Abstract
Background
Within the ongoing AGEhIV Cohort Study in Amsterdam, we prospectively compared the incidence of and risk factors for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection between human immunodeficiency virus (HIV)–positive and HIV-negative participants. Moreover, we compared SARS-CoV-2 nucleocapsid antibody levels between participants with incident infection from both groups.
Methods
Starting in September 2020, consenting HIV-positive and HIV-negative participants were assessed every 6 months for incident SARS-CoV-2 infection, using combined immunoglobulin (Ig) A/IgM/IgG SARS-CoV-2 nucleocapsid antibody assay. Cumulative incidence of SARS-CoV-2 infection and associated risk factors were assessed from 27 February 2020 through 30 April 2021, using complementary log-log regression. In those with incident SARS-CoV-2 infection, nucleocapsid (N) antibody levels were compared between groups using linear regression.
Results
The study included 241 HIV-positive (99.2% virally suppressed) and 326 HIV-negative AGEhIV participants. The cumulative SARS-CoV-2 incidence by April 2021 was 13.4% and 11.6% in HIV-positive and HIV-negative participants, respectively (P = .61). Younger age and African origin were independently associated with incident infection. In those with incident infection, only self-reported fever, but not HIV status, was associated with higher N antibody levels.
Conclusions
HIV-positive individuals with suppressed viremia and adequate CD4 cell counts had similar risk of SARS-CoV-2 acquisition and similar SARS-CoV-2 N antibody levels after infection compared with a comparable HIV-negative cohort.
Clinical Trial Registration
Keywords: SARS-CoV-2, HIV, COVID-19, incidence, serology
Human immunodeficiency virus (HIV)–positive individuals with suppressed viremia and adequate CD4 cell counts, compared with a comparable HIV-negative cohort, were not at increased risk of severe acute respiratory syndrome coronavirus 2 infection and had similar postinfection nucleocapsid antibody levels.
Since the first case of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was diagnosed in the Netherlands on 27 February 2020, >2.0 million Dutch people have become infected and >18 000 have died of coronavirus disease 2019 (COVID-19) as of 12 October 2021 [1]. Established risk factors for polymerase chain reaction (PCR)–confirmed SARS-CoV-2 infection include older age, male sex, obesity, comorbid conditions, such as diabetes, hypertension, and other cardiovascular diseases, as well as certain conditions characterized by immunodeficiency [2–4].
With respect to people with human immunodeficiency virus (HIV) (PWH), studies investigating the acquisition of SARS-CoV-2 infection and COVID-19 severity conducted in different parts of the world have reported contrasting findings [5–18]. Most studies report a similar [8, 10, 11, 14, 15] or even lower [9, 13] incidence of PCR-confirmed SARS-CoV-2 infection among HIV-positive compared with HIV-negative groups. However, some studies have compared the incidence in PWH with data from the general population [8, 9, 13], whereas others have compared it with data from individuals without HIV using population-based surveillance registers [10, 11, 14]. Only one study has compared SARS-CoV-2 incidence in PWH with that in a group of age-, race-, sex- and site-matched HIV-negative people [15].
In the general population, the majority of people with symptomatic SARS-CoV-2 infection, with or without PCR confirmation, develop detectable antibodies against the nucleocapsid (N), spike (S) and receptor-binding domain protein [19–21]. N antibodies are detectable in >91% of individuals after a symptomatic SARS-CoV-2 infection [22, 23]. Vaccination with the currently European Medicines Agency–approved vaccines all trigger an immune response to the SARS-CoV-2 S-protein but do not elicit N antibodies [24–27]. SARS-CoV-2 N antibodies are thus an appropriate marker to detect past SARS-CoV-2 infection, including infection acquired despite partial or complete vaccination. Several studies found that SARS-CoV-2 N antibodies remained detectable for ≥8 months after infection, although levels may decline over time [28, 29].
SARS-CoV-2 S- and N antibody titers are each correlated with disease severity, titers being higher in patients with moderate to severe COVID-19 than in those with asymptomatic or mild symptomatic infection [21, 29–31]. Moreover, one general population study reported that older age and higher body mass index (BMI) were also associated with higher N antibody titers [32]. Few studies have addressed the potential impact of HIV on the antibody response to SARS-CoV-2 infection. One small cross-sectional study reported no significant difference in SARS-CoV-2 N antibody titers between 47 PWH and 35 HIV-negative healthcare workers [33]. Another small study in 28 PWH found no difference in SARS-CoV-2 immunoglobulin (Ig) G N antibody titers between PWH with CD4 cell counts ≥500/µL or <500/µL [8].
To our knowledge, no study has prospectively compared the acquisition of symptomatic or asymptomatic SARS-CoV-2 and the antibody response to infection between people with well-controlled HIV and comparable HIV-negative individuals. We therefore conducted a study of this design nested within our ongoing AGEhIV cohort study in Amsterdam.
METHODS
Study Design and Participants
The AGEhIV Cohort Study is a prospective observational cohort study assessing the prevalence and incidence of age-related comorbid conditions and their risk factors in HIV-positive and HIV-negative participants aged ≥45 years. Between 2010 and 2012, HIV-positive participants were recruited at the outpatient HIV clinic of the Amsterdam University Medical Centers, Academic Medical Center location, and HIV-negative participants from either the sexual health clinic or the Amsterdam Cohort Studies on HIV/AIDS at Public Health Service Amsterdam, resulting in a control group with highly similar sociodemographic and behavioral characteristics. At baseline and every 2 years thereafter, patients undergo standardized screening for age-related comorbid conditions, and collection of blood, urine, and stool samples for cryopreservation. Details have been described elsewhere [34].
In August 2020, after the first SARS-CoV-2 epidemic wave in the Netherlands, all AGEhIV Cohort participants in active follow-up and residing in the Netherlands were asked to participate in a COVID-19 substudy, which includes 5 planned study visits at 6-month intervals between September 2020 and October 2022. During each visit, a blood sample is obtained to assess SARS-CoV-2 humoral and cellular immune responses, and participants complete a standardized study questionnaire.
For the current analysis, data from the first and second study visits (September–October 2020 and March–April 2021) were used. These data capture approximately up to 14 months of possible exposure to SARS-CoV-2 in the Netherlands: from 27 February 2020 (when the first case of COVID-19 was identified in the Netherlands) until 30 April 2021 (end of second COVID-19 substudy visit). Written informed consent was obtained from all participants. The study was approved by the ethics committee of the Amsterdam University Medical Centers, Academic Medical Center location, and is registered at www.clinicaltrials.gov (NCT01466582).
Data Collection
Participant Characteristics
Date of birth, sex at birth, and ethnic origin obtained at the time of enrollment into the AGEhIV Cohort Study were used for all participants. Other baseline characteristics were obtained from the last available parent cohort study visit before 27 February 2020 and included data on number of prevalent comorbid conditions, lifestyle (ie, smoking, alcohol use, recreational drug use, and other behavioral characteristics), BMI, CD4 and CD8 cell count measurements, last HIV test result for HIV-negative participants, and antiretroviral treatment (ART) and HIV-1 RNA for HIV-positive participants. BMI (calculated as weight in kilograms divided by height in meters squared) was categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0). Undetectable HIV-1 plasma viral load was defined as <50 copies/mL, while viral blips up to 200 copies/mL were also considered undetectable.
Questionnaire Data
At each substudy visit, participants were asked to complete a standardized questionnaire (Supplementary Data 1). This questionnaire assessed whether participants had, since the start of the pandemic or the previous study visit, possibly experienced any particular COVID-19–related symptoms and/or had been tested for or received a diagnosis of SARS-CoV-2 infection. Furthermore, questions were included on changes in substance use, sexual behavior and use of combination ART (cART) in HIV-positive participants or preexposure prophylaxis (PrEP) for HIV in HIV-negative individuals since the start of the coronavirus outbreak and the implementation of social distancing measures in March 2020. Finally, participants were also asked about their self-perceived adherence to and experiences with social distancing measures.
SARS-CoV-2 N Antibody Measurements
SARS-CoV-2–specific N antibodies were measured to determine which participants had become infected with SARS-CoV-2. At each substudy visit, SARS-CoV-2 N antibody levels were measured using the semiquantitative INgezim COVID-19 double recognition assay (Eurofins Ingenasa), which captures the combined IgA, IgM, and IgG antibody response to the SARS-CoV-2 nucleocapsid protein (sensitivity, 100%; specificity, 98.2% [35]). N antibody levels were expressed as a ratio of the sample to positive control for each sample, calculated as follows: [(OD) sample − OD blank)/(OD positive control − OD blank)) × 10, where OD represents optical density. A sample–positive control ratio ≥6 was considered a positive SARS-CoV-2 N antibody response, in accordance with the manufacturer’s instructions, and was used to define SARS-CoV-2 infection.
Statistical Analysis
Baseline was defined as 27 February 2020. Follow-up continued until the date of the last available SARS-CoV-2 N antibody measurement, loss to follow-up, or death, whichever occurred first. Baseline characteristics and clinical course of SARS-CoV-2 infection were compared between HIV-positive and HIV-negative participants using Pearson χ2, Fisher exact, or Wilcoxon rank sum tests, as appropriate.
The cumulative incidence of SARS-CoV-2 infection was estimated during 2 time intervals (from 27 February to 31 October 2020 and from 1 November 2020 to 30 April 2021) and was compared between HIV-positive and HIV-negative participants using a log-rank test. Hazard ratios comparing the incidence of SARS-CoV-2 infection across levels of risk factors, along with their 95% confidence intervals, were estimated using complementary log-log regression with discrete-time survival, while accounting for within-participant correlation with a clustered variance estimator. Included risk factors were age, sex, ethnic origin, BMI, number of concomitantly prevalent comorbid conditions, possible COVID-19–related symptoms, substance use (smoking, alcohol, and recreational drugs), number of sexual contacts, number of household contacts, self-reported compliance with social distancing measures, baseline CD4 and CD8 cell counts and CD4/CD8 ratio, use of PrEP in HIV-negative participants, and HIV-negative participants, and HIV-specific parameters in HIV-positive participants (nadir CD4 count, years since HIV diagnosis and years since start of first ART). The multivariable log-log regression model was built using a backward stepwise selection procedure, including all variables associated with a P value <.20 in univariable analyses and subsequently removing all those with a P value ≥.05. Biologically plausible interactions between significant variables in the final multivariable model and HIV status were also assessed.
Differences in SARS-CoV-2 N antibody levels across levels of risk factors and their 95% confidence intervals were estimated using linear regression. Risk factors considered in this analysis were HIV status, age, sex, ethnic origin, BMI, number of comorbid conditions, presence of COVID-19–related symptoms, hospitalization for COVID-19, baseline CD4 and CD8 cell counts and CD4/CD8 ratio, use of PrEP in HIV-negative participants, and HIV-specific parameters in HIV-positive participants (nadir CD4 count and years since HIV diagnosis and since start of first ART). The multivariable linear regression model was built using a backward stepwise selection procedure, including all variables associated with a P value <.20 in univariable analyses and subsequently removing all those with a P value ≥.05. Statistical significance was defined as a 2-sided P value <.05. Statistical analyses were carried out using Stata/IC software (version 15.1; StataCorp).
RESULTS
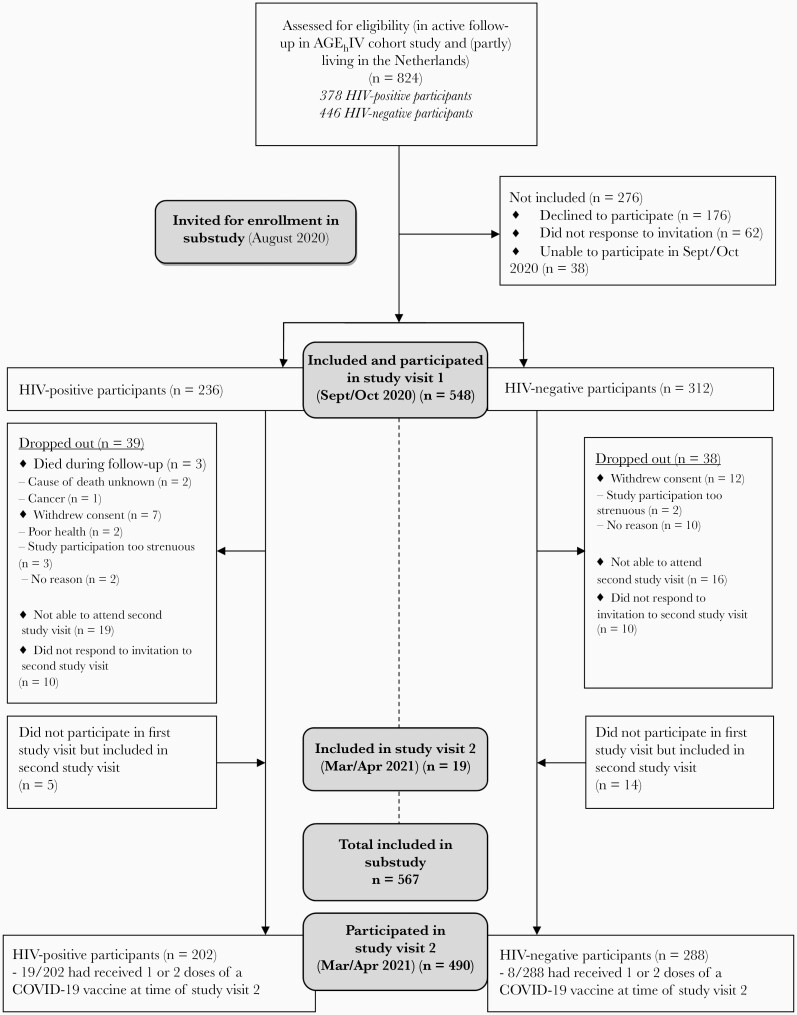
A total of 824 participants were eligible for participation in the AGEhIV COVID-19 substudy and were invited to participate. Initially, 548 participants provided consent and were included in the first substudy visit. Between the first and second visits, 77 participants dropped out and 19 new consenting participants were included, resulting in 490 individuals participating in the second study visit (Figure 1). In total, 567 participants were included: 326 HIV-negative controls and 241 HIV-positive participants. Compared with AGEhIV participants who declined participation (n = 257), included participants were significantly more often HIV negative and white and had higher educational levels (Supplementary Table 1). Reasons for declining participation did not differ between HIV-positive and HIV-negative participants (Supplementary Table 2).
Figure 1.
Overview of inclusion and participation during the first (September–October 2020) and second (March–April 2021) study visits of the AGEhIV coronavirus disease 2019 (COVID-19) substudy. Abbreviation: HIV, human immunodeficiency virus.
The characteristics of included participants are shown in Table 1. The majority were white men (83.4%), and the median age was 60.9 years. HIV-positive participants were more often male and had more comorbid conditions. The median time since HIV diagnosis was 21.4 years, with a median CD4 cell count nadir of 190/µL. At baseline, all HIV-positive participants were receiving cART, except for 1 elite controller. Of those with an available HIV-1 viral load measurement (n = 237), 99.2% were virologically suppressed (<50 copies/mL, n = 235; 50–200 copies/mL, n = 1; >200 copies/mL, n = 1). The current median CD4 cell count of HIV-positive participants was 680/µL; 79.7% had counts ≥500/µL.
Table 1.
Characteristics of Participants Included in the AGEhIV Coronavirus Disease 2019 Substudy (September–October 2020 and March–April 2021), by Human Immunodeficiency Virus Status
| Characteristica | Participants, No. (%)b | P Value | |
|---|---|---|---|
| HIV Negative (n = 326) | HIV Positive (n = 241) | ||
| Age, median (IQR), y | 60.3 (56.8–65.9) | 61.9 (57.7–66.9) | .06c |
| Age category | |||
| 53–59 y | 158 (48.5) | 97 (40.3) | .12d |
| 60–64 y | 77 (23.6) | 62 (25.7) | |
| 65–69 y | 44 (13.5) | 48 (19.9) | |
| ≥70 y | 47 (14.4) | 34 (14.1) | |
| Male sex at birth | 271 (83.1) | 221 (91.7) | .003d |
| MSM | 231/323 (71.5) | 204/240 (85.0) | <.001d |
| Ethnic origin | |||
| White | 309 (94.8) | 229 (95.0) | .08e |
| African | 9 (2.8) | 11 (4.6) | |
| Asian | 8 (2.5) | 1 (0.4) | |
| Educational level | |||
| Lower education (primary and secondary) | 130/319 (40.8) | 111/239 (46.4) | .12d |
| Higher vocational or university education | 184/319 (57.7) | 120/239 (50.2) | |
| Other | 5/319 (1.6) | 8/239 (3.4) | |
| BMI categoryf,g | |||
| Underweight | 2 (0.6) | 2 (0.8) | .64e |
| Normal weight | 163 (50.0) | 133 (55.2) | |
| Overweight | 124 (38.0) | 81 (33.6) | |
| Obese | 37 (11.4) | 25 (10.4) | |
| Total comorbid conditionsg | <.001d | ||
| 0 | 197 (60.4) | 104 (43.2) | |
| 1–2 | 113 (34.7) | 113 (46.9) | |
| ≥3 | 16 (4.9) | 24 (9.9) | |
| Smokingg,h | 72 (22.1) | 46 (19.1) | .39d |
| Alcohol consumptiong,h | 283 (86.8) | 191 (79.3) | .02d |
| Recreational drug useg,h | 105/306 (34.3) | 74/225 (32.9) | .73d |
| Use of PrEPg,h | 31 (9.5) | NA | … |
| Time since HIV diagnosis, median (IQR), y | NA | 21.4 (15.1–26.9) | … |
| Time since ART initiation, median (IQR), y | NA | 18.6 (12.6–23.7) | … |
| Use of cARTg | NA | 240 (99.6) | … |
| Undetectable HIV-1 viral loadg | NA | 235/237 (99.2) | … |
| Cell count, median (IQR), cells/µLg | |||
| CD4 cell count | 840 (660–1130) | 680 (530–830) | <.001c |
| CD4 cell count nadir | NA | 190 (90–260) | … |
| CD8 cell count | 470 (320–630) | 730 (520–1000) | <.001c |
| CD4/CD8 cell count ratiog | 1.90 (1.36–2.51) | 0.93 (0.67–1.25) | <.001c |
Abbreviations: ART, antiretroviral therapy; BMI, body mass index; cART, combination ART; HIV, human immunodeficiency virus; MSM, men having sex with men; NA, not applicable; PrEP, preexposure prophylaxis.
Characteristics at time of invitation to enrollment in the coronavirus disease 2019 substudy (August 2020). ‘
Data represent no. (%) unless otherwise specified. Denominators are specified where they vary from those in the column heads.
P value based on Wilcoxon rank sum test.
P value based on Pearson χ2 test.
P value based on Fisher exact test.
BMIs were calculated as was calculated as weight in kilograms divided by height in meters squared and categorized as underweight (<18.5), normal weight (18.5–24.9), overweight (25.0–29.9), or obese (≥30.0).
Last available data before baseline (defined as 27 February 2020).
During the last 6 months.
At the time of their second study visit, 19 of 202 HIV-positive participants (9.4%) and 8 of 288 HIV-negative participants (2.8%) had received 1 or 2 doses of a COVID-19 vaccine. Because vaccination involves only the SARS-CoV-2 spike protein and does not affect the N antibody assay used in our study, vaccinated participants were not excluded from the analyses.
Cumulative Incidence of SARS-CoV-2 Infection
Between 27 February 2020 and 30 April 2021, a total of 61 participants had positive N antibody responses, indicative of incident SARS-CoV-2 infection. Three additional HIV-positive participants without a detectable N antibody response, but who reported a positive PCR result in the 6 months before the study visit (all 3 also reported possible COVID-19–related symptoms), were also considered to have acquired SARS-CoV-2 infection.
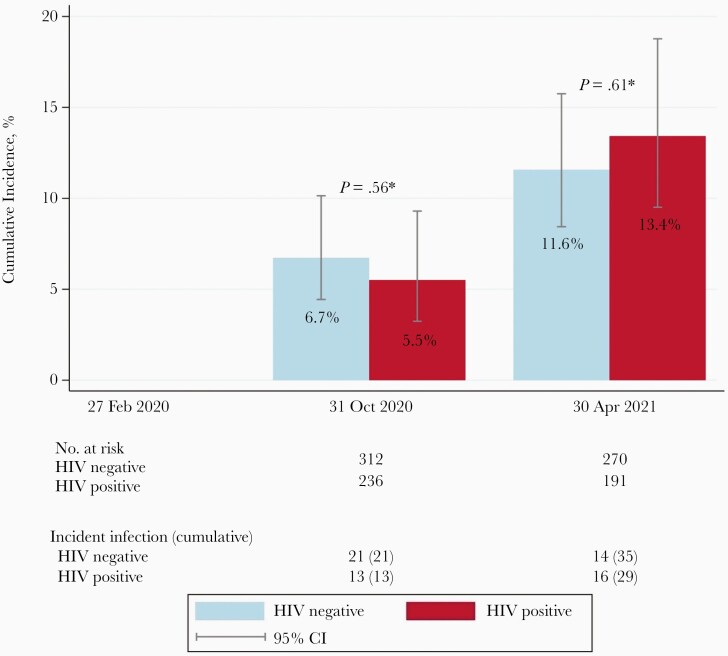
This resulted in an overall cumulative incidence of SARS-CoV-2 infection of 6.2% (n = 34) by 31 October 2020 and 12.3% (n = 64) by 30 April 2021. The cumulative incidence did not differ significantly between HIV-positive and HIV-negative participants (Figure 2). In a sensitivity analysis, in which we considered the 3 above-mentioned participants as not having acquired SARS-CoV-2 infection, the conclusions remained largely unchanged (Supplementary Data 2).
Figure 2.
Cumulative incidence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection from 27 February 2020 until 30 April 2021 among participants in the AGEhIV coronavirus disease 2019 substudy, Amsterdam. Follow-up started on 27 February 2020 and continued until the date of the last SARS-CoV-2 nucleocapsid antibody measurement, loss to follow-up, or death, whichever occurred first. Numbers of participants at risk and numbers with incident infection at the end of each time interval are shown. P values are based on log-rank test. Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Self-Reported SARS-CoV-2 Test Results and Potential COVID-19–Associated Signs and Symptoms
Study questionnaire data were available from 60 of the 64 participants with incident SARS-CoV-2 infection (32 HIV-negative and 28 HIV-positive participants) (Table 2). Of these 60 participants, 49 (81.7%) reported having experienced signs or symptoms since the start of the pandemic or the previous study visit, and 11 (18.3%) reported no signs or symptoms. Eighteen of the 60 (30%) had a positive PCR test result, 17 of whom also reported signs or symptoms. In the 49 symptomatic participants, the most frequently reported symptoms were fatigue, cough, rhinorrhea, muscle ache, headache, and fever. There was no significant difference between both groups, except for “confusion,” which was reported more often by HIV-positive participants. Only 2 participants, both HIV negative, reported that they had been admitted for >1 day to a general hospital ward for treatment of COVID-19 with supplemental oxygen.
Table 2.
Self-Reported PCR Test Results and Possible COVID-19-Associated Signs and Symptoms Since the Start of the COVID-19 Pandemic or the Previous Study Visit in 60 Participants of the AGEhIV COVID-19 Substudy
| Participants | |||
|---|---|---|---|
| Outcome or symptomsa | HIV-negative (n = 32) | HIV-positive (n = 28) | P value b |
| Self-reported SARS-CoV-2 | 9 (28.1%)c | 9 (32.1%)d | >.99 |
| PCR-positive test result | |||
| Symptomatic SARS-CoV-2 | .74 | ||
| Asymptomatic | 5 (15.6%) | 6 (21.4%) | |
| Symptomatic | 27 (84.4%) | 22 (78.6%) | |
| Experienced symptoms | |||
| Fever | 16 (50.0%) | 7 (25.0%) | .055 |
| Chills | 13 (40.6%) | 9 (32.1%) | .79 |
| Rhinorrhoea | 18 (56.3%) | 15 (53.6%) | >.99 |
| Ear pain | 2 (6.3%) | 3 (10.7%) | 0.83 |
| Cough | 18 (56.3%) | 15 (53.6%) | .79 |
| Phlegm | 12 (37.5%) | 11 (39.3%) | >.99 |
| Bloody phlegm | 0 (0.0%) | 0 (0.0%) | >.99 |
| Sore throat | 12 (37.5%) | 10 (35.7%) | .78 |
| Shortness of breath | 14 (43.8%) | 12 (42.9%) | >.99 |
| Loss of smell | 9 (28.1%) | 8 (28.6%) | .91 |
| Loss of taste | 9 (28.1%) | 7 (25.0%) | .89 |
| Fatigue | 16 (50.0%) | 19 (67.9%) | .26 |
| Muscle ache | 14 (43.8%) | 13 (46.4%) | .35 |
| Headache | 12 (37.5%) | 15 (53.6%) | .16 |
| Confusion | 0 (0.0%) | 5 (17.9%) | .03 |
| Nausea | 5 (15.6%) | 5 (17.9%) | >.99 |
| Vomiting | 1 (3.1%) | 2 (7.1%) | 0.79 |
| Abdominal pain | 5 (15.6%) | 5 (17.9%) | >.99 |
| Diarrhoea | 6 (18.8%) | 10 (35.7%) | .15 |
| Skin rash | 3 (9.4%) | 2 (7.1%) | >.99 |
| Chest pain | 5 (15.6%) | 4 (14.3%) | >.99 |
| Other | 5 (15.6%) | 0 (0.0%) | .06 |
| Admitted to the hospital | 2 (6.3%) | 0 (0.0%) | .50 |
Abbreviations: P, P-value; PCR, polymerase chain reaction.
Since the start of the COVID-19 pandemic or the previous study visit.
P values based on Fisher’s exact test.
Of 9 HIV-negative participants with PCR-positive test results, 8 were symptomatic.
Of 9 HIV-positive participants with PCR-positive test result, 9 were symptomatic.
Factors Associated With Incident SARS-CoV-2 Infection
HIV status was not independently associated with incidence of SARS-CoV-2 infection in both univariable (Supplementary Table 3) and multivariable (Table 3) analyses. In the univariable analysis, the association with self-reported compliance to social distancing did not reach statistical significance. In the multivariable analysis, incident SARS-CoV-2 infection was significantly associated with younger age and being of African origin, with none of these risk factors showing a statistically significant interaction with HIV status.
Table 3.
Factors Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Infection Acquired Between 27 February 2020 and 30 April 2021 Among 567 Participants of the AGEhIV COVID-19 Substudy
| Factor | Participants, No./Total (%)a | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| HR (95% CI) | P Value | aHR (95% CI)b | P Value | ||
| HIV status | |||||
| HIV negative | 35/326 (10.7) | Reference | .62 | Reference | .41 |
| HIV positive | 29/241 (12.0) | 1.14 (.69–1.86) | 1.23 (.75–2.03) | ||
| Agec | |||||
| 53–59 y | 39/246 (15.9) | 3.69 (1.32–10.32) | .01 | 3.61 (1.25–10.40) | .02 |
| 60–64 y | 16/143 (11.2) | 2.58 (.86–7.69) | 2.57 (.84–7.87) | ||
| 65–69 y | 5/95 (5.3) | 1.19 (.32–4.42) | 1.16 (.31–4.33) | ||
| ≥70 y | 4/83 (4.8) | Reference | Reference | ||
| Ethnic origin | |||||
| White | 57/538 (10.6%) | Reference | .008 | Reference | .02 |
| African | 6/20 (30.0%) | 3.71 (1.63–8.45) | 3.11 (1.40–6.90) | ||
| Asian | 1/9 (11.1%) | 1.06 (.16–7.10) | 1.76 (.29–10.60) | ||
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; HIV, human immunodeficiency virus; HR, hazard ratio.
Number and percentage of participants with incident infection per total amount of participants for each variable category.
aHRs are adjusted for HIV status, age, ethnic origin, and body mass index.
Age at time of severe acute respiratory syndrome coronavirus 2 nucleocapsid antibody test.
Factors Associated With SARS-CoV-2 N Antibody Levels
In the 61 participants with incident SARS-CoV-2 infection and detectable N antibody levels, HIV status was not independently associated with SARS-CoV-2 N antibody levels (P = .53) at the moment of the first N antibody positive test result. The median N antibody level was 34.2 (IQR, 17.8–37.7) in HIV-positive and 27.6 (15.7–36.0) in HIV-negative participants. In the 57 of 61 participants with available information on self-reported COVID-19 symptoms, HIV status was not associated with N antibody levels in univariable (Supplementary Table 4) or multivariable (Table 4) analyses. Experiencing fever in the 6 months before the positive SARS-CoV-2 N antibody test was the only variable significantly associated with a higher N antibody level in multivariable analysis.
Table 4.
Factors Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Nucleocapsid (N) Antibody Level at the Time of First N Antibody–Positive Measurement (September–October 2020 or March–April 2021) Among 57 Participants in the AGEhIV Coronavirus Disease 2019 Substudy
| Factor | Univariable Analysis | Multivariable Analysisa | ||
|---|---|---|---|---|
| Coefficient (95% CI) | P Value | Coefficient (95% CI) | P Value | |
| HIV status | ||||
| HIV negative | Reference | .53 | Reference | .25 |
| HIV positive | 2.03 (−4.46 to 8.52) | 3.67 (−2.66 to 10.00) | ||
| Self-reported feverb | ||||
| No | Reference | .005 | Reference | .003 |
| 9.73 (3.39–16.06) | ||||
| Yes | 9.07 (2.82– 15.33) | |||
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus.
Values represent regression coefficients with 95% confidence interval, adjusted for HIV status and reported fever.
In the 6 months prior to the first positive severe acute respiratory syndrome coronavirus 2 nucleocapsid antibody test.
DISCUSSION
During 14 months of possible SARS-CoV-2 exposure, we found no significant difference in the cumulative incidence of infection between HIV-negative participants in our study and those who were HIV-positive and—with few exceptions—were on cART with suppressed viremia and a reasonably high CD4 cell count. Having HIV also did not significantly impact the SARS-CoV-2 N antibody level, as measured by INgezim COVID-19 double recognition assay. Only 2 of our participants, both HIV negative, had been admitted to the hospital for COVID-19, with neither progressing to severe disease or death.
In our cohort, the cumulative incidences on 31 October 2020 and 30 April 2021 were 6.2% and 12.3%, respectively. This is similar to the SARS-CoV-2 seroprevalence for those 50–70 years old in the general Dutch population, which ranged between 4%–6% and 10%–15% in September 2020 and February 2021, respectively [36].
We found no difference in the incidence of SARS-CoV-2 infection between HIV-positive and HIV-negative participants. This is in line with findings from various cross-sectional studies comparing the prevalence of PCR-confirmed SARS-CoV-2 in PWH with that in the general population [8, 10, 11, 14, 18]. In contrast, a systematic review and meta-analysis of the epidemiology and outcomes of COVID-19 in PWH showed that the risk of a PCR-confirmed SARS-CoV-2 infection was significantly higher in HIV-positive than in HIV-negative individuals (risk ratio, 1.24) [37]. Importantly however, the influence of CD4 cell count, current ART use and the degree of HIV suppression on the incidence of COVID-19 in PWH could not be determined in this meta-analysis, as this information was not available for all included studies.
An important limitation of using PCR-based test results in those studies is the possibility that people with asymptomatic SARS-CoV-2 are missed. In a matched case-control observational study, Spinelli et al [38] found that the seroprevalence of SARS-CoV-2 was about 2 times lower among PWH than among HIV-negative people. Of note, participants were matched only for age and date of sampling; the 2 groups were not comparable with regard to sex, ethnic origin, and prevalence of comorbid conditions, which likely affects the observed difference in seroprevalence between the groups. In our study, participants in both groups were highly comparable, which might explain why we found no difference between the groups.
We found that living with HIV was not independently associated with an increased risk of SARS-CoV-2 infection. We did not find an association between incident SARS-CoV-2 infection and HIV-specific parameters. Of note, almost all HIV-positive participants in our study (99.2%) were virologically suppressed, with almost 80% having CD4 cells counts ≥500/µL and virtually none having clinically relevant immunodeficiency. This limits our ability to assess the associations between HIV viral load, CD4 cell count, and acquisition of SARS-CoV-2. However, other studies found no association between HIV viral load or current CD4 cell count and SARS-CoV-2 seropositivity [38, 39]. Moreover, most PCR-based prevalence studies also found no association between HIV-specific factors and SARS-CoV-2 infection [11, 13, 15, 16]. One study from Wuhan suggested a higher risk of SARS-CoV-2 infection in PWH who reported or were inferred to have had interrupted access to cART during the lockdown in the early stage of the epidemic [8].
Younger age and being of African origin were each associated with an increased risk of SARS-CoV-2 infection in our analysis, without a significant interaction between these factors and HIV status. These findings corroborate the higher seroprevalence of SARS-CoV-2 in younger adults observed in other studies [38, 40, 41] and are similar to observations in the general Dutch population [36]. A study among 6 ethnic groups living in Amsterdam likewise showed SARS-CoV-2 seroprevalence to be significantly higher in individuals of Ghanaian origin, compared with those of Dutch origin [42]. This increased risk in individuals of African origin might be associated with socioeconomic factors, including lower income, dependence on public transport, or work in a contact-based profession. Unfortunately, data on such factors were not available for our study.
In participants with an incident SARS-CoV-2 infection, N antibody levels did not differ significantly between HIV-positive and HIV-negative participants, similar to findings reported by another study in which HIV-positive participants (median age, 52 years) all had undetectable viral load [33]. Moreover, in that study antibody titers were similar in HIV-positive participants with CD4 cell counts ≥500/µL and those with counts <500/µL. In contrast, Spinelli et al [38] found significantly lower IgG receptor binding domain antibody levels in participants with HIV than in HIV-negative participants, after adjustment for age and sex. In HIV-positive participants, significantly lower titers were seen in those with CD4 cell counts <200/µL. Furthermore, Huang et al [8] observed lower SARS-CoV-2 S- and N antibody titers in HIV-positive participants with an HIV viral load >20 copies/mL than in those with a viral load ≤20 copies/mL. Inadequately treated HIV and thus possibly diminished immune responses might explain why both studies found lower SARS-CoV-2 antibody titers in PWH and why our study—in which 99.2% of HIV-positive participants were virologically suppressed—did not find a difference between HIV-positive and HIV-negative participants.
In the current study, participants who reported fever in the 6 months before their N antibody positive test had higher SARS-CoV-2 N antibody levels. These results are in line with observations in several other studies, where both fever [43] and disease severity were correlated with levels of SARS-CoV-2 antibodies [21, 29–31].
To our knowledge, the current study is the first prospective longitudinal systematic comparative assessment of SARS-CoV-2 incidence, irrespective of symptoms, comparing HIV-positive and HIV-negative individuals. A strength of our study is the inclusion of individuals >50 years old with significant comorbid conditions, which may increase their risk of symptomatic SARS-CoV-2 infection.
Furthermore, both HIV-positive and HIV-negative participants—highly comparable with respect to demographic, lifestyle, and behavioral characteristics—have been extensively characterized for the presence of comorbid conditions and their risk factors for ≥10 years. This allows for an unbiased assessment of the potential association between HIV-positive status and acquisition of SARS-CoV-2 infection. Moreover, the additional standardized collection of data on household size and self-reported compliance with social distancing measures allowed us to take these into account in the analysis.
However, the current study also has a number of limitations. First, our findings apply to HIV-positive participants in an urban setting with good access to healthcare and well-controlled HIV infection on cART with a reasonably high CD4 cell count. Thus, findings may not be generalizable to all individuals with HIV. Second, although reasons to decline participation in our COVID-19 substudy were similar for both groups of AGEhIV Cohort participants, those who declined were more often HIV positive, of African origin, and with a lower educational level. Individuals of African origin were underrepresented in our substudy and at greater risk of acquiring SARS-CoV-2, so our observed incidence may represent an underestimation. Third, with only 2 participants reporting hospitalization for COVID-19, we could not address the extent to which HIV-positive status in our cohort may affect the risk of COVID-19 disease severity. Finally, although we were able to consider many factors in our analysis, we cannot rule out potential unmeasured confounders, such as employment in professions which may have influenced the risk of SARS-CoV-2 exposure.
In conclusion, the risk of SARS-CoV-2 acquisition and N antibody levels after infection in our cohort of HIV-positive individuals with suppressed viremia and adequate CD4 cell counts were similar to those in a comparable cohort of HIV-negative people. This may be different in other populations and parts of the world, including in resource-limited settings, where significant numbers of PWH do not yet have access to or have less immune restoration on cART. Not only should this be investigated in such settings, but it also once more reinforces the urgency for global access to early diagnosis and treatment of HIV.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
AGEhIV Cohort Study Group. Scientific oversight and coordination: P. R. (principal investigator), F. W. N. M. W., M. v. d. V., E. Verheij, S. O. Verboeket, M. L. V., B. C. Elsenga, and C. J. van Eeden (Amsterdam University Medical Centers [UMC], Department of Global Health, University of Amsterdam, and Amsterdam Institute for Global Health and Development [AIGHD]); and M. Prins (co–principal investigator), M. F. S. v. d. L., L. del Grande, and I. Agard (Department of Infectious Diseases, Public Health Service of Amsterdam). Data management: S. Zaheri, M. M. J. Hillebregt, Y. M. C. Ruijs, D.P. Benschop, and A. el Berkaoui (HIV Monitoring Foundation). Statistical support: A. B. Central laboratory support: N. A. K., A.M. Harskamp-Holwerda, I. Maurer, M. M. Mangas Ruiz, A. F. Girigorie, and B. Boeser-Nunnink (Laboratory for Viral Immune Pathogenesis and Department of Experimental Immunology, Amsterdam UMC); and L. v. d. H. and M. B. (Department of Medical Microbiology and Infection Prevention, Laboratory of Experimental Virology, Amsterdam UMC). Project management and administrative support: W. Zikkenheiner and S. Nolst Trenité (AIGHD). Participating HIV physicians and nurses: S. E. Geerlings, A. Goorhuis, J. W. R. Hovius, F. J. B. Nellen, J. M. Prins, T. van der Poll, M. v. d. V., W.J. Wiersinga, M. van Vugt, G. de Bree, B. A. Lemkes, V. Spoorenberg, F. W. N. M. W., J. van Eden, A.M.H. van Hes, F. J. J. Pijnappel, A. Weijsenfeld, S. Smalhout, M. van Duinen, and A. Hazenberg (Division of Infectious Diseases, Amsterdam UMC). Other collaborators: P. G. Postema (Department of Cardiology, Amsterdam UMC), P. H. L. T. Bisschop, and M. J. M. Serlie (Division of Endocrinology and Metabolism, Amsterdam UMC), P. Lips (Amsterdam UMC), E. Dekker (Department of Gastroenterology, Amsterdam UMC), N. van der Velde and R. Franssen (Division of Geriatric Medicine, Amsterdam UMC), J. M. R. Willemsen and L. Vogt (Division of Nephrology, Amsterdam UMC), J. Schouten, P. Portegies, B. A. Schmand, and G. J. Geurtsen (Department of Neurology, Amsterdam UMC), F. D. Verbraak and N. Demirkaya (Department of Ophthalmology, Amsterdam UMC), I. Visser (Department of Psychiatry, Amsterdam UMC), A. Schadé (Department of Psychiatry, Amsterdam UMC), P. T. Nieuwkerk and N. Langebeek (Department of Medical Psychology, Amsterdam UMC), R. P. van Steenwijk and E. Dijkers (Department of Pulmonary Medicine, Amsterdam UMC), C. B. L. M. Majoie and M. W. A. Caan (Department of Radiology, Amsterdam UMC), H. W. van Lunsen and M. A. F. Nievaard (Department of Gynaecology, Amsterdam UMC), B. J. H. van den Born and E. S. G. Stroes (Division of Vascular Medicine, Amsterdam UMC), and W. M. C. Mulder and S. van Oorspronk (HIV Vereniging Nederland).
Disclaimer. None of the funding bodies had a role in the design or conduct of the study, the analysis and interpretation of the results, the writing of the report, or the decision to publish.
Financial support. This work was supported by ViiV Healthcare (investigator-initiated study grant). The parent AGEhIV Cohort Study was supported by the Netherlands Organization for Health Research and Development (ZonMW; grant 30002000), AIDS Fonds (grant 2009063), and unrestricted research grants from Gilead Sciences, ViiV Healthcare, Janssen Pharmaceuticals, and Merck Sharp & Dohme (MSD).
Potential conflicts of interest. F. W. N. M. W. has served on scientific advisory boards for ViiV Healthcare and Gilead Sciences. M. F. S. v. d. L. has received independent scientific grant support from Sanofi Pasteur, MSD Janssen Infectious Diseases and Vaccines, and Merck & Co; has served on advisory boards for GlaxoSmithKline and Merck & Co; and has received nonfinancial support from Stichting Pathologie Onderzoek en Ontwikkeling. M. v. d. V, through his institution, has received independent scientific grant support and consultancy fees from AbbVie, Gilead Sciences, MSD, and ViiV Healthcare, honoraria for which were all paid to his institution. P. R., through his institution, has received independent scientific grant support from Gilead Sciences, Janssen Pharmaceuticals, Merck & Co, and ViiV Healthcare and has served on scientific advisory boards for Gilead Sciences, ViiV Healthcare, and Merck & Co, honoraria for which were all paid to his institution. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 18th European AIDS Conference, London, UK, 27–30 October 2021 (abstract BPD3/8); 14th Netherlands Conference on HIV Pathogenesis, Epidemiology, Prevention and Treatment, virtual, 23 November 2021 (abstract O.02).
Contributor Information
Myrthe L Verburgh, Amsterdam University Medical Centers, Department of Infectious Diseases, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands; Department of Global Health, Amsterdam Institute for Global Health and Development, Amsterdam, the Netherlands.
Anders Boyd, HIV Monitoring Foundation, Amsterdam, the Netherlands; Department of Infectious Diseases, Public Health Service of Amsterdam, Amsterdam, the Netherlands.
Ferdinand W N M Wit, Amsterdam University Medical Centers, Department of Infectious Diseases, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands; HIV Monitoring Foundation, Amsterdam, the Netherlands.
Maarten F Schim van der Loeff, Amsterdam University Medical Centers, Department of Infectious Diseases, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands; Department of Infectious Diseases, Public Health Service of Amsterdam, Amsterdam, the Netherlands.
Marc van der Valk, Amsterdam University Medical Centers, Department of Infectious Diseases, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands; HIV Monitoring Foundation, Amsterdam, the Netherlands.
Margreet Bakker, Amsterdam University Medical Centers, Department of Medical Microbiology and Infection Prevention, Laboratory of Experimental Virology, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands.
Neeltje A Kootstra, Amsterdam University Medical Centers, Department of Experimental Immunology, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands.
Lia van der Hoek, Amsterdam University Medical Centers, Department of Medical Microbiology and Infection Prevention, Laboratory of Experimental Virology, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands.
Peter Reiss, Amsterdam University Medical Centers, Department of Infectious Diseases, Amsterdam Infection and Immunity Institute, Amsterdam, the Netherlands; Department of Global Health, Amsterdam Institute for Global Health and Development, Amsterdam, the Netherlands.
References
- 1. Rijksoverheid. Coronadashboard. 2021. https://coronadashboard.rijksoverheid.nl/. Accessed 12 October 2021.
- 2. de Lusignan S, Dorward J, Correa A, et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis 2020; 20:1034–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Laxminarayan R, Mohan B C, G VT, Arjun Kumar KV, Wahl B, Lewnard JA.. SARS-CoV-2 infection and mortality during the first epidemic wave in Madurai, south India: a prospective, active surveillance study. Lancet Infect Dis 2021; 21:1665–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mao B, Liu Y, Chai YH, et al. . Assessing risk factors for SARS-CoV-2 infection in patients presenting with symptoms in Shanghai, China: a multicentre, observational cohort study. Lancet Digit Health 2020; 2:e323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Geretti AM, Stockdale AJ, Kelly SH, et al. Outcomes of COVID-19 related hospitalization among people with HIV in the ISARIC WHO Clinical Characterization Protocol (UK): a prospective observational study. Clin Infect Dis 2020; 73:e2095–106. [Google Scholar]
- 6. Hadi YB, Naqvi SFZ, Kupec JT, Sarwari AR.. Characteristics and outcomes of COVID-19 in patients with HIV: a multicentre research network study. AIDS 2020; 34:F3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boulle A, Davies MA, Hussey H, et al. Risk factors for COVID-19 death in a population cohort study from the Western Cape Province, South Africa. Clin Infect Dis 2020; 73:e2005–15. [Google Scholar]
- 8. Huang J, Xie N, Hu X, et al. Epidemiological, virological and serological features of COVID-19 cases in people living with HIV in Wuhan City: a population-based cohort study. Clin Infect Dis 2020; 73:e2086–94. [Google Scholar]
- 9. Del Amo J, Polo R, Moreno S, et al. Incidence and severity of COVID-19 in HIV-positive persons receiving antiretroviral therapy: a cohort study. Ann Intern Med 2020; 173:536–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Braunstein SL, Lazar R, Wahnich A, Daskalakis DC, Blackstock OJ.. COVID-19 infection among people with HIV in New York City: a population-level analysis of linked surveillance data. Clin Infect Dis 2020; 72:e1021–9. [Google Scholar]
- 11. Friedman EE, Devlin SA, McNulty MC, Ridgway JP.. SARS-CoV-2 percent positivity and risk factors among people with HIV at an urban academic medical center. PLoS One 2021; 16:e0254994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun J, Patel RC, Zheng Q, et al. . COVID-19 disease severity among people with HIV infection or solid organ transplant in the United States: a nationally-representative, multicenter, observational cohort study. medRxiv [Preprint: not peer reviewed]. 28 July 2021. Available from: https://pubmed.ncbi.nlm.nih.gov/34341798/. [Google Scholar]
- 13. Inciarte A, Gonzalez-Cordon A, Rojas J, et al. Clinical characteristics, risk factors, and incidence of symptomatic coronavirus disease 2019 in a large cohort of adults living with HIV: a single-center, prospective observational study. AIDS 2020; 34:1775–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tesoriero JM, Swain CE, Pierce JL, et al. COVID-19 outcomes among persons living with or without diagnosed HIV infection in New York State. JAMA Netw Open 2021; 4:e2037069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Park LR, Sigel K, Rodriguez-Barradas M.. Presented at: AIDS 2020: 23rd International AIDS Conference Virtual; 6–10 July 2020. [Google Scholar]
- 16. Nomah DK, Reyes-Urueña J, Díaz Y, et al. . Sociodemographic, clinical, and immunological factors associated with SARS-CoV-2 diagnosis and severe COVID-19 outcomes in people living with HIV: a retrospective cohort study. Lancet HIV 2021; 34:e701–e710. [Google Scholar]
- 17. Yang X, Sun J, Patel RC, et al. . Associations between HIV infection and clinical spectrum of COVID-19: a population level analysis based on US National COVID Cohort Collaborative (N3C) data. Lancet HIV 2021; 8:e690–e700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown LB, Spinelli MA, Gandhi M.. The interplay between HIV and COVID-19: summary of the data and responses to date. Curr Opin HIV AIDS 2021; 16:63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 2020; 183:996–1012.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Carvalho T, Krammer F, Iwasaki A.. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol 2021; 21:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. den Hartog G, Vos ERA, van den Hoogen LL, et al. Persistence of antibodies to SARS-CoV-2 in relation to symptoms in a nationwide prospective study. Clin Infect Dis 2021; 73:2155–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wajnberg A, Amanat F, Firpo A, et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020; 1227:30. [Google Scholar]
- 24. Ebinger JE, Fert-Bober J, Printsev I, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021; 27:981–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020; 396:467–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Barouch DH, Stephenson KE, Sadoff J, et al. Durable humoral and cellular immune responses 8 months after Ad26.COV2.S vaccination. N Engl J Med 2021; 385:951–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dan JM, Mateus J, Kato Y, et al. . Immunological memory to SARS-CoV-2 assessed for up to eight months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. He Z, Ren L, Yang J, et al. Seroprevalence and humoral immune durability of anti-SARS-CoV-2 antibodies in Wuhan, China: a longitudinal, population-level, cross-sectional study. Lancet 2021; 397:1075–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen X, Pan Z, Yue S, et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct Target Ther 2020; 5:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hansen CB, Jarlhelt I, Perez-Alos L, et al. SARS-CoV-2 antibody responses are correlated to disease severity in COVID-19 convalescent individuals. J Immunol 2021; 206:109–17. [DOI] [PubMed] [Google Scholar]
- 32. Dorigatti I, Lavezzo E, Manuto L, et al. SARS-CoV-2 antibody dynamics and transmission from community-wide serological testing in the Italian municipality of Vo’. Nat Commun 2021; 12:4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alrubayyi A, Gea-Mallorquí E, Touizer E, et al. Characterization of humoral and SARS-CoV-2 specific T cell responses in people living with HIV. Nat Commun 2021; 12:5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schouten J, Wit FW, Stolte IG, et al. Cross-sectional comparison of the prevalence of age-associated comorbidities and their risk factors between HIV-infected and uninfected individuals: the AGEhIV Cohort Study. Clin Infect Dis 2014; 59:1787–97. [DOI] [PubMed] [Google Scholar]
- 35. Hoste ACR, Venteo A, Fresco-Taboada A, et al. Two serological approaches for detection of antibodies to SARS-CoV-2 in different scenarios: a screening tool and a point-of-care test. Diagn Microbiol Infect Dis 2020; 98:115167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. National Institute for Public Health and the Environment. PIENTER Corona study: results. 2021. Available at: https://www.rivm.nl/en/pienter-corona-study/results. Accessed 12 October 2021.
- 37. Ssentongo P, Heilbrunn ES, Ssentongo AE, et al. Epidemiology and outcomes of COVID-19 in HIV-infected individuals: a systematic review and meta-analysis. Sci Rep 2021; 11:6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spinelli MA, Lynch KL, Yun C, et al. SARS-CoV-2 seroprevalence, and IgG concentration and pseudovirus neutralising antibody titres after infection, compared by HIV status: a matched case-control observational study. Lancet HIV 2021; 8:e334–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Berenguer J, Diez C, Martin-Vicente M, et al. Prevalence and factors associated with SARS-CoV-2 seropositivity in the Spanish HIV Research Network Cohort. Clin Microbiol Infect 2021; 27:1678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bajema KL, Wiegand RE, Cuffe K, et al. Estimated SARS-CoV-2 seroprevalence in the US as of September 2020. JAMA Intern Med 2021; 181:450–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 2020; 396:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Coyer L, Boyd A, Schinkel J, et al. . Differences in SARS-CoV-2 infections during the first and second wave of SARS-CoV-2 between six ethnic groups in Amsterdam, the Netherlands: a population-based longitudinal serological study. Lancet Reg Health Eur 2022; 13:100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schlickeiser S, Schwarz T, Steiner S, et al. Disease severity, fever, age, and sex correlate with SARS-CoV-2 neutralizing antibody responses. Front Immunol 2020; 11:628971. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.