Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines are being administered on an unprecedented scale. Assessing the risks of side effects is needed to aid clinicians in early detection and treatment. This study examined the risk of inflammatory heart disease, including pericarditis and myocarditis, after SARS-CoV-2 vaccination.
Methods
Intermountain Healthcare patients with inflammatory heart disease from December 15, 2020 to June 15, 2021, and with or without preceding SARS-CoV-2 vaccinations, were studied. Relative rates of inflammatory heart disease were examined for vaccinated patients compared to unvaccinated patients.
Results
Of 67 patients identified with inflammatory heart disease, 21 (31.3%) had a SARS-Cov-2 vaccination within the previous 60 days. Overall, 914 611 Intermountain Healthcare patients received a SARS-CoV-2 vaccine, resulting in an inflammatory heart disease rate of 2.30 per 100 000 vaccinated patients. The relative risk of inflammatory heart disease for the vaccinated patients compared to the unvaccinated patients was 2.05 times higher rate within the 30-day window (P = .01) and had a trend toward increase in the 60-day window (relative rate = 1.63; P = .07). All vaccinated patients with inflammatory heart disease were treated successfully with 1 death related to a pre-existing condition.
Conclusions
Although rare, the rate of inflammatory heart disease was greater in a SARS-CoV-2-vaccinated population than the unvaccinated population. This risk is eclipsed by the risk of contracting coronavirus disease 2019 and its associated, commonly severe outcomes. Nevertheless, clinicians and patients should be informed of this risk to facilitate earlier recognition and treatment.
Keywords: COVID-19, myocarditis, pericarditis, SARS-CoV-2, vaccination
Inflammatory heart disease (pericarditis and/or myocarditis) is a rare post-SARS-CoV-2 vaccination event with a rate of 2.30 per 100 000 patients. The per-day rate for a 30-day postvaccine window is 2.05 times higher than for unvaccinated individuals.
Since the discovery of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), there have been over 263 million cases of coronavirus disease 2019 (COVID-19) resulting in over 5.2 million deaths worldwide [1]. Fortunately, the extent of human devastation has been matched by human ingenuity and sheer determination to develop medical treatments and vaccines in a brief time frame. Vaccinations have resulted in sharp decreases in cases, hospitalizations, and deaths among vaccinated patients. However, both in the medical literature and anecdotally, there have been reports of rare side effects from the vaccines. Careful identification of these risks in large populations is critical for clinicians to enable early detection and treatment and provide the public an accurate understanding of potential risks of vaccination.
Inflammatory heart disease, which includes inflammation of the pericardium (pericarditis) and the myocardium (myocarditis), is rare but has been reported after vaccination for several different diseases [2–5]. The rate of inflammatory heart disease after SARS-CoV-2 vaccination has only recently been described, and most of these reports indicate a greater risk of inflammatory heart disease after vaccinations when compared to historical rates [6–12]. However, in the COVID-19 era, there may be an increase in the diagnosis of inflammatory heart disease because SARS-CoV-2 infections are associated with a higher rate of inflammatory heart disease both in the acute phase [13] and postinfection [14–16]. In addition, there has been an overall change in healthcare utilization patterns [17, 18]. Therefore, the use of historical rates may not accurately reflect the current true risk of inflammatory heart disease after SARS-CoV-2 vaccination. Moreover, most attention was given to the diagnosis of myocarditis and less to the diagnosis of pericarditis or the combination of myocarditis and pericarditis (myopericarditis) [6, 7, 10–12].
Therefore, in a large integrated US healthcare system, this study investigated the risk of inflammatory heart disease after SARS-CoV-2 vaccination and provided comparisons with nonvaccine-related inflammatory heart disease during the same period. All the inflammatory heart disease cases were adjudicated because of concerns about misclassifications based on diagnosis codes alone. In addition, the clinical characteristics and outcomes of these cases were examined. These results provide clinicians with increased knowledge of inflammatory heart disease risk associated with SARS-CoV-2 vaccination and help facilitate early recognition and treatment.
METHODS
A cohort of adult patients (≥18 years old) seen at Intermountain Healthcare were studied. Intermountain Healthcare is an integrated, not-for-profit healthcare system throughout Utah and parts of Idaho and Nevada. From December 15, 2020 to June 15, 2021, Utah patients diagnosed with pericarditis or myocarditis were reviewed. The restriction to Utah patients was done due to the availability of SARS-CoV-2 vaccine information for the Utah population from the Utah Department of Health. Intermountain Healthcare has 23 hospitals and over 160 clinics in Utah.
We used the Brighton Collaboration criteria of definitive and probable pericarditis and myocarditis for our case definition [19]. Diagnoses of pericarditis and myocarditis were excluded if considered secondary to recent cardiac surgery, cardiac ischemia, electrophysiology procedures, cancer chemotherapy, or other secondary causes. To identify these cases, all patients with a diagnosis code for pericarditis or myocarditis were reviewed by 2 members of a group of experienced clinicians that included 3 cardiologists and 1 cardiovascular physician assistant. The clinician’s review of each patient ensured that each case included in the study met well defined diagnostic criteria for pericarditis and/or myocarditis. Differences in reviewers’ assessments of case status were adjudicated by consensus review among the 4 clinicians. The reviewers were not provided knowledge of the patient’s vaccination status, although the medical record was not redacted. Patients who meet the criteria for both pericarditis and myocarditis are referred to as myopericarditis.
The vaccination records for the patients were obtained through the Utah Statewide Immunization Information System, maintained by the Utah Department of Health. The initial date of evaluation (December 15, 2020) corresponds to the beginning of population-based vaccinations. This statewide immunization system captures vaccine histories from healthcare providers throughout Utah and contains the vaccination dates and manufacturer of the vaccine. Prior positive SARS-CoV-2 test results for the patients were obtained using Intermountain Healthcare testing and as noted during the clinician review of the cases. The majority of these test results were based on polymerase chain reaction testing. Finally, the patients’ demographic and clinical characteristics were obtained using electronic medical records from Intermountain Healthcare. Differences in demographic and clinical characteristics between the vaccinated and unvaccinated cases were examined using χ2 tests or Fisher’s exact tests for categorical data and Wilcoxon rank-sum tests for continuous data. The distribution of vaccine manufacturers in the inflammatory heart disease cases was compared with the entire Intermountain patient population using a χ2 goodness-of-fit test.
The rate of inflammatory heart disease cases within 60-days post-SARS-CoV-2 vaccination was compared to the rate of inflammatory heart disease cases in unvaccinated individuals. Comparisons were performed using cases with and without vaccination during the same time. This was done instead of using historical data because of concern that COVID-19 has affected the rate of patients seeking medical care [17, 18], and COVID-19 infections can have a lasting impact, including symptoms of pericarditis or myocarditis after the initial acute infection [14–16].
Adult Utah patients with at least 1 encounter at an Intermountain Healthcare clinic, emergency department, or hospital since December 15, 2018 were included in the rate calculations. The restriction to recent visits ensured a population most likely to seek treatment at an Intermountain facility. The rates were based on the per-patient day of exposure or nonexposure to the vaccine. Unadjusted relative rates per day for the vaccinated and unvaccinated patients were calculated with 95% confidence intervals (CIs), and mid-P 2-sided P values were reported. These comparisons were repeated using a 30-day vaccine exposure window as well as for 3 subgroups: (1) pericarditis and myopericarditis cases, (2) only myocarditis cases, and (3) inflammatory heart disease cases without prior COVID-19 infection.
A case-crossover design and analyses were performed to determine the odds of inflammatory heart disease by vaccine exposure and to ensure consistency in results based on different study designs. Case-crossover designs are commonly used for examining medical outcomes based on exposures and have been used for vaccine and medication side-effect studies [20, 21]. In a case-crossover design, each case acts as its own control by examining exposure on control dates when the patient did not have the disease event (ie, inflammatory heart disease). For each case, up to 4 control dates were randomly chosen to be on the same day of the week between December 15, 2020 and the date of the case’s inflammatory heart disease event. One case occurred in the first week of the study and was dropped from these analyses leaving 66 cases. There were 52 cases with 4 control dates, 7 with 3 control dates, 4 with 2 control dates, and 3 with 1 control date. A McNemar’s paired analysis was performed. Odds ratios, 95% confidence intervals, and P values are reported using the 66 cases with 1–4 controls and the 52 cases with exactly 4 controls.
Although the case-crossover design is appropriately powered for these analyses, there are limitations to this study design. Therefore, we also included a self-control case series (SCCS) often used in vaccine safety studies [22, 23]. This approach, like the case-crossover design, uses the pericarditis/myocarditis case as its own control and looks at risk periods after vaccine exposures compared to a nonexposed control period. Because only 1 control period is examined, the power of this approach can be limited. Given our sample size, we estimated that our study had a power of 0.66. Despite the under power of this approach, we did a SCCS analysis using the 2-dose exposure periods with 20 days and 7 days. We chose 20 days to eliminate overlap between the first-dose and second-dose exposure.
Patient Consent Statement
This study was approved by the Intermountain Healthcare Institutional Review Board with a waiver of consent.
RESULTS
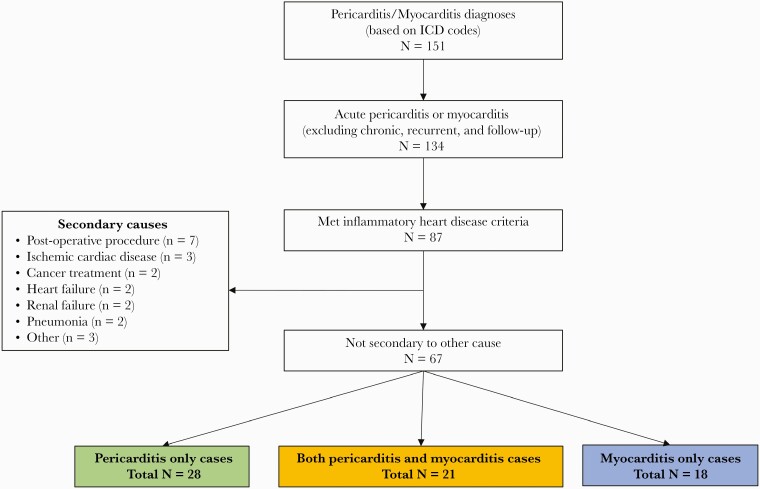
From a population of 1.7 million patients, 151 patients between December 15, 2020 and June 15, 2021 were diagnosed with pericarditis (n = 105) or myocarditis (n = 46) in the Intermountain Healthcare system. A total of 67 met diagnostic criteria for either pericarditis (28), myocarditis (18), or both myocarditis and pericarditis (myopericarditis; 21) (Figure 1). Of the 67 patients with inflammatory heart disease, the average age was 48 (interquartile range, 31–67), most were male (72%), and white (82%).
Figure 1.
Cases ascertainment diagram. ICD, International Classification of Diseases.
During the same period, 914 611 patients in the Intermountain Healthcare system had received at least 1 dose of the SARS-CoV-2 vaccine. Of the 67 inflammatory heart disease cases, 21 (31.3%) had received a SARS-Cov-2 vaccination within 60 days before the onset of their disease. This resulted in a rate of inflammatory heart disease postvaccine of 2.30 per 100 000 vaccinated patients. There were 780 903 unvaccinated patients during the same time period, and they have a 60-day rate of inflammatory heart disease of 1.96 per 100 000 patients. This results in a vaccine-attributable risk proportion of 14%. For the 30-day window, the rate of inflammatory heart disease was 1.96 per 100 000 vaccinated patients and 1.05 per 100 000 unvaccinated patients. This results in a substantial vaccine-attributed risk proportion of 47%.
The unvaccinated cases had statistically higher rates of prior COVID-19 diagnosis than the patients that had been vaccinated (37.0% vs 9.5%, respectively; P = .02). There were no statistically significant differences in the other baseline characteristics between the vaccinated and unvaccinated cases (Table 1). The distribution of vaccine type among the cases compared with all of Intermountain Healthcare-vaccinated patients was different, with more mRNA-1273 (Moderna) vaccines in the cases than expected: BNT162b2-mRNA (Pfizer-BioNTech) (23.8% of cases vs 52.1% of all vaccines), mRNA-1273 (Moderna) (71.4% of cases vs 39.4% of all vaccines), and Ad26.COV2.S (Janssen/Johnson & Johnson) (4.8%of cases vs 8.5% of all vaccines) (P = .01). Two thirds of the cases in the vaccinated cohort occurred after full vaccination, and almost half (47.6%) were within 7-days of a vaccine administration. Another 38.1% were between 7 and 30 days postvaccine administration, for a total of 86% within 30 days of the most recent vaccine.
Table 1.
Baseline Demographic and Clinical Characteristics for Inflammatory Heart Disease
| Characteristics | Vaccinated | Unvaccinated | |
|---|---|---|---|
| n = 21 | n = 46 | P Value∗ | |
| Age, median (IQR) | 56 (28–70) | 45 (32–65) | .39 |
| Gender | |||
| Male | 15 (71.4%) | 33 (71.7%) | .98 |
| Female | 6 (28.6%) | 13 (28.3%) | |
| Race/ethnicity | |||
| White | 19 (90.5%) | 36 (78.3%) | .64 |
| Hispanic | 1 (4.8%) | 6 (13.0%) | |
| Asian | 1 (4.8%) | 1 (2.2%) | |
| Native Hawaiian | 0 (0.0%) | 2 (4.4%) | |
| African American | 0 (0.0%) | 1 (2.2%) | |
| Prior COVID-19 diagnosis | 2 (9.5%) | 17 (37.0%) | .02 |
| Days From COVID-19 Positive Test Results | |||
| ≤30 days | 0 (0.0%) | 11 (64.7%) | .003 |
| 31–60 days | 0 (0.0%) | 5 (29.4%) | |
| >60 days | 2 (100.0%) | 1 (5.9%) | |
| Prior Disease History | |||
| Cancer | 3 (14.3%) | 4 (8.7%) | .67 |
| COPD | 7 (33.3%) | 14 (30.4%) | .81 |
| Depression | 5 (23.8%) | 10 (21.7%) | 1.00 |
| Diabetes | 3 (14.3%) | 9 (19.6%) | .74 |
| Drug Abuse | 5 (23.8%) | 4 (8.7%) | .13 |
| Heart Failure | 3 (14.3%) | 10 (21.7%) | .74 |
| Hypertension | 12 (57.1%) | 22 (47.8%) | .48 |
| Hypothyroidism | 4 (19.1%) | 8 (17.4%) | 1.00 |
| Liver Disease | 4 (19.1%) | 7 (15.2%) | .73 |
| MI | 2 (9.5%) | 5 (10.9%) | 1.00 |
| PVD | 4 (19.1%) | 6 (13.0%) | .71 |
| Renal Failure | 3 (14.3%) | 7 (15.2%) | 1.00 |
| Valvular Disease | 6 (28.6%) | 7 (15.2%) | .32 |
| Vaccinesa | |||
| BNT162b2-mRNA (Pfizer-BioNTech) | 5 (23.8%) | --- | |
| mRNA-1273 (Moderna) | 15 (71.4%) | --- | |
| Ad26.COV2.S (Janssen/Johnson & Johnson) | 1 (4.8%) | --- | |
| Vaccine Status | |||
| Partial | 7 (33.3%) | --- | |
| Full | 14 (66.7%) | --- | |
| Days Postvaccine | |||
| 0–7 days | 10 (47.6%) | --- | |
| 8–14 days | 3 (14.3%) | --- | |
| 15–30 day | 5 (23.8%) | --- | |
| >30 days | 3 (14.3%) | --- | |
Abbreviations: COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; IQR, interquartile range; MI, myocardial infarction; PVD, peripheral vascular disease.
NOTE: The vaccinated inflammatory heart disease cases occurred in ≤60 days postlast vaccine.
P values from χ2 tests (or Fisher exact tests) and Wilcoxon rank-sum tests.
The vaccine distribution for Intermountain patients was 52.1% BNT162b2-mRNA, 39.4% mRNA-1273, and 8.5% Ad26.COV2.S, and there was a significant difference in distribution from the general patient population and the vaccinated inflammatory heart disease cases (P = .011 based on a goodness-of-fit test).
Most of the cases were hospitalized (71% of the vaccinated and 70% of the unvaccinated cases), and 2 of the cases (1 vaccinated and 1 unvaccinated) were rehospitalized related to their inflammatory heart disease. More vaccinated cases were treated with colchicine than the unvaccinated cases (90.5% vs 60.9%, P = .01) (Table 2). Although not statistically significant, the unvaccinated cases had a higher rate of only myocarditis (34.8% vs 9.5%), and most of these (10 of 16, 62.5%) were COVID-related myocarditis. Pericardiocentesis was performed on 2 vaccinated cases and 2 unvaccinated cases (P = .58). There were 3 deaths: 1 among the vaccinated patients and 2 among the unvaccinated patients. The death after vaccination was in 1 male 65–84 years old who had a history of chronic myelogenous leukemia with resultant transformation to acute myeloid leukemia requiring treatment complicated by severe thrombocytopenia and anemia. He died from uncontrollable bleeding. Of the unvaccinated mortality cases, both had COVID-related myocarditis; however, one, a 65- to 84-year-old male, died within 5 days of diagnosis, and the other, an 18- to 24-year-old male, died 125 days later of unreported causes but not suspected to be due to COVID or myocarditis.
Table 2.
Inflammatory Heart Disease Criteria, Hospitalization, Treatment, and Outcomes
| Criteria, Treatments, and Outcomes | Cases | ||
|---|---|---|---|
| Vaccinated | Unvaccinated | ||
| n = 21 | n = 46 | P Value∗ | |
| Pericarditis only | 10 (47.6%) | 18 (39.1%) | .08 |
| Myocarditis only | 2 (9.5%) | 16 (34.8%) | |
| Myopericarditis | 9 (42.9%) | 12 (26.1%) | |
| Pericarditis Criteria | |||
| Chest pain | 19 (100%) | 32 (100%) | 1.00 |
| EKG changes | 14 (73.7%) | 18 (62.1%) | .40 |
| Pericardial effusion | 10 (52.6%) | 17 (60.7%) | .58 |
| Pericardial rub | 2 (10.5%) | 2 (6.7%) | .64 |
| CMR | .49 | ||
| None done | 12 (57.1%) | 32 (69.6%) | |
| Positive for both | 4 (19.1%) | 3 (6.5%) | |
| Positive for pericarditis | 2 (9.5%) | 4 (8.7%) | |
| Positive for myocarditis | 3 (14.3%) | 7 (15.2%) | |
| Negative for both | 0 (0.0%) | 0 (0.0%) | |
| Peak troponin, median (IQR) | 0.02 (<0.01–3.39) | 0.14 (0.02–0.53) | .73 |
| Peak Troponin | .05 | ||
| <0.04 | 7 (35.0%) | 16 (36.4%) | |
| 0.04–0.07 | 4 (20.0%) | 2 (4.6%) | |
| 0.08–0.99 | 2 (10.0%) | 17 (38.6%) | |
| 1.0–9.99 | 4 (20.0%) | 5 (11.4%) | |
| ≥10 | 3 (15.0%) | 4 (9.1%) | |
| Minimum LVEF, mean ± std | 55.7 ± 9.6 | 56.5 ± 10.7 | .71 |
| LVEF <50% | 4 (21.1%) | 6 (13.6%) | .47 |
| Hospitalized | 15 (71.4%) | 32 (69.6%) | .88 |
| Hospital LOS, median (IQR) | 1.9 (1.3–2.8) | 2.1 (1.7–4.8) | .48 |
| Colchicine (within 30 days) | 19 (90.5%) | 28 (60.9%) | .01 |
| Pericardiocentesis | 2 (9.5%) | 2 (4.2%) | .58 |
| Rehospitalized for inflammatory heart disease | 1 (4.8%) | 1 (2.2%) | .53 |
| Death | 1 (4.8%) | 2 (4.2%) | 1.00 |
| Days to death | 112 days | 5 and 125 days | |
Abbreviations: CMR, cardiac magnetic resonance; EKG, electrocardiogram; IQR, interquartile range; LOS, length of stay; LVEF, left ventricle ejection fraction; std, standard deviation.
NOTE: The vaccinated pericarditis cases occurred in <60 days postlast vaccine.
P values from χ2 tests (or Fisher exact tests) and Wilcoxon rank-sum tests.
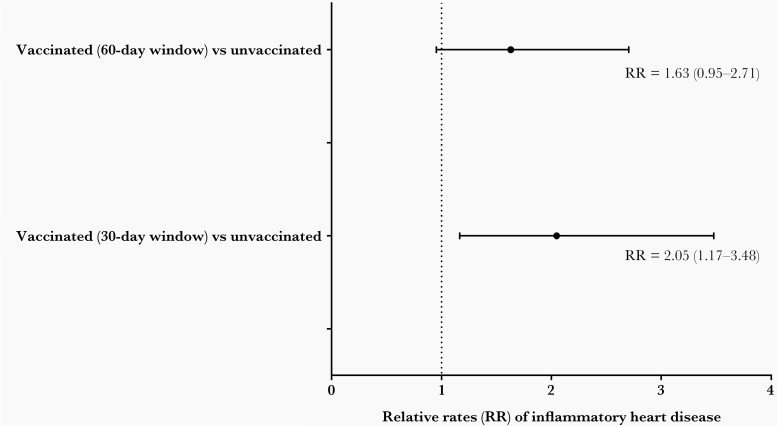
To quantitatively assess the rate of inflammatory heart disease in the vaccinated versus unvaccinated population, the disease incidence was evaluated using cases per 10 million patient-days. Within a 60-day postvaccination window, the rate of inflammatory heart disease was 3.06 per 10 million patient-days in the vaccinated patients. A total of 780 903 Intermountain patients were never vaccinated during the study period, yielding 141 724 835 patient-days. This, combined with the days outside the 60-day window for the vaccinated patients (98 951 481 days), resulted in a total of 240 676 316 unvaccinated patient-days. The rate of diagnosis of inflammatory heart disease in unvaccinated patients was 1.91 per 10 million patient-days. Thus, there was a 1.63 times higher rate of inflammatory heart disease in vaccinated patients compared to the unvaccinated individuals (P = .07). Changing to a 30-day postvaccination window resulted in a slightly higher relative rate of 2.05 for inflammatory heart disease in vaccinated patients compared to unvaccinated patients (P = .01) (Figure 2). Pericarditis or myopericarditis rates were higher in the vaccinated than in unvaccinated patients (Table 3). In contrast, although not statistically significant, vaccinated patients had a lower rate of myocarditis alone compared to the unvaccinated patients (Table 3). Finally, removal of cases with a prior COVID-19 diagnosis still resulted in higher rates of inflammatory heart disease in the vaccinated patients than the unvaccinated cases (Table 3).
Figure 2.
Relative rates of inflammatory heart disease.
Table 3.
Relative Rates and Odds Ratios for Inflammatory Heart Disease and Subgroups
| Vaccinated Cases | Unvaccinated Cases | Cohort Comparisons | Case-Crossover Comparisonsa |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Inflammatory Heart Disease Group | PostVaccine Window | n | Rateb | n | Rateb | Relative Rate (95% CI) | P Value | Odds Ratio (95% CI) | P Value |
| All inflammatory heart disease | 60-day | 21 | 3.11 | 46 | 1.91 | 1.63 (0.95–2.71) | .07 | 4.36 (2.27–8.40) | <.001 |
| 30-dayc | 18 | 3.84 | 49 | 1.87 | 2.05 (1.17–3.48) | .01 | 4.18 (2.17–8.07) | <.0001 | |
| Pericarditis and myopericarditis | 60-day | 19 | 2.81 | 30 | 1.25 | 2.26 (1.25–4.00) | .008 | 4.00 (2.07–7.74) | <.0001 |
| 30-dayc | 16 | 3.41 | 33 | 1.26 | 2.70 (1.45–4.87) | .002 | 3.91 (2.02–7.58) | <.0001 | |
| Myocarditis only | 60-day | 2 | 0.30 | 16 | 0.67 | 0.45 (0.07–1.69) | .28 | NAd | |
| 30-day | 2 | 0.43 | 16 | 0.61 | 0.70 (0.11–2.64) | .69 | NAd | ||
| Inflammatory heart disease excluding those with previous COVID | 60-day | 19 | 2.81 | 29 | 1.20 | 2.33 (1.29–4.16) | .006 | 3.73 (1.92–7.25) | <.0001 |
| 30-dayc | 16 | 3.41 | 32 | 1.22 | 2.79 (1.50–5.05) | .002 | 3.55 (1.82–6.92) | <.0001 | |
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; NA, not applicable.
The case-crossover analysis was done with up to 4 controls. Restricting to cases that had 4 controls gives similar odds ratios with <0.05 decreases and no changes in significance.
Rates are based on 10 million patient-days.
Sample size too small to calculate these.
Three pericarditis cases that occurred between 31 and 60 days postvaccination are included in the unvaccinated cases for the 30-day postvaccine window comparisons.
The case-crossover analyses showed a postvaccine odds ratio (OR) of inflammatory heart disease that was significantly higher than the unvaccinated interval for both vaccine windows (60-day window, OR = 4.36; 30-day window, OR = 4.18; both P < .0001) (Table 3). Significance in the case-crossover analyses was maintained for the subgroups (Table 3).
The results of the SCCS are provided in Supplementary Table 1. Although not statistically significant, the relative incidence for the first dose was 1.65 (95% CI, .73–3.71) and 1.97 (95% CI, .90–4.28), for the 20-day and 7-day windows, respectively. These are similar to the relative rates and odds ratios for the cohort rates and case-crossover design analyses. The second dose of the vaccine did not show an increase in risk.
DISCUSSION
In the Intermountain Healthcare population, inflammatory heart disease was rare in SARS-CoV-2 vaccine recipients. However, the risk of inflammatory heart disease was significantly higher shortly after the vaccination. Almost all the postvaccine cases were due to pericarditis and myopericarditis, with only 2 being myocarditis only. All patients with inflammatory heart disease associated with the SARS-CoV-2 vaccine were treated successfully, with only 1 death related to a severe pre-existing hematologic malignancy.
The rate of inflammatory heart disease was 2.3 per 100 000 vaccinated patients, which was only slightly higher than the rate of myocarditis reported among the active military of 1.9 [6]. However, this was 6 times the rate in the Vaccine Adverse Event Reporting System (VAERS), which was 0.41 per 100 000 [7]. This discrepancy suggests underreporting in this passive registry and highlights the need for additional research efforts into vaccine safety. However, the present study’s rate was slightly lower than reported in the Providence Health Care system for the combined rate of myocarditis and pericarditis [8]. A recent study in Israel reported that myocarditis was the leading side effect after SARS-CoV-2 vaccinations and did not find a higher risk of pericarditis [9]. However, a healthcare system in Israel reported 2.13 cases of myocarditis per 100 000 vaccinated patients [11]. In another study from Israel, the rate ratio for the comparison of the incidence of myocarditis between vaccinated and unvaccinated persons was 2.35 [12]. They did not include cases of pericarditis alone in the case counts [12]. It is interesting to note that the majority of our cases had pericarditis or myopericarditis. This indicates that pericardial involvement is a predominant manifestation of vaccine-induced inflammatory heart disease in our population.
The disparities in rates may be related to identification of cases using different methods such as CPT codes versus chart review, etc. We observed that only 40 (38%) of the 105 patients with a pericarditis diagnosis code met the criteria for probable or definitive pericarditis, and only 23 (50%) of the 46 patients with myocarditis diagnosis codes met the criteria for probable or definitive myocarditis based on Brighton Collaboration criteria.
Despite this apparent underreporting of postvaccination inflammatory events in the VAERS, this system allows the comparison of the risk for pericarditis and myocarditis after SARS-CoV-2 vaccination to vaccinations for other viruses. A greater risk of pericarditis or myocarditis follows smallpox vaccination (4 per 1000 doses), and this risk is well documented and well accepted [3, 4, 24]. There are some indications that the rate of pericarditis and myocarditis is also higher with typhoid, Japanese encephalitis, and anthrax vaccines [4]. However, based on a recent VAERS report, the risk of pericarditis and myocarditis after the SARS-CoV-2 vaccine might be higher than seen for influenza vaccinations, which were <0.1 per 1 000 000 doses [24]. Therefore, these new findings add to the growing evidence that several vaccines may cause an immune system response that results in pericardial and myocardial inflammation. The reason for the increased risk of inflammatory heart disease is not fully known. Still, a recent in vivo murine study of the SARS-CoV-2 vaccine did suggest that inadvertent intravenous injection may contribute to the rare incidence [25].
The cases in this study were mostly in patients who were male, middle-aged, and had hypertension as the leading comorbid condition. These characteristics were similar to those reported for myocarditis and pericarditis cases in other analysis after vaccinations [8, 11, 12]. Although most of the comorbidities were not statistically different between the vaccinated and unvaccinated cases in the current paper, there were potentially important clinical differences. The vaccine cases had double the rate of drug abuse reported compared to the unvaccinated cases (24% vs 9%), and they were slightly more likely to have a history of valvular disease than unvaccinated cases (29% vs 15%). Whether these differences are due to chance, given the small numbers or other reasons, is unclear and warrant further investigation. Almost half (48%) of the postvaccine-related inflammatory heart disease events occurred within the first 7 days. During this time frame, the patients are often dealing with general malaise from the vaccination. Therefore, clinicians should be aware of the symptoms and diagnostic criteria of pericarditis and myocarditis.
It is significant that post-SARS-COV-2 vaccine-related inflammatory heart disease responded well to treatment, and no one died due to the vaccination or had lasting effects postvaccine. In contrast, contracting COVID-19 can result in lengthy hospitalizations, severe organ complications, and death. The present study included 1 unvaccinated COVID-19-related myocarditis case that resulted in death. In a previous study, the risk of myocarditis after SARS-COV-2 vaccination was 3 per 100 000 patients compared with 11 per 100 000 patients for COVID-19 infections [9]. A cardiac magnetic resonance (CMR) study of 100 COVID-19 patients reported that 20% had a pericardial effusion, and 32% had myocardial late gadolinium enhancement [26]. A recent study of the English population provided direct comparisons of myocarditis and pericarditis rates after SARS-COV-2 vaccinations and after a positive COVID-19 infection [27]. In this study, there was no significant increase in myocarditis or pericarditis at day 0 for the vaccines first or second dose. In contrast, at day 0 for the COVID-19 infections, the incident rate ratios (IRRs) were 78 and 35 for myocarditis and pericarditis, respectively. After the first dose of vaccines, the 1–28 days myocarditis risk was increased for both vaccines (IRR = 2.97 for mRNA-1273 and IRR = 1.31 for BNT162b2); however, these IRRs were significantly lower than the IRR for COVID-19 infections (IRR = 9.79). It is interesting to note that the IRR for myocarditis in the 1–28 days after the second dose of mRNA-1273 (IRR = 9.84) was similar to the 1–28 days post-COVID-19 infection myocarditis rate (IRR = 9.79). Pericarditis was not significantly increased in the 1–28 days postvaccine (either first or second dose), whereas there was a significant increase 1–28 days after COVID-19 infections (IRR = 2.79). Thus, reported pericarditis and myocarditis rates post-COVID-19 infection are substantially higher than those after vaccination. In addition, SARS-CoV-2 vaccines are highly effective at preventing COVID-19 or reducing the severity of the disease [23, 28–31].
There are some limitations to this study. First, this is an observational study; therefore, no direct causation can be determined. Moreover, significant associations could be spurious or confounded by other factors not accounted for in the analyses. The misclassification of vaccinated cases due to possible incomplete capture of vaccine history could bias the effect of the vaccine towards no increase of risk. To minimize this possible bias, we did use Utah state’s vaccine registry linked to our patient’s medical records. Furthermore, although it was not the focus for the chart review, if vaccines were mentioned in the medical record the reviewer noted this. Thus, we think the bias due to under ascertainment of vaccination is minimal in the cases reviewed, and the finding of an elevated risk of inflammatory heart disease in vaccinated individuals is a conservative estimate. The case-crossover analysis was included to ensure results were consistent across study designs. However, due to the rollout of the vaccines, vaccine availability and, therefore, the possibility of exposure would vary for each patient. The impact of this on the case-crossover results is not known. However, the odds ratios and confidence intervals for these analyses overlap the relative rates from the cohort analyses and relative incident estimates from the self-control case study, thus providing similar results and consistent conclusions. The inclusion of COVID-19 cases could bias the risk toward the null; however, we chose to include these cases in the overall analysis because they represent the true background rate of inflammatory heart disease during the same time period. We believe that this allows for a more conservative estimate of risk. However, we did include an analysis where the COVID-19 cases were removed. Tissue or CMR data were not available on all patients diagnosed with inflammatory heart disease; however, when performed on the cases, CMR was always positive for inflammatory heart disease. Finally, due to the limited sample size, we were unable to provide stratified risk estimates by age, gender, or other factors.
CONCLUSIONS
In conclusion, acute inflammatory heart disease is a rare post-SAR-CoV-2 vaccination event, but the risk is elevated for the first 30-days compared to unvaccinated individuals. Most of the elevated risk is due to pericarditis or myopericarditis and not myocarditis in the absence of pericarditis. Moreover, the small risk of poor outcomes post-SARS-CoV-2 vaccine-related inflammatory heart disease is eclipsed by the risk of contracting COVID-19 and, with it, the associated, severe consequences. Nevertheless, clinicians should be informed of this risk to facilitate earlier recognition and treatment of post-SARS-CoV-2 vaccine-associated inflammatory heart disease.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
Financial support. This work was funded by the Dell Loy Hansen Heart Foundation.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. World Health Organization. WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int/. Accessed 4 December 2021.
- 2. de Meester A, Luwaert R, Chaudron JM.. Symptomatic pericarditis after influenza vaccination: report of two cases. Chest 2000; 117:1803–5. [DOI] [PubMed] [Google Scholar]
- 3. Eckart RE, Love SS, Atwood JE, et al. Incidence and follow-up of inflammatory cardiac complications after smallpox vaccination. J Am Coll Cardiol 2004; 44:201–5. [DOI] [PubMed] [Google Scholar]
- 4. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E.. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int J Cardiol 2018; 273:183–6. [DOI] [PubMed] [Google Scholar]
- 5. Mei R, Raschi E, Poluzzi E, Diemberger I, De Ponti F.. Recurrence of pericarditis after influenza vaccination: a case report and review of the literature. BMC Pharmacol Toxicol 2018; 19:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Montgomery J, Ryan M, Engler R, et al. Myocarditis following immunization with mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol 2021; 6:1202–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bozkurt B, Kamat I, Hotez PJ.. Myocarditis with COVID-19 mRNA vaccines. Circulation 2021; 144:471–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diaz GA, Parsons GT, Gering SK, Meier AR, Hutchinson IV, Robicsek A.. Myocarditis and pericarditis after vaccination for COVID-19. JAMA 2021; 326:1210–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barda N, Dagan N, Ben-Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med 2021; 385:1078–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Verma AK, Lavine KJ, Lin CY.. Myocarditis after Covid-19 mRNA vaccination. N Engl J Med 2021; 385:1332–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. D’Angelo T, Cattafi A, Carerj ML, et al. Myocarditis after SARS-CoV-2 vaccination: a vaccine-induced reaction? Can J Cardiol 2021; 37:1665–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid-19 in Israel. N Engl J Med 2021; 385:2140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Knowlton KU. Pathogenesis of SARS-CoV-2 induced cardiac injury from the perspective of the virus. J Mol Cell Cardiol 2020; 147:12–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bajaj R, Sinclair HC, Patel K, et al. Delayed-onset myocarditis following COVID-19. Lancet Respir Med 2021; 9:e32–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nicol M, Cacoub L, Baudet M, et al. Delayed acute myocarditis and COVID-19-related multisystem inflammatory syndrome. ESC Heart Fail 2020; 7:4371–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaminski A, Albus M, Mohseni M, Mirzan H, Harrison MF.. A delayed case of pericarditis following recovery from COVID-19 infection. Cureus 2021; 13:e14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lange SJ, Ritchey MD, Goodman AB, et al. Potential indirect effects of the COVID-19 pandemic on use of emergency departments for acute life-threatening conditions - United States, January-May 2020. Am J Transplant 2020; 20:2612–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Czeisler ME, Marynak K, Clarke KEN, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sexson Tejtel SK, Munoz FM, Al-Ammouri I, et al. Brighton Collaboration. Available at: https://brightoncollaboration.us/myocarditis-case-definition-update. Accessed 2 December 2021.
- 20. Delaney JA, Suissa S.. The case-crossover study design in pharmacoepidemiology. Stat Methods Med Res 2009; 18:53–65. [DOI] [PubMed] [Google Scholar]
- 21. Confavreux C, Suissa S, Saddier P, Bourdes V, Vukusic S; Vaccines in Multiple Sclerosis Study G. Vaccinations and the risk of relapse in multiple sclerosis. Vaccines in multiple sclerosis study group. N Engl J Med 2001; 344:319–26. [DOI] [PubMed] [Google Scholar]
- 22. Whitaker HJ, Farrington CP, Spiessens B, Musonda P.. Tutorial in biostatistics: the self-controlled case series method. Stat Med 2006; 25:1768–97. [DOI] [PubMed] [Google Scholar]
- 23. Petersen I, Douglas I, Whitaker H.. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ 2016; 354:i4515. [DOI] [PubMed] [Google Scholar]
- 24. Su JR, McNeil MM, Welsh KJ, et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990-2018. Vaccine 2021; 39:839–45. [DOI] [PubMed] [Google Scholar]
- 25. Li C, Chen Y, Zhao Y, et al. Intravenous injection of COVID-19 mRNA vaccine can induce acute myopericarditis in mouse model. Clin Infect Dis 2021; 73:2372–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Puntmann VO, Carerj ML, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from Coronavirus Disease 2019 (COVID-19). JAMA Cardiol 2020; 5:1265–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patone M, Mei XW, Handunnetthi L, et al. Risks of myocarditis, pericarditis, and cardiac arrhythmias associated with COVID-19 vaccination or SARS-CoV-2 infection. Nat Med 2021. doi:10.1038/s41591-021-01630-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haas EJ, Angulo FJ, McLaughlin JM, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet 2021; 397:1819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW.. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021:eabm0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Q, Qin C, Liu M, Liu J.. Effectiveness and safety of SARS-CoV-2 vaccine in real-world studies: a systematic review and meta-analysis. Infect Dis Poverty 2021; 10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenberg ES, Dorabawila V, Easton D, et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med 2022; 386:116–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.