Abstract
Background
Health care workers (HCWs) are on the frontline, playing a crucial role in the prevention of infection and treatment of patients.
Aims
This study was aimed to evaluate the prevalence of hospital-acquired coronavirus disease 2019 (COVID-19) infection at work and related factors at the University Hospital of Trieste workers exposed to COVID-19 patients.
Methods
From March 1 to May 31, of 4216 employees, 963 were in contact with COVID-19 patients or colleagues and were followed up. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in nasopharyngeal swabs was determined every 3 days, by RT-PCR.
Results
During the follow-up period, 193 workers were positive for COVID-19 (5%), and 165 of these (86%) were symptomatic. We identified five major cluster outbreaks of COVID-19 infection in Trieste Hospitals, four of which occurred before the implementation of universal masking for HCWs and patients (1–14 March 2020). COVID-19 infection was significantly higher in high-risk ward workers (Infectious Diseases, and Geriatric and Emergency Medicine, odds ratio [OR] 13.4; 95% confidence interval [CI] 5.8–31), in subjects with symptoms (OR 5.4; 95% CI 2.9–10) and in those with contacts with COVID-19 patients and colleagues (OR 2.23; 95% CI 1.01–4.9).
Conclusions
Hospital workers were commonly infected due to contact with COVID-19 patients and colleagues, mainly in the first 15 days of the pandemic, before the implementation of universal mask wearing of HCWs and patients. Repetitive testing and follow-up permitted the identification of COVID-19 cases before symptom onset, obtaining better infection prevention and control.
Keywords: COVID-19, epidemiology, health care workers
Key learning points.
What is already known about this subject:
Health care workers are at higher risk of developing COVID-19 due to contact with positive patients.
They have an important role to prevent the spread of the infection.
What this study adds:
Contract tracing of cases and periodical screening of health care workers for SARS-CoV-2 RNA detection in nasopharyngeal swabs with reverse transcription-polymerase chain reaction techniques permitted the identification of new cases mainly before the onset of symptoms.
During the follow-up period, 85% of health care workers developed symptoms, mainly involving the upper respiratory tract, and 15% remained asymptomatic.
We identified five major cluster outbreaks of COVID-19 at the University Hospital of Trieste, four of which occurred in the first 2 weeks of March.
What impact this may have on practice or policy:
Contact tracing and periodical screening of workers are crucial for reducing the spread of the infection.
Source control is crucial also in non-COVID-19 departments.
Introduction
The novel coronavirus (severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2]), which started in China in December 2019, caused the coronavirus disease 2019 (COVID-19) pandemic, which has severely affected health care workers (HCWs). HCWs are on the frontline and play a crucial role in the prevention of infection and treatment of patients. They are subject to psychological distress and fatigue in addition to the high risk of infection during their work tasks in hospitals [1–5]. To prevent infections among HCWs, hospitals periodically tested all HCWs and implemented infection control measures to detect early cases and to reduce the spread of infection to patients and colleagues [2]. HCWs can acquire the infection not only from patients but also from colleagues, when protection measures are reduced during meetings or coffee breaks [6].
The prevalence of HCWs tested positive for SARS-CoV-2 is ranging from approximately 10% (95% CI 5.3–14.9), but with symptoms milder than the general population [7], due to younger age compared to patients. Very little data are available for Italy, and the deep analysis of COVID-19 clusters in HCWs is crucial to understand the diffusion of the infection to prevent it [8].
The objective of this study was to report the epidemiological, clinical and laboratory characteristics of COVID-19 infections in HCWs at the University Hospital of Trieste during the first outbreak of COVID-19 from 1 March to 31 May 2020, and to investigate the related factors.
Methods
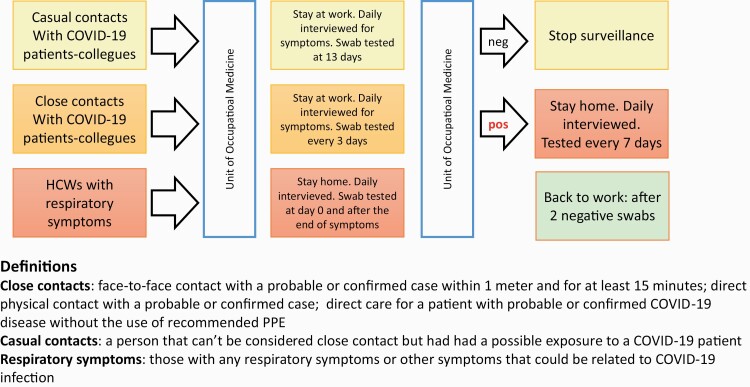
During the COVID-19 pandemic, the Unit of Occupational Medicine at the University Hospital of Trieste implemented infection prevention and control (IPC) measures involving specific surveillance for hospital workers and universal masking of all workers and patients. The use of personal protective equipment (PPE) was compulsory for the workforce according to the Istituto Superiore di Sanità Guidelines [9]. In case of exposure without suitable protection to a COVID-19 patient or colleague, the head nurse sent an e-mail describing the circumstances of the infection to the Unit of Occupational Medicine, which subsequently activated the surveillance protocol [9,10]. The workers received a phone interview to investigate primary and secondary contacts, and contacts were classified into close or casual contacts depending on exposure characteristics. Close contacts were defined according to the WHO guidelines: (i) face-to-face contact with a probable or confirmed case within 1 m and for at least 15 min, (ii) direct physical contact with a probable or confirmed case and (iii) direct care for patients with probable or confirmed COVID-19 without the use of recommended PPE. [9,11] Casual contacts were defined when an HCW had been exposed to a COVID-19 patient without matching the definition for close contact. According to Italian regulations, asymptomatic exposed staff were allowed to continue working and were advised to wear surgical masks at work as well as at home. If the patient was symptomatic or positive for SARS-CoV-2 detection in nasopharyngeal and oropharyngeal swabs by reverse transcriptase-polymerase chain reaction (RT-PCR), they were immediately restricted from work. Close contacts had to monitor and report body temperature twice a day and were interviewed daily to verify their health status. They were swab tested every 3 days from contact time. Casual contacts were interviewed daily to verify their health status, and they had to monitor and report their body temperature twice a day and underwent a swab test 13 days after contact (Figure 1). In case of symptom onset, HCWs were immediately tested, stopped working and remained quarantined at home with daily monitoring by phone call. In case of worsening of existing symptoms, they had to contact their general practitioner or the emergency number to be admitted to the hospital where appropriate. Upper respiratory tract symptoms were defined as sore throat, anosmia, loss of taste and cough. Lower respiratory tract symptoms were defined as cases of dyspnoea, bronchitis or pneumonia. Nasopharyngeal and oropharyngeal specimens were collected using the swab technique by Occupational Medicine Unit staff, and RNA was extracted and determined by RT-PCR targeting the E, N and RdRp genes of SARS-CoV-2 according to the Centers for Disease Control and Prevention (CDC) and Charité laboratory protocols [12]. The cycle threshold values of RT-PCR were used as indicators of viral load of SARS-CoV-2 RNA, with lower cycle threshold values corresponding to higher viral copy numbers. A cycle threshold value of less than 30 was considered positive for SARS-CoV-2 RNA.
Figure 1.
Layout of the study and definitions used.
Data analysis was performed using STATA™ software (version 14.0; Stata Corp., LP, College Station, TX, USA).
Categorical data were cross-tabulated into k × k contingency tables and statistically tested using the chi-squared test. Continuous data were reported as mean and standard deviation and statistically tested using the Kruskal–Wallis test. COVID-19 as an outcome was analysed by univariate logistic regression analysis, with sex, age (as a continuous variable), occupation (residence, nurse, nurse aid, others and physician as reference), wards (high risk, medium risk and low risk as reference), contacts (with HCWs, with patients and HCWs, contact of contact with patients as reference), use of PPE, comorbidity and symptoms as independent variables. Factors associated with COVID-19 infection in univariate logistic regression analysis were investigated using multivariate regression analysis. Odds ratios (ORs) and 95% confidence intervals (CIs) were estimated from the coefficients and standard errors of the logistic regression. Workers with missing data for relevant variables were excluded from the analysis. A P value of <0.05 was established as the limit of statistical significance.
The local Ethical committee approved the study (CEUR- 2020-Os-072) on 16 March 2020.
Results
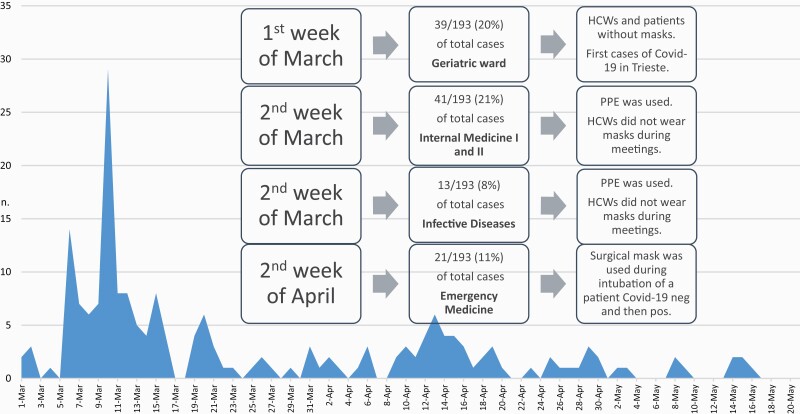
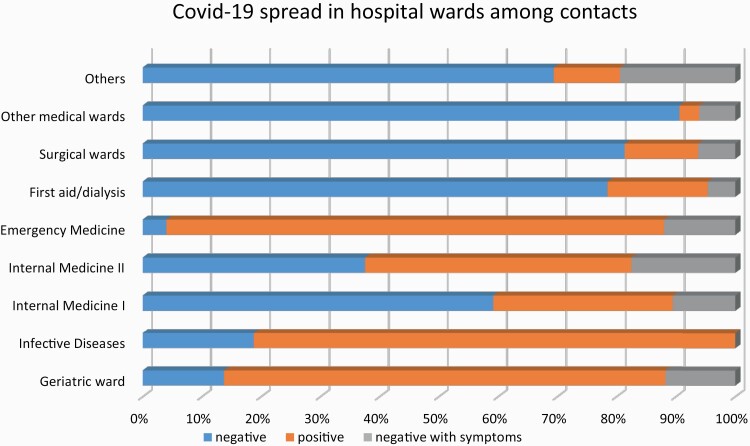
Nine hundred and sixty-three of 4216 HCWs employed in Trieste University Hospitals reported exposure to patients and/or colleagues known to have COVID-19 between 1 March and 31 May 2020. Their main characteristics are presented in Table 1. One hundred and ninety-three (5%) were COVID-19 confirmed cases. Of the 193 positive HCWs, 165 (86%) were symptomatic, with a median (interquartile range [IQR]) time from contact to symptom onset of 4 (2–8) days. HCWs with SARS-CoV-2 had different job titles, mostly nurses and physicians, and the vast majority were employed in medical wards. Of the symptomatic positive cases, 114 (69%) were female, with a mean (SD) age of 43 (11.2) years. Instead, the percentage of females (n) and the mean (SD) age appeared slightly lower among asymptomatic infected subjects (61% [n = 17] and 39.5 [11.8] years). Most affected subjects had contact with an infected patient (n = 103; 53%), 32 reported exposures only to colleagues (17%), 51 to both patients and colleagues (26%), and 7 to casual contact or contact with COVID-19 cases (4%). Most cases reported the use of PPE (n = 172; 89%): 101 (52%) wearing surgical masks and 71 (37%) wearing FFP2 or FFP3 masks, in accordance with safe routine procedures [9,13]. We identified five major cluster outbreaks of COVID-19 infection at the University hospital of Trieste, four of which occurred before implementation of universal masking of HCWs and patients (1–14 March 2020) (Figure 2). The first cluster of exposure occurred on 6 March 2020, in a Geriatric ward, at which time both HCWs and patients were without masks. The second and third clusters of contacts occurred in two internal medicine wards, where two other patients not suspected of having SARS-CoV-2 infection were admitted. At that time, the PPE was used, but HCWs did not wear masks during meetings and breaks. The fourth cluster was in Infectious Diseases ward, and the infection spread among co-workers during meetings. Finally, the fifth cluster of exposure occurred after implementation of universal masking and compliance with IPC measures in the Emergency Medicine ward (EMW). On 10 April 2020, a patient who underwent two consecutive negative RT-PCR tests over 24 h was admitted to the EMW, but a few days after he complained of fever >37.5°C and respiratory symptoms. Thus, the patient was tested again, and the test yielded positive results. The closure of the EMW was planned for 15 days, as the infection had largely spread among personnel. In that case, only the surgical mask was used during intubation of the patient. In total, 59% (n = 114) of the positive cases occurred within these five major cluster outbreaks at the University hospital of Trieste. The COVID-19 spread in the remaining hospital departments was much more prevalent, with some sporadic cases in other medical wards and very few cases in surgical wards (Figure 3).
Table 1.
Main characteristics of 963 HCWs reporting contacts with COVID-19 patients and/or colleagues at the University Hospital of Trieste
| SARS-CoV-2 negative | SARS-CoV-2 positive | Total | P | |||
|---|---|---|---|---|---|---|
| Asymptomatic | Symptomatic | Asymptomatic | Symptomatic | |||
| n (%) | 665 (69) | 105 (11) | 28 (3) | 165 (17) | 963 | |
| Age (years), mean (SD) | 44.3 (12) | 45.7 (10) | 39.5 (12)c | 43 (11) | 44 (12) | 0.04 |
| Women, n (%) | 467 (70) | 82 (78) | 17 (61) | 114 (69) | 680 (71) | NS |
| Characteristics of contact, n (%) | ||||||
| With patients | 240 (36) | 45 (43) | 16 (57) | 87 (53) | 388 (40) | 0.000 |
| With colleagues | 113 (17) | 30 (27) | 5 (18) | 27 (16) | 175 (18) | |
| With both | 11 (2) | 17 (16) | 5 (18) | 46 (28) | 79 (8) | |
| Contact of contact or casual contact | 301 (45) | 13 (12) | 2 (7) | 5 (3) | 321 (33) | |
| Occupation, n (%) | ||||||
| Physician | 174 (26) | 18 (17) | 14 (14) | 40 (24) | 236 (25) | NS |
| Residenta | 38 (6) | 7 (7) | 1 (4) | 13 (8) | 59 (6) | |
| Nurse | 283 (43) | 46 (44) | 13 (46) | 72 (44) | 414 (43) | |
| Nurse aid | 81 (12) | 16 (15) | 4 (14) | 26 (16) | 127 (13) | |
| Otherb | 89 (13) | 18 (17) | 6 (21) | 14 (8) | 127 (13) | |
| Use of PPE, n (%) | 642 (96) | 78 (74) | 27 (96) | 145 (88) | 892 (93) | 0.000 |
| Surgical mask | 634 (100) | 73 (94) | 15 (56) | 86 (59) | 808 (91) | |
| FFP2/FFP3 mask | 8 (1) | 5 (6) | 12 (44) | 59 (41) | 84 (9) | |
| Start of symptoms after contact (days), median (IQR) | – | 4 (2) | – | 4 (3) | 4 (3) | NS |
| Symptoms, n (%) | – | – | 270 | |||
| Upper respiratory tract | 82 (78) | 128 (78) | 210 (78) | NS | ||
| Cough | 52 (49) | 70 (43) | 122 (45) | NS | ||
| Loss of smell and taste | 7 (7) | 67 (41) | 74 (27) | 0.000 | ||
| Lower respiratory tract | 1 (1) | 14 (8) | 15 (6) | 0.008 | ||
| Fever >37.5°C | 36 (34) | 85 (52) | 121 (45) | 0.006 | ||
| Diarrhoea | 19 (18) | 13 (8) | 32 (12) | 0.001 | ||
NS, non-significant.
aPhysician during postgraduate course.
bTechnicians and clerks.
cKruskal–wallis test.
Figure 2.
Time trends in hospital contacts among positive HCWs from March 1 to the end of May, 2020.
Figure 3.
COVID-19 spread in different hospital wards among HCWs with exposure to positive patients and/or colleagues.
Of the 193 positive cases, 128 (78%) had limited, mild upper respiratory tract symptoms, while 14 (9%) suffered from a more severe disease with lower respiratory tract symptoms, which were observed significantly more often in COVID-19 HCWs (P < 0.05), as well as loss of smell and taste (P < 0.05), and fever >37.5°C (P < 0.05), compared to symptomatic SARS-CoV-2-negative workers (Table 1). No one died. Factors associated with COVID-19 infection are reported in Table 2, using univariate and multivariate regression analyses. ORs were higher for workers employed in high-risk wards (Infectious Diseases, Geriatric and Emergency Medicine, OR 13.4; 95% CI 5.8–31), subjects with symptoms (OR 5.4; 95% CI 2.9–10), and those with contacts with COVID-19 patients and colleagues (OR 2.23; 95% CI 1.01–4.9). Compliance with the use of PPE was high in COVID-19 HCWs (OR 3.7; 95% CI 1.6–8.2).
Table 2.
Univariate and multivariate logistic regression analysis of factors involved in COVID-19 positivity in HCWs
| Univariate analysis OR (95% CI) |
Multivariate analysis OR (95% CI) |
|
|---|---|---|
| Age | 0.98 (0.99–0.99) | 0.98 (0.96–1.01) |
| Men versus women | 1.14 (0.75–1.7) | 1.69 (0.9–3.2) |
| Occupation | ||
| Physician | 1 | |
| Residenta | 1.36 (0.78–2.69) | |
| Nurse | 1.12 (0.75–1.69) | |
| Nurse aid | 1.39 (0.83–2.35) | |
| Otherb | 1.01 (0.6–2.00) | |
| Wards | ||
| Low risk: surgical and medical wards | 1 | 1 |
| Medium risk: Internal Medicine I and II | 4.6 (2.9–7.2) | 1.88 (0.97–3.6) |
| High risk: Infectious Diseases, Geriatric and Emergency Medicine | 30.8 (17.0–53.2) | 13.4 (5.8–31.0) |
| Contacts | ||
| With patients | 1 | 1 |
| With HCWs | 0.62 (0.4–0.96) | 1.39 (0.64–3.0) |
| With both | 5.13 (3.1–8.6) | 2.23 (1.01–4.9) |
| Contact with contact or causal contacts | 0.61 (0.03–0.13) | 1.40 (0.28–6.9) |
| Use of PPE | 2.9 (1.63–5.1) | 3.7 (1.6–8.2) |
| Use of N95 mask | 7.8 (4.0–15.2) | |
| Comorbidity | 0.88 (0.31–2.51) | |
| Symptoms | 37.3 (23.8–58.5) | 5.4 (2.9–10.0) |
| Lower respiratory symptoms | 9.7 (1.26–74.9) | |
| Fever >37.5°C | 2.03 (1.26–74.9) | |
| Cough | 0.76 (0.46–1.2) | |
| Loss of smell and taste | 9.7 (4.2–22.1) | |
| Diarrhoea | (0.18–0.83) |
Significant associations are highlighted in bold.
aPhysician during postgraduate course.
bTechnicians and clerks.
Discussion
We investigated the COVID-19 first outbreak among HCWs that occurred at the University Hospital of Trieste (northeastern Italy). Between 1 March and 31 May 2020, the Trieste Hospital surveillance system was notified of 963 workers who reported contact with COVID-19 patients and/or colleagues. Among such contacts, 193 cases were COVID-19 confirmed, representing 4.6% of all occupied workers in our hospital and 14% of total COVID-19 cases in the province of Trieste as of May 31 [14]. The incidence of the infection in the general population in Trieste was 59 cases/10 000 inhabitants from 1 March 2020 to 31 May 2020 [14].
Higher prevalence of infected HCWs was observed in the USA, with 19% of COVID-19 cases among HCWs registered by CDC [15], and 9.6% by Los Angeles County Department of Public Health [16]. In a recent meta-analysis, Sahu et al. [7] reported a prevalence of infection of 10.1% (CI 95% 5.3–14.9). In general, western countries reported higher values of COVID-19 infection in HCWs than in Chinese data [17], probably because in the first period of the pandemic, the diffusion of infection was underestimated between HCWs and risk perception was lower in western countries, probably due to less experience with the diffusive virus epidemic.
Our data on the prevalence of infection in HCWs are similar to those reported in Milan hospitals (4.2–5.3% in HCWs) in which all workers with close contacts were screened as in our study [8]. However, in a study conducted in a Madrid hospital [18], only symptomatic workers were screened, and the prevalence of infection was 11%. These data suggest that symptom-based screening does not permit a quick identification of infected people, resulting in the spread of infection [19].
The analysis of the clusters of COVID-19 infections that occurred during the lockdown in Italy revealed that more than 50% of cases were found in five wards, mainly in the first 2 weeks of March. The spread of infection was due to the lack of protective measures with patients initially tested negative for SARS-CoV-2 or during meetings and coffee breaks with colleagues. Universal masking for HCWs and patients was implemented after half of March, but patients with respiratory symptoms were allowed not to wear a mask. This is what happened in the last cluster in EMW, in which patients that were COVID-19 positive did not wear a mask, while HCWs wear surgical masks, not enough to be protected against SARS-CoV-2 spread. During the first 14 days of March, awareness of the novel biological hazard and hospital masking policies were limited. Thus, improper use of PPE, especially during HCW meetings, was found to play a crucial role in the amplification of early outbreaks among co-workers. Moreover, the use of proper PPE and disinfection habits need to be associated with efficient ventilation to ensure good air quality in the workplace [20].
In addition, exposure to infected colleagues may be another reason for the infection of SARS-CoV-2 in HCWs. Sporadic spread was observed in other medical departments, and few infected HCWs were found in surgical wards. Interestingly, there were no cases that originated from the COVID-19 wards (excluding the accident in the Infectious Diseases ward) or intensive care units, showing better adherence to IPC measures and use of contact and droplet precautions by HCWs caring for patients with SARS-CoV-2.
Investigations among infected HCWs in China suggest that COVID-19 was predominantly acquired in the community within the household [21]. The proper preparedness of Oriental countries mindful of the previous SARS and Middle East respiratory syndrome coronavirus outbreaks seems to have significantly contributed to better prevention and management of COVID-19 infection in health care settings [22,23].
In addition, the data recording process and the classification of occupational and non-occupational diseases may also explain some differences found in the proportion of HCWs infected by COVID-19 in Italy compared to Oriental countries [24].
Finally, it is very likely that the number of infected HCWs at the University Hospital of Trieste was due to the execution, based on an accurate contact tracing, of a large number of swab tests, resulting in the diagnosis of many pauci-symptomatic and asymptomatic subjects.
Sex-based differences in COVID-19 infection are known, and men are more likely to be affected and experience severe symptoms than women [25]. At the University Hospital of Trieste, male HCWs affected by SARS-CoV-2 were 32% (n = 62), higher than expected considering that they represent the 25% of the health care workforce.
The accurate surveillance activities put in place at the University Hospital of Trieste led to the identification of 128 (78% of symptomatic positive cases) who had limited, mild upper respiratory tract symptoms. A significant number of COVID-19 HCWs suffered from fever >37.5°C (52%) and loss of smell and taste (41%), and few subjects had lower respiratory tract symptoms (9%) and diarrhoea (8%), in agreement with other investigations [26]. Our data strongly emphasize the importance of paying attention to symptoms such as fever and loss of smell and taste that are often the first or only ones present. However, 28 (15%) infected HCWs did not show any typical symptoms of SARS-CoV-2.
One of the first studies conducted on health workers in Italian hospitals observed that 44% of the infected workers had no fever or respiratory symptoms, and approximately one in three of the HCWs who tested positive never manifested any symptoms [27].
Pre-symptomatic and asymptomatic transmission contributes to the pandemic and symptom screening alone is not sufficient to identify individuals with COVID-19 [8].
The time between exposure to COVID-19 and the moment when symptoms start is commonly around 4–5 days but can range from 1 to 14 days [28], which is perfectly in line with our finding (median time of 4 days with an IQR of 2–8 days).
The time-trend analysis of contacts and cases onset showed a progressive improvement in the spread of nosocomial infections after the identification of the early four main clusters. Most transmission of COVID-19 occurred before the implementation of universal masking of HCWs and patients (1–14 March 2020), and largely among personnel employed in medical wards. Active surveillance also allowed 105 (38.9%) symptomatic HCWs to return to work as they tested negative for SARS-CoV-2, thus reducing workforce depletion.
Some limitations of our study need to be noted. First, it is possible that an initial unclear definition of contact, in addition to the fear of contagion, led to a large number of subjects reporting exposure; therefore, our results may be affected by selection bias. Second, the progressive reduction in HCW infections can be related to better adherence to protective measures, but also to a progressive decrease in COVID-19 infections in the general population.
Despite these potential limitations, our study aimed to provide the epidemiological, clinical and laboratory characteristics derived from the surveillance data of HCWs in Trieste Hospital during the COVID-19 outbreak in order to improve the IPC measures in health care settings. Accurate contact tracing and active surveillance allowed the isolation of a large number of HCWs who had initially acquired the infection because of an inadequate risk perception [29], and therefore to reduce the insidious spread from asymptomatic or mild cases. Finally, the incidence of COVID-19 infection in our HCWs decreased before the decline in the general population.
Universal mask wearing, when implemented together with strict employee surveillance and contact tracing, reduced nosocomial transmission of SARS-CoV-2 and strengthened the health care workforce. Disinfection procedures, use of other PPE, and proper ventilation of workplaces can further contribute to reducing the onset of COVID-19 clusters in HCWs.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Competing interests
None declared.
Data availability
The data underlying this article are available on request.
References
- 1. Adams JG, Walls RM. Supporting the health care workforce during the COVID-19 global epidemic. J Am Med Assoc 2020;323:1439–1440. [DOI] [PubMed] [Google Scholar]
- 2. Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association between universal masking in a health care system and SARS-CoV-2 positivity among health care workers. J Am Med Assoc 2020;324:703–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hunter E, Price DA, Murphy E et al. First experience of COVID-19 screening of health-care workers in England. Lancet 2020;395:e77–e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Black JRM, Bailey C, Przewrocka J, Dijkstra KK, Swanton C. COVID-19: the case for health-care worker screening to prevent hospital transmission. Lancet 2020;395:1418–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Klompas M, Morris CA, Sinclair J, Pearson M, Shenoy ES. Universal masking in hospitals in the COVID-19 era. N Engl J Med 2020;382:e63. [DOI] [PubMed] [Google Scholar]
- 6. Schneider S, Piening B, Nouri-Pasovsky PA, Krüger AC, Gastmeier P, Aghdassi SJS. SARS-coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control 2020;9:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sahu AK, Amrithanand VT, Mathew R, Aggarwal P, Nayer J, Bhoi S. COVID-19 in health care workers—a systematic review and meta-analysis. Am J Emerg Med 2020;38:1727–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mandić-Rajčević S, Masci F, Crespi E et al. Source and symptoms of COVID-19 among hospital workers in Milan. Occup Med (Lond) 2020;70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Istituto Superiore di Sanità. Indicazioni ad interim per un utilizzo razionale delle protezioni per infezione da SARS-CoV-2 nelle attività sanitarie e sociosanitarie (assistenza a soggetti affetti da COVID-19) nell’attuale scenario emergenziale SARS-CoV-2. Rapporto ISS n.2/29 Marzo 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/health-workers (7 July 2021, date last accessed). [Google Scholar]
- 10. World Health Organization (WHO). Risk Assessment and Management of Exposure of Health Care Workers in the Context of COVID-19: Interim Guidance. https://www.who.int/publications/i/item/risk-assessment-and-management-of-exposure-of-health-care-workers-in-the-context-of-covid-19-interim-guidance (10 December 2020, date last accessed). [Google Scholar]
- 11. World Health Organization (WHO). Contact Tracing in the Context of COVID 19. https://www.who.int/publications/i/item/contact-tracing-in-the-context-of-covid-19 (10 March 2020, date last accessed). [Google Scholar]
- 12. World Health Organization (WHO). Coronavirus Disease (COVID-19) Technical Guidance: Laboratory Testing for 2019-nCoV in Humans. 2020. https://www.who.int/publications/i/item/laboratory-testing-strategy-recommendations-for-covid-19-interim-guidance (30 October 2021, date last accessed). [Google Scholar]
- 13. World Health Organization (WHO). Advice on the Use of Masks in the Community, During Home Care and in Health Care Settings in the Context of the Novel Coronavirus (2019-nCoV) Outbreak. Interim Guidance. 2020. https://apps.who.int/iris/bitstream/handle/10665/330987/WHO-nCov-IPC_Masks-2020.1-eng.pdf?sequence=1&isAllowed=y (2 March 2020, date last accessed). [Google Scholar]
- 14. Dipartimento della Protezione Civile, Italy. Open Data Repository. 2020. http://opendatadpc.maps.arcgis.com/apps/opsdashboard/index.html#/b0c68bce2cce478eaac82fe38d4138b1 (10 December 2020, date last accessed). [Google Scholar]
- 15. CDC COVID-19 Response Teams. Characteristics of health care personnel with COVID-19—United States, February 12–April 9, 2020. MMWR Morb Mortal Wkly Rep 2020;68:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hartmann S, Rubin Z, Sato H et al. Coronavirus 2019 (COVID-19) infections among healthcare workers, Los Angeles County, February–May 2020. Clin Infect Dis 2021;73:e1850–e1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. J Am Med Assoc 2020;323:1239–1242. [DOI] [PubMed] [Google Scholar]
- 18. Folgueira MD, Munoz-Ruiperez C, Alonso-Lopez MA, Delgado R. SARS-CoV-2 infection in health care workers in a large public hospital in Madrid, Spain, during March 2020. medRxiv 2020, preprint: not peer reviewed. doi: 10.1101/2020.04.07.20055723 [DOI] [Google Scholar]
- 19. Kimball A, Hatfield KM, Arons M et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep 2020. ;69:377–381.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller SL, Nazaroff WW, Jimenez JL et al. Transmission of SARS-CoV-2 by inhalation of respiratory aerosol in the Skagit Valley Chorale superspreading event. Indoor Air 2021;31:314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tan LF. Preventing the transmission of COVID-19 amongst healthcare workers. J Hosp Infect 2020;105:364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hsin DH, Macer DR. Heroes of SARS: professional roles and ethics of health care workers. J Infect 2004;49:210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yen MY, Lin YE, Lee CH et al. Taiwan’s traffic control bundle and the elimination of nosocomial severe acute respiratory syndrome among healthcare workers. J Hosp Infect 2011;77:332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J Am Med Assoc 2020;323:1775–1776. [DOI] [PubMed] [Google Scholar]
- 25. Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med 2020;8:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lechien JR, Chiesa-Estomba CM, De Siati DR et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Magnavita N, Tripepi G, Di Prinzio RR. Symptoms in health care workers during the COVID-19 epidemic. A cross-sectional survey. Int J Environ Res Public Health 2020;17:5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. World Health Organization (WHO). Q&A on Coronaviruses (COVID-19). 17 April 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/question-andanswers-hub/q-a-detail/q-a-coronaviruses (20 April 2020, date last accessed). [Google Scholar]
- 29. Piapan L, De Michieli P, Ronchese F et al. COVID-19 outbreak in healthcare workers in hospitals in Trieste, North-east Italy. J Hosp Infect 2020;106:626–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available on request.