Abstract
This study demonstrated a favorable short-term safety profile after a third dose of the BNT162b2 vaccine among healthcare workers (HCWs). There were more frequent local reactions and less systemic reactions compared to the second dose. The HCWs who reported reactions had higher prebooster titer of anti-S1 antibodies compared to those who reported no reactions.
Keywords: antibodies, BNT162b2, booster, COVID-19, side-effects
A 2-dose series of the BNT162b2 mRNA vaccine was shown to prevent symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection [1–4], with an acceptable safety profile [1–5]. Waning vaccine effectiveness, coupled with a surge of infections due to the Delta SARS-CoV-2 variant, prompted the Israeli Ministry of Health to recommend a third (booster) dose of the vaccine for adults aged 60 years and above as well as healthcare workers (HCWs) above the age of 30 years [6]. However, the safety of repeat BNT162b2 dosing in immunocompetent individuals has not been systematically assessed. In this study, we report on the short-term incidence of adverse events after BNT162b2 booster vaccination among HCWs.
METHODS
This study was conducted at the Tel Aviv Sourasky Medical Center, a tertiary medical center in Tel Aviv Israel. Healthcare workers aged 30 years or older were included in this study if they had previously completed a 2-dose BNT162b2 vaccination series, had no documented previous infection with SARS-CoV-2, and were not immunocompromised. Informed consent was obtained by means of an electronic form. The BNT162b2 dose (30 mcg in 0.3 mL delivered to the deltoid muscle) was identical to primary series doses. Adverse events were monitored using a structured 13-item questionnaire administered after the second and third doses of the vaccine. The questionnaire was sent to participants 3–7 days after vaccination.
Antispike receptor binding domain (S1 RBD) immunoglobulin (Ig)G antibodies titer (“pre-booster antibody titer”) was measured up to 7 days before immunization using indirect chemiluminescent microparticle immunoassay on the ADVIA Centaur XP System (Siemens, Tarrytown, NY). The SARS-CoV-2 IgG (sCOVG) assays are reported in index values. A conversion factor between index values and the World Health Organization (WHO) binding antibody units (BAU/mL) has been established. A total of 1.00 index on the sCOVG assay would have a WHO BAU/mL value of 21.8.
Patient Consent Statement
Comparison With Rate of Adverse Events After Second Dose
For comparison with reported rates of local and systemic reactions after the second dose, we relied on historical anonymous records from our institution of similar questionnaires screening for adverse events.
Statistical Analysis
All statistical analyses were performed using R software version 4.0.3. Statistical significance for continuous variables was determined using Wilcoxon rank-sum test (one-tailed) and for categorical variables using a hypergeometric test (one-tailed). P values were corrected for multiple testing where applicable using Holm’s method.
RESULTS
Between August 1, 2021 and August 21, 2021, 2979 HCWs received a third BNT162b2 dose as part of the hospital’s vaccination campaign. Median participant age was 52 years (interquartile range [IQR], 42–62). Overall, 890 HCWs (30%) responded to the adverse event questionnaire and contributed to the safety cohort. The median age in the safety cohort was 47 years (IQR, 39–55), 204 (22.9%) were older than 55 years, and 588 (66%) were female. In 97.2% of participants the time interval between second and third vaccine dose was at least 5 months. Prebooster anti-S1 IgG titers were available for 536 HCWs (60%). The historical safety cohort included 2256 HCWs who responded to the adverse event questionnaire after the second vaccine dose (Supplemental Table 1).
Reactions Reported After the Third (Booster) Vaccine Dose
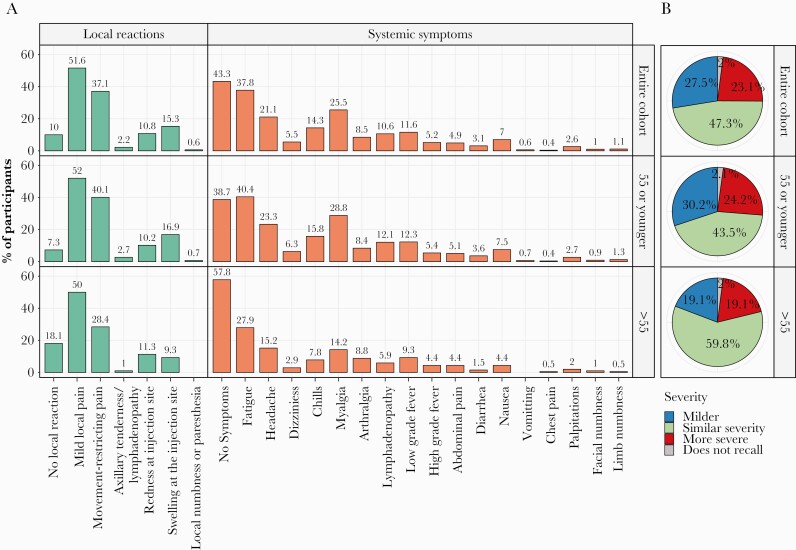
Local injection site reactions were reported by 778 HCWs (87.4%) (Supplemental Table 2). The most common local reaction was pain at the injection site reported by 461 HCWs (52%). Local reactions were significantly more frequent among persons aged 55 years or younger than among persons older than 55 years: 92.7% versus 81.9% (P < .001) (Figure 1A).
Figure 1.
(A) Third vaccine dose reactions. (B) Reaction to third dose vaccine in comparison to second vaccine dose (for the same vaccine participants).
Systemic symptoms were reported by 505 HCWs (56.7%). The most frequent symptoms were fatigue (37.8%), myalgia (25.5%), and headache (21.1%) (Figure 1A). Systemic symptoms were significantly more frequent among persons aged 55 years or younger than among persons older than 55 years: 61.3% versus 42.2% (P < .001) (Figure 1A).
No participant required emergency treatment for local or systemic reactions and no immediate type allergic reactions were reported. Seven participants (0.8%) reported a systemic rash.
Comparing their own overall experience after the third vaccine dose with the experience they recalled after the second dose, 206 participants (23.1%) reported more severe reactions after the third dose, 245 (27.5%) reported milder reactions after the third dose, and 421 (47.3%) reported similar degree of reactions. Eighteen participants (2%) responded that they did not recall and could not compare their experience (Figure 1B).
Comparison Between Reactions Reported After the Third Dose (Current Cohort) and After Second Dose (Historical Cohort) Vaccine
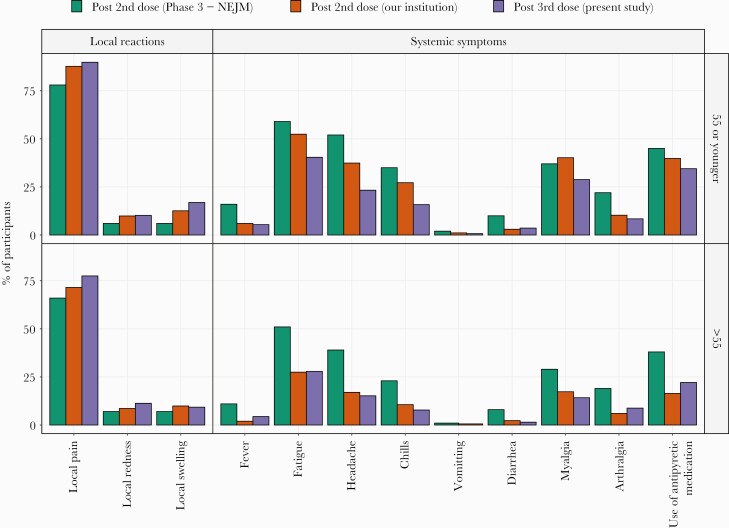
Compared with local reactions reported after the second vaccine dose, reactions were more frequent after the third dose: 92.7% versus 89.6% for persons aged 55 years or younger (P = .039) and 81.9% versus 75.3% for persons older than 55 years (P = .058) (Figure 2). Systemic symptoms were less frequent after the third vaccine dose compared to the second dose: 61.3% versus 71.8% for persons aged 55 years and younger (P < .001) and 42.2% versus 48.6% for persons older than 55 years (P = .06) (Figure 2).
Figure 2.
Local and systemic reactions in the study cohort in comparison to reactions reported after the second dose.
Prebooster Titer of Anti-S1 Immunoglobulin G Antibodies and Association to Reported Reactions to Third Dose (Booster) Vaccine
The median prebooster titer of anti-S1 IgG antibodies was significantly higher for participants who reported local and systemic reactions compared with those who reported no reactions after the booster dose (4.61 [IQR, 2.89–8.24] vs 3.35 [IQR, 1.79–5.12], P < .0001) (Supplemental Figure S1 and Supplemental Table S3). There was no significant difference in the anti-S1 IgG antibody titers between the age groups with a median titer of 4.24 (IQR, 2.72–7.63) in persons 55 years or younger and 4.42 (IQR, 1.89–8.33) in persons older than 55 years
DISCUSSION
In this observational study of immunocompetent HCWs who received a third (booster) vaccine dose BNT162b2, the local and systemic reactions within 1 week after vaccination were mild and were similar to those observed in previous studies that evaluated the safety of the BNT162b2 primary series [1, 7]. There were no severe adverse events. As noted for the primary BNT162b2 series, local reactions and systemic symptoms were more frequent in persons 55 years or younger than in older individuals [1, 7].
It is significant that more local but fewer systemic reactions were reported after the third versus the second vaccine dose. The median prebooster anti-S1 IgG titers were higher in persons who reported local and systemic reactions than for those who reported no symptoms, as was also recently reported by Rechavi et al [8]. This finding suggests that humoral immune priming is associated with local and systemic symptoms after booster vaccination. Previous studies have shown that the humoral response to an mRNA vaccine persisted longer in the regional lymph nodes than in peripheral blood. Turner et al [9] found that although circulating IgG and IgA-secreting plasmablasts in peripheral blood that target the S protein declined and became undetectable 3 weeks after the second immunization, S-binding B cells and plasmablasts persisted in the germinal centers of draining lymph nodes on the side of immunization for at least 12 weeks after immunization.
There are several limitations to our study. First, the study was questionnaire based with relatively low response rate, and therefore it was subjected to recall and report bias. However, the questionnaire was sent to participants up to 1 week after receiving the booster dose to minimize this effect. Second, only short-term reactions occurring up to 7 days after vaccination were assessed. Third, the cohort size was small with predominance of young participants, as expected from a cohort of HCWs, and was not powered to detect rare adverse events such as myocarditis and anaphylaxis.
CONCLUSIONS
In conclusion, a third dose of BNT162b2 was associated with generally mild short-term local and systemic reactions, which were more frequent among younger vaccine recipients. Compared to the second vaccine dose, the third dose was associated with more local reactions and fewer systemic symptoms. No severe adverse events were encountered. Monitoring of late-onset adverse events is ongoing.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
The study was approved by the Tel Aviv Sourasky Medical Center Institutional Review Board (approval numbers 0565-21-TLV and 0530-21-TLV). The Helsinky Committee has exempted us from obtaining written informed consent of the patients involved in our manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Angel Y, Spitzer A, Henig O, et al. Association between vaccination with BNT162b2 and incidence of symptomatic and asymptomatic SARS-CoV-2 infections among health care workers. JAMA 2021; 325:2457–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021; 385:1078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Indications for third mRNA vaccine against COVID-19, 4th update. Available at: https://www.gov.il/he/departments/policies/epi-546597221. Accessed 10 August 2021.
- 7. Menni C, Klaser K, May A, et al. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis 2021; 21:939–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rechavi Y, Shashar M, Lellouche J, Yana M, Yakubovich D, Sharon N.. Occurrence of BNT162b2 vaccine adverse reactions is associated with enhanced SARS-CoV-2 IgG antibody response. Vaccines (Basel) 2021; 21:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turner JS, O’Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021; 596:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.