Abstract
Background
Cancer-related cognitive decline (CRCD) is an important clinical problem, but limited research exists on assessment of cognitive function in patients with lymphoma.
Methods
The overall objective of this nationwide, prospective, observational study conducted in the National Cancer Institute Community Clinical Oncology Research Program (NCORP) was to assess changes in memory, attention, and executive function in patients with lymphoma from pre- (A1) to postchemotherapy (A2) and to 6 months postchemotherapy (A3). Individuals without cancer served as noncancer controls, paired to patients by age and sex, and assessed at the same time-equivalent points. Longitudinal linear mixed models (LMM) including A1, A2, and A3 and adjusting for age, education, race, sex, cognitive reserve score, baseline anxiety, and depressive symptoms were fit. We assessed changes in patients compared with control participants without cancer and assessed differences in cognitive function in those patients with Hodgkin vs non-Hodgkin disease and by disease subtype. All statistical tests were 2-sided.
Results
Patients with lymphoma (n = 248) and participants without cancer serving as controls (n = 212) were recruited from 19 NCORP sites. From pre- to postchemotherapy and from prechemotherapy to 6 months follow-up, patients reported more cognitive problems over time compared with controls (Functional Assessment of Cancer-Therapy-Cognitive Function [FACT-Cog] perceived cognitive impairment effect size (ES) = 0.83 and 0.84 for A1 to A2 and A1 to A3, respectively; P < .001; single-item cognitive symptoms ES range = 0.55 to 0.70 inclusive of A1 to A2 and A1 to A3; P < .001); the complaints were more pronounced in women with lymphoma compared with men with lymphoma (FACT-Cog Perceived Cognitive Impairment (PCI) score group-by-time-by-sex interaction, P = .007). Patients with lymphoma also performed statistically significantly less well on tests of verbal memory and delayed recall, attention and executive function, and telephone-based category fluency.
Conclusion
Patients with lymphoma experience worse patient-reported and objectively assessed cognitive function from prechemotherapy to 6-month follow-up compared with age- and sex-paired controls without cancer assessed at similar time intervals.
Cancer-related cognitive decline (CRCD) is an important clinical problem with a negative impact on quality of life (1-5). CRCD has been fairly well characterized in patients with common solid tumors, showing that chemotherapy exacerbates CRCD (5–21); however, less is known about how chemotherapy affects cognitive function in patients with hematologic malignancies (22). Post-treatment cross-sectional studies have shown that survivors of lymphoma have long-term cognitive deficits compared with individuals without cancer or normative scores (23–25). One longitudinal study in patients with lymphoma, without a pretreatment assessment or control group, has suggested that chemotherapy may negatively impact cognitive function over time (26). Because the disease biology of lymphoma is systemic and affects the immune system, it is important to consider the pretreatment impact of disease on cognition (27,28). It is also not clear if CRCD affects men and women differently as few studies have been able to systematically address sex differences, although the results of a large study of patients with colorectal cancer showed that men had a greater risk of cognitive decline than women on some cognitive outcomes (21).
The primary aim of this study was to assess longitudinal changes in cognition in patients with lymphoma from pre- to postchemotherapy and from prechemotherapy to 6 months postchemotherapy compared with age- and sex-paired controls assessed at the same time intervals. We evaluated baseline effects and trajectories of cognitive complaints measured by self-report as well as performance on objective neurocognitive tests of memory, attention, and executive function.
We also investigated and controlled for several factors that are known or thought to influence cognitive function including age, sex, race, education (29), cognitive reserve (30), lymphoma type (31,32), chemotherapy regimen, and anxiety and depression symptoms (16,18–20,32). We hypothesized that patients with lymphoma scheduled to receive chemotherapy would experience more cognitive problems than noncancer controls at baseline and that these changes would persist up to 6 months postchemotherapy.
Methods
Participants
Patients with lymphoma and individuals without cancer serving as noncancer controls were recruited from 19 National Cancer Institute Community Oncology Research Program (NCORP) locations nationwide. Eligibility included the following: 1) diagnosis of intermediate or high-grade lymphoma defined by the treating physician, 2) scheduled for a standard course of chemotherapy (with or without biologics), 3) chemotherapy naïve, 4) 21 years of age or older and able to speak and read English, 5) no confirmed central nervous system disease, 6) no neurodegenerative disease diagnosis, 7) no recent major psychiatric illness leading to hospitalization within the past year, 8) life expectancy greater than 10 months, and 9) no plan to receive concurrent radiation during chemotherapy. Control participants without cancer were the same age (within 5 years) and sex as the patients and met eligibility criteria 3-8. This study was approved by the institutional review boards of each NCORP and the University of Rochester Cancer Center (URCC) NCORP Research Base; all participants provided written informed consent. This cohort study was conducted concurrently with another cohort of patients with breast cancer and control participants without cancer, which finished accrual prior to this cohort; we published the results of the breast cancer study previously (16,33). Additional details are provided in the Supplementary Methods (available online) and in previous reports (16,33). We are presenting for the first time the results of the lymphoma cohort study.
Cognitive Assessments and Covariates
All cognitive assessments were completed predominately at the following timepoints for patients: 1) prechemotherapy baseline assessment within 7 days prior to the first chemotherapy administration (A1), 2) postchemotherapy assessment within 1 month of the last chemotherapy administration (A2), and 3) follow-up assessment at 6 months following assessment 2 (A3). Participants serving as controls also completed the same assessments at the same time intervals as patients.
All study staff were formally trained. A standardized cognitive assessment manual was used. Computerized testing was conducted, followed by paper-based testing, and then self-report measures. The phone-based measures were administered following the in-person assessments by URCC staff. We used the same testing paradigm as described in detail in our previous publication; these measures are summarized briefly (16,33).
Computerized Neuropsychological Assessments
Computerized tests were from the Cambridge Neuropsychological Test Automated Battery (CANTAB) and included the Delayed Match to Sample test (12-second delay), verbal recognition memory (VRM), rapid visual processing, and One Touch Stockings of Cambridge (34).
Paper-Based Neuropsychological Assessments
Paper-based assessments included the Hopkins Verbal Learning and Memory Test-Revised (HVLT-R) (35–37), the Trail Making Test (TMT) A (ie, Comprehensive TMT 1) and B (ie, Comprehensive TMT 5) (38–40), and the Controlled Oral Word Association test (COWA) (41,42).
Phone-Based Cognitive Assessments
The Brief Test of Adult Cognition by Telephone included the Rey Auditory Verbal Learning Test (RAVLT) immediate and delayed (based on 1 list learning trial), digits backward, category fluency, and backward counting (43).
FACT-Cog
Perceived cognitive function was assessed by the Functional Assessment of Cancer-Therapy-Cognitive Function (FACT-Cog) Version 2 (44). The FACT-Cog has 4 subscales: perceived cognitive impairment (PCI), perceived cognitive abilities, impact of perceived cognitive impairment on quality of life, and comments from others. Additionally, a total score can be computed summing all 4 subscales. Smaller values on these scales imply greater cognitive difficulties. A ½ SD as a cutoff for a minimal clinically important difference has been identified for this measure (45).
Single-Item
On a Likert-type scale (0 to 10), participants rated their level of difficulty over 7 days on 3 single items for remembering things, paying attention, and multitasking as part of a modified symptom inventory (46).
Covariate Measures
Participants self-identified age, sex, and race. Patient medical and treatment information was obtained from the medical record. Chemotherapy was categorized into 4 regimen types: rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP); bendamustine and rituximab (BR); doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD); or other agents. Baseline reading ability, a proxy for cognitive reserve, was assessed with the Wide Range Assessment Test-4th Edition (WRAT-4) reading subscale (47). Anxiety was assessed with the Spielberger Trait Anxiety Inventory (48), and depressive symptoms were measured by an item from the Multidimensional Fatigue Symptom Inventory (49).
Statistical Analyses
For comparison of baseline characteristics for the patients and controls, t tests were used for continuous variables, and χ2 tests were used for categorical variables.
The primary goal of this study was to assess trajectories of change in cognitive function from A1 to A2 and from A1 to A3 using longitudinal linear mixed modeling (LMM) adjusting for important a priori baseline covariates. We aimed to accrue 200 evaluable patients and 200 evaluable controls. Statistical computations were performed using R Version 3 (www.r-project.org) and SAS Version 9.4 (SAS Institute, Cary, NC). A 2-sided P-value of no more than .05 was considered statistically significant.
For LMM analyses, the fixed effects were time (assessments 1, 2, and 3 treated as nominal), group (patient or control), sex, age, education (less than high school, high school or general equivalency diploma [GED], college or graduate), race (Black, White, Other), cognitive reserve (WRAT), anxiety and depressive symptoms, and the following interactions: group-by-time, time-by-sex, group-by-sex, and group-by-time-by-sex. The random effect was subject-specific A1, A2, and A3 means with an unstructured covariance matrix. Based on graphical examination, for FACT-Cog total, we allowed group-specific covariance matrices; this was not necessary for the other measures. Estimation was performed using restricted maximum likelihood, and inferences were performed using the Kenward-Roger procedure (50). Appropriate contrasts were used to quantify the mean changes from A1 to A2 and from A1 to A3 (in addition to means for each time) by group and sex (for self-report measures). We provided estimates for both men and women computed from these models for those with statistically significant sex-related interactions.
We also fit similarly constructed models for the patients to assess the effects of lymphoma type and regimen type on cognitive outcomes. In women, we explored the effect of baseline menopausal status (ie, pre- vs peri- or postmenopausal) on cognitive outcomes.
All analyses were based on all available data. Missing data were assumed to be missing at random (51). We used general linear modeling to assess whether FACT-Cog PCI at A1 was predictive of dropout. The distribution of the TMT was highly skewed; values were log10-transformed. We also adjusted for multiple comparisons across all outcome measures with the Benjamini-Hochberg false discovery rate (FDR) and report the adjusted P values as indicated in the tables (52).
Results
Baseline Characteristics
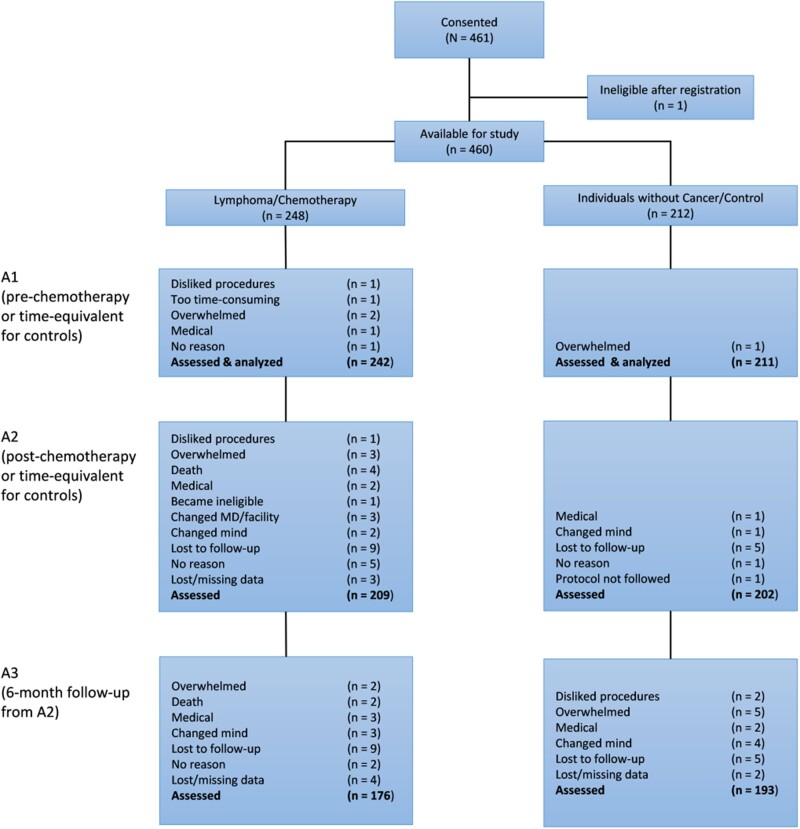
In total, 460 eligible participants consented to the study including 248 patients (62.5% male) and 212 controls (59.0% male). The patient and control groups were balanced with respect to age, race, ethnicity, WRAT-4 reading score (cognitive reserve), and marital status (Table 1). Groups based on sex were balanced with respect to age, race, education, ethnicity, and WRAT-4 reading score, but female patients showed higher anxiety and depressive symptoms (Supplementary Table 1, available online). Retention from A1 to A2 was 86.4% in the lymphoma group and 95.7% in noncancer controls. Retention was 72.7% in the lymphoma patient group at A3 and 91.5% in the noncancer control group (Figure 1). Baseline FACT-Cog PCI score was not predictive of those who dropped out at A2 or A3 by group or by sex. Of 248 patients with lymphoma, 186 had non-Hodgkin lymphoma, and of all lymphoma subtypes, 39.5% of patients had diffuse large B-cell lymphoma. The mean interval from A1 to A2 for those on ABVD was 113 (95% confidence interval [CI] = 99 to 127) days, BR was 132 (95% CI = 117 to 147) days, and R-CHOP was 103 (95% CI = 95 to 111) days.
Table 1.
Lymphoma patient and noncancer control participant characteristics and demographics
| Characteristic | Lymphoma/Chemotherapy | Control | Total | P |
|---|---|---|---|---|
| (n = 248) | (n = 212) | (N = 460) | ||
| Age, y | .30b | |||
| Mean (SD) | 55.40 (14.70) | 53.96 (14.98) | 54.74 (14.83) | |
| Range | 21.00-83.00 | 18.00-84.00 | 18.00-84.00 | |
| Sex, No. (%) | .44a | |||
| Female | 93 (37.5) | 87 (41.0) | 180 (39.1) | |
| Male | 155 (62.5) | 125 (59.0) | 280 (60.9) | |
| Race, No. (%) | .44a | |||
| White | 232 (93.6) | 197 (92.9) | 429 (93.3) | |
| Black | 11 (4.4) | 7 (3.3) | 18 (3.9) | |
| Other | 5 (2.0) | 8 (3.8) | 13 (2.8) | |
| Ethnicity, No. (%) | .84a | |||
| Hispanic/Latino | 5 (2.0) | 6 (2.8) | 11 (2.4) | |
| Not Hispanic/Latino | 238 (96.0) | 201 (94.8) | 439 (95.4) | |
| Unknown | 5 (2.0) | 5 (2.4) | 10 (2.2) | |
| Education, No. (%) | .02a | |||
| < High school | 7 (2.8) | 1 (0.5) | 8 (1.7) | |
| High school/GED | 51 (20.6) | 27 (12.7) | 78 (17.0) | |
| Partial College and Higher | 189 (76.2) | 184 (86.8) | 373 (81.1) | |
| Unknown | 1 (0.4) | 0 (0.0) | 1 (0.2) | |
| Marital status, No. (%) | .48a | |||
| Married | 181 (73.0) | 149 (70.3) | 330 (71.7) | |
| Divorced | 16 (6.4) | 18 (8.5) | 34 (7.4) | |
| Long-term relationship | 18 (7.3) | 9 (4.2) | 27 (5.9) | |
| Separated | 3 (1.2) | 2 (0.9) | 5 (1.1) | |
| Single | 23 (9.3) | 23 (10.8) | 46 (10.0) | |
| Widowed | 7 (2.8) | 11 (5.1) | 18 (3.9) | |
| WRAT 4 Reading, mean (SD) | 62.86 (6.16) | 63.67 (4.83) | 63.24 (5.59) | .12b |
| Anxiety (STAI), mean (SD) | 34.95 (12.58) | 27.46 (9.21) | 31.48 (11.74) | <.001b |
| Depression item, mean (SD) | 0.64 (0.97) | 0.30 (0.61) | 0.48 (0.84) | <.001b |
| Lymphoma type, No. (%) | ||||
| Hodgkin | 51 (20.6) | — | — | |
| Non-Hodgkin | 186 (75.0) | — | — | |
| Unknown | 11 (4.4) | — | — | |
| Lymphoma subtype, No. (%) | ||||
| NHL subtypes | ||||
| DLBCL | 98 (39.5) | — | — | |
| Follicular | 41 (16.5) | — | — | |
| Other B cell | 29 (11.7) | |||
| T cell | 8 (3.2) | — | — | |
| HL subtype | — | — | ||
| Classical | 45 (18) | — | — | |
| Lymphocyte predominant | 3 (1.2) | — | — | |
| Unknown subtype | 24 (9.7) | — | — | |
| Lymphoma regimen, No. (%) | ||||
| ABVD | 42 (16.9) | — | — | |
| BR | 35 (14.1) | — | — | |
| Other | 26 (10.5) | — | — | |
| R-CHOP | 116 (46.8) | — | — | |
| Unknown | 29 (11.7) | — | — |
Two-sided Pearson χ2 test. ABVD = doxorubicin, bleomycin, vinblastine, and dacarbazine; BR = bendamustine and rituximab; DLBCL = diffuse large B-cell lymphoma; GED = general equivalency diploma; NHL = non-Hodgkin lymphoma; R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone; STAI = Spielberger Trait Anxiety Inventory; WRAT-4 = Wide Range Achievement Test, 4th edition; — = not applicable.
Welch t test. All tests were 2-sided.
Figure 1.
Participant flowchart. A = assessment.
Perceived Cognitive Impairment
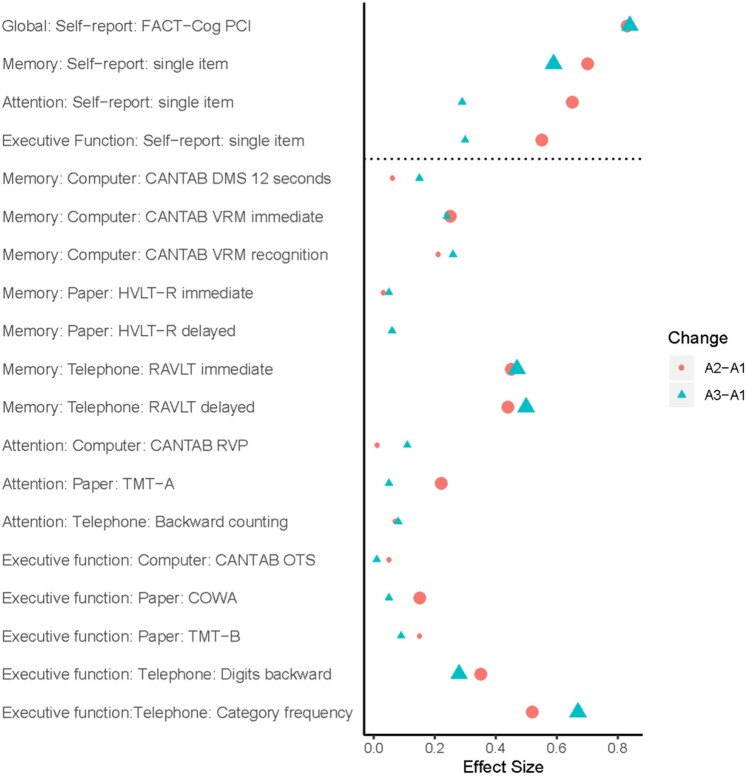
Cronbach α values for FACT-Cog PCI scores were 0.96 and 0.94 for patients and controls, respectively, and 0.96 overall. Cronbach α values for FACT-Cog total scores were 0.92 and 0.87 for patients and controls, respectively, and 0.91 overall. At baseline, patients with lymphoma self-reported statistically significantly more problems on the FACT-Cog (PCI and total scores) compared with sex-paired controls (P < .05; Table 2). Patients reported statistically significantly more attentional difficulty compared with controls (P = .01; Table 2). From A1 to A2 and from A1 to A3, patients with lymphoma reported statistically significantly greater perceived cognitive impairment (FACT-Cog PCI, FACT-Cog total, single item) compared with controls over time (P < .05; Table 2). After adjusting for multiple comparisons, all associations remained statistically significant except for the associations of attention and multitasking ability single items from A1 to A3. The FACT-Cog PCI effect size (ES) was 0.83 and 0.84 for A1 to A2 and A1 to A3, respectively (FDR-adj. P < .001), and the single item cognitive symptoms ES ranged from 0.55 and 0.70 inclusive of A1 to A2 and A1 to A3 (FDR-adj. P < .001; Figure 2; Supplementary Table 2, available online).
Table 2.
Self-report, memory, attention, and executive function measures at baseline (prechemotherapy [A1], postchemotherapy [A2], and 6 months follow-up [A3]) and changes from pre- to postchemotherapy and changes from prechemotherapy to 6 months follow-up in patients with lymphoma and age- and sex-paired controls assessed at the same equivalent times
| Domain and test/measure | Outcome | Better score | Chemotherapy |
Control |
Baseline: chemotherapy (A1) - control (A1) |
Pre- to post- chemotherapy: chemotherapy (A2-A1) - control (A2-A1) |
Pre-Chemotherapy to 6 months follow-up: chemotherapy (A3-A1) - control (A3-A1) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A1 | A2 | A3 | Adjusted β (SE) | P a | Adjusted β (SE) | P a | FDR-adj. Pb | Adjusted β (SE) | P a | FDR-adj. Pb | |||
| Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | (95% CI) | (95% CI) | (95% CI) | ||||||||
| Self-reported cognitive problems | ||||||||||||||||
| FACT-Cog PCI | Perceived cognitive impairment | Higher | 91.20 (2.82) | 80.74 (2.89) |
|
94.32 (2.87) | 94.08 (2.93) | 95.12 (2.97) |
|
.03 |
|
<.001 | <.001 |
|
<.001 | <.001 |
| FACT-Cog total score | Perceived cognitive difficulties | Higher | 149.88 (4.28) | 134.98 (4.54) | 136.51 (4.69) | 157.79 (4.17) | 157.34 (4.18) | 157.93 (4.20) |
|
<.001 |
|
<.001 |
|
<.001 | ||
| Single item: memory | Perceived memory difficulty | Lower |
|
|
|
|
|
|
|
.05 |
|
<.001 | <.001 |
|
<.001 | <.001 |
| Single item: attention | Perceived attention difficulty | Lower |
|
|
|
|
|
|
|
.01 |
|
<.001 | <.001 |
|
.045 | .10 |
| Single item: executive function | Perceived multitasking difficulty | Lower |
|
|
|
|
|
|
|
.07 |
|
<.001 | <.001 |
|
.02 | .05 |
| Memory | ||||||||||||||||
| Computer: CANTAB delayed match to sample | Percent correct at the 12 second delay | Higher | 78.99 (2.72) | 81.60 (2.72) |
|
79.97 (2.77) | 83.56 (2.74) | 76.72 (2.89) |
|
.57 |
|
.65 | .72 |
|
.32 | .48 |
| Computer: CANTAB verbal recognition memory | Total correct (immediate recall) | Higher |
|
|
|
|
|
|
|
.12 |
|
.01 | .03 |
|
.02 | .05 |
| Computer: CANTAB verbal recognition memory | Recognition memory | Higher | 23.25 (0.19) | 23.36 (0.19) |
|
23.14 (0.19) | 23.48 (0.19) | 23.40 (0.19) |
|
.36 |
|
.09 | .15 |
|
.05 | .10 |
| Paper: Hopkins Verbal Learning Test-Revised | Total correct (immediate recall) | Higher |
|
|
|
|
|
|
|
.38 |
|
.73 | .77 |
|
.58 | .64 |
| Paper: Hopkins Verbal Learning Test-Revised | Total correct (delayed recall) | Higher |
|
|
|
|
|
|
|
.44 |
|
.47 | .58 |
|
.52 | .6 |
| Phone: Rey Auditory Verbal Learning (Trial 1) | Total correct (immediate recall) | Higher |
|
|
|
|
|
|
|
.36 |
|
<.001 | <.001 |
|
<.001 | <.001 |
| Phone: Rey Auditory Verbal Learning (Trial 1) | Total correct (delayed recall) | Higher |
|
|
|
|
|
|
|
.35 |
|
<.001 | <.001 |
|
<.001 | <.001 |
| Attention | ||||||||||||||||
| Computer: CANTAB rapid visual processing speed | Total correct | Higher | 242.63 (2.15) | 246.39 (2.15) | 246.84 (2.16) | 241.44 (2.19) | 245.40 (2.17) | 247.25 (2.18) |
|
.33 |
|
.86 | .86 |
|
.17 | .29 |
| Paper: Trail Making Test-A (CTMT 1) | Total time (note: values log transformed) | Lower |
|
|
|
|
|
|
|
.40 |
|
.02 | 0.04 |
|
.65 | .69 |
| Phone: backward counting | Final number | Lower | 63.69 (2.17) | 63.26 (2.19) |
|
64.58 (2.20) | 63.49 (2.22) | 61.98 (2.24) |
|
.40 |
|
.32 | .44 |
|
.33 | .48 |
| Executive function | ||||||||||||||||
| Computer: CANTAB One Touch Stockings of Cambridge | Mean choice to correct response | Lower |
|
|
|
|
|
|
|
.13 |
|
.49 | .58 |
|
.95 | .95 |
| Paper: Controlled Oral Word Association | Total correct words (Avg) | Higher | 11.73 (0.62) | 11.68 (0.62) |
|
12.28 (0.63) | 12.76 (0.63) | 13.05 (0.64) |
|
.09 |
|
.02 | .04 |
|
.51 | .62 |
| Paper: Trail Making Test-B (CTMT 5) | Total time (note: values log transformed) | Lower |
|
|
|
|
|
|
|
.35 |
|
.10 | .15 |
|
.37 | .50 |
| Phone: digits backward | Total correct | Higher |
|
|
|
|
|
|
|
.46 |
|
.001 | .002 |
|
.01 | .04 |
| Phone: category fluency | Total correct | Higher | 15.87 (0.51) | 14.79 (0.52) |
|
15.59 (0.52) | 15.88 (0.52) | 16.29 (0.52) |
|
.34 |
|
<.001 | <.001 |
|
<.001 | <.001 |
Adjusted means, standard errors, 95% confidence intervals (CI), and β estimates are from the longitudinal linear mixed models after adjustment for age, education, race, reading score, baseline anxiety, and baseline depression. Estimates are based on group-by-time interaction. All P values are based on 2-sided tests. A = assessment; adj. = adjusted; CANTAB = Cambridge Neuropsychological Test Automated Battery; CTMT = Comprehensive Trail Making Test; FACT-Cog = Functional Assessment of Cancer Therapy—Cognitive Function; FDR = false discovery rate; PCI = perceived cognitive impairment.
FDR-adjusted P values are adjusted for all cognitive outcomes. All P values are based on 2-sided tests.
Figure 2.
Effect sizes for changes on cognitive measures in patients compared to controls. To determine the effect size (ES), we used a Cohen d approach, where the effect estimates (β) were divided by the estimated standard deviation of the population (ie, all subjects at baseline). Statistically significant effect sizes (after FDR adjustment) are expressed as larger circles for assessment 1 (A1) to assessment 2 (A2) and larger triangles for assessment 1 (A1) to assessment 3 (A3). CANTAB = Cambridge Neuropsychological Test Automated Battery; COWA = Controlled Oral Word Association Test; DMS = delayed match to sample; FACT-Cog = Functional Assessment of Cancer-Therapy-Cognitive Function; HVLT-R = Hopkins Verbal Learning and Memory Test-Revised; RAVLT = Rey Auditory Verbal Learning Test; RVP = Rapid Visual Processing; TMT = Trail Making Test; VRM = verbal recognition memory.
Memory
At baseline, we did not observe any statistically significant differences between patients and controls on the objective memory tests (Table 2). From A1 to A2, patients with lymphoma performed statistically significantly less well relative to controls on the CANTAB VRM immediate recall (ie, patients declined whereas controls improved; P = .01). From A1 to A3, patients performed less well than controls, showing less of an improvement over time (P = .02). From A1 to A2 and from A1 to A3, patients with lymphoma performed statistically significantly less well than controls on the phone-based RAVLT immediate and delayed recall (ie, patients declined, whereas controls improved; P < .001). After adjusting for multiple comparisons, all statistically significant results remained significant (P < .05) except VRM immediate recall from A1 to A3. The ES range for all statistically significant memory tests (after FDR adjustment) was 0.25 to 0.50 (Figure 2).
Attention
At baseline, there were no statistically significant differences between patients and controls on any attention tests (Table 2). From A1 to A2, patients performed less well than controls, (ie, they did not change, whereas controls improved) on the TMT-A (P = .02; ES = 0.22), even after controlling for multiple comparisons.
Executive Function
At baseline, there were no statistically significant differences between patients and controls on any executive function tests (Table 2). From A1 to A2, we observed that patients performed statistically significantly less well on the COWA compared with controls (P = .02). From A1 to A2 and from A1 to A3, patients performed statistically significantly less well than controls on the phone-based digits backward (P < .001 for A1 to A2 and P = .01 for A1 to A3) and category fluency tests (all P < .001). All statistically significant results remained statistically significant after adjusting for multiple comparisons. The ES range on statistically significant executive function tests (after FDR adjustment) was 0.15 to 0.67 inclusive of A1 to A2 and A1 to A3.
Predictors of Cognitive Change
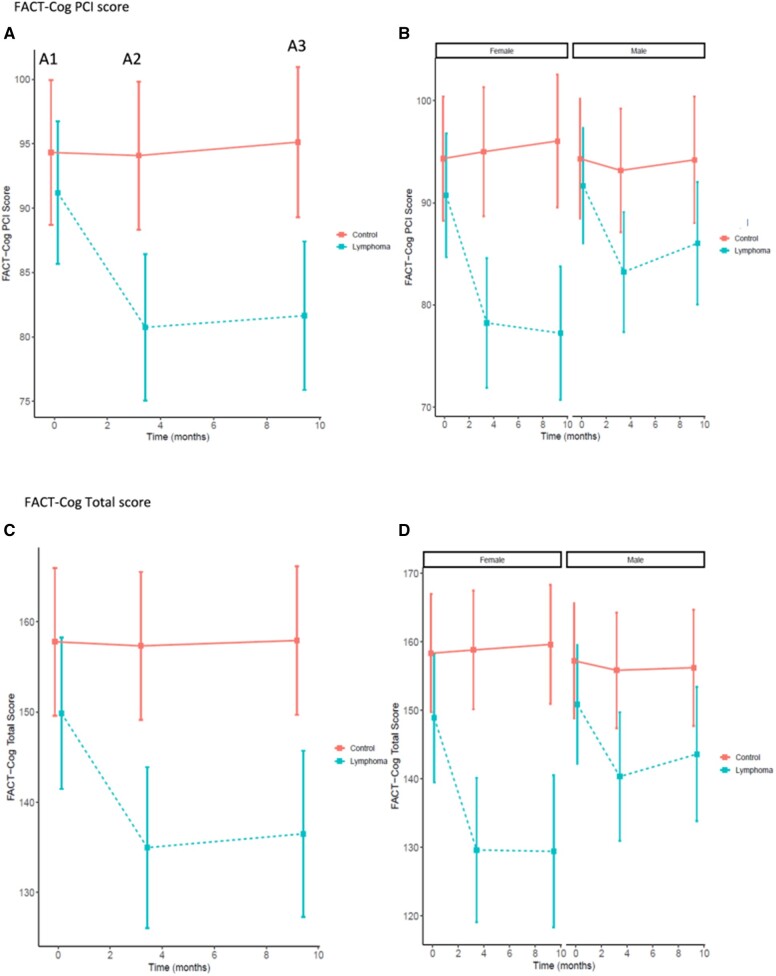
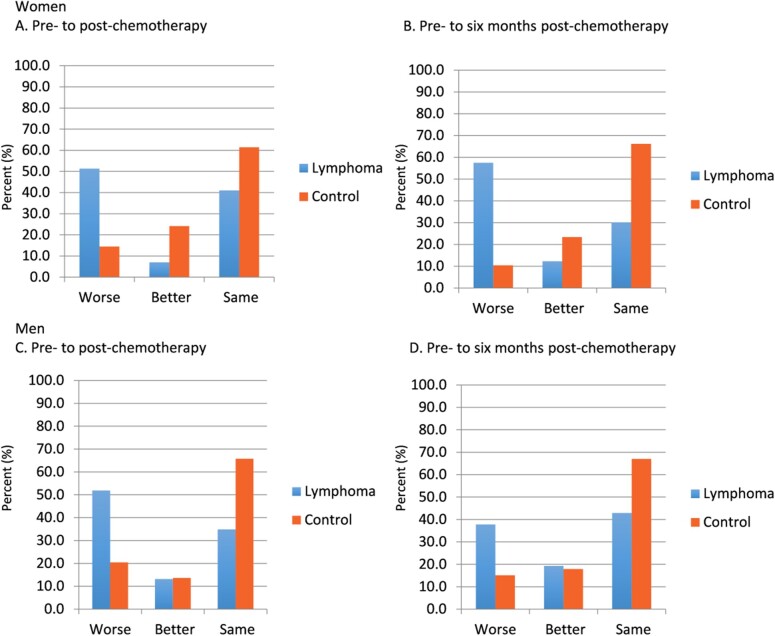
The decrease in FACT-Cog PCI score, indicative of increased perceived impairment, was statistically significantly greater for women with lymphoma compared with men with lymphoma as compared with controls from A1 to A2 and from A1 to A3 revealed by a statistically significant group-by-time-by-sex interaction (P = .007; Table 3 and Figure 3; Supplementary Table 3, available online). In women with lymphoma, 51.3% reported a perceived decline in FACT-Cog PCI scores compared with 14.5% of women who were controls (P < .001; Figure 4). From prechemotherapy to 6 months follow-up, representing almost 1 year later, 57.5% of women with lymphoma reported decline in FACT-Cog scores (based on ½ SD at A1) compared with 10.4% of female controls (P < .001). In men with lymphoma, 51.9% reported a perceived decline in FACT-Cog scores compared with 20.5% of men who were controls (P < .001; Figure 4). From prechemotherapy to 6 months follow-up, representing almost 1 year later, 37.8% of men with lymphoma reported decline in FACT-Cog scores compared with 15.1% of male controls (P < .001). Similar patterns were observed for FACT-Cog total scores (Figure 3;Supplementary Figure 2, available online). Additionally, we observed a statistically significant group-by-sex interaction where women with lymphoma reported statistically significantly more difficulty compared with men on the memory and attention difficulty items. We also observed an overall group effect where men performed less well than women on verbal memory tests (VRM, RAVLT, and HVLT-R) regardless of group.
Table 3.
Self-report measures at baseline (prechemotherapy [A1], post-chemotherapy [A2], and 6 months follow-up [A3]) and changes from pre- to postchemotherapy and changes from prechemotherapy to 6 months follow-up in men and women with lymphoma and age- and sex-paired controls assessed at the same equivalent times
| Self-report | Outcome | Better score | Chemotherapy |
Control |
Baseline: chemotherapy (A1) -control (A1) |
Pre- to postchemotherapy: chemotherapy (A2-A1) - control (A2-A1) |
Prechemotherapy to 6 months follow-up: chemotherapy (A3-A1) - control (A3-A1) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | A3 | A1 | A2 | A3 | Adjusted β (SE) | P a | Adjusted β (SE) | P a | Adjusted β (SE) | P a | |||
| Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | Adjusted mean (SE) | (95% CI) | (95% CI) | (95% CI) | ||||||
| Women | ||||||||||||||
| FACT-Cog PCI | Perceived cognitive impairment | Higher | 90.72 (3.08) |
|
|
|
|
|
|
.11 |
|
<.001 |
|
<.001 |
| FACT-Cog total score | Perceived cognitive difficulties | Higher |
|
|
|
|
|
|
|
.005 |
|
<.001 |
|
<.001 |
| Single item: memory | Perceived memory difficulty | Higher |
|
|
|
|
|
|
|
.10 |
|
<.001 |
|
.002 |
| Single item: attention | Perceived attention difficulty | Higher |
|
|
|
|
|
|
|
.03 |
|
<.001 |
|
.045 |
| Men | ||||||||||||||
| FACT-Cog PCI | Perceived cognitive impairment | Higher |
|
83.23 (2.98) |
|
|
|
|
|
.14 |
|
<.001 |
|
.004 |
| FACT-Cog total score | Perceived cognitive difficulties | Higher |
|
|
|
|
|
|
|
.02 |
|
<.001 |
|
.04 |
| Single item: memory | Perceived memory difficulty | Higher |
|
|
|
|
|
|
|
.13 |
|
.02 |
|
.04 |
| Single item: attention | Perceived attention difficulty | Higher |
|
|
|
|
|
|
|
.21 |
|
.05 |
|
.48 |
Adjusted means, standard errors, 95% confidence intervals (CI), and β estimates are from the longitudinal linear mixed models after adjustment for age, education, race, reading score, baseline anxiety, and baseline depression. Estimates are based on group-by-time-by-sex interaction. All P values are based on 2-sided tests. FACT-Cog = Functional Assessment of Cancer Therapy—Cognitive Function; PCI = perceived cognitive impairment.
Figure 3.
Fact-Cog self-report scores in women and men with lymphoma and controls. Assessments (A) are prechemotherapy (A1), postchemotherapy (A2), and 6 months following chemotherapy (A3; or time equivalent for control participants). Smaller values imply greater perceived cognitive impairment. Mean adjusted scores on each test and the corresponding 95% confidence intervals are presented for A1 (0 months; approximately within 7 days prior to chemo), A2 (approximately 3.3 months from A1; postchemotherapy), and A3 (approximately 9.3 months from A1; 6-month follow-up) in panels A and C (stratified by patients with lymphoma receiving chemotherapy (dashed blue line) vs control participants (solid red line)) and panels B and D (additionally stratified by female vs male). Panels A and B include adjusted PCI scores, and panels C and D include adjusted total scores. Unadjusted plots are provided in Supplementary Figure 1 (available online). FACT-Cog = Functional Assessment of Cancer-Therapy-Cognitive Function.
Figure 4.
Prevalence of overall perceived cognitive impairment on the FACT-Cog PCI score based on clinically important differences from pre- to postchemotherapy and prechemotherapy to 6 months following completion of chemotherapy in women (A and B) and men (C and D). “Better” is defined as a minimal clinically important difference (based on ½ SD of controls at baseline) increase of 6.19 or more in the FACT-Cog, and “worse” is defined as a decrease of 6.19 or more. The y axis represents the percentage of participants meeting each threshold or who stayed the same. FACT-Cog = Functional Assessment of Cancer Therapy-Cognitive Function; PCI = perceived cognitive impairment. Blue bars = lymphoma and orange bars = control.
On a majority of assessments, older age was statistically significantly associated with worse performance on objective tests as well as perceived cognitive impairment (Supplementary Table 3, available online). In general, those with a some college or greater education were associated with statistically significantly better performance on a majority of tests. In general, lower WRAT-4 reading score was associated with worse performance. Higher baseline anxiety and depressive symptoms were associated with worse performance on some cognitive tests as well as perceived cognitive impairment.
In additional exploratory LMMs, we did not observe any consistent pattern in differences in cognitive outcomes in those with Hodgkin compared with non-Hodgkin lymphoma or any consistent differences in cognitive performance by those receiving R-CHOP, BR, or ABVD chemotherapy regimens (data not shown). We also did not observe differences in those who were pre- vs peri- or postmenopausal at baseline (data not shown).
Discussion
In this nationwide study, we showed that patients with lymphoma perform statistically significantly worse over time on objective assessments of cognitive function compared with sex- and age-paired participants serving as noncancer controls assessed at the same time intervals as patients. Based on ES estimates, these mean changes of group differences are in the mild to moderate range. Additionally, patients with lymphoma report statistically significantly more perceived impairments over time compared with controls. Of note, we found that women with lymphoma report statistically significantly more perceived impairment compared with men with lymphoma and controls.
The overall trajectory of cognitive complaints assessed by the FACT-Cog in patients with lymphoma is similar to the pattern of those in our breast cancer study (16) and other studies (53). Also, similar to the breast cancer study, in this study, we observed overall similar trajectories of cognitive function scores on objective tests in patients with lymphoma who performed less well over time compared with controls from A1 to A2 and from A1 to A3 demonstrating either decline over time relative to controls or less improvement over time (33). With the smaller sample size of the lymphoma cohort, we did not have the same power to detect longitudinal group differences on some measures as we did with the larger breast cancer study. For example, in the breast cancer study, we saw statistically significantly worse performance on the rapid visual processing sustained attention test over time in patients compared with controls, with patients improving less over time compared with controls (33). In the current lymphoma study, patients show the same overall pattern compared with controls; however, these were not statistically significant.
Of note, in the breast cancer cohort from our previous publication (16), there was only a trending difference in patients compared with controls on the FACT-Cog total score after controlling for covariates at baseline. Herein, we observed statistically significant baseline effects on the FACT-Cog total score in patients with lymphoma compared with controls even after controlling for covariates suggesting that there may be an impact of disease from lymphoma at the prechemotherapy time point on perceived functioning. There were also trending baseline differences in executive function in patients with lymphoma compared with noncancer controls. It is possible that the biology of lymphoma and other hematologic malignancies may play a unique role in CRCD. Indeed, those with higher risk chronic lymphocytic leukemia have greater problems in verbal memory and executive function, irrespective of treatment status (54). However, many factors can contribute to pretreatment cognitive functioning including comorbid conditions (55) and coping ability (56). The interaction of these factors and their impact on CRCD in lymphoma and other diseases needs to be further studied.
Although exploratory, we did not see differences in cognitive function outcomes between patients by lymphoma type or treatment regimen, possibly because of the small relative sample sizes in these subgroups. Future studies should be designed to address differences of the impact of disease subtypes and treatment on cognitive function, including those receiving more targeted therapies and immunotherapy. We also did not observe changes in cognitive function between women who were premenopausal vs peri- or postmenopausal at baseline. We did not assess changes in menopausal status during chemotherapy and how that influences cognitive function, which is worthy of further study. A limitation of our research is that we only included a single item to assess depression, which did not allow for a comprehensive assessment. Another limitation is that our study lacked racial and ethnic diversity.
This study is, to our knowledge, the first comprehensive longitudinal study assessing the impact of chemotherapy on cognitive function in patients with lymphoma. Our nationwide study increases generalizability on diverse factors such as geographic location and individuals with varying levels of education. The use of a study-specific control group was critical to identifying changes over time between groups, particularly for the objective assessments, where practice effects were observed on some assessments. Having both age- and sex-paired controls also allowed us to precisely assess sex effects. Our results are limited to 6 months postchemotherapy; research on more long-term cognitive effects of chemotherapy is needed as has been investigated in other studies. Interventions to alleviate cognitive problems in patients with lymphoma should be developed.
Funding
Funding was provided by NCI U10CA037420 Supplement (MCJ), NCI UG1CA189961 (GRM, KMM), DP2195765 (MCJ), NCI K07CA1688 (MCJ), and NCI R01CA231014 (MCJ).
Notes
Role of the funders: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: There are no disclosures to report by any authors related to any information in this manuscript.
Author contributions: Conceptualization: MJ, GM, TA, CH. Data curation: MJ, MM, SD, JG, TA, GM, MM. Formal analysis and investigation: MJ, MM, LP, AM, EB, CK, LM, PR, SM, GM, TA, CH. Writing—original draft: MJ, CH. Writing—review and editing: All authors.
Acknowledgements: The authors thank the participants in this study and all staff at the URCC NCI Community Oncology NCORP Research Base and our NCORP affiliate sites who recruited and followed participants. We thank the NCI CCOP and NCORP programs for their funding and support of this project. NCORP was formerly known as the Community Clinical Oncology Program (CCOP) when this study started in 2011. The following CCOP/NCORPs participated in this study: Aurora, Heartland (formerly Central Illinois), Columbus, CRCWM (formerly Grand Rapids and Kalamazoo), Dayton, Delaware, Greenville, HOACNY, Kansas City, MMCORP (formerly Metro Minnesota), Nevada, Northwell (formerly North Shore), PCRC (formerly WORC), SCOR (formerly SCCC), Upstate Carolina, Virginia Mason, Wichita, and WiNCORP (formerly Marshfield). We also thank the staff of the Cancer Control and Supportive Care Research Program and the Cancer Control and Psychoneuroimmunology Lab for their assistance with this research project. Observational study registered as NCT01382082.
Data Availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary Material
References
- 1. Mayo SJ, Lustberg M, Dhillon HM, et al. Cancer-related cognitive impairment in patients with non-central nervous system malignancies: an overview for oncology providers from the MASCC Neurological Complications Study Group. Support Care Cancer 2021;29(6):2821–2840. [DOI] [PubMed] [Google Scholar]
- 2. Wefel JS, Lenzi R, Theriault RL, et al. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: results of a prospective, randomized, longitudinal trial. Cancer. 2004;100(11):2292–2299. [DOI] [PubMed] [Google Scholar]
- 3. Reid-Arndt SA, Yee A, Perry MC, et al. Cognitive and psychological factors associated with early posttreatment functional outcomes in breast cancer survivors. J Psychosoc Oncol. 2009;27(4):415–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bradley CJ, Neumark D, Bednarek HL, et al. Short-term effects of breast cancer on labor market attachment: results from a longitudinal study. J Health Econ. 2005;24(1):137–160. : [DOI] [PubMed] [Google Scholar]
- 5. Hurria A, Zuckerman E, Panageas KS, et al. A prospective, longitudinal study of the functional status and quality of life of older patients with breast cancer receiving adjuvant chemotherapy. J Am Geriatr Soc. 2006;54(7):1119–1124. [DOI] [PubMed] [Google Scholar]
- 6. Brezden CB, Phillips KA, Abdolell M, et al. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18(14):2695–2701. [DOI] [PubMed] [Google Scholar]
- 7. Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jansen CE, Dodd MJ, Miaskowski CA, et al. Preliminary results of a longitudinal study of changes in cognitive function in breast cancer patients undergoing chemotherapy with doxorubicin and cyclophosphamide. Psychooncology. 2008;17(12):1189–1195. [DOI] [PubMed] [Google Scholar]
- 9. Hurria A, Rosen C, Hudis C, et al. Cognitive function of older patients receiving adjuvant chemotherapy for breast cancer: a pilot prospective longitudinal study. J Am Geriatr Soc. 2006;54(6):925–931. [DOI] [PubMed] [Google Scholar]
- 10. Collins B, Mackenzie J, Stewart A, et al. Cognitive effects of chemotherapy in post-menopausal breast cancer patients 1 year after treatment. Psychooncology. 2009;18(2):134–143. [DOI] [PubMed] [Google Scholar]
- 11. Jenkins V, Shilling V, Deutsch G, et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br J Cancer. 2006;94(6):828–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahles TA, Saykin AJ, McDonald BC, et al. Cognitive function in breast cancer patients prior to adjuvant treatment. Breast Cancer Res Treat. 2008;110(1):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stewart A, Collins B, Mackenzie J, et al. The cognitive effects of adjuvant chemotherapy in early stage breast cancer: a prospective study. Psychooncology. 2008;17(2):122–130. [DOI] [PubMed] [Google Scholar]
- 14. Ouimet LA, Stewart A, Collins B, et al. Measuring neuropsychological change following breast cancer treatment: an analysis of statistical models. J Clin Exp Neuropsychol. 2009;31(1):73–89. [DOI] [PubMed] [Google Scholar]
- 15. Jansen CE, Cooper BA, Dodd MJ, et al. A prospective longitudinal study of chemotherapy-induced cognitive changes in breast cancer patients. Support Care Cancer. 2011;19(10):1647–1656. [DOI] [PubMed] [Google Scholar]
- 16. Janelsins MC, Heckler CE, Peppone LJ, et al. Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: an analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol. 2017;35(5):506–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahles TA, Root JC, Ryan EL.. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol. 2012;30(30):3675–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Merriman JD, Sereika SM, Brufsky AM, et al. Trajectories of self-reported cognitive function in postmenopausal women during adjuvant systemic therapy for breast cancer. Psychooncology. 2017;26(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bender CM, Merriman JD, Gentry AL, et al. Patterns of change in cognitive function with anastrozole therapy. Cancer. 2015;121(15):2627–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ganz PA, Petersen L, Castellon SA, et al. Cognitive function after the initiation of adjuvant endocrine therapy in early-stage breast cancer: an observational cohort study. J Clin Oncol. 2014;32(31):3559–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vardy JL, Dhillon HM, Pond GR, et al. Cognitive function in patients with colorectal cancer who do and do not receive chemotherapy: a prospective, longitudinal, controlled study. J Clin Oncol. 2015;33(34):4085–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams AM, Zent CS, Janelsins MC.. What is known and unknown about chemotherapy-related cognitive impairment in patients with hematological malignancies and areas of needed research. Br J Hematol Invited Rev. 2016;174(6):835–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahles TA, Saykin AJ, Furstenberg CT, et al. Quality of life of long-term survivors of breast cancer and lymphoma treated with standard-dose chemotherapy or local therapy. J Clin Oncol. 2005;23(19):4399–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zimmer P, Mierau A, Bloch W, et al. Post-chemotherapy cognitive impairment in patients with B-cell non-Hodgkin lymphoma: a first comprehensive approach to determine cognitive impairments after treatment with rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone or rituximab and bendamustine. Leuk Lymphoma. 2015;56(2):347–352. [DOI] [PubMed] [Google Scholar]
- 25. Trachtenberg E, Mashiach T, Ben Hayun R, et al. Cognitive impairment in Hodgkin lymphoma survivors. Br J Haematol. 2018;182(5):670–678. [DOI] [PubMed] [Google Scholar]
- 26. Khan MA, Garg K, Bhurani D, et al. Early manifestation of mild cognitive impairment in B-cell non-Hodgkin’s lymphoma patients receiving CHOP and rituximab-CHOP chemotherapy. Naunyn Schmiedebergs Arch Pharmacol. 2016;389(12):1253–1265. [DOI] [PubMed] [Google Scholar]
- 27. Dantzer R, Kelley KW.. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao HM, Hong JS.. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29(8):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matthews F, Marioni R, Brayne C; for the Medical Research Council Cognitive Function and Ageing Study. Examining the influence of sex, education, social class and birth cohort on MMSE tracking over time: a population-based prospective cohort study. BMC Geriatr. 2012;12(45):1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ahles TA, Saykin AJ, McDonald BC, et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 2010;28(29):4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Janelsins MC, Mustian KM, Palesh OG, et al. Differential expression of cytokines in breast cancer patients receiving different chemotherapies: implications for cognitive impairment research. Support Care Cancer. 2012;20(4):831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kesler SR, Blayney DW.. Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol. 2016;2(2):185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Janelsins MC, Heckler CE, Peppone LJ, et al. Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol. 2018;36(32):3231-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Robbins TW, James M, Owen AM, et al. Cambridge Neuropsychological Test Automated Battery (CANTAB): a factor analytic study of a large sample of normal elderly volunteers. Dementia. 1994;5(5):266–281. [DOI] [PubMed] [Google Scholar]
- 35. Shapiro AM, Benedict RH, Schretlen D, et al. Construct and concurrent validity of the Hopkins Verbal Learning Test-revised. Clin Neuropsychol. 1999;13(3):348–358. [DOI] [PubMed] [Google Scholar]
- 36. Rasmusson DX, Bylsma FW, Brandt J.. Stability of performance on the Hopkins Verbal Learning Test. Arch Clin Neuropsychol. 1995;10(1):21–26. [PubMed] [Google Scholar]
- 37. Brandt JB. Hopkins Verbal Learning Test-Revised Professional Manual. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- 38. Gaudino EA, Geisler MW, Squires NK.. Construct validity in the Trail Making Test: What makes Part B harder? J Clin Exp Neuropsychol. 1995;17(4):529–535. [DOI] [PubMed] [Google Scholar]
- 39. Goul WR, Brown M.. Effects of age and intelligence on Trail Making Test Performance and validity. Percept Mot Skills. 1970;30(1):319–326. [DOI] [PubMed] [Google Scholar]
- 40. Reynolds CR: Comprehensive Trail-Making Test Professional Manual. Austin, TX: PRO-ED; 2002. [Google Scholar]
- 41. Bentonal H, Sivan AB.. Controlled Oral Word Association Multilingual Aphasia Examination Professional Manual. Lutz, FL: Psychological Assessment Resources; 1978. [Google Scholar]
- 42. Lezak M. Neuropsychological Assessment 4th ed. Oxford, United Kingdom: Oxford University Press; 2004. [Google Scholar]
- 43. Tun PA, Lachman ME.. Telephone assessment of cognitive function in adulthood: the brief test of adult cognition by telephone. Age Ageing. 2006;35(6):629–632. [DOI] [PubMed] [Google Scholar]
- 44. Wagner L, Sweet J, Butt Z, Lai J, Cella D.. Measuring patient self-reported cognitive function: development of the functional assessment of cancer therapy-cognitive function instrument. J Support Oncol. 2009;7(6):32–39. [Google Scholar]
- 45. Cheung YT, Foo YL, Shwe M, et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol. 2014;67(7):811–820. [DOI] [PubMed] [Google Scholar]
- 46. Cleeland CS, Mendoza TR, Wang XS, et al. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory . Cancer. 2000;89(7):1634–1646. [DOI] [PubMed] [Google Scholar]
- 47. Wilkinson GS. Wide Range Achievement Test 4 (WRAT4) Professional Manual. Lutz, FL: Psychological Assessment Resources; 2006.
- 48. Spielberger CD, Sydeman SJ, Owen AE, Marsh BJ, Measuring anxiety and anger with the State-Trait Anxiety Inventory (STAI) and the State-Trait Anger Expression Inventory (STAXI). In: Maruish ME, ed. The Use of Psychological Testing for Treatment Planning and Outcomes Assessment 7th ed.Mahwah, NJ: Lawrence Erlbaum Associates, Inc; 1999:993–213. [Google Scholar]
- 49. Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 50. Kenward MG, Roger JH.. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983–997. [PubMed] [Google Scholar]
- 51. Little R, Rubin DB.. Statistical Analysis with Missing Data. Hoboken, NJ: Wiley; 2002. [Google Scholar]
- 52. Benjamini Y, Hochberg Y.. Controlling the false discovery rate: a practical and powerful approach for multiple testing. J Roy Stat Soc Ser B. 1995; 57(1):289–300. [Google Scholar]
- 53. Wagner LI, Gray RJ, Sparano JA, et al. Patient-reported cognitive impairment among women with early breast cancer randomly assigned to endocrine therapy alone versus chemoendocrine therapy: results from TAILORx. J Clin Oncol. 2020;38(17):1875–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Williams AM, van Wijngaarden E, Seplaki CL, et al. Cognitive function in patients with chronic lymphocytic leukemia: a cross-sectional study examining effects of disease and treatment. Leuk Lymphoma. 2020;61(7):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: Is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32(18):1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Cimprich B, Ronis DL.. Attention and symptom distress in women with and without breast cancer. Nurs Res. 2001;50(2):86–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.