Abstract
Background
Antibiotics use may increase colorectal cancer (CRC) risk by altering the gut microbiota, with suggestive evidence reported. Our study aims to investigate antibiotics use in relation to subsequent CRC risk.
Methods
This is a nationwide, population-based study with a matched case-control design (first primary CRC cases and 5 matched, cancer-free controls). Complete-population data, extracted from Swedish national registers for the period 2005-2016, were used to calculate odds ratios and 95% confidence intervals.
Results
We included 40 545 CRC cases and 202 720 controls. Using the full dataset, we found a positive association between more frequent antibiotics use and CRC, excluding antibiotics prescribed within 2 years of diagnosis attenuated results toward the null. In site-specific analyses, excluding the 2-year washout, the positive association was confined to the proximal colon (adjusted odds ratio for very high use vs no use = 1.17, 95% confidence interval = 1.05 to 1.31). For rectal cancer, an inverse association, which appears to be driven by women, was observed. Quinolones and sulfonamides and/or trimethoprims were positively associated with proximal colon cancer, whereas a more general inverse association, across antibiotics classes, was observed for rectal cancer. We found no association between methenamine hippurate, a urinary tract antiseptic not affecting the gut microbiota, and CRC risk.
Conclusions
This register-based study covering the entire population of Sweden found a robust association between antibiotics use and higher risk of proximal colon cancer and an inverse association with rectal cancer in women. This study strengthens the evidence from previous investigations and adds important insight into site-specific colorectal carcinogenesis.
Colorectal cancer (CRC) is a multifactorial disease. Extensive epidemiological research has identified several lifestyle and medical risk factors for CRC (1,2), but the etiology is still partly unknown. A continued effort to identify risk factors for CRC is imperative, because reducing even minor risk factors at the population level could have a substantial impact on the incidence of CRC (3,4).
The composition and function of the gut microbiome are believed to have a role in CRC development (5). A structural segregation of the gut microbiome between colorectal carcinoma and benign colorectal mucosa has been reported (6,7) and evidence supports a pathogenic role of certain microbes, such as Fusobacterium nucleatum, in colorectal carcinogenesis (8-10). Mima et al. (11) reported that the proportion of colorectal cancers enriched with F. nucleatum decreases gradually from caecum to rectum, suggesting a site-specific effect of the gut microbiome in carcinogenesis.
Many established CRC risk factors, including excess body fat and dietary factors, could alter the gut microbiome (12,13). However, use of antibiotics can have a more disruptive effect (14,15). For example, treatment with antibiotics can alter the microbial balance in the gut resulting in intestinal overgrowth of toxin-producing Clostridium difficile bacteria (16), causing diarrhea and inflammation. Antibiotic-induced dysbiosis may disrupt the anti-inflammatory effects of some microbiota and increase pathogenic bacteria, influencing CRC tumorigenesis (7,17). Previous investigations of antibiotics use and CRC have generally indicated a positive association (18-23). However, most studies had limited information or insufficient power for extensive analyses of aspects such as type, dose, or duration of antibiotics and tumor stage and site. Recently, a large-scale study conducted in the United Kingdom reported that antibiotics use was associated with a higher risk of colon cancer but a lower risk of rectal cancer (24). These observations warrant swift validation.
In this study, we used data from the comprehensive Swedish national population registers to investigate antibiotics use in relation to CRC risk. The large sample size made it possible to conduct well-powered subgroup analyses on antibiotics type and clinical factors such as disease stage and tumor site.
Methods
Study Design
A matched case-control study was conducted using data from Swedish population registers (study period July 1, 2005, to December 31, 2016) (see Figure 1). Sweden’s unique personal identity numbers allow for multiregister linkage and matching (25). In brief, CRC cases were identified using the Swedish Colorectal Cancer Register, controls were matched using the Total Population Register, data on antibiotics use were extracted from the Swedish Prescribed Drug Register, and other variables of interest were taken from the Swedish Inpatient Register and the Longitudinal Integration Database for Health Insurance and Labor Market Studies (LISA by Swedish acronym). Full descriptions of the Swedish national registers included in the study can be found in the Supplementary Methods (available online).
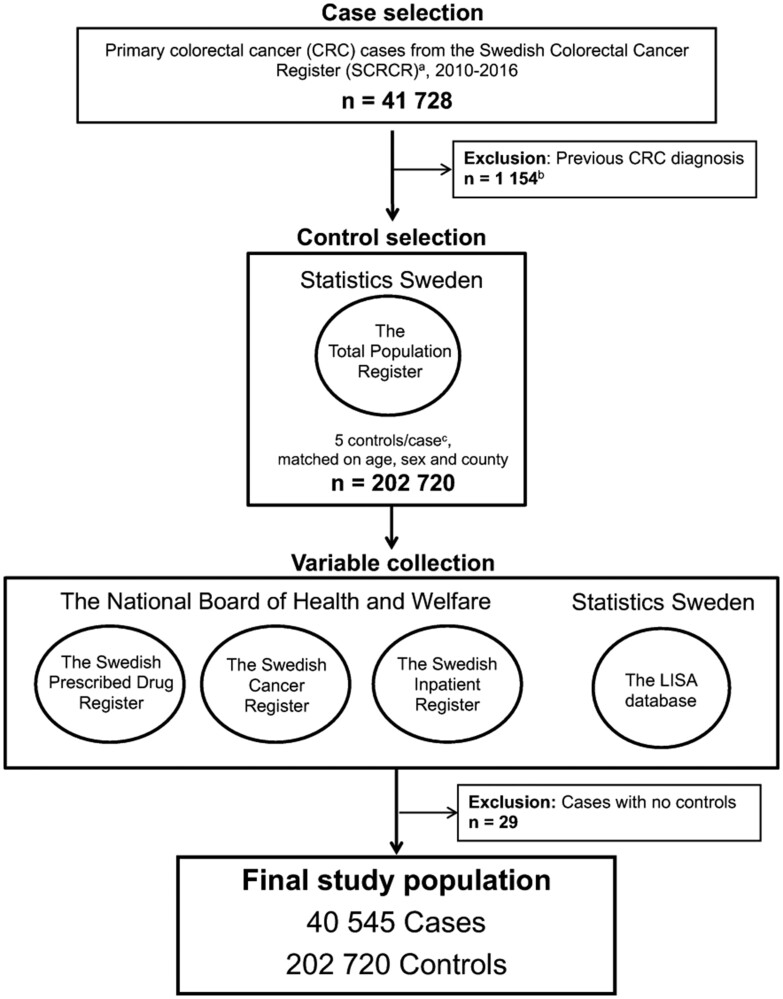
Figure 1.
Flowchart of case and control selection. aThe Swedish colorectal cancer register was initiated in 1995 for rectal cancer and in 2007 for colon cancer. bCase first diagnosed before the start of the Swedish Colorectal Cancer Register. cThree cases had fewer than 5 eligible controls.
Selection of Cases and Controls
All primary CRC cases (International Classification of Diseases, 10th edition, codes: C18.0, C18.2-18.9, C19.9, and C20.9) diagnosed between January 1, 2010, and December 31, 2016, were selected from the Swedish Colorectal Cancer Register. Cases were classified as proximal colon cancer (caecum, ascending colon, hepatic flexure, transverse colon, or splenic flexure), distal colon cancer (descending or sigmoid colon), or rectal cancer (rectosigmoidal junction or rectum). Stages of CRC were categorized as early stage (stage I-II) and late stage (stage III-IV) based on TNM Classification of Malignant Tumours, 8th edition (26).
For each CRC case, 5 controls were selected from the Total Population Register. Incidence density sampling was used to minimize bias when using cases and controls with different follow-up times (27). The final dataset included 40 545 CRC cases and 202 720 controls (see Figure 1). A full description of the case and control selection can be found in the Supplementary Methods (available online).
Exposure Variables and Covariates
The study population was linked to the Swedish Prescribed Drug Register to extract information on dispensed antibiotics under Anatomical Therapeutic Chemical codes J01 and J04 (anti-infective agents for systemic use) from July 1, 2005 (start of the register), until December 31, 2016. We also obtained data on other drug groups with antibiotic effects under Anatomical Therapeutic Chemical codes: A07A (intestinal anti-infective agents) and P01 (anti-protozoal that could have antibacterial effects). Antibiotics use reported as defined daily doses, a unit of comparison for drug statistics (28), was categorized as no use (no reported use of antibiotics during the study period), low (1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days) use. A binary variable for antibiotics use “no use” vs “any use” and a variable for total number of prescriptions were also constructed. Classification of antibiotics can be found in the Supplementary Methods (available online) and Supplementary Table 1 (available online).
Additional covariates were considered based on previously established associations with CRC risk and their availability in the registers. They included socioeconomic factors (level of education, country of birth, and marital status retrieved from the LISA database) and health-care utilizations (number of specialist visits and hospitalizations from the Swedish Inpatient Register), the latter as a surrogate for potentially relevant comorbidities and health-care–seeking behavior. Detailed descriptions of these covariates can be found in the Supplementary Methods (available online).
Statistical Analysis
Tests for differences in characteristics between cases and controls were performed using a Pearson χ2 test and 2-sample t test. Conditional logistic regression, adjusting for selected covariates as potential confounders, was used to investigate associations between antibiotics use and risk of CRC, reported as odds ratios (ORs) with 95% confidence intervals (CIs). The reference category for antibiotics use was “no use.” To evaluate any potential dose-response effect, we conducted tests for trends in which categorical antibiotics exposures were expressed as a continuous variable. Trend tests were conducted for all analyses except the analyses with binary antibiotics exposures. Multiplicative interaction terms were introduced to assess effect modification by sex, and Q-statistics with 1 degree of freedom were used to test for heterogeneity of estimates between men and women (using binary categories of antibiotics use). Based on the study hypothesis, a number of prespecified subgroup analyses (sex, age, and anatomical tumor site) and sensitivity analyses (excluding the 2 years prior to case diagnosis) were performed, which are described in detail in the Supplementary Methods (available online). All statistical tests, 2-sided with a statistical significance level of .05, were performed using Stata/MP 16.1 (Stata Corp, College Station, TX).
Ethical Approval
The study was approved by the regional ethical review board in Umeå, Sweden (Dnr: 2017/338-31), and was conducted in accordance with the Declaration of Helsinki.
Results
The study population consisted of 40 545 cases and 202 720 matched controls (52.9% men and 47.1% women; Table 1). During the study period, 18.7% of the cases and 22.4% of the controls had no antibiotics prescribed, and 20.8% of cases and 19.3% of controls had used antibiotics for more than 2 months (P < .001). The mean age at CRC diagnosis was 72 years (Supplementary Table 2, available online). Among all cases, 36.4% had proximal colon cancer, 29.3% had distal colon cancer, and 33.0% had rectal cancer. The median follow-up time was 8 years.
Table 1.
Characteristics of the study population
| Characteristics | Cases (n = 40 545) | Controls (n = 202 720) | P a |
|---|---|---|---|
| Sex, No. (%)b | |||
| Men | 21 458 (52.9) | 107 285 (52.9) | |
| Women | 19 087 (47.1) | 95 435 (47.1) | 1.00 |
| County, No. (%)b | |||
| Region Stockholm | 6 995 (17.3) | 34 974 (17.3) | |
| Region Skåne | 5 481 (13.5) | 27 403 (13.5) | |
| Region Västra Götaland | 7 060 (17.4) | 35 300 (17.4) | |
| Other regionsc | 21 009 (51.8) | 105 043 (51.8) | 1.00 |
| Country of birth, No. (%) | |||
| Sweden | 35 391 (87.3) | 175 288 (86.5) | |
| Rest of Europe | 4 090 (10.1) | 20 662 (10.2) | |
| Non-European country | 936 (2.3) | 6 757 (3.3) | <.001 |
| Unknown | 128 (0.3) | 13 (<0.01) | |
| Education, No. (%) | |||
| Primary school up to 9 years | 15 031 (37.1) | 74 912 (37.0) | |
| Secondary school | 16 028 (39.5) | 78 030 (38.5) | |
| Postsecondary school | 8 672 (21.4) | 44 618 (22.0) | <.001 |
| Unknown | 814 (2.0) | 5 160 (2.5) | |
| Marital status, No. (%) | |||
| Married/Living with partner | 21 382 (52.7) | 105 802 (52.2) | |
| Widower/Widow | 7 653 (18.9) | 38 702 (19.1) | |
| Unmarried | 5 018 (12.4) | 25 740 (12.7) | |
| Divorced | 6 492 (16.0) | 32 475 (16.0) | .14 |
| Unknown | 0 (<0.01) | 1 (<0.01) | |
| Specialist visits, mean (SD) | |||
| All specialist visits within the study period | 28.6 (29.5) | 15.6 (25.5) | <.001 |
| Specialist visits up to 2 years before case diagnosisd | 4.2 (7.9) | 3.0 (7.1) | <.001 |
| Specialist visits more than 2 years before case diagnosisd | 8.2 (15.6) | 7.2 (13.5) | <.001 |
| Hospitalizations, mean (SD) | |||
| All hospitalizations within the study period | 5.6 (5.1) | 2.7 (4.4) | <.001 |
| Hospitalizations up to 2 years before case diagnosisd | 1.1 (1.7) | 0.5 (1.4) | <.001 |
| Hospitalizations more than 2 years before case diagnosisd | 1.2 (2.5) | 1.1 (2.4) | <.001 |
| Antibiotics exposure, No. (%) | |||
| No use | 7 568 (18.7) | 45 427 (22.4) | |
| Low (1-10 days) | 5 847 (14.4) | 28 106 (13.9) | |
| Moderate (11-60 days) | 18 695 (46.1) | 90 005 (44.4) | |
| High (61-180 days) | 6 685 (16.5) | 31 269 (15.4) | |
| Very high (>180 days) | 1 750 (4.3) | 7 913 (3.9) | <.001 |
P value for Pearson χ2 test (categorical variables) in which missing categories were excluded or 2-sample t test (continuous variables). All tests were 2-sided.
Matching variables.
All other counties in Sweden.
For the matched controls, the diagnosis date of the index case was used.
Antibiotics use was positively associated with CRC for moderate use (OR = 1.15, 95% CI = 1.12 to 1.18) and very high use (OR = 1.17, 95% CI = 1.10 to 1.24) vs no use (Ptrend < .001; Table 2). However, in the analysis excluding all antibiotics use occurring 2 years before CRC diagnosis (and for the controls, the 2 years before diagnosis of their index case) to account for reverse causation, the association was attenuated and not statistically significant for very high vs no use (OR = 1.02, 95% CI = 0.95 to 1.09; Ptrend = .97). Consequently, this 2-year exclusion was applied to all analyses.
Table 2.
Associations between antibiotics use and risk of colorectal cancer by tumor site
| Tumor site and antibiotics usea | Including all antibiotics use before diagnosis |
Excluding antibiotics use during the 2 years preceding CRC diagnosis |
||
|---|---|---|---|---|
| No. of cases/controls | Adjusted OR (95% CI)b | No. of cases/controls | Adjusted OR (95% CI)b | |
| Colorectum | ||||
| No use | 9 728/54 641 | 1 (Referent) | 13 714/70 136 | 1 (Referent) |
| Low | 4 209/21 044 | 1.11 (1.07 to 1.15) | 4 745/23 468 | 1.02 (0.98 to 1.06) |
| Moderate | 18 316/88 401 | 1.15 (1.12 to 1.18) | 16 536/81 914 | 1.01 (0.98 to 1.04) |
| High | 6 554/30 774 | 1.17 (1.13 to 1.21) | 4 414/21 982 | 0.98 (0.94 to 1.02) |
| Very high | 1 738/7 860 | 1.17 (1.10 to 1.24) | 1 136/5 220 | 1.02 (0.95 to 1.09) |
| Ptrendc | <.001 | .97 | ||
| Colon | ||||
| No use | 6 019/35 848 | 1 (Referent) | 8 739/46 146 | 1 (Referent) |
| Low | 2 727/14 144 | 1.13 (1.08 to 1.19) | 3 138/15 874 | 1.03 (0.98 to 1.07) |
| Moderate | 12 459/59 432 | 1.23 (1.19 to 1.27) | 11 356/55 108 | 1.06 (1.02 to 1.09) |
| High | 4 721/21 130 | 1.28 (1.23 to 1.34) | 3 128/15 230 | 1.02 (0.97 to 1.07) |
| Very high | 1 248/5 316 | 1.27 (1.19 to 1.37) | 813/3 512 | 1.07 (0.98 to 1.17) |
| Ptrendc | <.001 | .009 | ||
| Proximal colon | ||||
| No use | 3 129/18 991 | 1 (Referent) | 4 492/24 557 | 1 (Referent) |
| Low | 1 520/7 707 | 1.18 (1.10 to 1.26) | 1 707/8 638 | 1.07 (1.00 to 1.14) |
| Moderate | 6 685/32 436 | 1.23 (1.17 to 1.29) | 6 218/30 191 | 1.09 (1.05 to 1.14) |
| High | 2 663/11 725 | 1.30 (1.22 to 1.38) | 1 840/8 452 | 1.10 (1.03 to 1.17) |
| Very high | 768/2 966 | 1.35 (1.23 to 1.49) | 508/1 987 | 1.17 (1.05 to 1.31) |
| Ptrendc | <.001 | <.001 | ||
| Distal colon | ||||
| No use | 2 771/16 128 | 1 (Referent) | 4 051/20 682 | 1 (Referent) |
| Low | 1 174/6 153 | 1.10 (1.02 to 1.19) | 1 385/6 902 | 1.00 (0.94 to 1.07) |
| Moderate | 5 526/25 810 | 1.23 (1.17 to 1.29) | 4 925/23 813 | 1.03 (0.98 to 1.08) |
| High | 1 941/8 974 | 1.25 (1.17 to 1.34) | 1 212/6 465 | 0.93 (0.86 to 1.00) |
| Very high | 452/2 255 | 1.16 (1.03 to 1.30) | 291/1 458 | 0.96 (0.84 to 1.10) |
| Ptrendc | <.001 | .56 | ||
| Rectum | ||||
| No use | 3 709/18 793 | 1 (Referent) | 4 975/23 990 | 1 (Referent) |
| Low | 1 482/6 900 | 1.08 (1.01 to 1.15) | 1 607/7 594 | 1.01 (0.94 to 1.07) |
| Moderate | 5 857/28 969 | 1.02 (0.97 to 1.07) | 5 180/26 806 | 0.92 (0.88 to 0.96) |
| High | 1 833/9 644 | 0.96 (0.90 to 1.03) | 1 286/6 752 | 0.91 (0.84 to 0.97) |
| Very high | 490/2 544 | 0.98 (0.88 to 1.09) | 323/1 708 | 0.91 (0.80 to 1.04) |
| Ptrendc | .44 | <.001 | ||
Antibiotics use was categorized as no use (no prescriptions during the study period), low (1-10 days), moderate (11-60 days), high (61-180 days), and very high (>180 days) use, using defined daily doses. CI = confidence interval; CRC = colorectal cancer; OR = odds ratio.
Odds ratios, conditioned on matching factors (age, sex, county) and adjusted for socioeconomic factors (level of education, country of birth, marital status) and health-care utilizations prior the 2 years preceding colorectal cancer diagnosis (number of specialist visits and hospitalizations).
The Ptrend represents a trend test in which the 5 categories of antibiotics use were included in the model as a continuous variable.
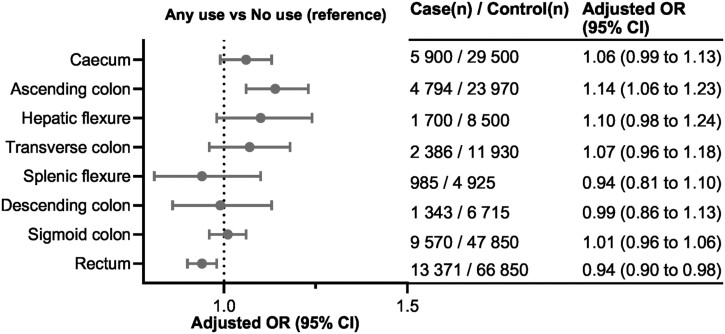
In analyses stratified by tumor site in the colorectum (Table 2), the dose-response association between antibiotics use and CRC risk was mostly confined to proximal colon cancer for moderate use (OR = 1.09, 95% CI = 1.05 to 1.14) and for very high use (OR = 1.17, 95% CI = 1.05 to 1.31) vs no use (Ptrend < .001). Associations were close to null for distal colon cancer and slightly inverse for rectal cancer (Table 2), with odds ratios of 0.96 (95% CI = 0.84 to 1.10; Ptrend = .56) and 0.91 (95% CI = 0.80 to 1.04), respectively, for very high use vs no use (Ptrend < .001). Stratification by tumor subsites, in the analysis of any use vs no use of antibiotics, revealed a risk gradient along the colorectal continuum (Figure 2), with the strongest positive association in the ascending colon and an inverse association in the rectum.
Figure 2.
Associations between antibiotics use and risk of colorectal cancer (CRC) by tumor subsites. Odds ratios (OR), conditioned on matching factors (age, sex, county) and adjusted for socioeconomic factors (level of education, country of birth, marital status) and health-care utilizations prior the 2 years preceding CRC diagnosis (number of specialist visits and hospitalizations). Antibiotics use during the 2 years preceding CRC diagnosis was excluded to account for possible reverse causation. CI = confidence interval.
In analyses of all antibiotics use, further stratified by sex (Supplementary Table 3, available online), an inverse association for rectal cancer was observed in women only, with an odds ratio of 0.86 (95% CI = 0.80 to 0.92) for moderate use and 0.84 (95% CI = 0.76 to 0.94) for high use vs no use (Ptrend < .001). A statistically significant interaction was found between antibiotics use and sex for rectal cancer (Pinteraction = .002) but not for proximal or distal colon cancer (Pinteraction = .81 and .33, respectively). Similarly, tests for heterogeneity showed statistically significant differences between men and women for rectal cancer (Phet = .004) but not for proximal or distal colon cancer (Phet = 1.00 and .35, respectively).
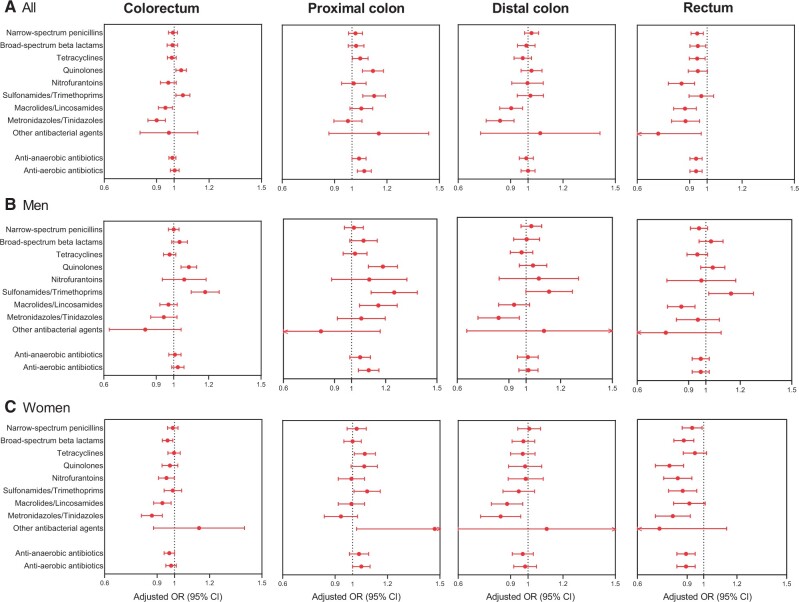
We further examined the association between antibiotics use and risk of CRC separately in 9 classes of antibiotics (Supplementary Table 1, available online). In these analyses, quinolones and sulfonamides and/or trimethoprims were associated with increased risk of proximal colon cancer (Figure 3; Supplementary Table 4, available online), whereas nitrofurantoins, macrolides and/or lincosamides, and notably, metronidazoles and/or tinidazoles (which exclusively inhibit anaerobic bacteria) were inversely associated with rectal cancer (Figure 3). Antibiotics grouped according to effect on anaerobic bacteria or primarily effecting aerobic bacteria showed roughly similar associations (Figure 3;Supplementary Figure 1, available online). Associations for rectal cancer in women were consistently inverse across antibiotics classes.
Figure 3.
Associations between antibiotics classes and risk of site-specific colorectal cancer, stratified by sex. Odds ratios (OR), conditioned on matching factors (age, sex, county) and adjusted for socioeconomic factors (level of education, country of birth, marital status) and health-care utilizations prior the 2 years preceding colorectal cancer (CRC) diagnosis (number of specialist visits and hospitalizations). Antibiotics use during the 2 years preceding CRC diagnosis was excluded to account for possible reverse causation. Antibiotics with effect on both anaerobic and aerobic bacteria, and metrodinazoles and/or tinidazoles (which only affect anaerobic bacteria) were categorized as anti-anaerobic antibiotics. Antibiotics that primarily or only affect aerobic bacteria were categorized as anti-aerobic antibiotics. Any use of specific antibiotics class was compared with no use of the specific antibiotics class (reference category) during the study period. Results for all participants (A), men (B), and women (C) are shown. CI = confidence interval.
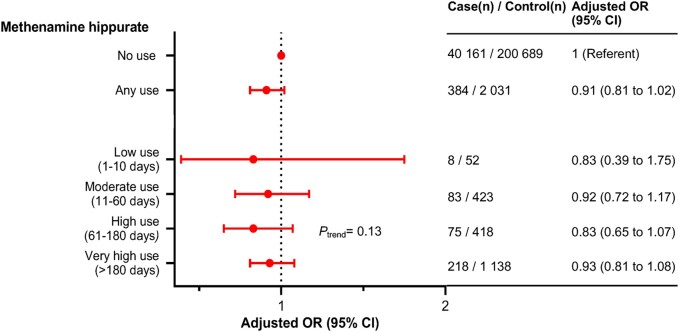
For methenamine hippurate, a urinary tract antiseptic with no known effects on gut microbiota, the associations with overall risk of CRC were null (OR for very high use vs no use = 0.93, 95% CI = 0.81 to 1.08; Ptrend = .13) (Figure 4). Methenamine hippurate use was related to women, higher age, and higher antibiotics use (respective P < .001) (data not shown).
Figure 4.
Associations between methenamine hippurate use and risk of colorectal cancer (CRC). Odds ratios (OR), conditioned on matching factors (age, sex, county) and adjusted for socioeconomic factors (level of education, country of birth, marital status) and health-care utilizations prior the 2 years preceding CRC diagnosis (number of specialist visits and hospitalizations). Methenamine hippurate use during the 2 years preceding CRC diagnosis was excluded to account for possible reverse causation. The Ptrend represents a trend test in which the 5 categories of antibiotics use were included in the model as a continuous variable. CI = confidence interval.
Results stratified by age at diagnosis are presented in Supplementary Table 5 (available online), and further analyses stratified by both age and sex are in Supplementary Tables 6 and 7 (available online). The association between antibiotics use and risk of proximal colon cancer was clearer among participants aged 50 years and older at the time of diagnosis, compared with participants aged younger than 50 years.
In analyses stratified by tumor stage, the positive association between antibiotics use and risk of proximal colon cancer was more pronounced in stage I-II cancer compared with stage III-IV cancer (Supplementary Table 8, available online). In contrast, the inverse association in rectal cancer was limited to stage III-IV.
We also performed analyses based on follow-up time from exposure to diagnosis (Supplementary Table 9, available online). Only individuals with a single antibiotic prescription were included to eliminate potential confounding by frequent prescriptions. The analysis confirmed the presence of reverse confounding. Analyses based on the total number of antibiotics prescriptions showed similar patterns of association as for defined daily doses (Supplementary Table 10, available online).
Discussion
In this nationwide analysis of more than 40 000 CRC cases, we found a robust association between antibiotics use and higher risk of proximal colon cancer, consistent with previous investigations (18–23,29). We also provide important and timely confirmation of the inverse association between antibiotics use and rectal cancer risk recently reported (24). By adding stratification on sex, we show that this inverse association occurs primarily in women. Furthermore, we report results based on fine anatomic detailing of the colorectum, using stratification by follow-up time and stage at diagnosis to explore the role of timing of antibiotics exposure in the carcinogenic process. Finally, a null association between use of methenamine hippurate, a urinary antiseptic acting locally in the urinary bladder, and CRC risk provides indirect support for dysbiosis of the gut flora as a mechanism behind the association between antibiotics use and colonic carcinogenesis.
In our study, analyses stratified by specific anatomical tumor subsites demonstrated that the association was most pronounced in the ascending colon, after which it diminished, supporting the concept of the colon as a continuum (30). The observed risk gradient along the colorectal continuum is consistent with a high microbial impact in the proximal colon and a decreasing concentration of short-chain fatty acids along the colon, resulting in higher bacterial activity, biofilm formation, and fermentation in the proximal compared with the distal colon and rectum (31–34). Moreover, the positive associations between antibiotics use and proximal colon cancer began at the lowest level of antibiotics use, providing a potential justification for reducing antibiotics prescriptions in clinical practice.
The weak inverse association between antibiotics use and rectal cancer risk has been previously reported, with sexually transmitted infection suggested as a possible explanation (24). In our study, sex-stratified analyses revealed a clear sex difference with an inverse association with rectal cancer, mostly pronounced in women. Interaction and heterogeneity tests also supported the potential effect modification by sex for rectal cancer. Though speculative, sexually transmitted infections may be a possible explanation. Infection of the rectum such as Chlamydia infection occurs frequently in women as a secondary infection because of the closer proximity to the primary infection site (vagina vs male urethra) (35). Chlamydia infections have malignant potential (36) and can persist, triggering inflammation and reducing apoptosis in infected cells (37).
In the analyses stratified by antibiotics classes, quinolones and sulfonamides and/or trimethoprims were associated with increased risk of proximal cancer in both sexes, possibly reflecting the effect of these antibiotics on bacterial diversity (14). The limited effect of these antibiotics on anaerobic bacteria would favor anaerobic bacteria such as Fusobacteria species and Bacteroidetes species, which may have a role in CRC development (8–11,38). On the other hand, nitrofurantoins, macrolides and/or lincosamides, and metronidazoles and/or tinidazoles were inversely associated with rectal cancer. The potent effect of metronidazoles and/or tinidazoles and lincosamides on anaerobic bacteria could reduce Fusobacteria and Bacteroidetes. This is in line with the hypothesis that a gut flora with more abundant Fusobacteria and Bacteroidetes may contribute to CRC development. Our findings support the existence of heterogeneity in antibiotics effects along the colorectum, as concluded by Zhang et al. (24).
Use of methenamine hippurate was associated with higher antibiotics use. This was expected because methenamine hippurate is used for recurrent urinary tract infections, which previously have been treated with antibiotics. Yet, the association between methenamine hippurate use and CRC risk was null, strengthening the interpretation of an etiological role for antibiotics in CRC mediated through the gut microbiome. However, we acknowledge that even though this supports the idea that antibiotics use is causally related indirectly to an increase in the risk of proximal colon cancer, any interpretation of causality should be done with caution.
To understand at what phase of the carcinogenic process the putative role of antibiotics occurs, we conducted subgroup analyses by disease stage and follow-up time from exposure to CRC diagnosis. For proximal colon cancer, antibiotics use was associated with higher risk of stage I-II, but not III-IV disease, possibly suggesting an early role for antibiotics. However, compared with other studies (20,23,24), the follow-up time in our dataset is relatively short, between 2.5 and 9.5 (a median of 6) years after excluding antibiotics prescribed within 2 years prior to diagnosis. Therefore, we were unable to address the role of antibiotics prescribed more than 10 years prior to diagnosis, probably the most critical time period for CRC initiation, which generally takes more than 10 years to develop. No previous study has investigated associations between antibiotics use and CRC risk stratified by tumor stage, and datasets with longer follow-up times will be required to confirm our findings. Furthermore, the inverse association for rectal cancer was strongest for stage III-IV disease, suggesting that antibiotics might play a role in slowing tumor progression in the rectum. A caveat with respect to follow-up time is the possibility of reverse causation, if symptomatic undiagnosed CRC leads to increased antibiotics use in the late prediagnostic phase, but excluding exposures in the 2 years before diagnosis should minimize this issue.
A major limitation in our study is the possibility of unmeasured confounding such as diet, anthropometric measurements, medical comorbidities such as diabetes or inflammatory bowel disease, and use of other medications. To account for confounding, we adjusted our final model for socioeconomic factors, number of specialist visits, and number of hospitalizations. These factors tend to correlate with CRC risk factors such as diet, lifestyle, and body size (39,40), and the variables for specialist visits and hospitalizations should capture relevant comorbidities to a substantial degree (41–44). These adjustments also account for potential confounding by a health-care–seeking behavior (ie, extensive use of health-care services) resulting in a higher likelihood of being prescribed antibiotics, as well as being referred to colonoscopy for gastrointestinal symptoms. Previous studies that accounted for numerous dietary and lifestyle factors (20) and specific comorbidities such as inflammatory bowel disease (24) reported similar risk estimates to ours, with no material attenuations. Furthermore, confounding by unmeasured CRC risk factors would be expected to yield false-positive associations between antibiotics use and a higher risk of CRC. However, for rectal cancer, an inverse association was observed. For these reasons, although we acknowledge the lack of data on some potential confounders, including specific comorbidities, as a limitation of this study, we do not believe that confounding due to specific comorbidities can explain our findings.
Many countries have implemented CRC screening programs. This might bias results in studies of CRC risk, as some individuals are more likely to comply with the screening program. However, at the time of our study, there was no national CRC screening in Sweden. On the local level, organized screening programs have been in place in Stockholm and Gotland counties since 2008 (45). In the current study, cases and controls are matched on county of residence, and any influence of population-based CRC screening on the observed associations is likely to be minimal.
Other limitations included lack of data on antibiotics administered during inpatient care and data on antibiotics use before 2005 (the start of the pharmaceutical register) resulting in less than 10 years follow-up time. Despite this, the size of our dataset allowed us to identify clear associations, which, in light of previous findings (18–24,29,46), seem likely to be strengthened in the future when more time has passed. We could not account for patient compliance. However, compliance is generally considered high for antibiotics (47), and the register data in our study are for antibiotics not just prescribed but actually dispensed from the pharmacy (and includes primarily oral antibiotics). Finally, the extensive analyses in our study resulted in 32 statistical comparisons, raising the issue of chance findings because of multiple testing. Applying a Bonferroni-corrected P value threshold of .002 (α value divided by the number of hypotheses = 0.05/32), our main findings remain statistically significant.
The main strength of this investigation was the use of high-quality, nationwide, registry-based data, allowing us to conduct the largest and most comprehensive original research study to date on antibiotics and CRC risk. More than 98% of all diagnosed CRC cases have been reported to the Swedish Colorectal Cancer Register, making it a reliable register for research (48,49). The Swedish Prescribed Drug Register, one of the largest pharmaco-epidemiological databases in the world, provides complete national data on dispensed drugs in the Swedish population (50). The LISA database and the Swedish Inpatient Register also have high validity (51,52). Our results are, hence, generalizable and comparable with other northern European countries. The large sample size further allowed us to conduct well-powered and detailed subgroup analyses with great precision.
In conclusion, we observed a consistent association between antibiotics use and higher subsequent risk of proximal colon cancer and an inverse association for rectal cancer in women. Our findings strengthen prior evidence and provide new insights into site-specific carcinogenesis as well as indirect support for the role of gut microbiota.
Funding
This work was supported by the Lion’s Cancer Research Foundation, Umeå University (LP 172154), and Region Västerbotten (RV 932777).
Notes
Role of the funders: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures: All authors declare no support from any organization for the submitted work. Competing interests are reported for 3 authors. BVG reports grants from Region Västerbotten during the conduct of the study, RM reports personal fees form AstraZeneca (outside the submitted work), ÅG reports other from Public Health Agency of Sweden, grants from Swedish research council, grants from Swedish Government Fund for Clinical Research, other from Norrlands University hospital, nonfinancial support from Institut Français de Suède, outside the submitted work; and unpaid medical advisor in the small biotech company Quretec Bio AB. There are no other relationships or activities that could appear to have influenced the submitted work.
Author contributions: SSML: Data Curation; Formal Analysis; Investigation; Methodology; Visualization; Writing-Original Draft; Writing-Review & Editing. ZM: Data Curation; Formal Analysis; Investigation; Writing-Review & Editing. CH: Methodology; Writing-Review & Editing. RM: Data Curation; Methodology; Writing-Review & Editing. EL: Data Curation; Writing-Review & Editing. ÅG: Data Curation; Methodology; Writing-Review & Editing. BVG: Conceptualization; Funding acquisition; Methodology; Resources; Supervision; Writing-Review & Editing. SH: Conceptualization; Data Curation; Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing-Review & Editing.
Acknowledgements: We thank Dr Jennifer Stewart Williams and Professor Stig Wall from the Department of Epidemiology and Global Health, Umeå University, for reviewing our initial manuscript. Our gratitude also goes to reviewers for their time and expertise, which helped improve the manuscript.
Prior presentations: Presentation number-1055, American Association for Cancer Research (AACR) annual meeting (June 22-24, 2020).
Data Availability
The data that support the findings of this study may be requested from the included Swedish registers. Ethical and legal restrictions may apply to the availability of these data, and study-specific ethical permissions are required.
Supplementary Material
References
- 1. Marley AR, Nan H.. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7(3):105–114. [PMC free article] [PubMed] [Google Scholar]
- 2. Brenner H, Kloor M, Pox CP.. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. [DOI] [PubMed] [Google Scholar]
- 3. Aleksandrova K, Pischon T, Jenab M, et al. Combined impact of healthy lifestyle factors on colorectal cancer: a large European cohort study. BMC Med. 2014;12(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chan AT, Giovannucci EL.. Primary prevention of colorectal cancer. Gastroenterology. 2010;138(6):2029–2043.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petra L, Georgina LH, Harry JF.. The gut microbiota, bacterial metabolites and colorectal cancer. Nat Rev Microbiol. 2014;12(10). [DOI] [PubMed] [Google Scholar]
- 6. Raskov H, Burcharth J, Pommergaard H-C.. Linking gut microbiota to colorectal cancer. J Cancer. 2017;8(17):3378–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tingting W, Guoxiang C, Yunping Q, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2011;6(2):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saus E, Iraola-Guzmán S, Willis JR, et al. Microbiome and colorectal cancer: roles in carcinogenesis and clinical potential. Mol Aspects Med. 2019;69:93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dahmus JD, Kotler DL, Kastenberg DM, et al. The gut microbiome and colorectal cancer: a review of bacterial pathogenesis. J Gastrointest Oncol. 2018;9(4):769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mima K, Cao Y, Chan AT, et al. Fusobacterium nucleatum in colorectal carcinoma tissue according to tumor location. Clin Transl Gastroenterol. 2016;7(11):e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bouter KE, van Raalte DH, Groen AK, et al. Role of the gut microbiome in the pathogenesis of obesity and obesity-related metabolic dysfunction. Gastroenterology. 2017;152(7):1671–1678. [DOI] [PubMed] [Google Scholar]
- 13. Singh R, Chang H, Yan D, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ianiro G, Tilg H, Gasbarrini A.. Antibiotics as deep modulators of gut microbiota: between good and evil. Gut. 2016;65(11):1906–1915. [DOI] [PubMed] [Google Scholar]
- 15. Bhalodi AA, van Engelen TSR, Virk HS, et al. Impact of antimicrobial therapy on the gut microbiome. J Antimicrob Chemother. 2019;74(suppl 1):i6–i15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Slimings C, Riley TV.. Antibiotics and hospital-acquired Clostridium difficile infection: update of systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(4):881–891. [DOI] [PubMed] [Google Scholar]
- 17. Abreu MT, Peek RM.. Gastrointestinal malignancy and the microbiome. Gastroenterology. 2014;146(6):1534–1546.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dik V, Oijen M, Smeets H, et al. Frequent use of antibiotics is associated with colorectal cancer risk: results of a nested case-control study. Dig Dis Sci. 2016;61(1):255–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang JL, Chang CH, Lin JW, et al. Infection, antibiotic therapy and risk of colorectal cancer: a nationwide nested case-control study in patients with type 2 diabetes mellitus. Int J Cancer. 2014;135(4):956–967. [DOI] [PubMed] [Google Scholar]
- 20. Cao Y, Wu K, Mehta R, et al. Long-term use of antibiotics and risk of colorectal adenoma. Gut. 2018;67(4):672–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kilkkinen A, Rissanen H, Klaukka T, et al. Antibiotic use predicts an increased risk of cancer. Int J Cancer. 2008;123(9):2152–2155. [DOI] [PubMed] [Google Scholar]
- 22. Armstrong D, Dregan A, Ashworth M, et al. The association between colorectal cancer and prior antibiotic prescriptions: case control study. Br J Cancer. 2020;122(6):912–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boursi B, Haynes K, Mamtani R, et al. Impact of antibiotic exposure on the risk of colorectal cancer. Pharmacoepidemiol Drug Saf. 2015;24(5):534–542. [DOI] [PubMed] [Google Scholar]
- 24. Zhang J, Haines C, Watson AJM, et al. Oral antibiotic use and risk of colorectal cancer in the United Kingdom, 1989-2012: a matched case-control study. Gut. 2019;68(11):1971–1978. [DOI] [PubMed] [Google Scholar]
- 25. Ludvigsson J, Otterblad-Olausson P, Pettersson B, et al. The Swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brierley JD, Gospodarowicz MK, Wittekind C.. TNM Classification of Malignant Tumours. Chicester, UK: John Wiley & Sons, Inc; 2017. [Google Scholar]
- 27. Lubin JH, Gail MH.. Biased selection of controls for case-control analyses of cohort studies. Biometrics. 1984;40(1):63–75. [PubMed] [Google Scholar]
- 28.World Health Organization. WHO collaborating center for drug statistics methodology: defined daily dose definitions and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/. Accessed April 19, 2019.
- 29. Wan Q-Y, Zhao R, Wang Y, et al. Antibiotic use and risk of colorectal cancer: a meta-analysis of 412 450 participants. Gut. 2020;69(11):2059–2060. [DOI] [PubMed] [Google Scholar]
- 30. Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61(6):794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Christine MD, Elizabeth CW, Elizabeth MH, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci. 2014;111(51):18321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnoldini M, Cremer J, Hwa T.. Bacterial growth, flow, and mixing shape human gut microbiota density and composition. Gut Microbes. 2018;9(6):559–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Den Besten G, van Eunen K, Groen A, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res .2013;54(9):2325–2340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136(1):65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heijne JCM, van Liere GAFS, Hoebe CJPA, et al. What explains anorectal chlamydia infection in women? Implications of a mathematical model for test and treatment strategies. Sex Transm Infect. 2017;93(4):270–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chumduri C, Gurumurthy R K, Zadora P K, et al. Chlamydia infection promotes host DNA damage and proliferation but impairs the DNA damage response. Cell Host Microbe. 2013;13(6):746–758. [DOI] [PubMed] [Google Scholar]
- 37. Fischer SF, Vier J, Kirschnek S, et al. Chlamydia inhibit host cell apoptosis by degradation of proapoptotic BH3-only proteins. J Exp Med. 2004;200(7):905–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshino Y, Kitazawa T, Ikeda M, et al. Clinical features of Bacteroides bacteremia and their association with colorectal carcinoma. J Infect Dis. 2012;40(1):63–67. [DOI] [PubMed] [Google Scholar]
- 39. Doubeni CA, Laiyemo AO, Major JM, et al. Socioeconomic status and the risk of colorectal cancer: an analysis of more than a half million adults in the National Institutes of Health-AARP Diet and Health Study (Report). Cancer. 2012;118(14):3636–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Anu M. The contribution of lifestyle factors to socioeconomic differences in obesity in men and women: a population-based study in Sweden. Eur J Epidemiol. 2003;18(3):227–234. [DOI] [PubMed] [Google Scholar]
- 41. Balasubramaniam K, Elnegaard S, Rasmussen S, et al. Lifestyle, socioeconomic status and healthcare seeking among women with gynaecological cancer alarm symptoms: a combined questionnaire-based and register-based population study. BMJ Open. 2018;8(7):e021815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tomic K, Ventimiglia E, Robinson D, et al. Socioeconomic status and diagnosis, treatment, and mortality in men with prostate cancer. Nationwide population-based study. Int J Cancer. 2018;142(12):2478–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hughes AE, Tiro JA, Balasubramanian BA, et al. Social disadvantage, healthcare utilization, and colorectal cancer screening: leveraging longitudinal patient address and health records data. Cancer Epidemiol Biomarkers Prev. 2018;27(12):1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mark C, Robin U, Cynthia K, et al. Impact of comorbidity and healthcare utilization on colorectal cancer stage at diagnosis: literature review. Cancer Causes Control. 2012;23(2):213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Basu P, Ponti A, Anttila A, et al. Status of implementation and organization of cancer screening in the European Union Member States—summary results from the second European screening report. Int J Cancer. 2018;142(1):44–56. [DOI] [PubMed] [Google Scholar]
- 46. Simin J, Fornes R, Liu Q, . et al. Antibiotic use and risk of colorectal cancer: a systematic review and dose-response meta-analysis. Br J Cancer. 2020;123(12):1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Axelsson M. Report on personality and adherence to antibiotic therapy: a population-based study. BMC Psychol . 2013;1(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kodeda K, Nathanaelsson L, Jung B, et al. Population‐based data from the Swedish Colon Cancer Registry. Br J Surg. 2013;100(8):1100. [DOI] [PubMed] [Google Scholar]
- 49. Moberger P, Sköldberg F, Birgisson H.. Evaluation of the Swedish colorectal cancer registry: an overview of completeness, timeliness, comparability and validity. Acta Oncologica (Stockholm, Sweden. 2018;57(12):1611–1621. [DOI] [PubMed] [Google Scholar]
- 50. Wettermark B, Hammar N, Michaelfored C, et al. The new Swedish Prescribed Drug Register—opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735. [DOI] [PubMed] [Google Scholar]
- 51. Ludvigsson J, Svedberg P, Olén O, et al. The longitudinal integrated database for health insurance and labour market studies (LISA) and its use in medical research. Eur J Epidemiol. 2019;34(4):423–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11(1):450–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study may be requested from the included Swedish registers. Ethical and legal restrictions may apply to the availability of these data, and study-specific ethical permissions are required.