Abstract
BACKGROUND
Because of the progressive nature of twin-to-twin transfusion syndrome, difficulties in healthcare access during the COVID-19 pandemic may lead to delayed diagnosis and referral to fetal surgery centers, which may have repercussions on outcomes.
OBJECTIVE
This study aimed to assess the clinical impact of the COVID-19 pandemic on pregnancies complicated with twin-to-twin transfusion syndrome.
STUDY DESIGN
A retrospective cohort study of consecutive monochorionic diamniotic twin pregnancies complicated with twin-to-twin transfusion syndrome evaluated in our national referral fetal surgery center at Queretaro, Mexico, for possible surgical fetoscopy was conducted. Maternal-fetal characteristics and perinatal outcomes of cases evaluated during the first year of the World Health Organization's COVID-19 pandemic declaration (March 11, 2020 to March 10, 2021) were retrospectively compared with outcomes of cases evaluated during the same period in the previous year (March 11, 2019 to March 10, 2020).
RESULTS
Overall, 109 consecutive twin-to-twin transfusion syndrome cases were evaluated during the 2-year study period, 54 during the COVID-19 pandemic and 55 in the previous year. In the former group, a higher proportion of cases with fetal surveillance interval longer than 2 weeks (70.4% vs 47.3%; P=.01); twin-to-twin transfusion syndrome complications precluding laser therapy, such as intrauterine fetal demise, preterm rupture of membranes, or cervical dilatation with prolapsed amniotic membranes (18.5% vs 1.8%; P<.01); advanced twin-to-twin transfusion syndrome (53.7% vs 36.4%; P=.07); preoperative short cervix (25.9% vs 10.9%; P<.05); and lower overall perinatal survival (56.9% vs 80.0% [P=.01; at least 1 twin] and 39.2% vs 56.4% [P=.08; both twins], respectively) were observed. A significantly lower number of cases were selected for fetoscopic laser therapy during the pandemic (75.9% vs 92.7%; P=.01), with similar postoperative outcomes seen in both study periods.
CONCLUSION
In pregnancies with twin-to-twin transfusion syndrome, the COVID-19 pandemic has shown an adverse impact involving suboptimal fetal surveillance, advanced stages at diagnosis, poorer survival rates, and higher number of complications that preclude fetoscopic laser therapy.
Key words: coronavirus, COVID-19, fetal surgery, fetoscopy, pandemic, placental laser, pregnancy, twin-to-twin transfusion syndrome
AJOG Global Reports at a Glance.
Why was this study conducted?
This study aimed to demonstrate that twin-to-twin transfusion syndrome (TTTS) must be conceived as an emergency diagnosis and as such therapy must not be delayed, regardless of the COVID-19 pandemic.
Key findings
During the first year of the COVID-19 pandemic, compared with the prepandemic period, pregnancies complicated with TTTS had worse outcomes, such as higher rates of advanced disease, short cervical length, and complications that hinder fetal therapy, that led to lower overall survival rates.
What does this add to what is known?
Our study contributes information concerning the importance of stringent prenatal monitoring in monochorionic twin pregnancies even during a pandemic. During this period, we demonstrated a longer prenatal surveillance interval than suggested, and this could be a reason that explains the higher number of complications, inoperable cases, and overall lower survival rates.
Introduction
Twin-to-twin transfusion syndrome (TTTS) is a disease exclusive of monochorionic (MC) twin pregnancies associated with a mortality rate of approximately 80% to 100% with expectant management.1,2 The pathophysiological mechanism for the development of TTTS is related to the presence of placental vascular anastomoses that allow an unbalanced progressive volume transfusion between both twins. The first line of treatment has long been fetoscopic coagulation of placental anastomoses, a procedure that is associated with 90% survival rate of at least 1 twin and 70% to 80% of dual survival.3, 4, 5, 6 Because up to 20% of the MC twin pregnancies may develop TTTS, fortnightly prenatal follow-up has been recommended as the optimal surveillance protocol for timely diagnosis.7 In addition, opportune referral to fetal surgery centers is essential to improve prognosis, as delayed therapy may lead to advanced stages, intrauterine fetal demise, preterm premature rupture of membranes (PPROM), or preterm delivery.8, 9, 10, 11
On March 11, 2020, COVID-19 was declared a global pandemic by the World Health Organization (WHO),12 which was associated with elevated mortality mainly secondary to pneumonia. Different risk populations were reported, including pregnant women, which showed a 2-fold higher risk of pneumonia, admission to the intensive care unit, and death than nonpregnant women at similar ages, especially in low- and middle-income countries.13, 14, 15 However, the burden of disease of COVID-19 is not only limited to those pregnant women with SARS-CoV-2 infection; as during this period, excess in the maternal mortality rate, stillbirths, and ruptured ectopic pregnancies have been demonstrated.16
In Mexico, the first case of COVID-19 was diagnosed on February 27, 2020, and thereafter, the government recommended lockdown, and some international medical societies suggested decreasing the number of antenatal visits to prevent infection and spread of the virus in this susceptible population.17,18 The impact of suboptimal antenatal follow-up during a pandemic has not been previously assessed in the population of MC twin pregnancies at risk of TTTS.
This study aimed to assess the maternal-fetal characteristics and survival outcomes of monochorionic diamniotic (MCDA) twin pregnancies complicated with TTTS during the first year of the COVID-19 pandemic and to compare outcomes with those cases evaluated during the same period in the previous year.
Material and Methods
Subjects
Between March 11, 2019 and March 10, 2021, a retrospective cohort study of consecutive MCDA twin pregnancies with confirmed TTTS that were evaluated in a single tertiary fetal surgery center at Medicina Fetal México in Queretaro, Mexico (the main national referral center for fetal surgery), was conducted. The inclusion criteria for fetoscopy were MCDA pregnancies with TTTS defined according to the Eurofoetus criteria,19 which include polyhydramnios in the recipient twin with the deepest vertical amniotic fluid pocket of at least 8.0 cm before 20 weeks of gestation and 10.0 cm thereafter, along with oligohydramnios in the donor twin with the deepest vertical amniotic fluid pocket of <2.0 cm. TTTS was classified according to the severity staging system proposed by Quintero et al.20 Early-stage disease was defined as those TTTS cases in stage I or II, whereas advanced-stage disease was defined as those TTTS cases in stage III or V.
The cervical length was measured immediately before fetal intervention in the operating room by transvaginal ultrasound according to the technique described by Burger et al.21 A short cervix was defined as that <25 mm.
Maternal-fetal characteristics and perinatal outcomes of cases evaluated during the first year of the WHO's COVID-19 pandemic declaration (March 11, 2020 to March 10, 2021) were retrospectively compared with outcomes of cases evaluated during the same period in the previous year (March 11, 2019 to March 10, 2020).
The exclusion criteria for fetoscopy were (1) PPROM, (2) cervical dilatation with intact amniotic membranes, (3) congenital structural malformations, and (4) intrauterine fetal demise. All ultrasound examinations were performed with either a Voluson E8 Expert BT12 (General Electric Healthcare, Zipf, Austria) or a Voluson E10 BT18" to Voluson E10 BT18 (General Electric Healthcare, Zipf, Austria) equipment with a 6- to 2-MHz linear curved array transducer by the same medical group within both periods. The surgical protocol was approved by the hospital ethics committee, and patients provided written informed consent.
During the COVID-19 pandemic, our fetal surgery group developed a protocol, including a COVID-19 screening test by nasopharyngeal swabbing for quantitative polymerase chain reaction in symptomatic patients. In asymptomatic pregnant patients and as recommended by international societies, in the setting of emergency interventions performed under local anesthesia, such as laser therapy for TTTS, procedures were performed without a screening test.22,23 Considering the absence of viral testing and the poor negative predictive value of the COVID-19 test,24 patients were managed as suspected COVID-19 carriers, and therefore, all patients, visitors, and healthcare workers had to wear surgical masks as suggested by the WHO.
Fetoscopic therapy
Selective laser coagulation of the placental anastomoses on the chorionic plate was performed by the same fetal surgery team in both study periods as previously described25 and involved percutaneous insertion of 1.2- to 3.0-mm semirigid endoscopes through operative fetoscopic sheaths and trocars with an external diameter of 8F to 10F under ultrasound guidance. Intertwin anastomoses were identified and coagulated systematically along the intertwin vascular equator with a nontouch technique using a diode laser with a 600-nm fiber at power settings of 35 W. To conclude the fetoscopic procedure, amniodrainage was performed until the deepest vertical pocket was <8 cm on ultrasound examination. The use of N95 or air-purifying respirator was not mandatory as all fetoscopies were performed under maternal local anesthesia and therefore considered as low-risk aerosol-generating procedures. According to our protocol, the number of persons in the operating room was restricted to 5 during the pandemic (the pregnant women, the fetoscopic surgeon, a surgical assistant, an ultrasonographer, and a nurse). Prophylactic tocolysis with 100 mg indomethacin was administrated, and amniodrainage was completed within 24 hours. Patients were usually discharged within 24 to 72 hours.
After initial follow-up at the fetal surgery center, patients were referred back to their local obstetrician or maternal-fetal medicine specialist for further follow-up. Information regarding perinatal outcomes was collected in all cases, including PPROM, intrauterine fetal demise of 1 or both twins, gestational age at delivery, and neonatal death.
Statistical analysis
The Student t test and Pearson chi-squared test were used to compare the quantitative and qualitative data within the study group, respectively. All tests were 2-tailed, and a probability value of <.05 was considered statistically significant. Statistical calculations were performed using the Statistical Package for the Social Sciences software (version 25.0; SPSS Inc, Chicago, IL).
Results
A total of 109 consecutive MCDA twin pregnancies with TTTS were referred to our fetal surgery center during the 2-year study period (55 patients in 2019 [pre-COVID-19 pandemic] and 54 patients in 2020 [during the first pandemic year]). According to Quintero's staging system, 28 (25.7%), 32 (29.4%), 26 (23.9%), 18 (16.5%), and 5 (4.6%) cases were classified as stage I, II, III, IV, and V, respectively.
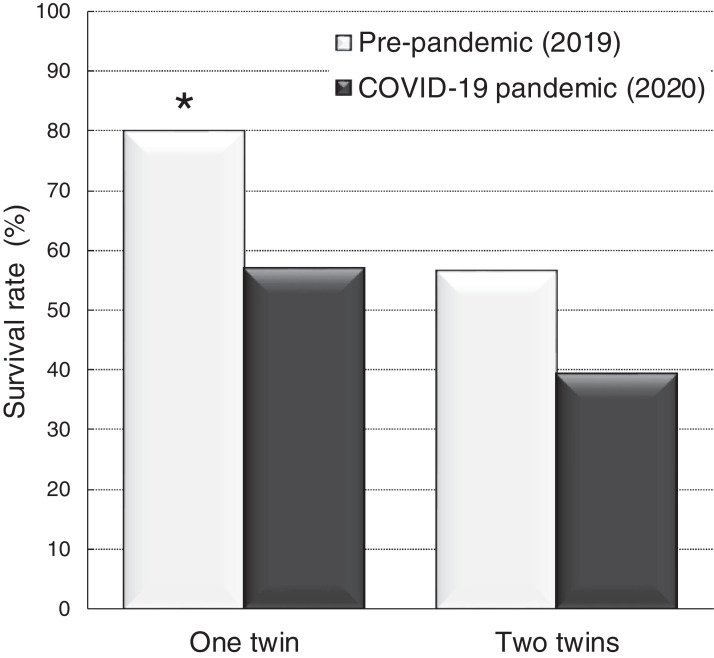
Table 1 displays the data on the baseline maternal-fetal clinical characteristics in TTTS pregnancies evaluated before and during the pandemic. A similar number of cases refused fetoscopic therapy in both study periods (7.4% vs 5.5%; P=.68). During the COVID-19 pandemic, compared with the previous year, the proportion of cases with ultrasound surveillance interval longer than 2 weeks was significantly higher (70.4% vs 47.3%; P=.01) and a considerably higher proportion of cases showed TTTS complications precluding laser therapy, such as intrauterine fetal demise, PPROM, or cervical dilatation with intact amniotic membranes (18.5% vs 3.6%; P=.01), advanced TTTS stages (53.7% vs 36.4%; P=.07), and higher frequency of short cervix at diagnosis (25.9% vs 10.9%; P<.05). Furthermore, lower overall perinatal survival (56.9% vs 80.0% [P=.01; at least 1 twin] and 39.2% vs 56.4% [P=.08; both twins]) were observed during the COVID-19 pandemic (Figure 1).
Table 1.
Maternal and neonatal clinical characteristics for twin-to-twin transfusion syndrome cases evaluated during the first year of the COVID-19 pandemic (2020) and the same period of the previous year
| Characteristic | 2019 (n=55) | 2020 (n=54) | P valuea |
|---|---|---|---|
| Maternal age (y) | 29.9 (6.0) | 29.3 (5.5) | .64 |
| BMI (kg/m2) | 26.0 (5.4) | 27.9 (6.4) | .11 |
| Primiparity | 38.2 | 42.6 | .64 |
| Fortnightly antenatal visits | 52.7 | 29.6 | .01 |
| TTTS staging | |||
| I | 30.9 | 20.4 | .21 |
| II | 32.7 | 25.9 | .44 |
| III | 20.0 | 27.8 | .34 |
| IV | 14.5 | 18.5 | .58 |
| V | 1.8 | 7.4 | .16 |
| Late TTTS stage (III–V) | 36.4 | 53.7 | .07 |
| Refused fetoscopic laser therapy | 5.5 | 7.4 | .68 |
| TTTS complications precluding fetoscopy | 3.6 | 18.5 | .01 |
| TTTS stage V | 1.8 | 7.4 | .16 |
| PPROM before laser | 0.0 | 3.7 | .15 |
| Cervical dilatation | 1.8 | 5.6 | .30 |
| Fetoscopic laser therapy | 90.9 | 75.9 | .03 |
| Short cervix at diagnosis | 10.9 | 25.9 | .04 |
| GA at delivery (wk) | 29.9 (5.8) | 29.2 (10.3) | .68 |
| Preterm delivery<32 wk | 43.6 | 45.1 | .88 |
| Overall survival | |||
| 1 twin | 80.0 | 56.9 | .01 |
| 2 twins | 56.4 | 39.2 | .08 |
Data are presented as mean (standard deviation) or percentage, unless otherwise indicated.
BMI, body mass index; GA, gestational age; PPROM, preterm premature rupture of membranes; TTTS, twin-to-twin transfusion syndrome.
The Student t test for independent samples or the Pearson chi-squared test was used.
López-Briones. Twin-to-twin transfusion syndrome and COVID-19. Am J Obstet Gynecol Glob Rep 2022.
Figure 1.
Survival outcomes of TTTS between study groups
Adverse perinatal outcome was significantly different between the groups (P<.05).
TTTS, twin-to-twin transfusion syndrome.
López-Briones. Twin-to-twin transfusion syndrome and COVID-19. Am J Obstet Gynecol Glob Rep 2022.
Compared with the year before the pandemic, a significantly lower number of cases that fulfilled the criteria for fetoscopy were observed during the first year of the COVID-19 pandemic (41/54 [75.9%] vs 50/55 [90.9%]; P<.05). During the 2-year study period, fetoscopic laser therapy was successfully performed at a mean gestational age of 22.7±3.3 weeks in 91 cases. Table 2 shows the baseline maternal-fetal clinical characteristics and surgical procedure of the cases treated with fetoscopy during both study periods. There was no significant difference in preoperative cervical length, gestational age at fetoscopy, total fluid drained on amniodrainage, or procedure duration.
Table 2.
Maternal and neonatal clinical characteristics for twin-to-twin transfusion syndrome cases selected for fetoscopic laser therapy before (2019) and during the COVID-19 pandemic (2020)
| Characteristic | 2019 (n=50) | 2020 (n=41) | P valuea |
|---|---|---|---|
| Maternal age (y) | 30.0 (6.0) | 29.5 (5.5) | .67 |
| BMI (kg/m2) | 26.4 (5.5) | 27.8 (6.6) | .30 |
| Primiparity | 38.0 | 46.3 | .42 |
| TTTS staging | |||
| I | 30.0 | 22.0 | .39 |
| II | 34.0 | 29.3 | .63 |
| III | 20.0 | 34.0 | .13 |
| IV | 16.0 | 14.6 | .86 |
| Late TTTS stage (III–IV) | 36.0 | 48.8 | .22 |
| Cervical length (mm) | 29.3 (11.1) | 28.5 (10.0) | .74 |
| Short cervix | 8.0 | 14.6 | .31 |
| GA at fetoscopy (wk) | 22.8 (3.3) | 22.6 (3.3) | .78 |
| Drained amniotic fluid (mL) | 1727 (1625) | 1986 (1446) | .43 |
| Surgery time (min) | 25.0 (12.8) | 24.6 (21.9) | .92 |
Data are presented as mean (standard deviation) or percentage, unless otherwise indicated.
BMI, body mass index; GA, gestational age; TTTS, twin-to-twin transfusion syndrome.
The Student t test for independent samples or the Pearson chi-squared test was used.
López-Briones. Twin-to-twin transfusion syndrome and COVID-19. Am J Obstet Gynecol Glob Rep 2022.
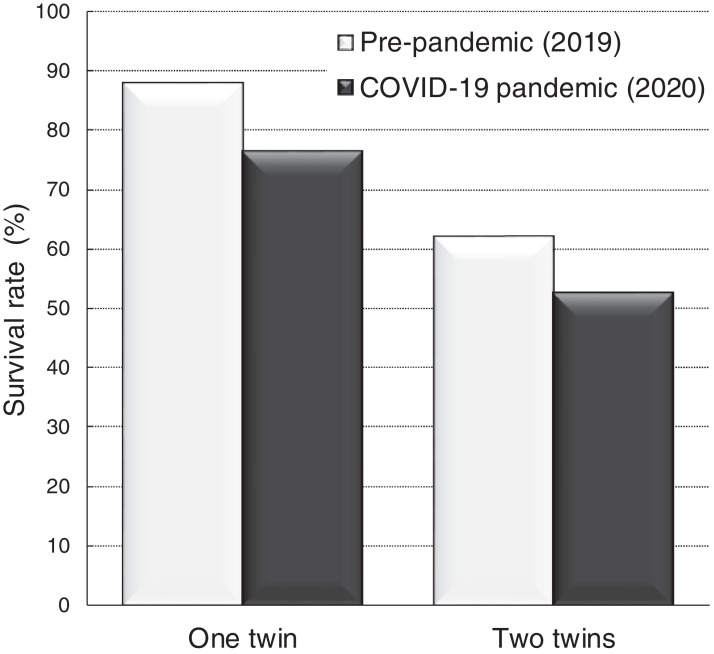
Figure 2 and Table 3 illustrate the perinatal outcomes of the 88 TTTS pregnancies treated with fetoscopy between the 2 study periods that were delivered. Surgical outcomes were similar despite the COVID-19 pandemic, considering that both groups showed similar proportion of PPROM (31.6% vs 22.0%; P=.31), preterm delivery before 32 weeks of gestation (50.0% vs 44.0%; P=.58), and survival rates (76.3% vs 88.0% [P=.15; at least 1 twin] and 52.6% vs 62.0% [P=.38; both twins]).
Figure 2.
Outcomes of TTTS with laser therapy during the study periods
TTTS, twin-to-twin transfusion syndrome.
López-Briones. Twin-to-twin transfusion syndrome and COVID-19. Am J Obstet Gynecol Glob Rep 2022.
Table 3.
Perinatal outcomes of twin-to-twin transfusion syndrome cases treated with fetoscopic laser therapy during the first year of the COVID-19 pandemic (2020) and the same period of the previous year (2019)
| Outcome | 2019 (n=50) | 2020 (n=38) | P valuea |
|---|---|---|---|
| Interval between fetoscopy and delivery (wk) | 8.0 (5.2) | 8.6 (11.5) | .75 |
| PPROM | 22.0 | 31.6 | .31 |
| GA at delivery (wk) | 30.6 (5.5) | 31.4 (11.0) | .67 |
| Preterm delivery<32 wk | 44.0 | 50.0 | .58 |
| Overall survival | |||
| 1 twin | 88.0 | 76.3 | .15 |
| 2 twins | 62.0 | 52.6 | .38 |
Data are presented as mean (standard deviation) or percentage, unless otherwise indicated.
GA, gestational age; PPROM, preterm premature rupture of membranes.
The Student t test for independent samples or the Pearson chi-squared test was used.
López-Briones. Twin-to-twin transfusion syndrome and COVID-19. Am J Obstet Gynecol Glob Rep 2022.
Comment
Principal findings
This study reported an adverse clinical impact during the COVID-19 pandemic, compared with the prepandemic period, in MCDA twin pregnancies complicated with TTTS involving a trend for higher rates of advanced stages at diagnosis and a considerably higher rate of short CL, higher frequency of exclusion criteria for fetoscopy, and lower overall survival rates.
Results in the context of what is known
Previous studies have demonstrated that MCDA twin pregnancies with TTTS may show a progression of hemodynamic changes during pregnancy, which leads to the deterioration of both twins and a higher probability of either single or dual intrauterine fetal demise.26, 27, 28 Furthermore, TTTS may show a progressive increase in the amount of the recipient's amniotic fluid, leading to a higher risk of either maternal respiratory morbidity, PPROM, or preterm delivery, with a detrimental effect on neonatal survival.2,29 Considering that up to 20% of the MC twins may develop this progressive lethal condition, the International Society of Ultrasound in Obstetrics and Gynecology has published guidelines for the management of twin pregnancies recommending a fortnightly surveillance for all MC twins from 16 weeks of gestation to delivery and even a closer follow-up with weekly ultrasound assessment for those showing amniotic fluid discordance.7 Previous studies have reported poorer perinatal outcomes of TTTS pregnancies in the setting of suboptimal prenatal surveillance. McDonald et al10 showed that longer intervals between the last ultrasound evaluation and diagnosis of TTTS were associated with advanced TTTS stage and higher risk of fetal demise. Similarly, Thorson et al11 evaluated 108 MC twins and found advanced stages and higher risk of hydrops in pregnancies with ultrasound intervals longer than 2 weeks than those with closer surveillance.
Selected TTTS cases for fetoscopy showed similar disease stages and survival rates during the 2 studied periods, which suggested that the main population affected by the COVID-19 pandemic were those MCDA pregnancies that arguably had a less stringent prenatal monitoring and thus assessed at the fetal surgical center when the disease was inoperable.
These findings were in line with previous studies published during the COVID-19 pandemic, showing considerably reduced visits for prenatal ultrasound screening and decreased number of diagnostic procedures, such as chorionic villous sampling and amniocentesis, during the pandemic compared with the previous year.30 Regarding fetal interventions, the fetal surgery group from the Mayo Clinic compared outcomes of 9 cases undergoing fetal intervention during the first 2.5 months of the COVID-19 pandemic with 8 cases intervened in the same period a year before. The authors suggested that the COVID-19 pandemic had minimal impact on survival outcomes of cases selected for fetal intervention.31 However, in such study, cases that did not undergo intervention (7/15 [46.7%] before the pandemic and 13/22 [59%] during the pandemic) were excluded for analysis, and only a small number of cases complicated with TTTS were evaluated within the study period (2 before the pandemic and 5 during the pandemic); therefore, the true impact of the pandemic on MC twin pregnancies complicated with TTTS may have been underestimated.
Clinical implications
Our study provides evidence that in the context of a pandemic, MC twin pregnancies with TTTS could be affected by the restrictions applied aiming to control the disease. During such a period, pregnant women with TTTS were referred to our center at advanced stages mainly because of delayed prenatal diagnosis secondary to longer intervals between antenatal visits. The clinical relevance of prompt detection and referral of these cases should not be underestimated, as according to our findings, overall survival rates during the pandemic were considerably lower than the preceding year. Considering that some consequences of late diagnosis may represent an exclusion criterion for laser therapy, timely diagnosis and opportune referral can decrease the probability of being excluded for the benefit of fetoscopic laser coagulation of the placental anastomosis. In keeping with this argument, the North American Fetal Therapy Network and other international societies have recommended that even during a pandemic process, nonexperimental emergency fetal interventions that have demonstrated profits for lethal conditions in randomized controlled trials, such as laser therapy for TTTS, should still be offered and performed during the pandemic.32
However, we recognize that during a pandemic process, where a potential maternal infection can increase maternal morbidity and mortality, pregnant women should be advised about the potential higher risk of COVID-19 and prenatal surveillance with fetal ultrasound should be performed with caution as recommended in all at-risk cases.33
Research implications
From a clinical point of view, our recommendations may be extrapolated to other fetal pathologies for fetal intervention that can evolve to intrauterine fetal demise when expectantly managed, such as fetal hydrothorax, solid and cystic lung anomalies associated with massive pleural effusions, and fetal anemia.34 Similarly, a suboptimal and delayed prenatal surveillance in other progressive fetal conditions may impair the prognosis, such as cases prenatally diagnosed with low urinary tract obstruction leading to either bladder rupture or bilateral renal failure35,36 or those with critical aortic stenosis evolving to left heart hypoplasia.37 Similar to TTTS, in advanced stages of all these fetal pathologies, the prognosis and survival outcomes worsen despite fetal intervention, and therefore, such cases should be diagnosed and referred to experienced fetal surgery centers promptly to have maximal benefit.38, 39, 40 Future research studies are required to address the clinical impact of a pandemic process in such fetal pathologies.
Strengths and limitations
The main strength of our study was that it included a well-selected cohort of all consecutive pregnancies complicated with TTTS referred for possible fetoscopic laser therapy to our fetal surgery center, which is considered the most experienced referral center in Mexico.3, 4, 5, 6 This means that the main population of TTTS cases from our country has been included in our series, and therefore, a potential selection bias, such as referral of the most complex cases, was unlikely.
Because a delay in TTTS management may result in intrauterine fetal demise, the main limitation of our study was that none of the asymptomatic operated pregnant patients at our Institution had COVID-19 screening test before fetoscopy. However, careful selection of case candidates for fetoscopy was essential to minimize the risk of COVID-19 exposure to the mother, their fetuses, and healthcare workers; moreover, this was ameliorated with the correct use of personal protective equipment.
Conclusions
During the COVID-19 pandemic, MCDA twins complicated with TTTS were evaluated at advanced stages and showed a higher incidence of exclusion criteria for fetoscopic laser surgery and lower survival rates. This information may be relevant for clinicians during future similar lockdowns and highlight the need to implement other strategies, such as telemedicine, in an attempt to avoid delayed antenatal visits of MC twin pregnancies, enhance early recognition of TTTS and timely referral to a fetoscopic center, and improve the prognosis and decrease the risk of fetal loss.
Acknowledgments
R.C. wishes to thank the Fetal Medicine Mexico Foundation for supporting the National Project of Fetal Surgery at Queretaro, México.
Footnotes
The authors report no conflict of interest.
Cite this article as: López-Briones H, Villalobos-Gómez R, Chávez-González E, et al. Twin-to-twin transfusion syndrome and coronavirus disease 2019: impact on diagnosis, referral, eligibility for fetoscopic laser therapy, and outcomes. Am J Obstet Gynecol Glob Rep 2022;2:100040.
References
- 1.Habli M, Lim FY, Crombleholme T. Twin-to-twin transfusion syndrome: a comprehensive update. Clin Perinatol. 2009;36:391–416. doi: 10.1016/j.clp.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Lewi L, Jani J, Blickstein I, et al. The outcome of monochorionic diamniotic twin gestations in the era of invasive fetal therapy: a prospective cohort study. Am J Obstet Gynecol. 2008;199 doi: 10.1016/j.ajog.2008.03.050. 514.e1–8. [DOI] [PubMed] [Google Scholar]
- 3.Salomon LJ, Ville Y. [Twin-to-twin transfusion syndrome: diagnosis and treatment] Bull Acad Natl Med. 2008;192:1575–1586. [PubMed] [Google Scholar]
- 4.Diehl W, Diemert A, Grasso D, Sehner S, Wegscheider K, Hecher K. Fetoscopic laser coagulation in 1020 pregnancies with twin-twin transfusion syndrome demonstrates improvement in double-twin survival rate. Ultrasound Obstet Gynecol. 2017;50:728–735. doi: 10.1002/uog.17520. [DOI] [PubMed] [Google Scholar]
- 5.Persico N, Fabietti I, D'Ambrosi F, Riccardi M, Boito S, Fedele L. Postnatal survival after endoscopic equatorial laser for the treatment of twin-to-twin transfusion syndrome. Am J Obstet Gynecol. 2016;214 doi: 10.1016/j.ajog.2015.10.020. 533.e1–7. [DOI] [PubMed] [Google Scholar]
- 6.Cruz-Martinez R, Lopez-Briones H, Luna-Garcia J, et al. Incidence and survival of MCDA twin pregnancies with TTTS presenting without amniotic fluid discordance due to spontaneous septostomy and treated with fetoscopy. Ultrasound Obstet Gynecol. 2021;58:488–489. doi: 10.1002/uog.23129. [DOI] [PubMed] [Google Scholar]
- 7.Khalil A, Rodgers M, Baschat A, et al. ISUOG Practice Guidelines: role of ultrasound in twin pregnancy. Ultrasound Obstet Gynecol. 2016;47:247–263. doi: 10.1002/uog.15821. [DOI] [PubMed] [Google Scholar]
- 8.Chmait RH, Kontopoulos EV, Korst LM, Llanes A, Petisco I, Quintero RA. Stage-based outcomes of 682 consecutive cases of twin-twin transfusion syndrome treated with laser surgery: the USFetus experience. Am J Obstet Gynecol. 2011;204 doi: 10.1016/j.ajog.2011.02.001. 393.e1–6. [DOI] [PubMed] [Google Scholar]
- 9.Duryea EL, Happe SK, McIntire DD, Dashe JS. Sonography interval and the diagnosis of twin-twin transfusion syndrome. J Matern Fetal Neonatal Med. 2017;30:640–644. doi: 10.1080/14767058.2016.1182976. [DOI] [PubMed] [Google Scholar]
- 10.McDonald R, Hodges R, Knight M, et al. Optimal interval between ultrasound scans for the detection of complications in monochorionic twins. Fetal Diagn Ther. 2017;41:197–201. doi: 10.1159/000448094. [DOI] [PubMed] [Google Scholar]
- 11.Thorson HL, Ramaeker DM, Emery SP. Optimal interval for ultrasound surveillance in monochorionic twin gestations. Obstet Gynecol. 2011;117:1227. doi: 10.1097/AOG.0b013e3182172c82. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization. WHO DirectorGeneral's opening remarks at the media briefing on COVID-19 - 12 . 2020. January 2022.https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---12-january-2022 Available at: Accessed Jan. 12, 2022. [Google Scholar]
- 13.Martinez-Portilla RJ, Smith ER, He S, et al. Young pregnant women are also at an increased risk of mortality and severe illness due to coronavirus disease 2019: analysis of the Mexican National Surveillance Program. Am J Obstet Gynecol. 2021;224:404–407. doi: 10.1016/j.ajog.2020.12.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez-Portilla RJ, Sotiriadis A, Chatzakis C, et al. Pregnant women with SARS-CoV-2 infection are at higher risk of death and pneumonia: propensity score matched analysis of a nationwide prospective cohort (COV19Mx) Ultrasound Obstet Gynecol. 2021;57:224–231. doi: 10.1002/uog.23575. [DOI] [PubMed] [Google Scholar]
- 15.Mullins E, Hudak ML, Banerjee J, et al. Pregnancy and neonatal outcomes of COVID-19: coreporting of common outcomes from PAN-COVID and AAP-SONPM registries. Ultrasound Obstet Gynecol. 2021;57:573–581. doi: 10.1002/uog.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chmielewska B, Barratt I, Townsend R, et al. Effects of the COVID-19 pandemic on maternal and perinatal outcomes: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e759–e772. doi: 10.1016/S2214-109X(21)00079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boelig RC, Saccone G, Bellussi F, Berghella V. MFM guidance for COVID-19. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The American College of Obstetricians and Gynecologists . 2020. Novel coronavirus 2019 (COVID-19): practice advisory.https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/03/novel-coronavirus-2019 Available at: Accessed Jul. 8, 2021. [Google Scholar]
- 19.Gratacós E, Deprest J. Current experience with fetoscopy and the Eurofoetus registry for fetoscopic procedures. Eur J Obstet Gynecol Reprod Biol. 2000;92:151–159. doi: 10.1016/s0301-2115(00)00440-1. [DOI] [PubMed] [Google Scholar]
- 20.Quintero RA, Morales WJ, Allen MH, Bornick PW, Johnson PK, Kruger M. Staging of twin-twin transfusion syndrome. J Perinatol. 1999;19:550–555. doi: 10.1038/sj.jp.7200292. [DOI] [PubMed] [Google Scholar]
- 21.Burger M, Weber-Rössler T, Willmann M. Measurement of the pregnant cervix by transvaginal sonography: an interobserver study and new standards to improve the interobserver variability. Ultrasound Obstet Gynecol. 1997;9:188–193. doi: 10.1046/j.1469-0705.1997.09030188.x. [DOI] [PubMed] [Google Scholar]
- 22.Moletta L, Pierobon ES, Capovilla G, et al. International guidelines and recommendations for surgery during Covid-19 pandemic: a systematic review. Int J Surg. 2020;79:180–188. doi: 10.1016/j.ijsu.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kovoor JG, Tivey DR, Williamson P, et al. Screening and testing for COVID-19 before surgery. ANZ J Surg. 2020;90:1845–1856. doi: 10.1111/ans.16260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly JC, Dombrowksi M, O'Neil-Callahan M, Kernberg AS, Frolova AI, Stout MJ. False-negative testing for severe acute respiratory syndrome coronavirus 2: consideration in obstetrical care. Am J Obstet Gynecol MFM. 2020;2 doi: 10.1016/j.ajogmf.2020.100130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Senat MV, Deprest J, Boulvain M, Paupe A, Winer N, Ville Y. Endoscopic laser surgery versus serial amnioreduction for severe twin-to-twin transfusion syndrome. N Engl J Med. 2004;351:136–144. doi: 10.1056/NEJMoa032597. [DOI] [PubMed] [Google Scholar]
- 26.Mari G, Roberts A, Detti L, et al. Perinatal morbidity and mortality rates in severe twin-twin transfusion syndrome: results of the International Amnioreduction Registry. Am J Obstet Gynecol. 2001;185:708–715. doi: 10.1067/mob.2001.117188. [DOI] [PubMed] [Google Scholar]
- 27.Manning N, Archer N. Cardiac manifestations of twin-to-twin transfusion syndrome. Twin Res Hum Genet. 2016;19:246–254. doi: 10.1017/thg.2016.20. [DOI] [PubMed] [Google Scholar]
- 28.Yoda H. Fetal and neonatal circulatory disorders in twin to twin transfusion syndrome (the secondary publication) J Nippon Med Sch. 2019;86:192–200. doi: 10.1272/jnms.JNMS.2019_86-301. [DOI] [PubMed] [Google Scholar]
- 29.Murgano D, Khalil A, Prefumo F, et al. Outcome of twin-to-twin transfusion syndrome in monochorionic monoamniotic twin pregnancy: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2020;55:310–317. doi: 10.1002/uog.21889. [DOI] [PubMed] [Google Scholar]
- 30.Ozalp M, Demir O, Akbas H, Kaya E, Celik C, Osmanagaoglu MA. Effect of COVID-19 pandemic process on prenatal diagnostic procedures. J Matern Fetal Neonatal Med. 2021;34:3952–3957. doi: 10.1080/14767058.2020.1815190. [DOI] [PubMed] [Google Scholar]
- 31.Narang K, Elrefaei A, Wyatt MA, et al. Fetal surgery in the era of SARS-CoV-2 pandemic: a single-institution review. Mayo Clin Proc Innov Qual Outcomes. 2020;4:717–724. doi: 10.1016/j.mayocpiqo.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahtiyar MO, Baschat A, Deprest J, et al. Fetal interventions in the setting of the coronavirus disease 2019 pandemic: statement from the North American Fetal Therapy Network. Am J Obstet Gynecol. 2020;223:281–284. doi: 10.1016/j.ajog.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Narang K, Ibirogba ER, Elrefaei A, et al. SARS-CoV-2 in pregnancy: a comprehensive summary of current guidelines. J Clin Med. 2020;9:1521. doi: 10.3390/jcm9051521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilad N, Furman Y, Weissmann-Brenner A. A retrospective clinical analysis of 20 cases of congenital lung masses. J Matern Fetal Neonatal Med. 2020:1–6. doi: 10.1080/14767058.2020.1836149. [DOI] [PubMed] [Google Scholar]
- 35.Berte N, Vrillon I, Larmure O, et al. Long-term renal outcome in infants with congenital lower urinary tract obstruction. Prog Urol. 2018;28:596–602. doi: 10.1016/j.purol.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 36.Lacher M, Stehr M, Schiessl B, Dietz HG. Fetal urinary bladder rupture and urinary ascites secondary to posterior urethral valves. A case report. Eur J Pediatr Surg. 2007;17:217–220. doi: 10.1055/s-2007-965148. [DOI] [PubMed] [Google Scholar]
- 37.Gardiner HM, Kovacevic A, Tulzer G, et al. Natural history of 107 cases of fetal aortic stenosis from a European multicenter retrospective study. Ultrasound Obstet Gynecol. 2016;48:373–381. doi: 10.1002/uog.15876. [DOI] [PubMed] [Google Scholar]
- 38.Di Mascio D, Khalil A, D'Amico A, et al. Outcome of twin-twin transfusion syndrome according to Quintero stage of disease: systematic review and meta-analysis. Ultrasound Obstet Gynecol. 2020;56:811–820. doi: 10.1002/uog.22054. [DOI] [PubMed] [Google Scholar]
- 39.Gámez-Varela A, Martínez-Rodríguez M, López-Briones H, et al. Preoperative cervical length predicts the risk of delivery within one week after pleuroamniotic shunt in fetuses with severe hydrothorax. Fetal Diagn Ther. 2021;48:297–303. doi: 10.1159/000514912. [DOI] [PubMed] [Google Scholar]
- 40.Ruano R, da Silva MM, Salustiano EM, Kilby MD, Tannuri U, Zugaib M. Percutaneous laser ablation under ultrasound guidance for fetal hyperechogenic microcystic lung lesions with hydrops: a single center cohort and a literature review. Prenat Diagn. 2012;32:1127–1132. doi: 10.1002/pd.3969. [DOI] [PubMed] [Google Scholar]