ABSTRACT
Background
Dialysis confers the highest risk of coronavirus disease 2019 (COVID-19) death among comorbidities predisposing to severe COVID-19. However, reports of COVID-19-associated mortality frequently refer to mortality during the initial hospitalization or first month after diagnosis.
Methods
In a prospective, observational study, we analysed the long-term (1-year follow-up) serological and clinical outcomes of 56 haemodialysis (HD) patients who were infected by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) during the first pandemic wave. COVID-19 was diagnosed by a positive polymerase chain reaction (PCR) test (n = 37) or by the development of anti-SARS-CoV-2 antibodies (n = 19).
Results
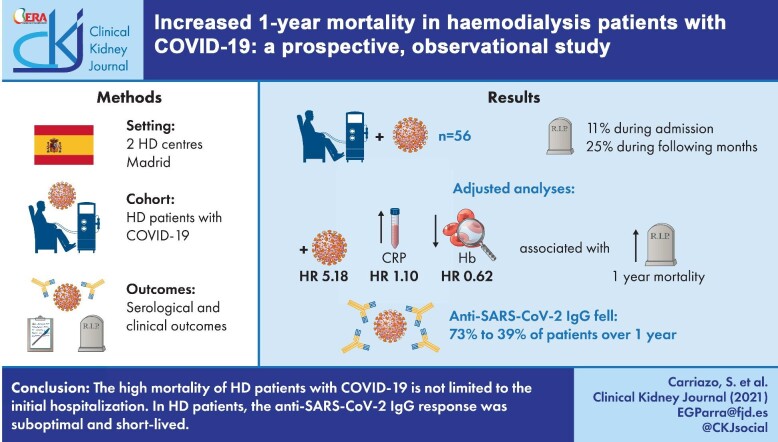
After >1 year of follow-up, 35.7% of HD patients infected by SARS-CoV-2 during the first pandemic wave had died, 6 (11%) during the initial admission and 14 (25%) in the following months, mainly within the first 3 months after diagnosis. Overall, 30% of patients died from vascular causes and 40% from respiratory causes. In adjusted analysis, a positive SARS-CoV-2 PCR test for diagnosis {hazard ratio [HR] 5.18 [interquartile range (IQR) 1.30–20.65], P = 0.020}, higher baseline C-reactive protein levels [HR 1.10 (IQR 1.03–1.16), P = 0.002] and lower haemoglobin levels [HR 0.62 (IQR 0.45–0.86), P = 0.005] were associated with higher 1-year mortality. Mortality in the 144 patients who did not have COVID-19 was 21 (14.6%) over 12 months [HR of death for COVID-19 patients 3.00 (IQR 1.62–5.53), log-rank P = 0.00023]. Over the first year, the percentage of patients having anti-SARS-CoV-2 immunoglobulin G (IgG) decreased from 36/49 (73.4%) initially to 27/44 (61.3%) at 6 months and 14/36 (38.8%) at 12 months.
Conclusions
The high mortality of HD patients with COVID-19 is not limited to the initial hospitalization. Defining COVID-19 deaths as those occurring within 3 months of a COVID-19 diagnosis may better represent the burden of COVID-19. In HD patients, the anti-SARS-CoV-2 IgG response was suboptimal and short-lived.
Keywords: anti-SARS-CoV-2 antibodies, chronic kidney disease, COVID-19, haemodialysis, mortality, outcomes
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) causes coronavirus disease 2019 (COVID-19) [1]. As of September 2021, COVID-19 has caused >4 million deaths worldwide (https://covid19.who.int/; accessed 30 September 2021). Risk factors for severe or fatal COVID-19 include age, male sex, non-European ancestry, obesity, diabetes mellitus, immunosuppression and cardiovascular disease [2]. However, persons with chronic kidney disease (CKD) represent the largest global population at high risk of severe COVID-19 [3, 4]. Furthermore, dialysis patients have the highest risk of COVID-19 death among comorbidities predisposing to COVID-19. In an English study including >17 million persons, transplant recipients and dialysis patients had the highest risk of COVID-19 death among comorbidities predisposing to COVID-19 and CKD category G4 was among the top 5 conditions conferring this highest risk of COVID-19-related death [3, 4]. A meta-analysis that included 3 867 367 patients from 12 studies reported that the mortality rate was higher among CKD patients with COVID-19 infection than among CKD patients without COVID-19 infection {odds ratio [OR] 5.81 [95% confidence interval (CI) 3.78–8.94, P < 0.00 001, I2 = 30%} [5]. Reasons associated with this increased risk are not completely elucidated. Kidney failure is considered an immunosuppressed state characterized by altered innate and adaptative immunity [6, 7]. The combination of a systemic pro-inflammatory state [8], impaired immune response and high cardiovascular risk [9] might contribute to this increased risk of severe COVID-19.
Reports of COVID-19-associated mortality frequently refer to mortality during the initial hospitalization or 28 days after diagnosis [10–12]. We previously reported an overall mortality rate of 10% in haemodialysis (HD) patients with COVID-19 during hospitalization in the first pandemic wave starting in March 2020 [13]. We further reported that 17% of SARS-CoV-2 PCR-positive HD patients failed to develop detectable antibodies against SARS-CoV-2 by 3 months of infection [14].
We have now analysed the long-term (1 year of follow-up) serological and clinical outcomes of HD patients who were infected by SARS-CoV-2 during the first pandemic wave and survived hospitalization or the initial COVID-19 episode and present data on overall 12-month mortality from the diagnosis of COVID-19.
MATERIALS AND METHODS
In a previous retrospective observational study [13] we reported on COVID-19 in patients from two HD units in Madrid, Spain, the Hospital Universitario Fundación Jiménez Díaz (UHFJD) HD unit and its associated centre Fundación Renal Centro Santa Engracia (FRCSE) that coordinate for patient care. Patients are dialysed at the hospital-based UHFJD HD unit when they have higher comorbidity or present intercurrent health issues. The present report relates to a prospective observational study of chronic HD patients diagnosed with COVID-19 during the first wave starting in March 2020 and up to May 2020 and followed for 1 year. At the start of the pandemic in Madrid, both centres had a total combined number of 200 adult patients on HD (58 in UHFJD and 142 in FRCSE). FRCSE patients with a diagnosis of COVID-19 were transferred to the UHFJD. The study was approved by the IIS-Fundación Jiménez Díaz Ethics Committee (PIOH036-20_FJD, 27 April 2020) and was performed in accordance with the Declaration of Helsinki and the European Union Clinical Trial Directive. Patients enrolled provided written informed consent.
COVID-19 diagnosis
Between March and May 2020, 56 HD patients were confirmed to have SARS-CoV-2 infection through SARS-CoV-2 quantitative reverse transcription polymerase chain reaction (PCR) (n = 37) or anti-SARS-CoV-2 antibody testing (n = 19) (Figure 1). The PCR test for SARS-CoV-2 consists of a nucleic acid extraction from nasopharyngeal swab samples and subsequent amplification by reverse transcription and PCR of the ORF1ab and N genes (VIASURE SARS-COV-2 RT-PCR, Certest, San Mateo de Gállego, Spain). Anti-SARS-CoV-2 immunoglobulin M (IgM) and IgG tests were performed according to FJD laboratory standards of the moment: In March and April, the antibody test used was Enzyme linked immunoassay-Chemiluminescence linked immunassay against spike (S) and nucleocapsid (N) antigens (VIRCELL, Granada, Spain). IgG positivity was set at >0.4 UA. Sensitivity and specificity were 82–88% (7 days after PCR positive) and 98.8% for IgM and 100% (19 days after PCR positive) and 98% for IgG. After May 2020, the test used was VITROS XT 7600 (Ortho), IgG against spike (S) protein. IgG positivity was set at >1.4 UA (sensitivity 90%, specificity 100%).
FIGURE 1:
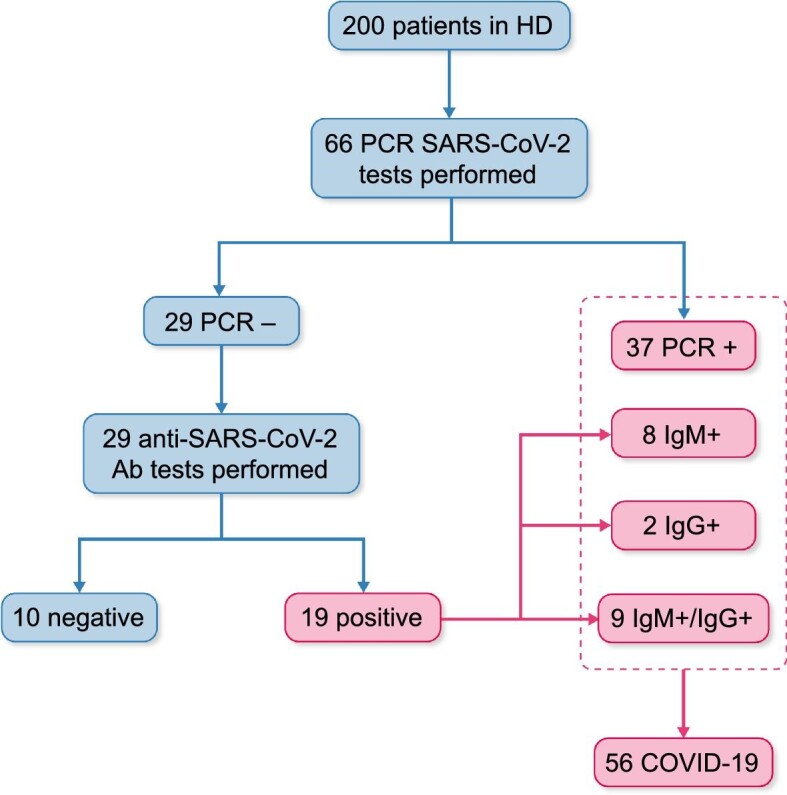
Flow chart of HD patients diagnosed with SARS-CoV-2 infection between March and May 2020.
Additional laboratory tests at baseline included haemoglobin (Hb), interleukin-6 (IL-6), ferritin, C-reactive protein (CRP) and D-dimer levels and lymphocyte count. Furthermore, the weekly doses of erythropoiesis-stimulating agents (ESAs) and erythropoietin resistance index (ERI) were also included in the analysis. ERI was calculated by dividing the weekly body weight–adjusted epoetin dose by the Hb concentration.
Initial treatment
SARS-CoV-2 PCR-positive patients underwent HD in a separate isolation room. Patients were hospitalized in the presence of pneumonia, oxygen saturation <94% or hypoxaemia evidenced in arterial blood and/or significant deterioration in general condition. Initial treatment for hospitalized patients and outpatients was administered according to the local protocol. In March 2020, this included hydroxychloroquine (200 mg/12 h for 5 days) and antibiotics (doxycycline 100 mg/12 h for 5 days or levofloxacin 250 mg/48 h for 5 days). In case of acute respiratory insufficiency and inflammatory signs, such as marked elevation of ferritin and CRP, glucocorticoids were added (methylprednisolone 250 mg/day for 3 days, followed by oral prednisone 40 mg/12h for 3–4 days). For those with analytical data of procoagulant status, i.e. D-dimer elevation or with markers of acute myocardial damage, prophylactic tinzaparin (3500 IU/day) was prescribed. However, the initial protocols did not include prophylactic heparin, which was eventually incorporated for 7 days for outpatients with respiratory infection with or without pneumonia, as well as patients with non-complicated disease with risk factors (previous history of venous thromboembolic disease, thrombophilia, active oncologic disease, recent surgery within the last 3 months, hormonal treatment, pregnancy and puerperium). For patients with bilateral pneumonia and/or oxygen saturation <94%, lopinavir–ritonavir (Kaletra 400 mg/12 h for 5 days) was added and patients with more severe COVID-19 could also receive tocilizumab 400 mg (maximum 2 doses in 48 h). The most severe cases were admitted to the intensive care unit (ICU). Severe cases were defined according to the protocol as partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) <150 mmHg or sepsis or septic shock at the emergency room or the need for >40% FiO2 of supplemental oxygen for maintaining an O2 saturation >92%.
Follow-up
A total of 56 HD patients with a COVID-19 diagnosis during the first wave (March–May 2020) were prospectively followed and anti-SARS-CoV-2 IgG antibodies were evaluated at 3, 6, 9 and 12 months. The primary clinical endpoint was all-cause death. Deaths were separated into those occurring during the first hospitalization and those occurring later. As a sensitivity analysis, early deaths were defined as those that occurred within the first 30 days after diagnosis of COVID-19. The secondary endpoint was a composite of hospitalization from any cause and events requiring therapeutic intervention even if there was no hospitalization. During the follow-up period, Spain suffered successive COVID-19 waves (Supplementary data, Figure S1). Thus re-infection was theoretically possible.
At the end of follow-up, 9 of 36 survivors had been vaccinated from January 2021 onwards, starting with the elderly. HD patients not yet vaccinated because of age were vaccinated between April and May 2021. The type of vaccine administered was decided by health authorities according to Spanish health authorities’ guidelines (messenger RNA vaccine) and availability by regional health authorities: all nine patients received BNT162b2 (Pfizer-BioNTech).
Statistical analysis
Data are presented as medians and interquartile ranges (IQRs) except when otherwise specified. The normality of the data was assessed using the Kolmogorov–Smirnov test. Then the Mann–Whitney U test or the Kruskal–Wallis rank test was employed for binary and multiple comparisons, respectively. Time to death among patients with COVID-19 diagnosis was explored with a log-rank test of Kaplan–Meier curves and HR. Contingency tables and Fisher’s exact test were performed to compare mortality between COVID-19-diagnosed patients and non-COVID-19 among HD patients. R version 4 (https://cran.r-project.org/) was used for statistical analysis and two-tailed P-values <0.05 were considered statistically significant. Libraries ggplot2, Jmv, finalfit, easyalluvial, vtree, tangram, car, afex and survival were employed for graphics and analysis. The association of risk factors with time to death was assessed in univariate analysis and parameters with P < 0.1 in univariate analysis were included in multivariate Cox proportional hazards regression models.
RESULTS
Study population
Overall, the 200 HD patients had a median age of 67.5 years (IQR 55.4–77.0) and 70.5% were male and had a dialysis vintage of 30.4 months (IQR 14.7–73.8). The two most common comorbidities were hypertension and diabetes (93% and 38%, respectively). There were no differences in age, gender, comorbidities or dialysis vintage among patients with or without COVID-19.
A total of 56 of 200 (28%) HD patients were diagnosed with COVID-19 during the first pandemic wave (March 2020–May 2020): 37 of 56 (66.1%) based on positive PCR and 19 of 56 (33.9%) based on anti-SARS-CoV-2 antibodies (Figure 1). Clinical characteristics are shown in Table 1. The median age was 72 year; a majority (64%) were male and comorbidities were common. Dialysis vintage was 36 months. Of COVID-19 patients, 29 of 56 (52%) required hospital admission for the COVID-19 episode (Figure 2).
Table 1.
Characteristics of patients with confirmed COVID-19 by positive PCR or serology
| Characteristics | COVID-19 (n = 56) | Death (n = 20) | Survival (n = 36) | P-value |
|---|---|---|---|---|
| Age (years), median (IQR) | 72.50 (57.4–80.6) | 76.5 (70–82.2) | 64.5 (54.0–78.7) | 0.041 |
| Male, n (%) | 36 (64) | 13 (65) | 23 (64) | 0.93 |
| Smoker, n (%) | 23 (41) | 9 (45) | 14 (39) | 0.99 |
| Comorbidities, n (%) | ||||
| Hypertension | 50 (89) | 18 (90) | 32 (89) | 0.99 |
| Diabetes | 21 (38) | 7 (35) | 14 (39) | 0.77 |
| Ischaemic heart disease | 20 (36) | 7 (35) | 13 (36) | 0.93 |
| History of immunosuppression | 14 (25) | 5 (25) | 9 (25) | 0.99 |
| Overweight/obesity | 13 (23) | 3 (15) | 10 (28) | 0.99 |
| Chronic heart failure | 12 (21) | 5 (25) | 7 (19) | 0.74 |
| Cancer | 10 (18) | 5 (25) | 5 (14) | 0.99 |
| Autoimmune disease | 9 (16) | 3 (15) | 6 (17) | 0.99 |
| COPD/asthma | 8 (14) | 4 (20) | 4 (11) | 0.99 |
| Peripheral arterial disease | 8 (14) | 0 (0) | 8 (22) | 0.041 |
| Immunocompromised | 7 (12) | 2 (10) | 5 (14) | 0.99 |
| Cirrhosis | 2 (3.6) | 1 (5.0) | 1 (2.8) | 0.99 |
| HIV | 1 (1.8) | 0 (0) | 1 (2.8) | 0.99 |
| Cause of CKD, n (%) | 0.422 | |||
| Unknown aetiology | 15 (27) | 6 (30) | 9 (25) | |
| Diabetic nephropathy | 13 (24) | 2 (10) | 11 (31) | |
| Glomerular disease | 12 (22) | 4 (20) | 8 (22) | |
| ADPKD | 4 (7.1) | 1 (5.0) | 3 (8.3) | |
| Other | 12 (22) | 7 (35) | 5 (14) | |
| Dialysis vintage (months), median (IQR) | 33.5 (17–75.2) | 25.5 (12.4–85.1) | 43.5 (22.0–75.2) | 0.20 |
| Diagnostic criteria for COVID-19, n (%) | ||||
| PCR | 37 (66) | 17 (85) | 20 (56) | 0.025 |
| Anti-SARS-CoV-2 positive | 19 (34) | 3 (15) | 16 (44) | |
| IgM positive | 8 (14) | 1 (5.0) | 7 (19) | |
| IgG | 2 (3.5) | 1 (5.0) | 1 (2.7) | |
| IgM + IgG | 9 (16) | 1 (5.0) | 8 (22) | |
| Symptoms, n (%) | ||||
| Asymptomatic | 15 (27) | 7 (35) | 8 (22) | 0.14 |
| Asthenia | 5 (8.9) | 1 (5.0) | 4 (11) | |
| Fever | 16 (29) | 3 (15) | 13 (36) | |
| Respiratory | 10 (18) | 6 (30) | 5 (14) | |
| Cardiovascular | 2 (3.5) | 1 (5.0) | 1 (2.7) | |
| Gastrointestinal | 8 (14) | 3 (15) | 5 (14) | |
| Chest X-ray, n/N (%) | ||||
| Normal | 22/52 (42) | 8/20 (40) | 14/32 (44) | |
| Unilateral pneumonia | 10/52 (19) | 5/20 (25) | 6/32 (19) | |
| Bilateral pneumonia | 19/52 (37) | 6/20 (30) | 12/32 (38) | |
| Analytical values, median (IQR) | ||||
| Hb (g/dL) | 11.1 (10.1–12.3) | 10.3 (9.1–11.4) | 11.5 (10.4–12.6) | 0.013 |
| Lymphocytes (/µL) | 900.0 (600.0–1200.0) | 850.0 (641.7–1000.0) | 900.0 (541.7–1258.0) | 0.813 |
| IL-6 (pg/mL) | 15.4 (5.1–42.7) | 20.5 (9.2–71.3) | 12.1 (3.3–27.2) | 0.083 |
| Ferritin (ng/mL) | 872.0 (406.2–1377.1) | 20.5 (9.2–71.3) | 12.1 (3.3–27.2) | 0.083 |
| CRP (mg/dL) | 1.6 (0.5–7.6) | 5.7 (2.4–17.1) | 1.3 (0.2–3.7) | <0.013 |
| D-dimer (µg/L) | 1442.0 (740.2–2056.0) | 1533.5 (914.5–3001.6) | 1345.0 (652.7–1976.2) | 0.303 |
| EPO dose (units/week) | 9500.0 (3000.0–18 000.0) | 8000.0 (4416.7–22 333.3) | 11 000.0 (2805.6–18 000.0) | 0.523 |
| ERI (UI/kg/week/Hb) | 11.7 (3.9–25.3) | 13.9 (3.6–25.2) | 10.4 (4.9–31.1) | 0.76 |
ADPKD, autosomal dominant polycystic kidney disease; COPD, chronic obstructive pulmonary disease; EPO, erythropoietin.
FIGURE 2:
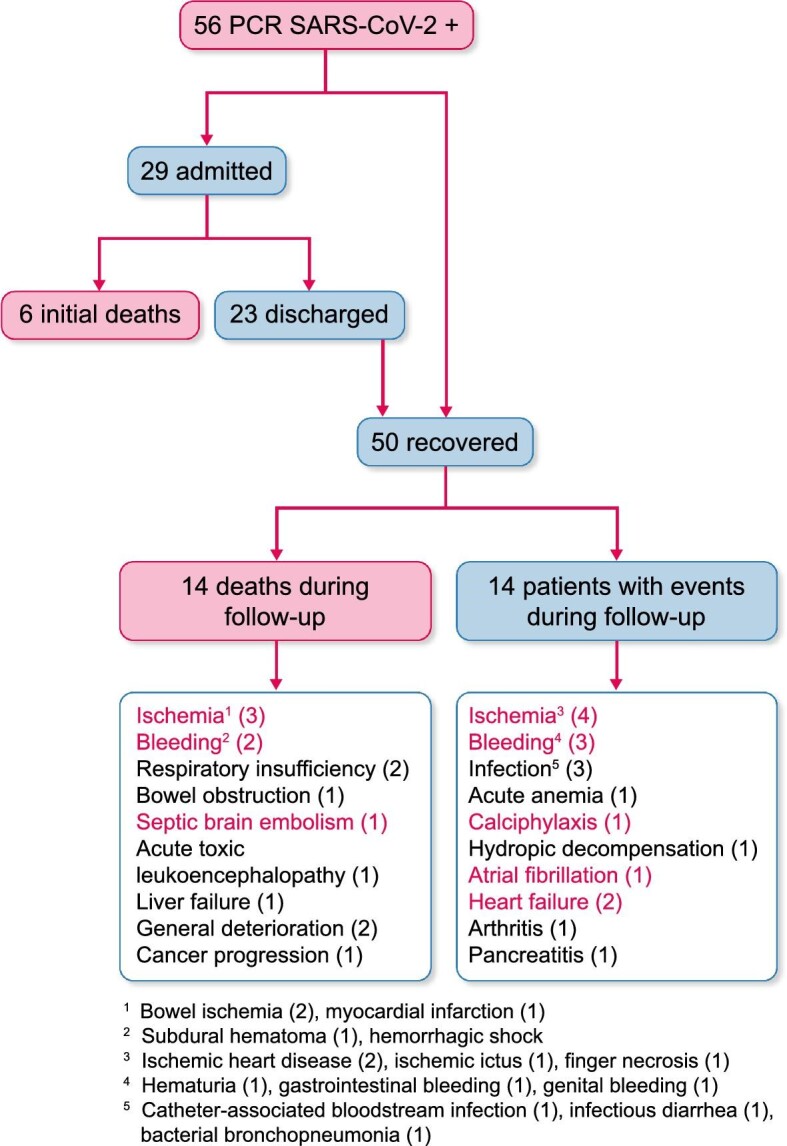
The 1-year outcomes of HD patients diagnosed with COVID-19 between March and May 2020. Some patients had more than one complication. Among causes of death and events, red is used to denote vascular causes.
1-year outcomes
The 12-month mortality between March 2020 and May 2021 was 35.7% (20 of 56 patients with COVID-19) (Figure 2): 6 of 56 (11%) died during the initial admission from respiratory failure, as previously reported [13], and 14 of 56 (25%) died in the following months. Of these 14 patients, 6 died from vascular causes and 2 from respiratory causes. Thus 6 of 20 (30%) deaths in COVID-19 HD patients occurred early in the course of the disease and 14 of 20 (70%) occurred later on, but mainly within 3 months of the diagnosis (Figure 3). Overall, 8 of 20 (40%) deaths were caused by respiratory failure and 6 of 20 (30%) by vascular causes. As a sensitivity analysis, early deaths were defined as those occurring within the first 30 days. This sensitivity analysis did not change the results, since one patient died after 37 days of hospitalization from respiratory failure and another patient died from respiratory failure within 30 days of diagnosis after having been discharged.
FIGURE 3:
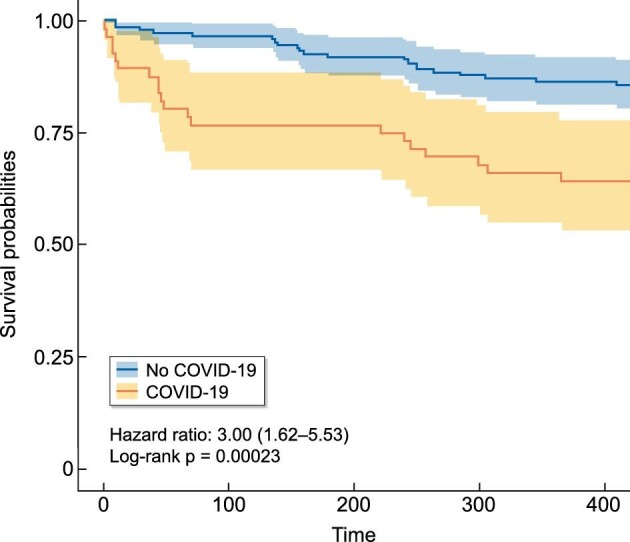
Kaplan–Meier survival curves of HD patients diagnosed with SARS-CoV-2 infection between March and May 2020 as compared with those not infected.
The 12-month mortality for non-COVID-19 HD patients was 21 of 144 (14.6%), which is in line with secular mortality trends both at our unit and within Spain [15] (Figure 3). The 12-month mortality was significantly higher for COVID-19 than for non-COVID-19 patients (P ≤ 0.001) and the HR for mortality in COVID-19 patients was 3.00 (95% CI 1.62–5.53; P < 0.001). While deaths in non-COVID-19 patients were distributed over the 12 months of follow-up, deaths among COVID-19 patients were concentrated into two distinct waves within the first 3 months after a COVID-19 diagnosis and thereafter ran parallel to those in non-COVID-19 patients.
COVID-19 patients who died were older than those who survived [76.5 years (IQR 70–82.2) versus 64.5 (54.0–78.7), respectively; P = 0.041] and more frequently had a positive PCR test as a diagnostic criterion for COVID-19 (Table 1). Interestingly, up to 35–40% of COVID-19 patients who died were initially asymptomatic or had a normal chest X-ray at baseline. There were no differences between the baseline clinical characteristics of COVID-19 patients who died during the initial COVID-19 hospitalization and those who died later.
Fourteen of the 36 (38.9%) survivors suffered at least one additional event during the 12-month follow-up. Some patients had more than one event. Overall, there were eight non-bleeding cardiovascular events, four bleeding events and two respiratory events (Figure 2).
We detected one reinfection during the follow-up period.
Risk factors for mortality at 1 year in multivariate analysis included having a positive SARS-CoV-2 PCR as a diagnostic criterion [HR 5.18 (95% CI 1.30–20.65), P = 0.020], higher baseline CRP levels [HR 1.10 (95% CI 1.03–1.16), P = 0.002] and lower baseline Hb levels [HR 0.62 (95% CI 0.45–0.86), P = 0.005] (Table 2). We did not find differences in lymphocyte counts, ESA weekly doses, ERI, IL-6, D-dimer or treatments between those who died and those who survived (Table 1).
Table 2.
Adjusted HRs (univariate and multivariate) of variables associated with 1-year mortality
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | 1.04 (1.00–1.07) | 0.028 | 1.02 (0.98–1.06) | 0.252 |
| Positive PCR | 3.02 (0.88–10.39) | 0.079 | 5.18 (1.30–20.65) | 0.020 |
| Haemoglobin (g/dL) | 0.66 (0.49–0.90) | 0.008 | 0.62 (0.45–0.86) | 0.005 |
| CRP (mg/dL) | 1.07 (1.02–1.12) | 0.003 | 1.10 (1.03–1.16) | 0.002 |
As deaths during the first 3 months were higher than in non-COVID-19 controls, we also evaluated the risk factors associated with mortality in this period. Like the 1-year analysis, PCR status, higher CRP levels and lower Hb levels were associated with higher mortality at 3 months [HR 9.11 (95% CI 1.53–54.28), P = 0.015; HR 1.09 (95% CI 1.04–1.14), P < 0.001 and HR 0.67 (95% CI 0.47–0.95), P = 0.025, respectively] (Table 3).
Table 3.
Adjusted HRs (univariate and multivariate) of variables associated with 3-month mortality
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age | 1.04 (1.00–1.08) | 0.063 | 1.02 (0.97–1.07) | 0.443 |
| Positive PCR | 3.31 (0.74–14.82) | 0.117 | 9.11 (1.53–54.28) | 0.015 |
| Haemoglobin (g/dL) | 0.67 (0.47–0.95) | 0.025 | 0.61 (0.41–0.90) | 0.013 |
| CRP (mg/dL) | 1.09 (1.04–1.14) | <0.001 | 1.14 (1.06–1.22) | <0.001 |
Anti-SARS-CoV-2 IgG over 12 months
Over the first year, the percentage of patients who had anti-SARS-CoV-2 IgG decreased progressively: from 36 of 49 (73.4%) initially to 28 of 46 (60.8%) and 27 of 44 (61.3%) at 3 and 6 months, respectively, and 14 of 36 (38.8%) at 12 months (Figure 4). Among survivors, only 30.6% of patients showed the continuous presence of anti-SARS-CoV-2 IgG from the initial assessment and up to 12 months (Figures 4 and 5). The largest decrease in anti-SARS-CoV-2 IgG positivity occurred between 6 and 12 months, while additional patients lost anti-SARS-CoV-2 IgG between baseline and 3 months. A few patients became negative and then became positive again during follow-up (Figure 4). This occurred in 3 of 36 (8%) patients (one of them had been vaccinated and also experienced reinfection; the second patient had been vaccinated before the end of the follow-up period and the third had not been vaccinated—whether this patient had experienced reinfection is unclear). In total, 9 of 32 patients were vaccinated before the end of the follow-up period: the two just described, four who were positive throughout the follow-up and three who never showed positive antibodies, even after vaccination.
FIGURE 4:
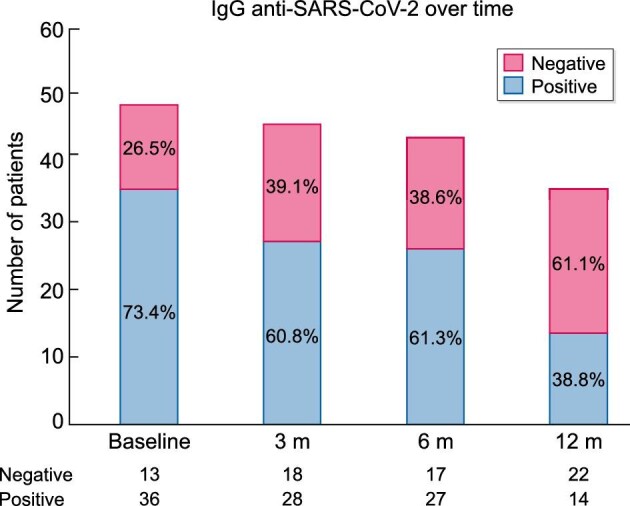
Percentage of HD COVID-19 patients with detectable IgG anti-SARS-CoV-2 antibodies during 1 year of follow-up.
FIGURE 5:
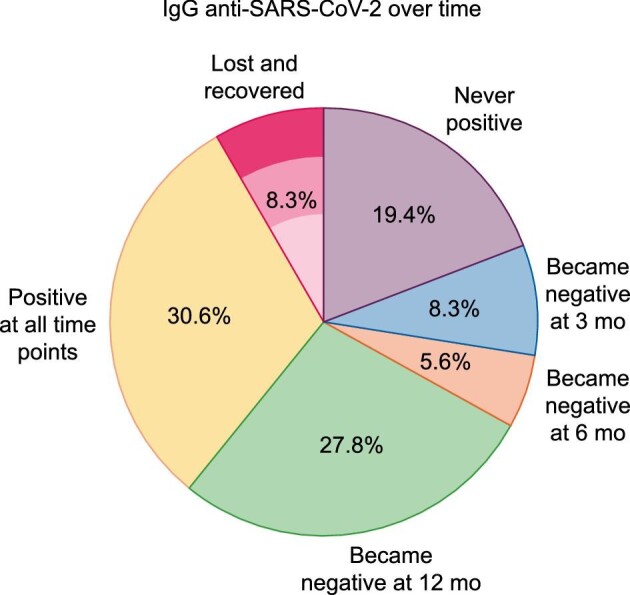
IgG anti-SARS-CoV-2 antibodies over 1 year of follow-up. (A) IgG anti-SARS-CoV-2 antibody trajectories. Y: positive IgG anti-SARS-CoV-2 antibodies; N: negative IgG anti-SARS-CoV-2 antibodies; exitus: patients who had died by this time point. (B) Percentage of patients with the different IgG anti-SARS-CoV-2 antibody trajectories during 1 year of follow-up. Percentage calculated for a total of 36 patients with assessments at all the different time points.
DISCUSSION
The present study has several main findings that may impact the care of HD patients with COVID-19. First, the 1-year mortality of HD patients with COVID-19 was high and a majority of the deaths (70%) occurred after discharge, especially within the first 3 months. Thus careful monitoring is required in the early post-COVID-19 period and prophylactic measures should be studied. Second, not all patients developed detectable IgG anti-SARS-CoV-2 antibodies and only 39% maintained positive antibodies 12 months after initial infection. Thus the mid- and long-term impact of vaccines on antibody development should be monitored to develop guidance as to the use of booster doses in HD patients. Third, a PCR-based diagnosis, lower baseline Hb levels and higher CRP levels were associated with higher 3-month and 1-year mortality.
In a large (17 million participants) study in England, patients on dialysis had the highest risk of death from COVID-19 in an adjusted analysis [16]. However, mortality reported in HD patients with COVID-19 has been highly variable and may have been influenced by criteria to diagnose COVID-19 (whether all patients in the unit were tested or only those that required hospitalization), by the availability of healthcare resources (some centres became overwhelmed and could not offer ICU care for all), comorbidities or other factors [17, 18]. In Madrid, mortality rates during the acute episode (defined as during hospitalization or within 28 days after diagnosis) in HD patients were 16.2% [19] to 30.5% [20]. In our unit, mortality during the initial admission was 11%. However, an unexpectedly high number of deaths was observed over 1 year of follow-up, as 36% of COVID-19 HD patients died. This was higher than the historical annual mortality at our centre of 9.2% per year [21] and higher than the mortality of HD patients in Spain, which has been relatively stable (ranging from 14.1% to 16.8%) from 2007 to 2019 [15]. Above all, the overall 12-month mortality in COVID-19 patients was higher than the mortality of HD patients not diagnosed with COVID-19 in our unit. This population represents the most appropriate controls, as they were exposed to the same pandemic-associated restrictions and potential limitations in access to healthcare. Although higher mortality would be expected for a potentially lethal condition, the higher mortality was observed for up to 3 months after diagnosis, illustrating a persistently high mortality risk. Only after this point did the mortality in COVID-19 patients run in parallel with that in non-COVID-19 patients.
COVID-19 can have long-term consequences [22, 23]. The most visible manifestations are symptoms such as asthenia or brain fog. However, in HD patients this may also include an increased mortality rate. The most frequent non-lethal and lethal episodes were related to ischaemia or bleeding. In this regard, persistently dysregulated haemostasis has been observed following acute COVID-19 and delayed catastrophic thrombotic events have been reported in young and asymptomatic post-COVID-19 patients [24, 25]. COVID-19 may also lead to long-lasting alterations of the heart, lungs and other organs [26].
The second observation refers to the long-term natural history of anti-SARS-CoV-2 antibodies in HD patients with COVID-19. In previously healthy people, most individuals with mild—moderate COVID-19 experienced robust IgG antibody responses against the viral spike protein. Titres are relatively stable for a period of about 5 months [27, 28] and could last for 10 months and support protective immunity in the recovered patients [29]. However, HD patients have suboptimal immune responses, a higher risk of death from infectious diseases and suboptimal responses to vaccines such as anti-hepatitis B virus [30]. In a recent multicentre study in HD patients, anti-SARS-CoV-2 IgG remained positive after 4 weeks in 90% of patients with symptomatic COVID-19 and in 52.5% of asymptomatic patients [31]. A single study to date has studied the immune response to COVID-19 in HD patients in the first 6 months post-infection and results were aligned with our longer study [32]: anti-SARS-CoV-2 nucleocapsid IgG developed in 89% of patients and 70% remained positive at 6 months, similar to the 61% positivity rate in our study at 6 months. We now expand these observations by describing a dramatic decrease in anti-SARS-CoV-2 at 12 months to 39%. Interestingly, some patients who lost anti-SARS-CoV-2 antibodies became positive later on. In this regard, successive waves of COVID-19 have been present in Spain continuously since the end of August 2020 and COVID-19 was diagnosed in one of three patients who showed positive anti-SARS-CoV-2 IgG at the end of follow-up but who had presented previous negative anti-SARS-CoV-2 IgG tests. A second patient became anti-SARS-CoV-2 IgG positive after the vaccination. Whether the third patient had an asymptomatic reinfection remains unclear, because this patient had not been vaccinated. The rapid loss of anti-SARS-CoV-2 antibodies is a cause of concern regarding the intensity and duration of the response to vaccination. Multiple studies are currently ongoing to define the immune response to SARS-CoV-2 vaccine in HD and the optimal vaccination schedule. Patients with kidney failure have significantly weaker antibody responses than controls [33, 34].
Factors associated with 3-month and 1-year mortality included low Hb and high CRP levels. Anaemia has also been associated with mortality in non-HD patients with COVID-19 [35–38]. In a prospective study, the presence of Hb <13 g/dL in hospitalized males and <12 g/dL in females with COVID-19 was associated with mortality [OR 1.68 (95% CI 1.10–2.57), P = 0.01], ventilator requirement [OR 1.74 (95% CI 1.19–2.54), P = 0.004] and risk of ICU admission [OR 2.06 (95% CI 1.46–2.90), P < 0.001] [35]. The association between anaemia and inflammation is well recognized [39]. In anaemic patients, low Hb levels may further impair tissue oxygenation [37], especially in patients with respiratory compromise [36]. HD patients have a high prevalence of anaemia, related to both erythropoietin deficiency and erythropoietin resistance caused by inflammation, iron deficiency and others [40]. Resistance to ESAs and the associated requirement for higher ESA doses have been associated with mortality in HD patients [41, 42]. Although we did not find an association between ERI and mortality, the study size was too small to draw definite conclusions.
Some limitations should be acknowledged. This was a single-centre study with a limited number of patients. Thus, the results should be confirmed in larger cohorts, such as national or international registries. However, our study provides some guidance for larger-scale analysis, as post-COVID-19 mortality should be followed and analysed for up to 1 year, comparing with mortality in non-COVID-19 patients. Additionally, HD patients in whom COVID-19 was diagnosed may have had pre-existing conditions, from medical to social, that predisposed to COVID-19 in the first place. In-depth analysis of confounders may also be performed in larger, multinational cohorts.
In conclusion, 1-year mortality in HD patients with COVID-19 was high and most deaths occurred after being discharged from the initial episode. Deaths among COVID-19 patients were concentrated into two distinct waves within the first 3 months after a COVID-19 diagnosis. Factors associated with mortality included low Hb levels and CRP levels at baseline and a diagnosis based on PCR rather than on serology. These data point to optimized anaemia management as a potential line of research to decrease COVID-19 mortality. In this regard, potentially preventable causes (ischaemia and bleeding) were the most common causes of death. The time course of the increased risk of death in dialysis patients with COVID-19 should be explored in larger databases and, if confirmed, preventive strategies should be developed. Therefore an increased risk of death should be considered part of the post-COVID-19 or long COVID-19 spectrum in dialysis patients. It was worrisome that the high percentage of dialysis patients did not have detectable anti-SARS-CoV-2 antibodies by 12 months after the episode. The duration of the immunological response to SARS-CoV-2 vaccine should be closely monitored in HD patients.
Supplementary Material
Contributor Information
Sol Carriazo, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Sebastian Mas-Fontao, Laboratorio de patología renal y diabetes, CIBERDEM, IIS-Fundación Jiménez Díaz UAM, Madrid, Spain.
Clara Seghers, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Jaime Cano, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Elena Goma, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Alejandro Avello, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Alberto Ortiz, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
Emilio Gonzalez-Parra, Servicio de Nefrología e Hipertensión, Fundación Jiménez Díaz, IIS-FJD UAM, Madrid, Spain.
FUNDING
This research received no external funding. The research groups of E.G.-P., S.M. and A.O. are funded by the Ministerio de Economia, Industria y competitividad: FIS/Fondos FEDER (PI16/01298, PI17/00257, PI18/01386, PI19/00588, PI19/00815, PI20/00487, PI21/01430), ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064 and PERSTIGAN AC18/00071, ISCIII-RETIC REDinREN RD016/-0009) and Sociedad Española de Nefrología, Comunidad de Madrid en Biomedicina (B2017/BMD-3686 CIFRA2-CM).
DATA AVAILABILITY STATEMENT
All data from the study are available upon request.
CONFLICT OF INTEREST STATEMENT
A.O. is the CKJ Editor-in-Chief. The other authors declare no conflicts of interest.
REFERENCES
- 1. Lu R, Zhao X, Li Jet al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williamson EJ, Walker AJ, Bhaskaran Ket al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Clark A, Jit M, Warren-Gash Cet al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Global Health 2020; 8: e1003–e1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. ERA-EDTA Council, ERACODA Working Group . Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant 2021; 36: 87–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cai R, Zhang J, Zhu Yet al. Mortality in chronic kidney disease patients with COVID-19: a systematic review and meta-analysis. Int Urol Nephrol 2021; 53: 1623–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alexiewicz JM, Smogorzewski M, Fadda GZet al. Impaired phagocytosis in dialysis patients: studies on mechanisms. Am J Nephrol 1991; 11: 102–111 [DOI] [PubMed] [Google Scholar]
- 7. Massry S, Smogorzewski M. Dysfunction of polymorphonuclear leukocytes in uremia: role of parathyroid hormone. Kidney Int Suppl 2001; 78: S195–S196 [DOI] [PubMed] [Google Scholar]
- 8. Carrero JJ, Stenvinkel P. Inflammation in end-stage renal disease—what have we learned in 10 years? Semin Dial 2010; 23: 498–509 [DOI] [PubMed] [Google Scholar]
- 9. García-Guimaraes M, Mojón D, Calvo Aet al. Influence of cardiovascular disease and cardiovascular risk factors in COVID-19 patients. Data from a large prospective Spanish cohort. REC CardioClinics 2021; 56: 108–117 [Google Scholar]
- 10. Turgutalp K, Ozturk S, Arici Met al. Determinants of mortality in a large group of hemodialysis patients hospitalized for COVID-19. BMC Nephrol 2021; 22: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen CY, Shao SC, Chen YTet al. Incidence and clinical impacts of COVID-19 infection in patients with hemodialysis: systematic review and meta-analysis of 396,062 hemodialysis patients. Healthcare (Basel) 2021; 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smolander J, Bruchfeld A. The COVID-19 epidemic: management and outcomes of hemodialysis and peritoneal dialysis patients in Stockholm, Sweden. Kidney Blood Press Res 2021; 46: 250–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stock da Cunha T, Gomá-Garcés E, Avello Aet al. The spectrum of clinical and serological features of COVID-19 in urban hemodialysis patients. J Clin Med 2020; 9: 2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pizarro-Sánchez MS, Avello A, Mas-Fontao Set al. Clinical features of asymptomatic SARS-CoV-2 infection in hemodialysis patients. Kidney Blood Press Res 2021; 46: 126–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Registro Español de Enfermos Renales (REER) . Informe de Diálisis y Trasplante 2019. https://www.senefro.org/contents/webstructure/INFORME_REER_SEN_2020_WEB_SEN.pdf (26 June 2021, date last accessed)
- 16. Williamson EJ, Walker AJ, Bhaskaran Ket al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020; 584: 430–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carriazo S, Kanbay M, Ortiz A. Kidney disease and electrolytes in COVID-19: more than meets the eye. Clin Kidney J 2020; 13: 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Quiroga B, Sánchez-Álvarez E, Ortiz Aet al. Suboptimal personal protective equipment and SARS-CoV-2 infection in nephrologists: a Spanish national survey. Clin Kidney J 2021; 14: 1216–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Albalate M, Arribas P, Torres Eet al. Alta prevalencia de COVID-19 asintomático en hemodiálisis. Aprendiendo día a día el primer mes de pandemia de COVID-19. Nefrología 2020; 40: 279–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goicoechea M, Sánchez Cámara LA, Macías Net al. COVID-19: clinical course and outcomes of 36 hemodialysis patients in Spain. Kidney Int 2020; 98: 27–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fernandez-Prado R, Peña-Esparragoza JK, Santos-Sánchez-Rey Bet al. Ultrafiltration rate adjusted to body weight and mortality in hemodialysis patients. Nefrologia 2021; 41: 426–435 [DOI] [PubMed] [Google Scholar]
- 22. Burke MJ, del Rio C. Long COVID has exposed medicine's blind-spot. Lancet Infect Dis 2021; 21: 1062–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Amin-Chowdhury Z, Ladhani SN. Causation or confounding: why controls are critical for characterizing long COVID. Nat Med 2021; 27: 1129–1130 [DOI] [PubMed] [Google Scholar]
- 24. Townsend L, Fogarty H, Dyer Aet al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemostasis 2021; 19: 1064–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fan BE, Umapathi T, Chua Ket al. Delayed catastrophic thrombotic events in young and asymptomatic post COVID-19 patients. J Thromb Thrombolysis 2021; 51: 971–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Del Rio C, Collins LF, Malani P. Long-term health consequences of COVID-19. JAMA 2020; 324: 1723–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wajnberg A, Amanat F, Firpo Aet al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 370: 1227–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ripperger TJ, Uhrlaub JL, Watanabe Met al. Orthogonal SARS-CoV-2 serological assays enable surveillance of low-prevalence communities and reveal durable humoral immunity. Immunity 2020; 53: 925–933.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng ML, Liu HY, Zhao Het al. Longitudinal dynamics of antibody responses in recovered COVID-19 patients. Signal Transduct Target Ther 2021; 6: 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chang YT, Guo CY, Tsai MSet al. Poor immune response to a standard single dose non-adjuvanted vaccination against 2009 pandemic H1N1 influenza virus A in the adult and elder hemodialysis patients. Vaccine 2012; 30: 5009–5018 [DOI] [PubMed] [Google Scholar]
- 31. Alcázar-Arroyo R, Portolés J, López-Sánchez Pet al. Rapid decline of anti-SARS-CoV-2 antibodies in patients on haemodialysis: the COVID-FRIAT study. Clin Kidney J 2021; 14: 1835–1844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sakhi H, Dahmane D, Attias Pet al. Kinetics of Anti–SARS-cov-2 IgG antibodies in hemodialysis patients six months after infection. J Am Soc Nephrol 2021; 32: 1033–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kolb T, Fischer S, Müller Let al. Impaired immune response to SARS-CoV-2 vaccination in dialysis patients and in kidney transplant recipients. Kidney360 2021; 2: 1491–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chauhan S, Meshram HS, Kute Vet al. SARS-CoV2 infection in chronic kidney disease vaccinated with Oxford/AstraZeneca COVID-19 vaccine: first report from India. Clin Kidney J 2021; 14: 2263–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dinevari MF, Somi MH, Majd ESet al. Anemia predicts poor outcomes of COVID-19 in hospitalized patients: a prospective study in Iran. BMC Infect Dis 2021; 21: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oh SM, Skendelas JP, Macdonald Eet al. On-admission anemia predicts mortality in COVID-19 patients: a single center, retrospective cohort study. Am J Emerg Med 2021; 48: 140–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen C, Zhou W, Fan Wet al. Association of anemia and COVID-19 in hospitalized patients. Future Virol 2021; 16: 459–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bergamaschi G, de Andreis F B, Aronico Net al. Anemia in patients with COVID-19: pathogenesis and clinical significance. Clin Exp Med 2021; 21: 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fishbane S, Hirsch JS. Erythropoiesis-stimulating agent treatment in patients with COVID-19. Am J Kidney Dis 2020; 76: 303–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Babitt JL, Lin HY. Mechanisms of anemia in CKD. J Am Soc Nephrol 2012; 23: 1631–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Szczech LA, Barnhart HX, Inrig JKet al. Secondary analysis of the CHOIR trial epoetin-α dose and achieved hemoglobin outcomes. Kidney Int 2008; 74: 791–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Solomon SD, Uno H, Lewis EFet al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med 2010; 363: 1146–1155 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data from the study are available upon request.