemolytic anemia (AIHA) may be associated with COVID-19.1-10
AIHA is a rare disorder, with an estimated incidence of 1 to 3 per 100,000 people annually. Approximately half of all AIHA cases are idiopathic, while secondary cases are often associated with underlying autoimmune or lymphoproliferative diseases.11 Reports of AIHA occurring in the context of several infectious diseases have been described, but a few pathogens have a well-described association with AIHA, including human immunodeficiency virus (HIV), Mycoplasma pneumoniae, and Epstein-Barr virus (EBV).11,12 Although, a definitive link between COVID-19 and AIHA has not been established, nor has an exact mechanism underlying this potential association been elucidated, several hypotheses have emerged, including immunologic hyperstimulation and molecular mimicry.13,14
Most of the evidence proposing an association between COVID-19 and AIHA is based on single case report data, much of which are limited in scope, design, and analysis. Given this overall paucity of data, this systematic review aimed to analyze the epidemiology, laboratory and blood bank parameters, therapeutic interventions, outcome, and AIHA subtype in patients with SARS-CoV-2 infection or vaccination.
MATERIALS AND METHODS
Case Selection
We performed a comprehensive literature review to identify all reports of SARS-CoV-2 infection or vaccination. We analyzed these studies to determine by keyword search any description of immune-mediated RBC destruction. The comprehensive list of keywords was as follows:
COVID-19: “COVID,” “COVID-19,” “COVID 19,” “coronavirus 19,” “coronavirus disease,” “novel coronavirus,” “SARS-CoV-2,” “SARS,” “severe acute respiratory syndrome,” “severe acute respiratory syndrome coronavirus 2,” “COVID-19 vaccine,” “COVID 19 vaccine,” “COVID-19 immunization,” “coronavirus vaccine,” “COVID vaccine”
AIHA: “autoimmune hemolytic anemia,” “hemolytic anemia,” “hemolysis,” “Coombs-positive anemia,” “Coombs hemolysis,” “AIHA,” “immune hemolysis,” “cold agglutinin,” “cold agglutinin disease,” “warm autoimmune anemia,” “mixed autoimmune hemolytic anemia,” “Evans Syndrome”
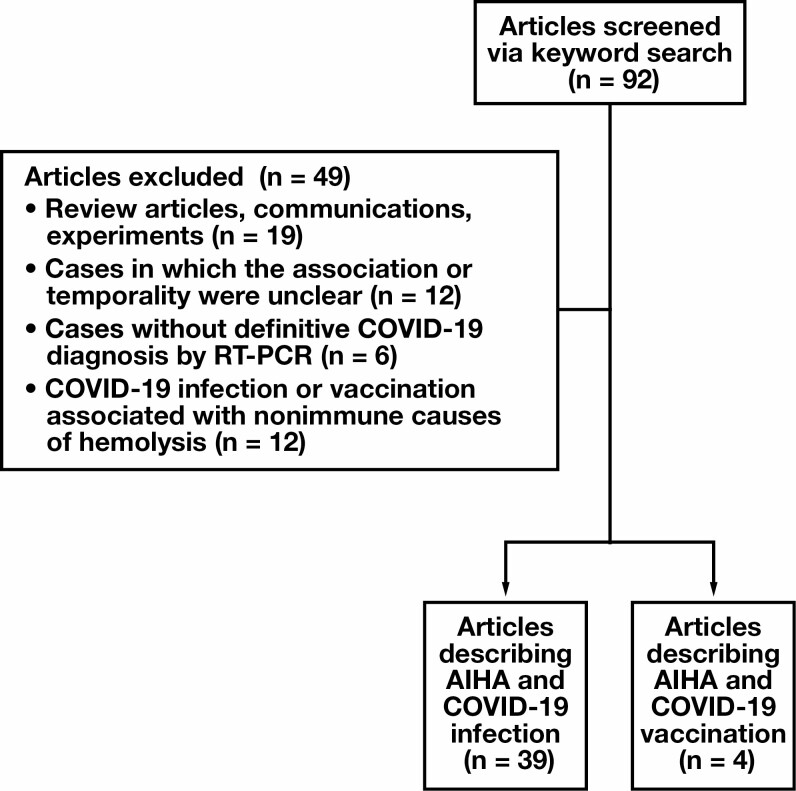
Searches were conducted across 4 scientific databases (PubMed, Web of Science, Scopus, Google Scholar) according to a standardized search protocol through September 24, 2021. Abstracts and titles were screened according to specific inclusion criteria. The search method is depicted in Figure 1 . All included publications were coded into relevant categories based on a standardized protocol.
Figure 1.
Study selection process. AIHA, autoimmune hemolytic anemia; COVID-19, coronavirus disease 2019; RT-PCR, reverse transcription polymerase chain reaction.
In addition to the multidatabase search, we queried the US Centers for Disease Control and Prevention (CDC) Vaccine Adverse Event Reporting System (VAERS) to look for reports of potential adverse events (AEs) associated with receipt of a SARS-CoV-2 vaccine.15
Inclusion Criteria
Inclusion criteria for this analysis were as follows: (1) COVID-19 confirmed by detection of SARS-CoV-2 nucleic acid by reverse transcription–polymerase chain reaction (RT-PCR) or receipt of 1 or more dose of a SARS-CoV-2 vaccine and (2) case reports, case series, and cohort studies describing 1 or more patients with a diagnosis of AIHA.
Exclusion Criteria
Exclusion criteria for this analysis were (1) duplicate publications, (2) articles not in English, (3) reviews or in vitro or in vivo experiments, (4) cases with concerns regarding temporality or unobscured associations between COVID-19 and AIHA, (5) cases without positive COVID-19 tests by RT-PCR, and (6) cases of COVID-19 and concurrent hemolysis secondary to nonimmune etiologies.
Data Analysis
We selected 92 articles for screening to assess their relevance for inclusion in the analysis. In total, 88 reports described COVID-19 infection, and 4 referenced SARS-CoV-2 vaccination. We included 43 reports in the study, 39 of which describe AIHA associated with COVID-19 and 4 of which describe AIHA associated with SARS-CoV-2 vaccination. The following is a comprehensive record of data categories extracted from the articles:
Bibliographic information: titles, authors, number of patients
Patient data: age, sex, comorbidities, symptoms of COVID-19 at presentation, time from COVID-19 symptom onset or SARS-CoV-2 vaccination to AIHA diagnosis, therapeutic interventions for AIHA, immunization status
Laboratory data: hemoglobin at presentation, hemoglobin nadir, lactate dehydrogenase (LDH) at AIHA diagnosis, haptoglobin at AIHA diagnosis, SARS-CoV-2 RT-PCR test result
Blood bank data: direct antiglobulin test (DAT) results, cold agglutinin evaluation, cold agglutinin titers, thermal amplitude test, other blood bank serologic results
Outcome: patient alive or deceased at the time of the report
The classification of AIHA subtype was determined by review of the authors’ reported diagnosis. In cases where the original authors did not explicitly classify the subtype of AIHA, we reported the diagnosis simply as “AIHA” in Table 1 and Table 2 . For these unclassified cases in which the clinical, laboratory, and blood bank evaluation were sufficient to clearly indicate a definitive AIHA classification, we included our AIHA classification in addition to the original unclassified diagnosis (see “Results”). To analyze outcomes, we used a binary parameter of either alive or deceased at the time of the report. If the original authors reported the suspected cause of death, we included the data for those patients reported to be deceased. All statistical analyses were conducted using Microsoft Excel, version 16.53 (Microsoft).
Table 1.
Cases of Confirmed Coronavirus Disease 2019 Infection Associated With Autoimmune Hemolytic Anemiaa
| Author | Patient Age, y | Patient Sex | Comorbidity | DAT | Reported Diagnosis | AIHA Treatment | RBC Transfusion (No. of Units) | Outcome |
|---|---|---|---|---|---|---|---|---|
| Capes et al1 | 62 | M | HTN, oropharyngeal SCC | IgG (−) C3b (+) | AIHA | Not reported | Yes (8) | Alive |
| Jacobs et al2 | 33 | F | Hypothyroidism | IgG (+) C3d (+) | Mixed AIHA | Corticosteroids, rituximab | Yes (11) | Alive |
| Maslov et al16 | 48 | M | HTN, IDDM, obesity, ESRD | Positiveb | Cold AIHA | Not reported | Yes (not reported) | Deceased (intracerebral insult) |
| Lazarian et al3 | 61 | M | HTN, CKD, CLL | IgG (+) C3d (+) | Warm AIHA | Corticosteroids | No | Alive |
| Lazarian et al3 | 89 | F | HTN, CKD, atrial fibrillation, MGUS | IgG (+) C3d (+) | Warm AIHA | Corticosteroids | No | Alive |
| Lazarian et al3 | 62 | F | HTN, hepatic cirrhosis, MZL | IgG (−) C3d (+) | Cold AIHA | Corticosteroids, rituximab | No | Alive |
| Lazarian et al3 | 69 | F | Obesity, MZL | IgG (+) C3d (+) | Cold AIHA | Corticosteroids | No | Alive |
| Lazarian et al3 | 61 | M | HTN, CKD, diabetes, hypercholesterolemia, prostate carcinoma | IgG (−) C3d (+) | Cold AIHA | None | Yes (not reported) | Alive |
| Lazarian et al3 | 61 | M | Diabetes | IgG (+) C3d (−) | Warm AIHA | Corticosteroids, rituximab | No | Alive |
| Lazarian et al3 | 75 | M | Diabetes, hypercholesterolemia, obesity, COPD, CLL | IgG (+) | Warm AIHA | None | Yes (not reported) | Alive |
| Hindilerden et al4 | 56 | M | HTN | IgG (+) C3d (+) | Warm AIHA | IVIG, corticosteroids | No | Alive |
| Wahlster et al17 | 17 | M | Chronic immune thrombocytopenia | IgG (+) C3 (+) | Warm AIHA | Corticosteroids | Not reported | Alive |
| Zagorski et al5 | 46 | F | ITP, asthma | IgG (+) C3 (+) | Cold AIHA | None | Yes (not reported) | Deceased (cardiac arrest) |
| Lopez et al6 | 46 | F | Congenital thrombocytopenia | IgG (+) C3 (+) | Warm AIHA | IVIG, corticosteroids | Yes (3) | Alive |
| Singhavi et al18 | 20 | M | None | Not reported | AIHA | Corticosteroids | Yes (not reported) | Alive |
| Patil et al19 | 51 | F | Breast carcinoma | IgG (−) C3 (+) | Cold AIHA | Corticosteroids | Yes (not reported) | Alive |
| Jawed et al7 | Early 50s | M | HTN | IgG (−) C3d (+) | AIHA | None | No | Alive |
| Woldie et al20 | 24 | M | AIHA in remission | IgG (+) C3 (+) | AIHA | Corticosteroids, cyclophosphamide | Not reported | Deceased (fulminant Cryptococcus infection) |
| Bae et al21 | 51 | M | None | IgG (−) C3 (−) | AIHA with DAT negative for IgG and C3 | IVIG for 3 d | Yes (1) | Alive |
| Vega Hernández et al22 | 13 | F | Psoriasis | IgG (+) C3 (−) | AIHA | Corticosteroids | No | Alive |
| Huda et al23 | 54 | M | Diabetes | IgG (+) | AIHA | Corticosteroids | Not reported | Not reported |
| Hsieh et al8 | 84 | M | Hypercholesterolemia | IgG (+) | Warm AIHA | Corticosteroids | Yes (5) | Alive |
| Raghuwanshi24 | 45 | M | Not reported | Positiveb | Cold AIHA | Not reported | Yes (2) | Not reported |
| Liput et al25 | 33 | F | None | IgG (−) C3 (+) | Warm AIHA | Corticosteroids | Yes (1) | Alive |
| Nesr et al26 | 80 | F | CLL | IgG (−) C3 (+) | Cold AIHA | Not reported | No | Alive |
| Huscenot et al9 | 43 | F | Obesity, multiple sclerosis | Positiveb | Cold AIHA | Not reported | Yes (not reported) | Not reported |
| Huscenot et al9 | 63 | M | HTN | IgG (+) C3 (+) | Cold AIHA | Not reported | Not reported | Not reported |
| Gupta et al27 | 77 | M | COPD, G6PD deficiency | C3d (+) | Cold AIHA | Corticosteroids | Yes (3) | Deceased (cardiovascular failure) |
| Renganathan et al28 | 42 | F | Not reported | Positiveb | Mixed AIHA | Corticosteroids | No | Not reported |
| Rosenzweig et al10 | 14 | F | Not reported | IgG (+) C3d (+) | Mixed AIHA | Corticosteroids, rituximab for 4 wk | Yes (not reported) | Alive |
| Ramos-Ruperto et al29 | 54 | M | None | IgG (−) C3d (+) | Cold AIHA | Corticosteroids, plasma exchange for 5 sessions | Not reported | Alive |
| Ramos-Ruperto et al29 | 72 | F | None | IgG (+) C3d (−) | Warm AIHA | Corticosteroids | Yes (not reported) | Alive |
| Ramos-Ruperto et al29 | 76 | F | HTN, hypothyroidism, CLL | IgG (+) C3d (−) | Warm AIHA | Corticosteroids | Yes (not reported) | Alive |
| Aldaghlawi et al30 | 69 | F | CLL | C3 (+) | Cold AIHA | Corticosteroids, IVIG for 2 d, rituximab for 4 wk | Not reported | Alive |
| McGregor et al31 | 75 | M | HTN, CKD, obesity | Positiveb | AIHA | Corticosteroids | Not reported | Deceased (multiorgan failure) |
| Pandey et al32 | 31 | M | Not reported | IgG (+) C3 (+) | AIHA | Corticosteroids | Yes (2) | Alive |
| Sujana et al33 | 59 | F | Not reported | Positiveb | AIHA | Corticosteroids | Yes (9) | Alive |
| Nair et al34 | 23 | M | Asthma | IgG (+) | Warm AIHA | Corticosteroids for 3 mo | Yes (2) | Alive |
| Saini et al35 | 94 | F | CKD, HFpEF | IgG (−) C3 (+) | Cold AIHA | Not reported | Yes (6) | Deceased (cardiopulmonary failure) |
| Ahmadnezhad et al36 | 49 | F | Thalassemia | IgG (−) C3 (+) | Cold AIHA | Not reported | Yes (not reported) | Deceased (not reported) |
| Hassanein et al37 | 48 | M | Not reported | IgG (−) C3d (+) | Cold AIHA | Not reported | Yes (not reported) | Alive |
| Campos-Cabrera et al38 | 35 | F | Not reported | IgG (+) C3d (+) | Warm AIHA | Corticosteroids, IVIG | Not reported | Alive |
| Campos-Cabrera et al38 | 58 | F | Not reported | IgG (+) C3d (+) | Warm AIHA | Corticosteroids, IVIG | Not reported | Alive |
| Li et al39 | 39 | M | Not reported | Positiveb | Evans syndrome | IVIG | Not reported | Alive |
| Gruden et al40 | 83 | F | HTN | IgG (−) C3 (−) | Evans syndrome, with DAT negative for IgG and C3d | Corticosteroids, IVIG for 5 d | Yes (1) | Alive |
| Georgy et al41 | 33 | M | Not reported | Positiveb | Evans syndrome | Corticosteroids | Not reported | Deceased (intracerebral hemorrhage) |
| Zama et al42 | 15 | M | None | IgG (+) C3 (+) | Evans syndrome | Corticosteroids for 32 d, IVIG for 3 d | Yes (6) | Alive |
| Zama et al42 | 2 | M | 6 mo afterHSCT for β-thalassemia major | IgG (+) C3 (+) IgA (+) IgM (+) | Cold AIHA | Corticosteroids for 42 d | Yes (not reported) | Alive |
| Kumarihamy et al43 | 50 | M | None | IgG (−) C3d (+) | Cold AIHA | Corticosteroids | Yes (5) | Alive |
| Hasan et al44 | 45 | M | Hypothyroidism | Positiveb | AIHA | Corticosteroids for 12 wk | Not reported | Alive |
AIHA, autoimmune hemolytic anemia; CKD, chronic kidney disease; CLL, chronic lymphocytic leukemia; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DAT, direct antiglobulin test; ESRD, end-stage renal disease; HFpEF, heart failure with preserved ejection fraction; HSCT, hematopoietic stem cell transplantation; HTN, hypertension; IDDM, insulin-dependent diabetes mellitus; Ig, immunoglobulin; ITP, immune thrombocytopenia purpura; IVIG, intravenous immunoglobulin G; MGUS, monoclonal gammopathy of undetermined significance; MZL, marginal zone lymphoma; RT-PCR, reverse-transcription polymerase chain reaction; SCC, squamous cell carcinoma.
aCOVID-19 infection was confirmed via RT-PCR. The outcome was reported as whether the patient was alive or deceased at the time of report. If deceased, the likely cause of death is included if reported by the original authors.
bOnly reported as positive; no details given.
Table 2.
Cases of Autoimmune Hemolytic Anemia Associated With Receipt of at Least 1 Dose of SARS-CoV-2 Vaccine
| Author | Patient Age, y | Patient Sex | DAT | Timing of Symptoms | AIHA Treatment | RBC Transfusion (No. of Units) | Vaccine Received | Diagnosis |
|---|---|---|---|---|---|---|---|---|
| Murdych45 | 84 | M | IgG (+) C3 (−) | 19 d after first dose | Corticosteroids | Yes (2) | BNT162b2 (Pfizer-BioNTech) | AIHA |
| Brito et al46 | 88 | F | IgG (+) C3 (+) | 2 d after second dose (23 d after first dose) | Corticosteroids | Yes (1) | mRNA vaccine | AIHA |
| Gaignard et al47 | 77 | M | IgG (+) C3 (+) | 5 d after first dose | Corticosteroids | Not reported | mRNA-1273 (Moderna) | Warm AIHA |
| Aoun et al48 | 45 | F | IgG (−) C3 (+) | 3 d after first dose | Rituximab weekly for 4 wk | Yes (not reported) | BNT162b2 (Pfizer-BioNTech) | Cold AIHA |
AIHA, autoimmune hemolytic anemia; DAT, direct antiglobulin test; Ig, immunoglobulin; mRNA, messenger RNA; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
RESULTS
COVID-19 and AIHA
A total of 50 patients with AIHA associated with SARS-CoV-2 infection Table 1 and 4 patients with AIHA associated with SARS-CoV-2 vaccination Table 2 were included in the analysis. The mean (SD) age of patients with SARS-CoV-2 infection and AIHA was 50.8 (21.6) years. In total, 83% (33/40) of patients with reported medical history had at least 1 comorbidity. Eighteen patients were diagnosed with cold AIHA, 14 with warm AIHA, 3 with mixed AIHA, 1 with DAT-negative AIHA, 1 with DAT-negative Evans syndrome, and 3 with Evans syndrome; in 10 cases, the original authors did not report the AIHA subtype. Following review of the 10 cases of AIHA that were not classified in the original reports, we subsequently classified 3 as probable cold AIHA and 4 as probable warm AIHA; 3 could not be classified because of insufficient data.
The median (interquartile range [IQR]) time frame from COVID-19 symptom onset to AIHA diagnosis was 7 (6.5) days, ranging from 0 to 20 days following development of COVID-19 symptoms. The mean (SD) hemoglobin value at AIHA diagnosis was 6.5 (2.8) g/dL (95% confidence interval [CI], 5.7-7.3 g/dL), the mean (SD) hemoglobin nadir was 5.7 (2.3) g/dL (95% CI, 4.6-6.8 g/dL), the mean (SD) LDH level was 1,124 (828) U/L (95% CI, 833-1,415 U/L), and the mean (SD) haptoglobin level was 27.3 (50.9) mg/dL (95% CI, 8.8-45.8 mg/dL). DAT was positive in 96% (48/50) of patients, a large proportion of whom were positive for C3 (30/38 [79%]), both with (16/38 [42%]) and without (14/38 [37%]) concurrent immunoglobulin G (IgG) Table 3 . Cold agglutinins were identified in all 14 patients tested, 64% (9/14) of whom had titers performed. In total, 78% (7/9) of cold agglutinin titers were considered clinically significant based on the titer (≥64), ranging from 8 to 16,384. Thermal amplitude testing was performed in 5 patients, and all 5 had clinically significant cold agglutinins because all reacted at 30°C (n = 2) or 37°C (n = 3).
Table 3.
Blood Bank Results
| DAT Results | Patients, No. | CA Titers | Patients, No. | Thermal Amplitude Reactivity | Patients, No. |
|---|---|---|---|---|---|
| IgG only | 8 | 8 | 1 | 37°C | 3 |
| C3 only | 14 | 32 | 1 | 30°C | 2 |
| IgG and C3 | 16 | 64 | 2 | – | – |
| Negative | 2 | 80 | 1 | – | – |
| Only reported as “positive” | 10 | 256 | 1 | – | – |
| – | – | 512 | 1 | – | – |
| – | – | 2,048 | 1 | – | – |
| – | – | 16,384 | 1 | – | – |
CA, cold agglutinin; DAT, direct antiglobulin test; Ig, immunoglobulin.
In total, 44% (22/50) of patients with COVID-19 and AIHA were female. There was no statistically significant difference in the mean hemoglobin level at AIHA diagnosis between female and male patients (6.6 g/dL vs 6.7 g/dL; P = .87), patients alive compared with those deceased (6.7 g/dL vs 6.3 g/dL; P = .72), or for those diagnosed with warm AIHA compared with cold AIHA (6.2 g/dL vs 7.1 g/dL; P = .38).
For patients with RBC transfusion therapy data reported, 74% (28/38) received at least 1 unit of RBCs. The number of transfused RBCs was documented for 25 patients, with a mean (SD) of 2.6 (3.2) units and a median (IQR) of 1 (0-5) unit transfused to these patients. Corticosteroids (n = 35), the most common of which was prednisone 1 mg/kg per day, were the most frequently used therapy for AIHA, while intravenous IgG (IVIG) 1 mg/kg per day (n = 9), rituximab 375 mg/m2 weekly (n = 5), and cyclophosphamide (n = 1) were employed in select patients. One patient with cold AIHA received 5 sessions of plasma exchange in addition to corticosteroids, but the replacement fluid type was not reported.
In total, 19% (8/42) of patients with reported outcomes were deceased. The mean (SD) age of the deceased patients was 55.8 (22.4) years (95% CI, 40.3-71.3 years). Of the deceased patients, 5 had cold AIHA and 1 had Evans syndrome; in 2 patients, the original authors did not classify the AIHA. In total, 88% (7/8) of deceased patients had multiple underlying medical comorbidities, including hemolytic risk factors such as a history of AIHA in remission, immune thrombocytopenic purpura in remission, thalassemia, and G6PD deficiency.
SARS-CoV-2 Vaccination and AIHA
New-onset AIHA was reported in 4 patients following receipt of a SARS-CoV-2 vaccine Table 2 . Two of these patients received the BNT162b2 (Pfizer-BioNTech) vaccine; 1 received the mRNA-1273 (Moderna) vaccine; and 1 received an mRNA vaccine, but the specific vaccine and manufacturer were not reported. Lot numbers were unavailable in all cases. In total, 75% (3/4) of patients developed AIHA after an initial dose of vaccine, while the fourth patient developed AIHA 2 days after the second dose. This patient had received the first dose of the same vaccine 21 days prior without complication. Warm AIHA occurred in 1 patient, and 1 patient developed cold AIHA. For the other 2 patients, the original authors did not report the AIHA subtype, but following review, both represent cases of probable warm AIHA.
The mean (SD) age of these patients was 73.5 (16.9) years (95% CI, 56.9-90.1 years), and only 2 of the 4 patients reported underlying medical conditions. Two patients underwent SARS-CoV-2 RT-PCR testing, and both tested negative. All 4 patients denied recent signs or symptoms of infection, and none endorsed historical SARS-CoV-2 infection. The median (IQR) time from vaccine administration to AIHA symptoms after 1 dose was 5 (3-19) days.
Corticosteroid therapy was instituted for 3 patients, and rituximab was used in 1 patient. Blood product transfusion data were available for 3 patients, all of whom were transfused with at least 1 unit of RBCs. One patient received 2 units, 1 patient received 1 unit, and the number of units was not reported for the third patient.
As of September 24, 2021, 219 reports had been submitted to the CDC VAERS describing AIHA, cold agglutinins, hemolysis, and warm AIHA as AEs associated with a SARS-CoV-2 vaccination. Details regarding the specific vaccine and symptom onset were not available.
Discussion
To date, 50 cases of AIHA associated with RT-PCR–confirmed COVID-19 have been reported. This number is miniscule compared with the more than 220 million reported cases of COVID-19 worldwide,49 but the significant case fatality rate (CFR) (19%) among patients with COVID-19–associated AIHA indicates a high risk of mortality in those who do develop this sequela. Although the small number of patients and the high rate of comorbidities in these patients may certainly influence the CFR, the overall mean age (50.8 years) of patients with COVID-19 and associated AIHA as well as the mean age (55.8 years) of deceased patients with AIHA is important. This finding highlights the severity of AIHA associated with COVID-19 and underscores the need for heightened awareness of and further investigation into this potentially fatal association.
The infectious agents known to be associated with AIHA predominantly induce either warm or cold AIHA, with only rare instances of a different AIHA subtype. In contrast, our findings demonstrate that COVID-19 is associated with both warm AIHA (28% of patients) and cold AIHA (38% of patients) for reasons that remain unclear.
Although the exact mechanism by which infectious pathogens induce AIHA has yet to be fully characterized, currently the most accepted hypothesis involves molecular mimicry between microbial epitopes and epitopes on RBCs.50 This mechanism has also been proposed as the predominant cause of AIHA associated with COVID-19 because studies have shown that epitopes on the SARS-CoV-2 spike protein share significant homology with ankyrin-1, an integral protein in the erythrocyte membrane.14 Because of the similarity between viral and erythrocyte proteins, antibodies targeting the spike protein on the SARS-CoV-2 virus may cross-react with erythrocytes, resulting in destruction of the RBC and subsequent anemia. Although this review and its associated epidemiologic findings are not designed to test this hypothesis, we demonstrate that the median time frame from COVID-19 symptom onset to AIHA diagnosis is approximately 7 days, indicating that AIHA typically presents during early active SARS-CoV-2 infection. This time frame corresponds to the period of immunoglobulin formation and supports the premise that these developing antibodies may cross-react with erythrocytes, leading to hemolytic anemia. Furthermore, the large proportion of patients with complement deposition on their RBCs may partially explain the severe hemolysis seen in these patients, as erythrocyte-bound complement in the setting of a hyperinflammatory immune response may predispose patients to a more severe form of intravascular hemolysis.
In addition to the 50 cases of AIHA associated with COVID-19, we identified 4 reported cases of AIHA in individuals recently vaccinated against SARS-CoV-2. Two of these 4 patients underwent testing for COVID-19 by RT-PCR following admission to the hospital for symptoms secondary to underlying hemolytic anemia. Both patients tested negative, while the other 2 had no respiratory symptoms but were not tested. None of the patients endorsed a history of COVID-19, and all denied symptoms before the development of AIHA. Notably, no cases of vaccine-associated AIHA were reported in the SARS-CoV-2 vaccine clinical trials, nor are there data on the package inserts for the Pfizer-BioNTech,51 Moderna,52 or Janssen53 vaccines. Despite this absence of data in clinical trials, there are rare cases of AIHA associated with other vaccines, including diphtheria-tetanus-pertussis54 and influenza55 vaccines, illustrating that vaccines have previously been associated with the development of AIHA. Thus, increased awareness of the potential for SARS-CoV-2 vaccination–induced AIHA is warranted.
This analysis is the first systematic review of AIHA associated with SARS-CoV-2 infection and vaccination, providing evidence that SARS-CoV-2 is associated with a subtype of warm or cold AIHA. We acknowledge several limitations to this study. Most importantly, most published cases lack comprehensive descriptions of the pertinent blood bank and serologic methods and results. Few authors described their techniques, reagents, and other essential details describing indirect and direct antiglobulin testing, elution studies, cold agglutinin titers, thermal amplitude testing, and the strength of positive reactions, all of which are crucial in the diagnosis and classification of immune-mediated hemolytic anemias. These scant data highlight the need for journals to implement standardized approaches requiring authors of manuscripts describing potential cases of hemolytic anemia to include their methods for evaluating and classifying AIHA as well as detailed test results. Despite these limitations, we found that complement is frequently deposited on erythrocytes in patients with COVID-19–associated AIHA irrespective of the AIHA subtype. This finding, in association with a mean hemoglobin under 7 g/dL at the time of diagnosis, indicates that COVID-19 may be associated with acute hemolysis, most commonly occurring within 10 days of symptom onset or vaccination. Increased awareness of this potential complication is warranted to mitigate morbidity and mortality in these patients.
Conclusions
It is accepted that COVID-19 is associated with significant disruptions to the normal hematologic physiology in a subset of patients. The association between COVID-19 and the development of potentially devastating AIHA, however, has not been systematically reviewed to date. We have characterized all documented cases of AIHA associated with either SARS-CoV-2 infection or vaccination, illustrating that SARS-CoV-2 should be considered an infectious agent capable of causing AIHA. Patients with COVID-19 should be monitored for this potential AE to mitigate morbidity and potential mortality.
References
- 1. Capes A, Bailly S, Hantson P, et al. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99:1679-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jacobs J, Eichbaum Q. COVID-19 associated with severe autoimmune hemolytic anemia. Transfusion. 2021;61:635-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lazarian G, Quinquenel A, Bellal M, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hindilerden F, Yonal-Hindilerden I, Akar E, et al. Severe autoimmune hemolytic anemia in COVID-19 infection, safely treated with steroids. Mediterr J Hematol Infect Dis. 2020;12:e2020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zagorski E, Pawar T, Rahimian S, et al. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19). Br J Haematol. 2020;190:e183-e184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez C, Kim J, Pandey A, et al. Simultaneous onset of COVID-19 and autoimmune haemolytic anaemia. Br J Haematol. 2020;190:31-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jawed M, Hart E, Saeed M. Haemolytic anaemia: a consequence of COVID-19. BMJ Case Rep. 2020;13:e238118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hsieh TC, Sostin O. Severe warm autoimmune hemolytic anemia in COVID-19 managed with least incompatible RBC product and glucocorticoids [published online ahead of print February 18, 2021]. Ann Hematol. doi: 10.1007/s00277-021-04457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huscenot T, Galland J, Ouvrat M, et al. ; APHP Lariboisière COVID Group . SARS-CoV-2-associated cold agglutinin disease: a report of two cases. Ann Hematol. 2020;99:1943-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenzweig JD, McThenia SS, Kaicker S. SARS-CoV-2 infection in two pediatric patients with immune cytopenias: a single institution experience during the pandemic. Pediatr Blood Cancer. 2020;67:e28503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liebman HA, Weitz IC. Autoimmune hemolytic anemia. Med Clin North Am. 2017;101:351-359. [DOI] [PubMed] [Google Scholar]
- 12. Brodsky RA. Warm autoimmune hemolytic anemia. N Engl J Med. 2019;381:647-654. [DOI] [PubMed] [Google Scholar]
- 13. Dotan A, Muller S, Kanduc D, et al. The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev. 2021;20:102792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Angileri F, Légaré S, Marino Gammazza A, et al. Is molecular mimicry the culprit in the autoimmune haemolytic anaemia affecting patients with COVID-19? Br J Haematol. 2020;190:e92-e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. About the Vaccine Adverse Event Reporting System (VAERS). Centers for Disease Control and Prevention Web site. http://wonder.cdc.gov/vaers.html. Accessed September 24, 2021.
- 16. Maslov DV, Simenson V, Jain S, et al. COVID-19 and cold agglutinin hemolytic anemia. TH Open. 2020;4:e175-e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wahlster L, Weichert-Leahey N, Trissal M, et al. COVID-19 presenting with autoimmune hemolytic anemia in the setting of underlying immune dysregulation. Pediatr Blood Cancer. 2020;67:e28382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Singhavi R, Sharma K, Desai HD, et al. A case of hemolytic anemia with acute myocarditis and cardiogenic shock: a rare presentation of COVID-19. Cureus. 2020;12:e10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Patil NR, Herc ES, Girgis M. Cold agglutinin disease and autoimmune hemolytic anemia with pulmonary embolism as a presentation of COVID-19 infection [published online ahead of print July 6, 2020]. Hematol Oncol Stem Cell Ther. doi: 10.1016/j.hemonc.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Woldie IL, Brown IG, Nwadiaro NF, et al. Autoimmune hemolytic anemia in a 24-year-old patient with COVID-19 complicated by secondary cryptococcemia and acute necrotizing encephalitis: a case report and review of literature. J Med Cases. 2020;11:362-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bae JY, Jeon JE, Hussein KI, et al. Coombs-negative hemolytic anemia in a male with COVID-19. Clin Case Rep. 2021;9:e04503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vega Hernández P, Borges Rivas Y, Ortega Sánchez E, et al. Autoimmune hemolytic anemia in a pediatric patient with severe acute respiratory syndrome coronavirus 2 infection. Pediatr Infect Dis J. 2020;39:e288. [DOI] [PubMed] [Google Scholar]
- 23. Huda Z, Jahangir A, Sahra S, et al. A case of COVID-19-associated autoimmune hemolytic anemia with hyperferritinemia in an immunocompetent host. Cureus. 2021;13:e16078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Raghuwanshi B. Serological blood group discrepancy and cold agglutinin autoimmune hemolytic anemia associated with novel coronavirus. Cureus. 2020;12:e11495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liput JR, Jordan K, Patadia R, et al. Warm autoimmune hemolytic anemia associated with asymptomatic SARS-CoV-2 infection. Cureus. 2021;13:e14101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nesr G, Koshy R, Foldes D, et al. Autoimmune haemolytic anaemia and a marked rise in the lymphocyte count associated with COVID-19 in a patient with treatment-naïve chronic lymphocytic leukaemia: a case report. Br J Haematol. 2020;190:e326-e328. [DOI] [PubMed] [Google Scholar]
- 27. Gupta R, Singh S, Anusim N, et al. Coronavirus disease 2019 and cold agglutinin syndrome: an interesting case. Eur J Case Rep Intern Med. 2021;8:002387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Renganathan V, Dhanalakshmi KS, Nanda A, et al. Severe haemolytic anaemia in COVID 19—a rare manifestation. Indian J Anaesth. 2021;65:489-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ramos-Ruperto L, García-Pérez E, Hernández-Maraver D, et al. A 3-case series of autoimmune haemolytic anaemia and COVID-19: is plasma exchange an alternative? [published online ahead of print April 14, 2021]. SN Compr Clin Med. doi: 10.1007/s42399-021-00884-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aldaghlawi F, Shammah A, Kio E. SARS-CoV-2 infection complicated with cold agglutinin disease and myositis. Clin Case Rep. 2021;9:2196-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McGregor DW, Nelson G, Bergolt C. Rapidly fatal autoimmune hemolytic anemia secondary to COVID-19. J Investig Med. 2021;69:416-416. [Google Scholar]
- 32. Pandey SK, Gupta MK, Butta SK, et al. Hemolytic anemia in a case of SARS CoV2 infection without respiratory involvement: a new dimension of COVID-19. Int J Res Med Sci. 2020;8:4497-4498. [Google Scholar]
- 33. Sujana IPS, Widiasari NPA, Arisanti NLPE, et al. Autoimmune hemolytic anemia as a novel complication of COVID-19 infection in Sanglah General Hospital Bali, Indonesia [published online ahead of print November 6, 2020]. Open Access Maced J Med Sci. doi: 10.3889/oamjms.2020.5484. [DOI] [Google Scholar]
- 34. Nair LJ, Regukumar A, Baalamurugan KT. COVID-19-associated severe autoimmune hemolytic anemia: a rare case report. Saudi J Med Sci. 2021;9:276-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saini AS, Shayani K, Schwartz J. Cold agglutinin hemolytic anemia induced by COVID-19. Am J Med Case Rep. 2021;9:328-330. [Google Scholar]
- 36. Ahmadnezhad M, Mosleh M, Ferdowsi S, et al. Cold agglutinin associated with COVID-19 infection in a thalassemia patient with multiple alloantibodies: a case of cold hemagglutinin disease (CAD) with complex antibody detection. Hematol Transfus Cell Ther. 2021;43:361-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hassanein H, Hajdenberg J. High thermal amplitude red blood cell agglutinating cold type autoantibodies in a case of severe acute respiratory syndrome coronavirus 2 pneumonia and multiorgan failure. J Med Cases. 2021;12:16-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campos-Cabrera G, Mendez-Garcia E, Mora-Torres M, et al. Autoimmune hemolytic anemia as initial presentation of COVID-19 infection. Blood. 2020;136(suppl 1):8.32614959 [Google Scholar]
- 39. Li M, Nguyen CB, Yeung Z, et al. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190:e59-e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gruden G, Beggiato E, Camerino E, et al. Treatment with eltrombopag of severe immune thrombocytopenia and hemolytic anemia associated with COVID-19 pneumonia: a case report. Ther Adv Hematol. 2021;12:20406207211011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Georgy JT, Jayakaran JAJ, Jacob AS, et al. Evans syndrome and immune thrombocytopenia in two patients with COVID-19. J Med Virol. 2021;93:2642-2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zama D, Pancaldi L, Baccelli F, et al. Autoimmune hemolytic anemia in children with COVID-19 [published online ahead of print September 7, 2021]. Pediatr Blood Cancer. doi: 10.1002/pbc.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumarihamy P, Gunaratne S, Ratnayake A, et al. A case of COVID-19 infection associated with severe cold agglutinin autoimmune haemolytic anaemia [published online ahead of print July 31, 2021]. Clin Med Rev Case Rep. doi: 10.23937/2378-3656/1410358. [DOI] [Google Scholar]
- 44. Hasan MN, Sami CA, Amin MR, et al. An elderly man presented with autoimmune haemolytic anaemia—a consequence of severe corona virus disease 19 (COVID-19). Bangabandhu Sheikh Mujib Med Univ J. 2021;14:57-59. [Google Scholar]
- 45. Murdych TM. A case of severe autoimmune hemolytic anemia after a receipt of a first dose of SARS-CoV-2 vaccine [published online ahead of print July 14, 2021]. Int J Lab Hematol. doi: 10.1111/ijlh.13653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brito S, Ferreira N, Mateus S, et al. A case of autoimmune hemolytic anemia following COVID-19 messenger ribonucleic acid vaccination. Cureus. 2021;13:e15035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gaignard ME, Lieberherr S, Schoenenberger A, et al. Autoimmune hematologic disorders in two patients after mRNA COVID-19 vaccine. Hemasphere. 2021;5:e618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Aoun SA, Motabi I. Cold agglutinin disease after COVID-19 vaccine [published online ahead of print June 27, 2021]. Br J Haematol. doi: 10.1111/bjh.17674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. WHO Coronavirus (COVID-19) dashboard. World Health Organization Web site. https://covid19.who.int/. Accessed September 22, 2021.
- 50. Barcellini W. New insights in the pathogenesis of autoimmune hemolytic anemia. Transfus Med Hemother. 2015;42:287-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Comirnaty (COVID-19 vaccine, mRNA) [package insert]. New York, NY: Pfizer and BioNTech Manufacturing; 2021.
- 52. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Moderna COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). US Food and Drug Administration Web site. https://www.fda.gov/media/144637/download. Accessed September 20, 2021.
- 53. Fact sheet for healthcare providers administering vaccine (vaccination providers): emergency use authorization (EUA) of the Janssen COVID-19 vaccine to prevent coronavirus disease 2019 (COVID-19). US Food and Drug Administration Web site. https://www.fda.gov/media/146304/download. Accessed September 20, 2021.
- 54. Johnson ST, McFarland JG, Kelly KJ, et al. Transfusion support with RBCs from an Mk homozygote in a case of autoimmune hemolytic anemia following diphtheria-pertussis-tetanus vaccination. Transfusion. 2002;42:567-571. [DOI] [PubMed] [Google Scholar]
- 55. Montagnani S, Tuccori M, Lombardo G, et al. Autoimmune hemolytic anemia following MF59-adjuvanted influenza vaccine administration: a report of two cases. Ann Pharmacother. 2011;45:e8. [DOI] [PubMed] [Google Scholar]