Abstract
Over the last decade, scientists have begun to model CNS development, function, and disease in vitro using human pluripotent stem cell (hPSC)-derived organoids. Using traditional protocols, these 3D tissues are generated by combining the innate emergent properties of differentiating hPSC aggregates with a bioreactor environment that induces interstitial transport of oxygen and nutrients and an optional supportive hydrogel extracellular matrix (ECM). During extended culture, the hPSC-derived neural organoids (hNOs) obtain millimeter scale sizes with internal microscale cytoarchitectures, cellular phenotypes, and neuronal circuit behaviors mimetic of those observed in the developing brain, eye, or spinal cord. Early studies evaluated the cytoarchitectural and phenotypical character of these organoids and provided unprecedented insight into the morphogenetic processes that govern CNS development. Comparisons to human fetal tissues revealed their significant similarities and differences. While hNOs have current disease modeling applications and significant future promise, their value as anatomical and physiological models is limited because they fail to form reproducibly and recapitulate more mature in vivo features. These include biomimetic macroscale tissue morphology, positioning of morphogen signaling centers to orchestrate appropriate spatial organization and intra- and inter-connectivity of discrete tissue regions, maturation of physiologically relevant neural circuits, and formation of vascular networks that can support sustained in vitro tissue growth. To address these inadequacies scientists have begun to integrate organoid culture with bioengineering techniques and methodologies including genome editing, biomaterials, and microfabricated and microfluidic platforms that enable spatiotemporal control of cellular differentiation or the biochemical and biophysical cues that orchestrate organoid morphogenesis. This review will examine recent advances in hNO technologies and culture strategies that promote reproducible in vitro morphogenesis and greater biomimicry in structure and function.
Keywords: tissue morphology, signaling centers, morphogenetic patterning, assembloids, vascularization, circuit formation, network maturation
1. Introduction
In 1998, James Thomson and colleagues reported the isolation of human embryonic stem cells (hESCs) from the inner cell mass of human blastocysts [1]. This generated excitement for the potential of hESC-derived cellular therapeutics. Yet, utilization of human pluripotent stem cells (hPSCs) for research purposes such as the study of cell fate acquisition, tissue development and physiology, disease modeling, and drug screening have been their most impactful applications to date. A decade after Thomson’s publication, Yoshiki Sasai demonstrated that 3D cellular aggregates of both mouse and human embryonic stem cells (hESCs) could spontaneously morph into polarized cortical tissues in vitro. These cortical tissues featured microscale cytoarchitectures reminiscent of the developing neocortex [2]. With this seminal demonstration, hPSC-derived neural organoid (hNO) culture, a practice that attempts to recapitulate central nervous system (CNS) morphogenesis in vitro, emerged as a new experimental modality to study early signaling processes that are essential to brain, retina, and spinal cord development, function, and disease. Since Sasai’s publication, organoids featuring cytoarchitectures and functions similar to a variety of other CNS tissues have been demonstrated. This includes the developing human telencephalon, optic cup, diencephalon, midbrain, cerebellum, and spinal cord [3, 4, 5, 6, 7, 8].
The importance of hNOs as an experimental model cannot be understated. Classical models of CNS morphogenesis were first derived from examination of non-mammalian vertebrate organisms [9, 10]. These early characterizations informed the development of non-human mammalian models [11]. However, those models are not generalizable to humans due to non-conserved CNS features between species of different vertebrate clades [12]. For instance, the developing human neocortex features both an inner and outer subventricular zone (iSVZ, oSVZ), with oSVZ radial glial cells dividing rapidly to increase the number and potentially the diversity of cortical neural cell fates [13, 14]. The oSVZ radial glial cell phenotype even differs between humans and other primates, and it likely has a role in the evolutionary expansion of the human neocortex [15, 16]. Thus, there are inherent limitations in using other vertebrate species to draw conclusion about human CNS development and disease pathologies. The use of hNOs enables the study of some facets of human CNS development while circumventing modeling and resource limitations associated with using animal or human fetal tissue.
Human neural organoids are distinct from traditional neurospheres and 3D tissue engineered constructs consisting of cells seeded on a 3D scaffold. As described by Marti-Figueroa et al. and others, organoids are derived from hPSCs or organ progenitors and form tissues that self-organize through cell sorting and spatially restricted lineage commitment in a biomimetic manner [17, 18, 19]. They initiate as an aggregate of stem cells receptive to biophysical and biochemical inputs. Over time, extrinsic inputs and intrinsic cellular signaling propel the aggregated stem cells to morph, i.e. differentiate and self-assemble, into a more complex tissue. This morphogenic process results in a level of biomimetic cell phenotypic diversity and microscale tissues cytoarchitecture that is not achievable by neurospheres and 3D tissue engineered constructs [20, 21].
Traditionally, there are two main strategies to culture neural organoids. One strategy utilizes an unguided approach wherein minimal cues coax cellular aggregates to differentiate and self-organize [22, 23]. Despite its minimalistic appearance, this method often generates ‘mixed’ organoids where the majority of the cells acquire various neuroectodermal fates but a small portion adopt phenotypes of other ectodermal, mesodermal, or endodermal lineages. Alternatively, directed culture strategies utilize more extrinsic cues and growth factors to pattern neural organoids towards a specific CNS regional fate [4, 6, 7, 24, 25, 26, 27]. This latter strategy reduces the semi-stochastic cell fate acquisition associated with the unguided approach but simultaneously reduces the diversity of neuroectodermal-derived tissue and cell phenotypes that emerge within the neural organoid. Despite their modeling superiority to standard cell culture methodologies, hNOs grown using the previously mentioned strategies have persistent biomimicry and standardization issues that could be rectified by enabling more precise spatiotemporal control of their in vitro morphogenesis.
Here, we review bioengineering approaches and methodologies that have been used to improve hNO recapitulation of CNS morphogenesis, tissue structure, and function. The review is not meant to be exhaustive, but instead, will highlight exemplars that encapsulate the various approaches found within the hNO field. In particular, the review focuses on the integration of bioengineering techniques to help improve the reproducibility of hNO cellular composition and anatomy [22, 28, 29], formation of signaling centers to produced stereotype morphogenetic patterning [30], generation of biomimetic circuits including inter-regional connectivity [28], and vascularization to enhance maturation [31] and reduce cell death [22]. Initially, hNO morphogenesis was thought to be an inherently variable, spontaneous process. However, bioengineering strategies are now being developed and integrated to effectively control such emergent behaviors.
2. Bioengineering hNO morphology
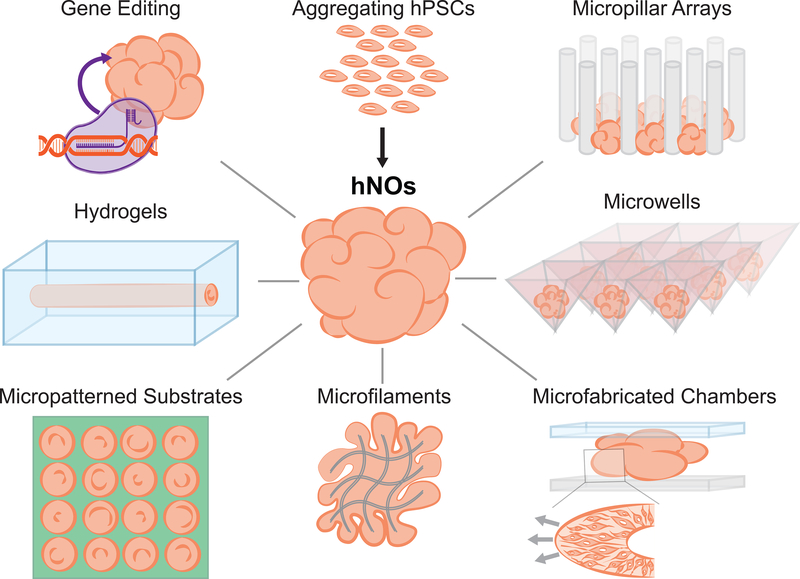
CNS morphogenesis is orchestrated in vivo by myriad, spatially and temporally discrete, biochemical and biophysical factors. Lack of control over such factors during hNO derivation is the source of organoid variability and failure, in some aspects, to recapitulate normal developmental processes. Application of biophysical constraints during the organoid derivation process is a tractable feat. Thus, researchers have begun integrating bioengineering approaches with hNO derivation protocols to more effectively control tissue morphology (Fig. 1).
Figure 1. Approaches to bioengineer human neural organoid (hNO) morphology.
Human pluripotent stem cell (hPSC) aggregate morphology can be regulated at the microscale using microfilaments, micropatterned substrates, micropillar arrays, microwells, molded or 3D printed hydrogels, and microfabricated chamber devices. Gene editing tools may also indirectly exert control over hNO morphology (e.g. folding) by regulating cell behavior (e.g. proliferation).
For over two decades, soft lithography techniques such as microcontact printing of alkanethiol self-assembled monolayers have been used to pattern cell adhesion at the microscale on optically transparent substrates [32]. Recently, Knight et al. used these techniques to investigate how a hPSC aggregate’s microscale morphology would influence subsequent neuroepithelial tissue emergence, i.e. the inception of hNO morphogenesis [33, 34]. They hypothesized that the spontaneous neurulation-like events that generate polarized neuroepithelial tissues, a.k.a. neural rosettes, within hNOs could be reproducibly induced in neurally differentiating hPSC aggregates of controlled microscale dimensions. In their experiments, hPSCs were seeded at cell densities sufficient to form a confluent monolayer on micropatterned regions, and over 5 days in neural induction media, the cells proliferated to form 3-D hemispherical tissues. They observed that hPSCs differentiated to a forebrain neuroepithelial fate and cultured on 250μm diameter circular micropatterns reproducibly formed tissues with a singularly polarized neural rosette, which is mimetic of the embryonic neural tube’s cytoarchitecture. Interestingly, reproducible formation of spinal neuroepithelial tissues with a singularly polarized rosette required smaller 150μm diameter circular micropatterns due to differences in the cells’ biomechanical properties [34]. Their observed effect of hPSC aggregate morphology on the emergence of polarized forebrain neuroepithelum was also reproduced by Haremaki et al. during derivation of micropatterned human ‘neuruloids’ [35]. In this study, reproducible and spatially stereotyped ectodermal morphogenesis, which entails emergence of neuroepithelial, neural crest, sensory placode and epidermal tissues, was achieved after forming hPSCs aggregates on 500μm diameter circular micropatterns and with exposure to bone morphogenic protein-4 (BMP4). These examples demonstrate that even in adherent cultures, inceptive tissue morphology is a biophysical characteristic that can be engineered to effectively regulate subsequent neural morphogenesis.
When deriving suspension hNOs, the initial spherical hPSC aggregate size has also been proven to be an important determinant for protocol reproducibility. For example, Zhu and colleagues engineered a micropillar array chip in which seeded hPSCs aggregate within the array’s inter-pillar regions to form spherical embryoid bodies (EBs) of uniform shape and size [36]. EB size can be varied by adjusting the micropillar array’s dimensions, and hNOs were most consistent when the micropillars were 800 μm in height with a 50 μm pitch. More commonly, researchers form cell aggregates from a specified number of hPSCs using low-attachment V-bottom well or AggreWell™ plates [37, 38]. In these platforms, cell aggregate size is indirectly controlled via cell seeding density and well dimensions. Subsequent dorsal forebrain patterning of such aggregates in suspension culture has been implemented at scale to generate cortical hNOs with neuronal subtype lamination similar to the inside-out patterning observed in vivo [24, 38, 39, 40]. At ~7 weeks post aggregation, astrogenesis can be observed within these hNOs, and at ~10-weeks post aggregation, cortical hNOs possess transcriptional profiles equivalent to the second trimester human fetal cortex [39]. Single-cell RNA-seq analysis proved that hNOs derived using such controlled conditions yielded highly reproducible cellular compositions, even across multiple hPSC lines [37]. Moreover, the diversity of constituent cell phenotypes and their transcriptional profile was similar to that of fetal human cortex at ~6 months of gestation [38]. While the described cortical hNO protocols begin with forming spheroidal cell aggregates of controlled, uniform size, they also exclude embedding the organoid within a xenogenic extracellular matrix (EMC) hydrogel during long-term culture. Hence, the protocols reproducibility may not be solely attributed to its inception via uniformly sized hPSC aggregates.
As an alternative approach to focusing on the initial spheroidal cell aggregate’s size, Lancaster and colleagues used poly(lactic-co-glycolic acid) microfilaments to seed the formation of elongated hPSCs aggregates with increased surface area-to-volume ratios [41]. They hypothesized that the increased surface-area-to-volume ratio would expose a larger fraction of the aggregate’s cells to its exterior surface resulting in more homogenous neural induction. Indeed, their elongated hPSCs aggregates preferentially differentiated into neuroepithelium with negligible presence of mesodermal and endodermal phenotypes. The resulting microfilament-engineered cerebral organoids (enCORs) displayed a significant and reproducible increase in the formation of forebrain tissues at the expense of more caudal midbrain and hindbrain tissues. The observed alteration in organoid composition due to the initial hPSC aggregate’s microfilament-guided increase in surface area-to-volume ratio further exemplifies the influence of the inceptive aggregate’s biophysical characteristics on organoid morphogenesis.
Regulating hNO morphology during latter stages of derivation protocols is challenging due to the near ubiquitous need for agitated suspension culture and the organoid’s constantly increasing size [22]. McNulty and Marti-Figueroa et al. attempted to address this issue using sacrificially molded alginate hydrogels to encapsulate hPSC aggregates and provide sustained imposition of a cylindrical morphology with defined microscale dimensions [42]. The combined rigidity and porosity of alginate hydrogels enabled stirred-tank bioreactor culture of the entire organoid-hydrogel composite, and robust neuroepithelium formation was observed over the course of 16 days. However, longer-term culture was not feasible due to the hydrogel cavity’s inability to expand with the growing neural organoid. Alternatively, Karzbrun and colleagues avoided agitated suspension culture altogether while using microfabricated culture chambers to constrain the morphology of developing hNOs [43]. This biophysical constraint induced compression forces within the organoid’s expanding neuroepithelium, and mechanical instabilities resulting from local changes in cell density as well as apical cytoskeletal contraction caused ‘wrinkling.’ The authors argue that this is a model of folding during normal human brain development [43]. However, a more convincing model of human brain folding was developed by Li et al.’s derivation of hNOs from hPSCs with PTEN loss of function mutations [44]. The loss of normal PTEN activity increased proliferation of the organoids’ neural progenitor cells, including that of HOPX+ outer radial glial cells in the sub-ventricular zone. Stresses generated by the resulting rapid tissue expansion induced folding of the emerging cortical plate [44]. Such genetic approaches for imposing biophysical factors that regulate organoid morphology can be instituted during long-term agitated suspension culture. Further integration of both genetic and previously discussed physical approaches could eventually enable constant control of biophysical factors throughout hNO morphogenesis.
3. Bioengineering symmetry breaking and morphogenetic patterning
Although inceptive hPSC aggregates are initially quite homogenous, the mere formation of a 3-D aggregate creates heterogenous cellular microenvironments that elicit spontaneous, anisotropic patterns of differentiation. This phenomenon is observed at the earliest stages of normal development. For example, cells on the surface of the 32-cell morula differentiate into trophectoderm while cells on the morula’s interior experience a different microenvironment and differentiate into an inner cell mass fate [45]. In neural organoid culture, this phenomenon induces spontaneous symmetry breaking and body axis-like patterning as powerfully exemplified by rostro-caudal, dorso-ventral, and medio-lateral tissue organizations observed in mESC-derived cerebral [46] and spinal organoids [47] and gastruloids [48]. Clearly organoids possess the potential for extensive morphogenetic patterning to generate tissues containing biomimetic body axis-like structure. The challenge is how to reproducibly engineer such morphogenetic patterning to increase the biomimicry of hNOs while maintaining consistency in each organoid’s anatomy.
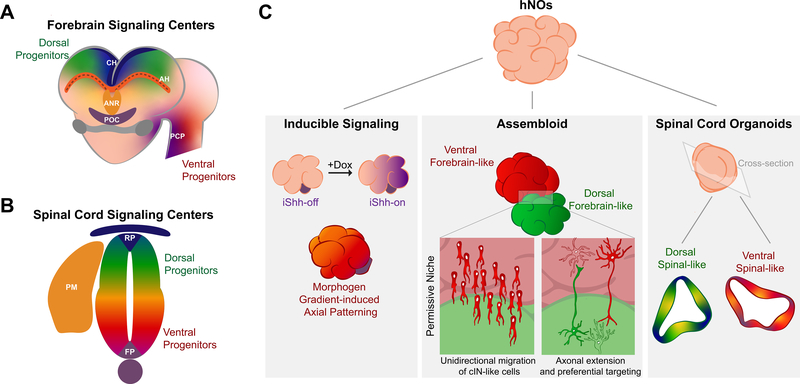
Within the developing CNS, morphogens emanating from signaling/organizing centers’ modulate local cellular differentiation to impart axial patterning. In 2013, Lancaster et al. demonstrated that a diverse spectrum of brain tissues could develop within hPSC-derived cerebral organoids (hCOs) [22]. This prompted the realization that such signaling centers could spontaneously arise within hNOs. In 2017, Renner et al. verified the presence of such signaling centers as well as coordinated axial pattering through a detailed analysis of hCO forebrain tissues [30]. In vivo, morphogenesis of the rostral telencephalon is orchestrated by fibroblast growth factors (FGFs) secreted from the rostral anterior neural ridge, Wnts and BMPs secreted from the dorsomedial cortical hem, and Wnt inhibitors, EGF, and other signaling molecules secreted from the antihem (AH; Fig. 2A) [49, 50, 51, 52]. The discrete spatial presence of these morphogenetic fields instructs dorso-ventral patterning of pallial and subpallial telencephalic domains. In their experiments, Renner et al. generated 53 hCOs with FGF-2 media supplementation during the initial embryoid body culture stage. They observed that 11 of the 53 hCOs generated both PSPB-like and cortical hem-like signaling centers. Specifically, the expression of Wnt-2b and BMP-6 overlapped with the presence of cortical hem tissues, and proper spatial abutment of choroid plexus-hem-dorsal pallium and lateral pallium-subpallial ganglionic eminence tissues was observed across serial cryosections [30, 53]. This suggests that morphogen molecules are present alongside signal center-like structures within hCOs.
Figure 2. Signaling centers and assembloids.
(A) The developing vertebrate forebrain is patterned by multiple signaling centers including the anterior neural ridge (ANR), the cortical hem (CH), the prechordal plate (PCP), and the antihem (AH, at dorsal-ventral telencephalon boundary). (B) The developing spinal cord is patterned by multiple signaling centers including the roof plate (RP), the floor plate (FP), and paraxial mesoderm (PM). It contains both dorsal and ventral progenitors. (C) Human neural organoids (hNOs) can be bioengineered as assembloids that contain inducible signaling centers (e.g. inducible Shh (iShh) domains). Moreover, interregional phenomena like unidirectional migration of cortical interneuron (cIN)-like cells or axonal extension and preferential targeting may also be observed. In spinal cord organoids, exposure to select growth factors induces spontaneous emergence of both dorsal and ventral signaling centers that morphogenetically pattern adjacent organoid regions.
Discrete signaling centers also form within hPSC-derived spinal organoids (hSOs) [8, 54]. In spinal cord development, rostro-caudal patterning of spinal neuroepithelial cell fate requires FGF, Wnt, and retinoic acid signaling [55]. Subsequently, dorso-ventral patterning of the spinal neuroepithelium is initiated by BMPs and Wnts secreted from the dorsal ectodermal roof plate and sonic hedgehog (Shh) secreted from the ventral mesodermal notochord (Fig. 2B) [56, 57]. In an analogous manner, caudalization of hPSCs to spinal neuroepithelial cell fates also requires FGF, Wnt, and retinoic acid signaling [58, 59]. In hSO culture, Ogura et al. demonstrated that roof-plate-like signaling centers can spontaneously emerge and secrete BMP4 that activates BMP and Wnt signaling within adjacent tissue regions (Fig. 2C) [8]. This induced axial patterning of the remaining dorsal progenitor domains within the hSO’s neuroepithelium. In a similar hSO approach, Duval et al. observed that application of exogenous BMP4 has the strongest dorsalizing effects at early exposure times suggesting the existence of an ideal temporal window for effective morphogenetic patterning [54]. Conversely, development of a ventral signaling center within hSO’s is not spontaneous, but requires media supplementation with a Shh small molecule agonists [8]. At specific levels of Shh activation, Ogura et al. demonstrated that floor plate-like signaling centers, which develop within the embryo’s spinal neuroepithelium immediately adjacent to the notochord and secrete Shh, were observed alongside other ventral progenitor domains in hSOs (Fig. 2C). Moreover, the floor plate and ventral progenitor domains were spatial organized in a manner consistent with morphogenetic patterning of the ventral spinal cord [8]. It remains to be demonstrated whether a complete dorso-ventral patterning axis can be established within a single hSO. This will likely require further integration of bioengineering methods.
In an effort to move from spontaneous to more instructed morphogenetic patterning of hCOs, Cederquist et al. used TALEN-mediated genome editing to engineer a doxycycline inducible Shh-expressing hPSC line (iShh) that could serve as a prechordal plate-like signaling center surrogate (Fig. 2A and C) [60]. Using round bottom microwells, a small iShh cell spheroid was formed first before subsequent aggregation as part of a larger hPSC spheroid. Upon further culture and doxycycline exposure, the iShh cells secreted Shh and acted as a signaling center to impart both dorso-ventral and rostro-caudal patterning of telencephalic and diencephalic forebrain tissues within the hCO and in a biomimetic distance dependent manner. This demonstration provides proof-of-concept that synthetic signaling centers can orchestrate hNO symmetry breaking and morphogenetic patterning. However, the use of cellular surrogates may not be ideal since their uncontrolled migration throughout the developing organoid confounds standardization of the morphogenetic process [60].
The previously discussed studies demonstrate the feasibility of standardizing long-range morphogenetic pattering of axial anatomic structure within hNOs by either inducing cellular signaling centers, i.e. via biochemical or optogenetic stimuli, or using artificial surrogates. However, reproducibility of signaling center formation and standardization of the spatial orientation and extent of axial pattern remains inconsistent from organoid-to-organoid. A major limitation to achieving more consistency is the requisite use of agitated suspension culture, which ensures sufficient interstitial nutrient and oxygen diffusion to sustain protracted organoid growth and maturation [8, 19, 39, 60]. If this limitation can be overcome, then microfluidic culture platforms can be used to reproducibly instruct hNO symmetry breaking and morphogenetic patterning. For example, Manfrin et al. recently developed a polydimethylsiloxane (PDMS)-based microfluidic device that uses simple Fickian diffusion to create stationary morphogen gradients across a micropatterned, geometrically restricted hPSC colony [61]. By applying counteracting gradients of BMP4 and Noggin– a BMP4 antagonist, they demonstrated controlled induction of differential germ layer fate acquisition across the colonies and in a direction parallel to the gradients. Integration of analogous microfluidic devices with hNO derivation protocols will be critical for scalable manufacture of tissues with reproducible and biomimetic anatomical structure.
4. Bioengineering interactions between organoid tissues of different CNS regions
Assembloids of intra- and inter-cephalic forebrain organoids have been developed as rudimentary models of inter-regional cellular migration and axonal targeting followed by synaptogenesis (Fig. 2C) [5]. The first assembloid studies characterized the fusion of dorsal and ventral telencephalic organoids [27, 62, 63]. Fusion was initiated by culturing prepatterned EBs side-by-side in a microcentrifuge tube/microwell [27, 63] or within a globule of Matrigel [62]. Within these intra-telencephalic assembloids, biomimetic emergent properties were observed such as unidirectional migration of nascent cortical interneurons from the ventral towards the dorsal organoid region. As a disease modeling exemplar, the assembloid’s ventral portion was derived from hiPSCs bearing a Timothy Syndrome (TS) gain-of-function mutation [27]. Distinct from their wild-type counterparts, cortical interneurons generated within mutant ventral tissues displayed abnormal migration patterns, which could be partially corrected using inhibitors of L-type calcium channels.
As an inter-cephanic assembloid example, Xiang et al. devised a patterning strategy to generate human thalamic-like organoids (hThOs) and fused them with human cortical organoids (hCrO) within a Matrigel droplet [5]. The thalamus emerges within the diencephalic vesicle and is an important routing and regulatory structure containing many primary and higher-order nuclei of differing functions. It is the major relay between the cortex and subcortical tissues [64]. Within hThO-CrO assembloids, Xiang et al. observed bundled axonal projections akin to reciprocal corticothalamic and thalamocortical projections. The reciprocal axonal projections preferentially targeted and synapsed with post-mitotic neuronal layers instead of progenitor regions within the assembloids, which is consistent with cortical-thalamic targeting in the developing forebrain [5, 65]. Despite obtaining a biomimetic axial organization and rudimentary circuitry, it remains to be examined as to whether assembloid fusion led to maturation of the hThO as evidenced by emergence of higher order thalamic-like nuclei. More generally, a potential limitation of the assembloid approach is the possible exclusion of critical inter-regional tissue structures generated via morphogenetic patterning during normal CNS development.
5. Bioengineering models of human circuit formation and maturation
Understanding the association between neuronal circuit function and neuropathology is a major objective of neuroscience research. Mechanisms underlying neuropsychiatric disorders such as Autism Spectrum Disorder, Epilepsy, Down Syndrome, and Schizophrenia indicate irregularities in developmental processes that govern circuit formation and network activity. These irregularities may cause imbalances in the inhibitory (GABAergic) and excitatory (glutamatergic) signaling within local networks [66, 67, 68, 69, 70, 71, 72, 73]. Despite the historical use of animal models to discern the properties of neuronal circuitry, there are significant concerns that some neuropsychiatric phenotypes are not conserved between model organisms and humans [73]. This has motivated efforts to develop hNOs as models of circuit formation and network activity.
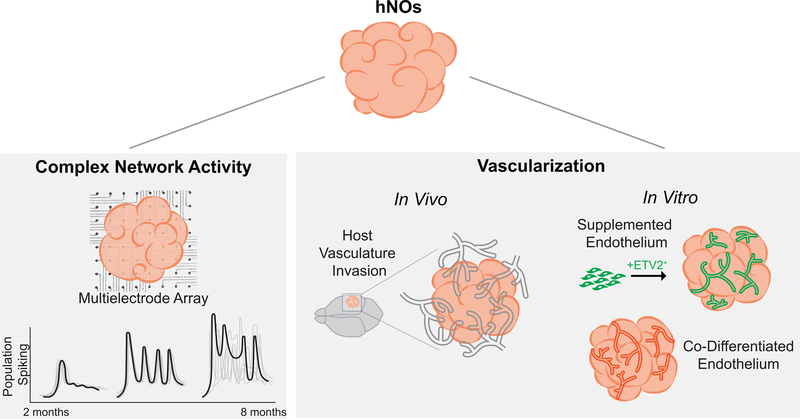
Extensive scRNA-sequencing has documented that the repertoire of fetal neuronal and glial subtypes required for biomimetic neuronal circuit formation exist within both cerebral [74] and cortical organoids [75] after ~6–8 months of culture. Substantial neuronal maturation occurs as evidenced by synapse and dendritic spine formation [74]. Moreover, both glutamatergic and GABAergic neurons contribute to the organoids’ patterns of electrical activity, which increase consistently with culture duration as measured by microelectrode arrays (Fig. 3). Analogous to human development, the spatiotemporal patterns of electrical activity evolve from discontinuous, non-correlated events (immature organoids) to periodic, synchronized bursting events (>6 mo. matured organoids). This progression indicates spontaneous formation of circuit networks within hNOs [74, 75, 76]. After 8 months of culture, retinal-like tissues within cerebral organoids contain photoreceptor-like cells that can be stimulated to fire action potentials using exogenous 530nm light [74]. After 10 months of culture, neural network firing patterns within cortical organoids demonstrate attributes of complex, oscillatory behaviors observed in human fetal electroencephalograms (EEGs). For example, human fetal EEGs at 7 months of gestation are characterized by intermittent burst of activity separated by periods of quiescence. Within these bursts, the superimposition of both low (e.g. 0.3–2Hz delta waves) and rhythmic, high frequency (e.g. 8–25Hz) activity can be detected [76]. Trujillo et al. documented an analogous superimposition within their cortical organoid’s oscillatory network activity (i.e. cross-frequency phase-amplitude coupling) using microelectrode arrays (MEA). However, as discussed by the study’s authors, multiple experimental and clinical variables like skull filtering properties, electrode placement, and potential neurological conditions limit direct EEG and MEA signal comparison [75]. Moreover, the non-stereotyped cytoarchitecture of hNOs makes it difficult to correlate the MEA’s local field potential measurements with specific, physiologically relevant CNS tissue structures or networks.
Figure 3. Circuit Network Formation and Vascularization.
Neuronal activity and maturation of circuits within hNOs can be measured using multielectrode arrays (MEAs) to detect the spatial and temporal patterns of neuronal activity. An increase in electrophysiological activity over culture duration and the presence of oscillatory waves indicates network formation and maturation. Human neural organoid (hNO) vascularization can be achieved upon invasion of host vasculature after implantation or using co-differentiated or supplemented endothelial cells in vitro.
Registration of intra-organoid circuits with a physiologically relevant network output will likely require integration of novel organoid culture and optogenetic techniques. For example, Giandomenico et al. combined the previously discussed enCOR method with air-liquid interface cerebral organoid slice culture (ALI-COs) [41, 77, 78]. This culture method reduced cell death and increased the number of cortical neuron populations as well as short and long-range axonal tract-like projections as compared to whole organoid controls [77]. Also, ALI-COs could be maintained in long-term culture to allow neuronal maturation and spontaneous network formation. Notably, the development of intracortical callosal-like projections that were responsive to endogenous axonal guidance cues and corticofugal-like projection tracts that extend away from the ALI-CO gross structure were observed. Using retrograde tracing, it was proven that CUX2+ (superficial layer) neurons accounted for the vast majority of internal callosal-like projections, whereas two-thirds of all exterior projecting neurons were CTIP2+ (deep layer) neurons. This distribution of CUX2+ and CTIP2+ neurons is mimetic of cortical and corticofugal projections in vivo, respectively. Moreover, Giandomenico et al. demonstrated that their corticofugal-like projections could synapse with explant mouse spinal cord tissue and generate contractions in mouse paraspinal muscle [77]. ALI-CO’ slice culture tissue morphology permits direct manipulation of constituents cells and circuits for facile integration of synthetic biology tools, e.g. optogenetics [79] and rabies virus-mediated monosynaptic retrograde tracing [80]. Such integration would help to further decipher and potentially modulate circuit network structure, dynamics, and maturation.
6. Bioengineering hNO vascularization
Poor gas and nutrient diffusion are a persistent issue with suspension hNO culture. It reduces cell survival within the organoids’ medulla during long-term culture [19]. Organoid culture within spinner flasks or oscillatory bioreactors improves gas and nutrients diffusion resulting in improved cell viability compared to stationary culture [22, 26]. However, agitating bioreactors still do not support sustained organoid expansion without formation of a hypoxic medulla [77]. The desire to expand hNO growth in the absence of hypoxia as well as include a critical factor of the neurogenic niche [81] and regulator of CNS physiology and disease [82] has motivated development of approaches to vascularize hNOs [79, 83, 84, 85].
Bioengineering approaches to achieve hNO vascularization have varied from co-derivation of cerebral organoids and blood vessels [83] to direct organoid implantation into rodents (Fig. 3) [79]. Ham et al. demonstrated that VEGF could be included within hCO derivation protocols to induce co-differentiate of endothelial-like cells (ECs) without inhibiting neural morphogenesis [83]. The ECs formed tubules within the organoid and surrounding Matrigel hydrogel and expressed blood-brain barrier (BBB) tight junction protein markers. Also, further supplementation with Wnt7a induced co-derivation of pericyte-like cells within the hCOs [83]. As an analogous co-derivation approach, Cakir et al. engineered doxycycline-inducible ETV2 expression into a hESC line [84]. Using an EB composed of 20% genome edited cells, ETV2-induced reprogramming could be used to generate hCOs containing ECs that formed perfusable vascular structures in vitro and in vivo. As a third approach, Pham et al. re-embedded day 34 hNOs in Matrigel containing iPSC-derived endothelial cells (ECs) [85]. After 20 days of in vitro culture and 2 weeks of implantation in an immunodeficient NSG mouse, human EC-based tubular structures penetrated the hNO’s core sustaining transplant viability in vivo compared to non-EC containing organoids [85]. The fourth approach entailed implantation of 40–50 day hCOs directly into the retrosplenial cortex of NOD-SCID mice [79]. In the absence of hPSC-derived ECs, host vasculature possessing BBB-like features invaded and perfused the organoids supporting long-term viability. Moreover, the formation of vascular structures enhanced neural differentiation as day 50 implanted hNOs exhibited greater numbers of NeuN+ cells compared to day 102, stage-matched, non-implanted controls. Interestingly, synaptic connectivity between the vascularized hCO and host brain tissues was observed via electrophysiological analysis and immunostaining [79]. Vascularization of hNOs is clearly feasible and several transplantation approaches can yield functional vascular networks with blood flow [79, 84]. However, reproducibility of the vascularization time course, vascular tree structure, and homogeneity of spatial distribution within the organoid would be improved by further incorporation of sacrificial molding [17, 42, 86] or 3D printing [87, 88] techniques.
7. Conclusion
The hNO experimental platform holds tremendous promise for modeling CNS development, physiology, and disease. However, as in all product development pipelines, standardization of hNO derivation protocols is critical to reproducibly bioengineer CNS morphogenesis ex vivo to create tissues with biomimetic cellular composition, cytoarchitecture, and anatomy. As reviewed previously [17], numerous tissue engineering methodologies can be applied to hNO culture to precisely control biochemical and biophysical microenvironmental facets. It is only via such integration that we will be able to decipher the biological rules and implement the necessary spatiotemporal control to fully bioengineer CNS development ex vivo. Here, we have provided exemplars of bioengineering approaches to control hNO tissue morphology, axial patterning via morphogens emanating from signaling centers or assembly of regionally distinct tissues, circuit formation and maturation, and vascularization. While these studies advance the field and respond to continued pressure to create ever more complex organoid systems, it is equally important for the practical application of hNO science to synthesize their collective biological findings and use this new knowledge to develop bioreactor systems and protocols capable of standardizing comprehensive hNO manufacture. After all, “organoid biology is developmental biology” [18]. If we can understand the rules of human CNS development, then we should be able to precisely bioengineer its morphogenesis.
Supplementary Material
Acknowledgments
This work is supported by EPA-G2013-STAR-L1 grant #83573701, NSF CAREER award #1651646, and NIH R33NS082618 and UG3TR003150 grants awarded to RSA. NJF and RSA equally contributed to the manuscript’s conceptualization and composition. NI aided in figure preparation and manuscript proofreading and is supported by NIH F32NS106740.
References
- [1].Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- [2].Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, et al. (2008). Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 3, 519–532. [DOI] [PubMed] [Google Scholar]
- [3].Mariani J, Simonini MV, Palejev D, Tomasini L, Coppola G, Szekely AM, et al. (2012). Modeling human cortical development in vitro using induced pluripotent stem cells. P. Natl. Acad. Sci. USA 109, 12770–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nakano T, Ando S, Takata N, Kawada M, Muguruma K, Sekiguchi K, et al. (2012). Self-Formation of Optic Cups and Storable Stratified Neural Retina from Human ESCs. Cell Stem Cell 10, 771–785. [DOI] [PubMed] [Google Scholar]
- [5].Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim K-Y, Sun P, et al. (2019). hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 24, 487–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Jo J, Xiao Y, Sun AX, Cukuroglu E, Tran H-D, Göke J, et al. (2016). Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem Cell 19, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y (2015). Self-Organization of Polarized Cerebellar Tissue in 3D Culture of Human Pluripotent Stem Cells. Cell Rep. 10, 537–550. [DOI] [PubMed] [Google Scholar]
- [8].Ogura T, Sakaguchi H, Miyamoto S, Takahashi J (2018). Three-dimensional induction of dorsal, intermediate and ventral spinal cord tissues from human pluripotent stem cells. Development 145, dev162214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Herrick CJ (1910). The morphology of the forebrain in Amphibia and Reptilia. J. Comp. Neurol 20, 413–547. [Google Scholar]
- [10].Orr HJ (1887). Contribution to the embryology of the lizard. J Morphol 1, 331–372. [Google Scholar]
- [11].Puelles L, Harrison M, Paxinos G, Watson C (2013). A developmental ontology for the mammalian brain based on the prosomeric model. Trends Neurosci 36, 570–578. [DOI] [PubMed] [Google Scholar]
- [12].Herculano-Houzel S (2011). Not all brains are made the same: new views on brain scaling in evolution. Brain Behav. Evolut 78, 22–36. [DOI] [PubMed] [Google Scholar]
- [13].Hansen DV, Lui JH, Parker PRL, Kriegstein AR (2010). Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 464, 554–561. [DOI] [PubMed] [Google Scholar]
- [14].Lui JH, Hansen DV, and Kriegstein AR (2011). Development and Evolution of the Human Neocortex. Cell 146, 18–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kriegstein A, Noctor S, Martínez-Cerdeño V (2006). Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci 7, 883–890. [DOI] [PubMed] [Google Scholar]
- [16].Pollen AA, Bhaduri A, Andrews MG, Nowakowski TJ, Meyerson OS, Mostajo-Radji MA, et al. (2019). Establishing Cerebral Organoids as Models of Human-Specific Brain Evolution. Cell 176, 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marti-Figueroa CR, and Ashton RS (2017). The case for applying tissue engineering methodologies to instruct human organoid morphogenesis. Acta Biomater 54, 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Huch M, Knoblich JA, Lutolf MP, Martinez Arias A (2017). The hope and the hype of organoid research. Development 144, 938–941. [DOI] [PubMed] [Google Scholar]
- [19].Lancaster MA, & Knoblich JA (2014). Generation of cerebral organoids from human pluripotent stem cells. Nat. Protoc 9, 2329–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Elson EL, & Genin GM (2016). Tissue constructs: platforms for basic research and drug discovery. Interface Focus 6, 20150095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pamies D (2017). A human brain microphysiological system derived from induced pluripotent stem cells to study neurological diseases and toxicity. Altex-Altern. Anim. Ex 34, 362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lancaster MA, Renner M, Martin C-A, Wenzel D, Bicknell LS, Hurles ME, et al. (2013). Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lindborg BA, Brekke JH, Vegoe AL, Ulrich CB, Haider KT, Subramaniam S, et al. (2016). Rapid Induction of Cerebral Organoids From Human Induced Pluripotent Stem Cells Using a Chemically Defined Hydrogel and Defined Cell Culture Medium: Cerebral Organoid Induction Using Defined Reagents. Stem Cell Transl. Med 5, 970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, et al. (2013). Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell–derived neocortex. P. Natl. Acad. Sci. USA 110, 20284–20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, et al. (2015). Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived dorsomedial telencephalic tissue. Nat. Commun 6, 8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. (2016). Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 165, 1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kelava I, & Lancaster MA. (2016). Dishing out mini-brains: Current progress and future prospects in brain organoid research. Dev. Biol 420, 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Brassard JA, & Lutolf MP (2019). Engineering Stem Cell Self-organization to Build Better Organoids. Cell Stem Cell 24, 860–876. [DOI] [PubMed] [Google Scholar]
- [30].Renner M, Lancaster MA, Bian S, Choi H, Ku T, Peer A, et al. (2017). Self-Organized developmental patterning and differentiation in cerebral organoids. Embo. J 36, 1316–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pașca SP. (2018). The rise of three-dimensional human brain cultures. Nature 553, 437–445. [DOI] [PubMed] [Google Scholar]
- [32].Mrksich M, Dike LE, Tien J, Ingber DE, Whitesides GM (1997). Using microcontact printing to pattern the attachment of mammalian cells to self-assembled monolayers of alkanethiolates on transparent films of gold and silver. Exp. Cell Res 235, 305–313. [DOI] [PubMed] [Google Scholar]
- [33].Knight GT, Sha J, and Ashton RS. (2015). Micropatterned, clickable culture substrates enable in situ spatiotemporal control of human PSC-derived neural tissue morphology. Chem. Commun 51, 5238–5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Knight GT, Lundin BF, Iyer N, Ashton LM, Sethares WA, Willett RM, et al. (2018). Engineering induction of singular neural rosette emergence within hPSC-derived tissues. ELife 7, e37549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Haremaki T, Metzger JJ, Rito T, Ozair MZ, Etoc F, Brivanlou AH (2019). Self-organizing neuruloids model developmental aspects of Huntington’s disease in the ectodermal compartment. Nat. Biotechnol. 37, 1198–1208. [DOI] [PubMed] [Google Scholar]
- [36].Zhu Y, Wang L, Yu H, Yin F, Wang Y, Liu H, et al. (2017). In situ generation of human brain organoids on a micropillar array. Lab Chip 17, 2941–2950. [DOI] [PubMed] [Google Scholar]
- [37].Yoon S-J, Elahi LS, Pașca AM, Marton RM, Gordon A, Revah O, et al. (2019). Reliability of human cortical organoid generation. Nat. Methods 16, 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, et al. (2019). Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 570, 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Paşca AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, et al. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Rakic P (2009). Evolution of the neocortex: a perspective from developmental biology. Nat. Rev. Neurosci 10, 724–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, et al. (2017). Guided self-organization and cortical plate formation in human brain organoids. Nat. Biotechn 35, 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].McNulty JD, Marti-Figueroa C, Seipel F, Plantz JZ, Ellingham T, Duddleston LJL, et al. (2019). Micro-injection molded, poly(vinyl alcohol)-calcium salt templates for precise customization of 3D hydrogel internal architecture. Acta Biomater 95, 258–268. [DOI] [PubMed] [Google Scholar]
- [43].Karzbrun E, Kshirsagar A, Cohen SR, Hanna JH, Reiner O (2018). Human brain organoids on a chip reveal the physics of folding. Nat. Phys 14, 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Li Y, Muffat J, Omer A, Bosch I, Lancaster MA, Sur M, et al. (2016). Induction of Expansion and Folding in Human Cerebral Organoids. Cell Stem Cell. 20, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Guo G, Huss M, Tong GQ, Wang C, Sun L Li., Clarke ND, et al. (2010). Resolution of Cell Fate Decisions Revealed by Single-Cell Gene Expression Analysis from Zygote to Blastocyst. Dev. Cell 18, 675–685. [DOI] [PubMed] [Google Scholar]
- [46].Takata N, Sakakura E, Eiraku M, Kasukawa T, Sasai Y (2017). Self-patterning of rostral-caudal neuroectoderm requires dual role of Fgf signaling for localized Wnt antagonism. Nat. Commun 8, 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Meinhardt A, Eberle D, Tazaki A, Ranga A, Niesche M, Wilsch-Bräuninger M, et al. (2014). 3D Reconstitution of the Patterned Neural Tube from Embryonic Stem Cells. Stem Cell Rep 3, 987–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Beccari L, Moris N, Girgin M, Turner DA, Baillie-Johnson P, Cossy AC, et al. (2018). Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature 562, 272–276. [DOI] [PubMed] [Google Scholar]
- [49].Grove EA, Tole S, Limon J, Yip L, Ragsdale CW (1998). The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development 125, 2315–2325. [DOI] [PubMed] [Google Scholar]
- [50].Fukuchi-Shimogori T, & Grove EA. (2001). Neocortex patterning by the secreted signaling molecule FGF8. Science 294, 1071–1074. [DOI] [PubMed] [Google Scholar]
- [51].Hébert JM, Mishina Y, and McConnell SK (2002). BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron 35, 1029–1041. [DOI] [PubMed] [Google Scholar]
- [52].Assimacopoulos S, Grove EA, and Ragsdale CW (2003). Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 23, 6399–6403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Chizhikov VV, Lindgren AG, Mishima Y, Roberts RW, Aldinger KA, Miesegaes GR, et al. (2010). Lmx1a regulates fates and location of cells originating from the cerebellar rhombic lip and telencephalic cortical hem. P. Natl. Acad. Sci. USA 107, 10725–10730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Duval N, Vaslin C, Barata T, Frarma Y, Contremoulins V, Baudin X, et al. (2019). BMP4 patterns Smad activity and generates stereotyped cell fate organisation in spinal organoids. Development dev 175430. [DOI] [PubMed] [Google Scholar]
- [55].Olivera-Martinez I & Storey KG. (2007). Wnt signals provide a timing mechanism for the FGF-retinoid differentiation switch during vertebrate body axis extension. Development 134, 2125–2135. [DOI] [PubMed] [Google Scholar]
- [56].Briscoe J & Ericson J (1999). The specification of neuronal identity by graded Sonic Hedgehog signalling. Semin. Cell Dev. Biol 10, 353–362. [DOI] [PubMed] [Google Scholar]
- [57].Zechner D, Müller T, Wende H, Walther I, Taketo MM, Crenshaw EB 3rd., et al. (2007). Bmp and Wnt/beta-catenin signals control expression of the transcription factor Olig3 and the specification of spinal cord neurons. Dev. Biol 303, 181–190. [DOI] [PubMed] [Google Scholar]
- [58].Gouti M, Tsakiridis A, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, et al. (2014). In vitro generation of neuromesodermal progenitors reveals distinct roles for wnt signalling in the specification of spinal cord and paraxial mesoderm identity. PLoS. Biol 12, e1001937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, et al. (2015). Deterministic HOX Patterning in Human Pluripotent Stem Cell-Derived Neuroectoderm. Stem Cell Rep 4, 632–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Cederquist GY, Asciolla JJ, Tchieu J, Walsh RM, Cornacchia D, Resh MD, et al. (2019). Specification of positional identity in forebrain organoids. Nat. Biotechn. 37, 436–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Manfrin A, Tabata Y, Paquet ER, Vuaridel AR, Rivest FR, Naef F, et al. (2019). Engineered signaling centers for the spatially controlled patterning of human pluripotent stem cells. Nat. Methods 16, 640–648. [DOI] [PubMed] [Google Scholar]
- [62].Bagley JA, Reumann D, Bian S, Levi-Strauss J, Knoblich JA (2017). Fused cerebral organoids model interactions between brain regions. Nat Methods 14, 743–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Xiang Y, Tanaka Y, Patterson B, Kang YJ, Govindaiah G, Roselaar N et al. (2017). Fusion of regionally specified hPSC-derived organoids models human brain development and interneuron migration. Cell Stem Cell 21 (3), 383–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Guillery RW, & Sherman SM (2002). Thalamic Relay Functions and Their Role in Corticocortical Communication: Generalizations from the Visual System. Neuron 33, 163–175. [DOI] [PubMed] [Google Scholar]
- [65].López-Bendito G, & Molnár Z (2003). Thalamocortical development: how are we going to get there? Nat. Rev. Neurosci 4, 276–289. [DOI] [PubMed] [Google Scholar]
- [66].Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW (2003). Abnormal neural synchrony in schizophrenia. J. Neurosci. 23, 7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Lewis DA, Hashimoto T, and Volk DW (2005). Cortical inhibitory neurons and schizophrenia. Nat. Rev. Neurosci 6, 312–324. [DOI] [PubMed] [Google Scholar]
- [68].Bartos M, Vida I, and Jonas P (2007). Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat. Rev. Neurosci 8, 45–56. [DOI] [PubMed] [Google Scholar]
- [69].Huo H-Q, Qu Z-Y, Yuan F, Ma L, Yao L, Xu M, et al. (2018). Modeling Down Syndrome with Patient iPSCs Reveals Cellular and Migration Deficits of GABAergic Neurons. Stem Cell Rep 10, 1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Brooks-Kayal A (2010). Epilepsy and autism spectrum disorders: Are there common developmental mechanisms? Brain Dev-JPN 32, 731–738. [DOI] [PubMed] [Google Scholar]
- [71].Zikopoulos B, & Barbas H (2013). Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci 7, 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].DeFelipe J (1999). Chandelier cells and epilepsy. Brain. 122, 1807–22. [DOI] [PubMed] [Google Scholar]
- [73].Nestler EJ, & Hyman SE (2010). Animal models of neuropsychiatric disorders. Nat. Neurosci 13, 1161–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Quadrato G, Nguyen T, Macosko EZ, Sherwood JL, Min Yang S, Berger DR, et al. (2017). Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545, 48–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Trujillo CA, Gao R, Negraes PD, Gu J, Buchanan J, Preissl S, et al. (2019). Complex Oscillatory Waves Emerging from Cortical Organoids Model Early Human Brain Network Development. Cell Stem Cell 25, 558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Khazipov R & Luhmann HJ. (2006). Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci 29, 414–418. [DOI] [PubMed] [Google Scholar]
- [77].Giandomenico SL, Mierau SB, Gibbons GM, Wenger LMD, Masullo L, Sit T, et al. (2019). Cerebral organoids at the air–liquid interface generate diverse nerve tracts with functional output. Nat. Neurosci. 22, 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Daza RAM, Englund C, Hevner RF (2007). Organotypic slice culture of embryonic brain tissue. CSH Protoc t4914. doi: 10.1101/pdb.prot4914. [DOI] [PubMed] [Google Scholar]
- [79].Mansour AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. (2018). An in vivo model of functional and vascularized human brain organoids. Nat. Biotechn.. 36, 432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Marshel JH, Mori T, Nielsen KJ, Callaway EM. (2010). Targeting single neuronal networks for gene expression and cell labeling in vivo. Neuron 67, 562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Palmer TD, Willhoite AR, and Gage FH (2000). Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol 425, 479–494. [DOI] [PubMed] [Google Scholar]
- [82].Sweeney MD, Kisler K, Montagne A, Toga AW, Zlokovic BV (2018). The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci 21, 1318–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ham O, Jin YB, Kim J, Lee M-O (2019). Blood vessel formation in cerebral organoids formed from human embryonic stem cells. Biochem. Bioph. Res. Co. Epub doi: 10.1016/j.bbrc.2019.10.079 [DOI] [PubMed] [Google Scholar]
- [84].Cakir B, Xiang Y, Tanaka Y, Kural MH, Maxime P, Kang YJ, et al. (2019). Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16, 1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. (2018). Generation of human vascularized brain organoids. Neuroreport 29, 588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Miller JS, Stevens KR, Yang MT, Baker BM, Nguyen DH, Cohen DM, et al. (2012). Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 11, 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue HJ, et al. (2015). Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv 1, e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Grigoryan B, Paulsen SJ, Corbett DC, Sazer DW, Fortin CL, Zaita AJ, et al. (2019). Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 364, 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.