Abstract
Background
Biological evidence indicates that smoking can influence macrophage functions and polarization, thereby promoting tumor evolution. We hypothesized that the association of smoking with colorectal cancer incidence might differ by macrophage infiltrates.
Methods
Using the Nurses’ Health Study and the Health Professionals Follow-up Study, we examined the association of smoking with incidence of colorectal cancer subclassified by macrophage counts. Multiplexed immunofluorescence (for CD68, CD86, IRF5, MAF, and MRC1 [CD206]) combined with digital image analysis and machine learning was used to identify overall, M1-polarized, and M2-polarized macrophages in tumor. We used inverse-probability–weighted multivariable Cox proportional hazards regression models to control for potential confounders and selection bias because of tissue data availability. All statistical tests were 2-sided.
Results
During follow-up of 131 144 participants (3 648 370 person-years), we documented 3092 incident colorectal cancer cases, including 871 cases with available macrophage data. The association of pack-years smoked with colorectal cancer incidence differed by stromal macrophage densities (Pheterogeneity = .003). Compared with never smoking, multivariable-adjusted hazard ratios (95% confidence interval) for tumors with low macrophage densities were 1.32 (0.97 to 1.79) for 1-19 pack-years, 1.31 (0.92 to 1.85) for 20-39 pack-years, and 1.74 (1.26 to 2.41) for 40 or more pack-years (Ptrend = .004). In contrast, pack-years smoked was not statistically significantly associated with the incidence of tumors having intermediate or high macrophage densities (Ptrend > .009, with an α level of .005). No statistically significant differential association was found for colorectal cancer subclassified by M1-like or M2-like macrophages.
Conclusions
The association of smoking with colorectal cancer incidence is stronger for tumors with lower stromal macrophage counts. Our findings suggest an interplay of smoking and macrophages in colorectal carcinogenesis.
Smoking is recognized as one of the most established risk factors for colorectal cancer (1). Colorectal cancer comprises heterogeneous tumors with complex interactions between neoplastic and immune cells in the tumor microenvironment (2-5). Accordingly, evidence indicates that the magnitude of the association of smoking with colorectal cancer incidence differs by tumor subtypes (6-12). For example, several studies have consistently shown that the association of smoking with colorectal cancer incidence is stronger for microsatellite instability (MSI)-high tumors compared with non–MSI-high tumors (10,12). A recent study has also reported that the association of smoking with colorectal cancer incidence was stronger for MSI-high and non-MSI-high tumors containing a higher density of CD3+ cells (8). These findings emphasize not only the importance of clarifying the heterogeneity of colorectal cancer but also the future potential of developing immune-based cancer prevention strategies (3,4,13).
Tumor-associated macrophages are among the most abundant types of immune cells in the tumor microenvironment and are known to influence tumor evolution (14-17). Two functional subgroups of macrophages namely, pro-inflammatory M1-like macrophages and anti-inflammatory M2-like macrophages—represent a phenotypic spectrum (18). In addition, macrophages have been shown to exhibit wide functional plasticity and heterogenous phenotypes in response to environmental stimuli (18-21). The abundance of macrophages has been associated with clinical outcomes in colorectal cancer patients (22,23).
Evidence suggests that smoking may influence macrophage functions and polarization, which could potentially promote tumor development (24). However, to our knowledge, no study has yet examined the effect of smoking on colorectal cancer incidence according to macrophage infiltration in cancer tissue. We therefore hypothesized that the association of smoking with colorectal cancer incidence might differ by macrophage counts. We tested this hypothesis using 2 large US prospective cohort studies that included data on incident colorectal cancer cases and macrophage counts and polarization determined by multiplex immunofluorescence assays.
Methods
Study Population
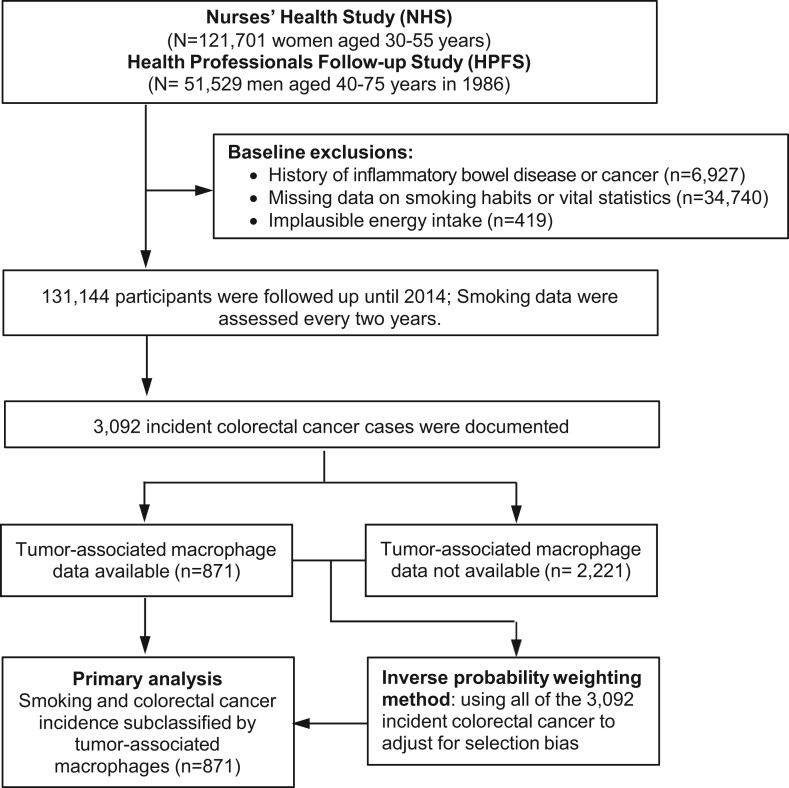
As shown in Figure 1, we used data from 2 large US prospective cohort studies: the Nurses’ Health Study (NHS; 121 701 women aged 30-55 years at enrollment followed-up since 1976) and the Health Professionals Follow-up Study (HPFS; 51 529 men aged 40-75 years at enrollment followed-up since 1986) (25). In both cohorts, participants were required to report their lifestyle factors, including smoking behavior and newly diagnosed diseases every 2 years and to report dietary data using the food frequency questionnaires every 4 years (26). The follow-up rate has been more than 90% for each follow-up questionnaire cycle in both cohorts. In this analysis, we excluded participants who met any of the following exclusion criteria at the baseline (1980 for the NHS and 1986 for the HPFS): 1) no data on smoking habits or vital statistics, 2) unreasonable total calorie intake (<600 or >3500 calories/d for women, and <800 or >4200 calories/d for men), and 3) history of inflammatory bowel disease or cancer (except for nonmelanoma skin cancer). Participants were followed-up until colorectal cancer diagnosis, death, loss to follow-up, or the end of follow-up (June 1, 2014, for the NHS; January 1, 2014, for the HPFS), whichever came first.
Figure 1.
Flow diagram of the study population in the Nurses’ Health Study and the Health Professionals Follow-up Study.
Informed consent was obtained from all participants at enrollment in the NHS and the HPFS, and additional consent for tissue analyses was obtained before tissue collection. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital and Harvard T.H. Chan School of Public Health. In addition, this study followed the state registry rules and policies for the use of cancer registry data for research.
Assessment of Smoking Behavior
The details of the assessment of smoking behavior were described previously (8,12). Briefly, smoking status and daily cigarette consumption have been reported by participants every 2 years since 1980 (for the NHS) and 1986 (for the HPFS). On the baseline questionnaires, they also reported their age when they began smoking and ceased smoking if applicable. Every 2 years, participants have updated their current smoking status and average daily cigarette consumption in the preceding 2 years. Information on cumulative pack-years smoked in each participant has been updated every 2 years.
Acquisition of Colorectal Cancer Cases
In both cohorts, incident colorectal cancer cases were identified based on biennial questionnaires. For nonrespondents, colorectal cancer–related deaths were ascertained through the National Death Index and US post office authorities. To confirm the diagnosis and to record tumor characteristics (eg, disease stage and primary tumor location), study physicians who were blinded to exposure data reviewed medical records of identified colorectal cancer cases. Formalin-fixed paraffin-embedded tissue specimens were retrieved from hospitals throughout the United States. The study pathologist (S.O.) confirmed diagnosis of colorectal cancer. We included both colon and rectal cancers based on the colorectal continuum model (27,28).
Multiplex Immunofluorescence and Tumor Analysis
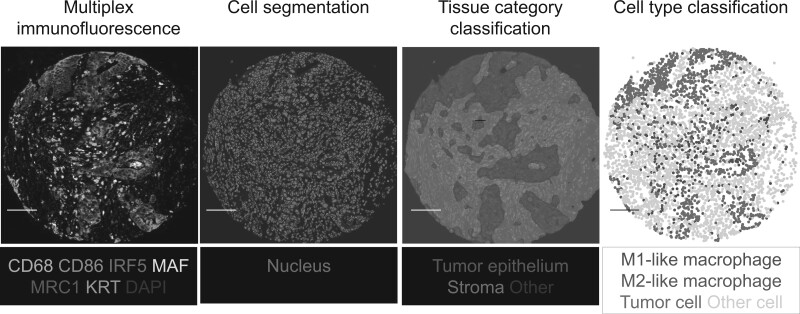
As previously described (29), we used multiplex immunofluorescence combined with digital image analysis and machine learning to identify and count M1-polarized and M2-polarized macrophages (Figure 2). Tissue microarray was made using 2-4 tissue cores from each tumor, as previously indicated (30). The multiplex immunofluorescence panel contained a pan-macrophage marker (CD68), 2 M1 phenotype markers (CD86, IRF5), 2 M2 phenotype markers [MAF, MRC1 (CD206)], a tumor epithelial cell marker [KRT (keratin, cytokeratin)], and a nuclear marker (4′,6-diamidino-2-phenylindole) following standardized protein nomenclature recommended by a panel of experts (31). We scanned the immunofluorescence slides using the Vectra 3.0 System (Akoya Biosciences, Hopkinton, MA, USA). The images were processed with pathologist-supervised machine learning algorithms within the inForm software package to perform tissue category segmentation, cell segmentation, and cell type classification. We calculated the M1:M2 polarization index using the formula “(CD86 × IRF5)/(MRC1 × MAF).” We defined the highest 30% of the index as M1-like macrophages and the lowest 30% as M2-like macrophages. We calculated a cell density measure (cells per square millimeter) in tumor intraepithelial and stromal regions separately. CD3+ cell density and tumor MSI status were determined as previously described (32).
Figure 2.
Representative images of the quantification of macrophage counts and polarization in the colorectal cancer microenvironment using a customized multiplex immunofluorescence assay. The multiplex immunofluorescence images were processed with pathologist-supervised image analysis algorithms to perform cell segmentation, tissue category classification, and cell type classification. The scale bar is 100 µm. The details were described in our previous article (26).
Statistical Analyses
Details of statistical analyses are described in the Supplementary Methods (available online). All statistical analyses were conducted using SAS software (version 9.4, SAS Institute, Cary, NC, USA). All P values were 2-sided, and we used the stringent α level of .005 as recommended by the expert statisticians (33). Our primary hypothesis testing was an assessment of heterogeneity in the associations of cumulative pack-years smoked (a continuous variable with a ceiling at 50 pack-years to eliminate outlier effect) with the incidence of colorectal cancer subgroups defined by macrophage density measures. All other assessments were secondary analyses.
We used multivariable Cox proportional hazards models to estimate the hazard ratio (HR) of colorectal cancer incidence. To assess differential associations of smoking variables with colorectal cancer subgroups by macrophage densities, we applied the duplication-method Cox regression model for competing risks (34). To test whether the strength of the exposure–outcome association might differ across the ordinal subtypes, we used the meta-regression method with a subtype-specific random effect term. The multivariable Cox regression models included body mass index (continuous with a ceiling at 35 kg/m2), family history of colorectal cancer in any first-degree relative (yes or no), physical activity (continuous with a ceiling at 50 metabolic equivalent task score-h/week), regular use of aspirin or nonsteroidal antiinflammatory drugs (yes or no), alcohol consumption (continuous with a ceiling at 30 g/d), red and processed meat intake (continuous with a ceiling at 14 servings/week), and folate intake (continuous with a ceiling at 1000 μg/d). For the NHS-only analyses, we additionally adjusted for menopausal hormone therapy (yes or no). To control for confounding by age, calendar time, and sex (ie, cohort), the Cox models were stratified by these factors using the “strata” option in SAS. Analyses were conducted in each stratum of combined statuses of age, calendar time, and sex (ie, cohort) and then summary hazard ratios were obtained. Proportional hazards assumptions were assessed by including an interaction term between cumulative pack-years and follow-up time and found to be justified. To control for selection bias due to macrophage density data availability, the inverse probability weighting (IPW) method (35) was integrated into multivariable Cox proportional hazards model using covariate data on the 3092 colorectal cancer. Proportional hazards assumptions were assessed by including an interaction term between cumulative pack-years smoked and follow-up time in the Cox model and found to be justified for analyses of all 3092 incident cases, 871 incident cases with available macrophages data, and 2221 incident cases without available macrophages data.
Use of Standardized Official Symbols
We used Human Genome Organisation–approved official symbols (or root symbols) for genes and gene products, including CD3, CD68, CD86, IRF5, KRT, MAF, and MRC1, all of which are described at www.genenames.org. The gene symbols are italicized to differentiate from nonitalicized gene product names.
Results
Age-standardized characteristics of participants in the prospective cohort studies according to cumulative pack-years smoked are shown in Table 1. During the follow-up of 131 144 participants (3 648 370 person-years), we documented 3092 incident colorectal cancer cases, including 871 cases with available tumor-associated macrophage data. Cumulative pack-years smoked were associated with the incidence of overall colorectal cancer, using the 3092 incident colorectal cancers, with a multivariable-adjusted hazard ratio of 1.28 (95% confidence interval [CI] = 1.15 to 1.42) for those who smoked 40 pack-years and more compared with never smokers (Supplementary Table 1, available online). This association was similarly apparent in analyses using the 871 cases with macrophage data and in analyses using the 2221 cases without macrophage data (Supplementary Table 1, available online). In further analyses, we used the IPW and all of the 3092 incident cases to adjust for selection bias because of macrophage data availability.
Table 1.
Age-standardized characteristics of participants according to cumulative pack-years smoked in the NHS (1980-2014) and the HPFS (1986-2014)
| Characteristica | Women (NHS) |
Men (HPFS) |
||||||
|---|---|---|---|---|---|---|---|---|
| Cumulative pack-years smoked |
Cumulative pack-years smoked |
|||||||
| 0 | 1-19 | 20-39 | ≥40 | 0 | 1-19 | 20-39 | ≥40 | |
| (n = 38 062) | (n = 26 603) | (n = 15 649) | (n = 6043) | (n = 21 193) | (n = 10 387) | (n = 8277) | (n = 4930) | |
| Mean age (SD), y | 61.7 (11.9) | 60.2 (11.9) | 60.5 (11.4) | 64.8 (9.8) | 63.9 (11.5) | 63.9 (11.3) | 66.0 (11.1) | 68.9 (9.8) |
| Family history of colorectal cancer, % | 13.6 | 13.8 | 13.8 | 12.9 | 12.7 | 12.5 | 12.7 | 12.3 |
| Mean BMI (SD), kg/m² | 25.5 (4.7) | 25.2 (4.6) | 25.2 (4.5) | 25.1 (4.4) | 25.6 (3.4) | 25.7 (3.1) | 26.3 (3.6) | 26.4 (3.6) |
| Postmenopausal status, % | 77.5 | 76.8 | 80.5 | 88.0 | — | — | — | — |
| Menopausal hormone therapy, % | 26.4 | 27.9 | 24.3 | 20.9 | — | — | — | — |
| History of colonoscopy/sigmoidoscopy, % | 41.5 | 44.7 | 39.9 | 36.2 | 56.0 | 57.7 | 53.8 | 49.0 |
| Regular use of aspirin, % | 34.2 | 35.0 | 36.4 | 37.2 | 46.1 | 49.0 | 49.6 | 50.6 |
| Regular use of other NSAIDs, % | 35.0 | 38.1 | 38.4 | 34.4 | 16.2 | 19.1 | 17.8 | 16.9 |
| Physical activity, mean (SD), METS-h/wk | 16.6 (16.8) | 18.1 (18.7) | 16.8 (17.7) | 13.3 (14.4) | 29.3 (24.7) | 29.6 (24) | 26.1 (22.7) | 20.7 (20.1) |
| Alcohol intake, mean (SD), g/d | 3.8 (6.9) | 6.9 (8.9) | 8.0 (10.6) | 9.9 (13.3) | 8.0 (11.1) | 12.5 (13.8) | 14.3 (15.8) | 16.6 (18.7) |
| Red and processed meat intake, mean (SD), servings/wk | 6.6 (3.7) | 6.3 (3.5) | 6.6 (3.6) | 7.1 (3.7) | 6.1 (4.3) | 6.1 (4.2) | 6.8 (4.6) | 7.9 (5.1) |
| Total folate intake, mean (SD), μg/d | 432 (212) | 442 (213) | 413 (204) | 389 (208) | 552 (253) | 563 (256) | 524 (251) | 494 (246) |
All variables other than age were standardized to age distribution of each cohort. Mean (SD) for continuous variables or percentages for categorical variables are presented. BMI = body mass index; HPFS = Health Professionals Follow-up Study; METS = metabolic equivalent task score; NHS = Nurses’ Health Study; NSAID = nonsteroidal anti-inflammatory drug.
In our primary hypothesis testing, the association of pack-years smoked with colorectal cancer incidence differed by the macrophage density in tumor stromal areas (Pheterogeneity = .003; Table 2). Compared with never smoking, multivariable-adjusted hazard ratios (95% CI) for tumors with low (tertile 1) macrophage densities were 1.32 (0.97 to 1.79) for 1-19 pack-years, 1.31 (0.92 to 1.85) for 20-39 pack-years, and 1.74 (1.26 to 2.41) for 40 and more pack-years (Ptrend = .004). In contrast, pack-years smoked were not statistically significantly associated with the incidence of tumors having intermediate (tertile 2) or high (tertile 3) macrophage densities (Ptrend > .009, with the α level of .005). We confirmed that similar results were obtained by a sensitivity analysis without IPW adjustment (Supplementary Table 2, available online). We did not observe a statistically significant difference in the associations with tumor subgroups by stromal M1 or M2 macrophage densities (Table 2) or tumor intraepithelial macrophage densities (Supplementary Table 3, available online).
Table 2.
Cumulative pack-years smoked and colorectal cancer incidence, overall and by stromal macrophage density
| Colorectal cancer subtype | Cumulative pack-years smoked |
P trend a | P heterogeneity b | |||
|---|---|---|---|---|---|---|
| 0 | 1-19 | 20-39 | ≥40 | |||
| Person-years | 1 695 634 | 985 798 | 579 878 | 387 060 | ||
| All colorectal cancer (N = 871) | ||||||
| No. | 361 | 209 | 149 | 152 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.09 (0.91 to 1.29) | 1.14 (0.94 to 1.38) | 1.38 (1.14 to 1.67) | <.001 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.08 (0.91 to 1.29) | 1.09 (0.90 to 1.33) | 1.27 (1.04 to 1.55) | .02 | — |
| Macrophage density | .003d | |||||
| Low (n = 288) | ||||||
| No. | 106 | 71 | 50 | 61 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.32 (0.98 to 1.80) | 1.36 (0.96 to 1.91) | 1.89 (1.37 to 2.60) | <.001 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.32 (0.97 to 1.79) | 1.31 (0.92 to 1.85) | 1.74 (1.26 to 2.41) | .004 | — |
| Intermediate (n = 294) | ||||||
| No. | 125 | 60 | 53 | 56 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 0.83 (0.61 to 1.13) | 1.09 (0.79 to 1.51) | 1.50 (1.09 to 2.05) | .002 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.83 (0.61 to 1.13) | 1.04 (0.75 to 1.44) | 1.38 (1.01 to 1.90) | .01 | — |
| High (n = 289) | ||||||
| No. | 130 | 78 | 46 | 35 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.12 (0.85 to 1.48) | 0.99 (0.70 to 1.38) | 0.89 (0.61 to 1.30) | .36 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.12 (0.84 to 1.48) | 0.95 (0.68 to 1.34) | 0.82 (0.56 to 1.20) | .17 | — |
| M1-like macrophage density | .63 | |||||
| Low (n = 289) | ||||||
| No. | 126 | 63 | 50 | 50 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 0.93 (0.68 to 1.26) | 1.04 (0.75 to 1.45) | 1.28 (0.92 to 1.79) | .10 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.93 (0.68 to 1.26) | 1.01 (0.72 to 1.40) | 1.19 (0.85 to 1.66) | .24 | — |
| Intermediate (n = 290) | ||||||
| No. | 112 | 71 | 50 | 57 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.20 (0.89 to 1.62) | 1.23 (0.88 to 1.72) | 1.74 (1.27 to 2.40) | .003 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.19 (0.88 to 1.61) | 1.17 (0.83 to 1.65) | 1.61 (1.17 to 2.23) | .02 | — |
| High (n = 288) | ||||||
| No. | 123 | 75 | 49 | 45 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.11 (0.83 to 1.47) | 1.12 (0.80 to 1.56) | 1.18 (0.84 to 1.65) | .27 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.10 (0.83 to 1.47) | 1.08 (0.77 to 1.51) | 1.08 (0.77 to 1.52) | .57 | — |
| M2-like macrophage density | .12 | |||||
| Low (n = 291) | ||||||
| No. | 113 | 66 | 56 | 56 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.25 (0.92 to 1.69) | 1.37 (0.99 to 1.89) | 1.65 (1.20 to 2.28) | .004 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.23 (0.90 to 1.67) | 1.31 (0.94 to 1.82) | 1.52 (1.10 to 2.10) | .02 | — |
| Intermediate (n = 292) | ||||||
| No. | 122 | 71 | 47 | 52 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 0.95 (0.71 to 1.28) | 1.04 (0.74 to 1.47) | 1.42 (1.03 to 1.96) | .02 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.96 (0.71 to 1.28) | 1.00 (0.71 to 1.41) | 1.32 (0.95 to 1.83) | .08 | — |
| High (n = 288) | ||||||
| No. | 126 | 72 | 46 | 44 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.05 (0.78 to 1.40) | 0.99 (0.71 to 1.39) | 1.12 (0.79 to 1.59) | .60 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.05 (0.78 to 1.40) | 0.96 (0.68 to 1.34) | 1.03 (0.73 to 1.47) | .99 | — |
P trend was calculated using pack-years as a continuous variable with a ceiling at 50 pack-years; 50 pack-years were used for pack-years greater than 50 to eliminate outlier effects. BMI = body mass index; CI = confidence interval; HR = hazard ratio.
P heterogeneity was calculated using the meta-regression method with a subtype-specific random effect term.
Inverse probability weighting was applied to reduce a potential selection bias because of the differential availability of macrophage density data (see “Statistical analyses” subsection in the Supplementary Methods, available online for details). The Cox models were stratified by age, calendar year of questionnaire cycle, and sex (ie, cohort). Multivariable models are adjusted for BMI (continuous with a ceiling at 35 kg/m2), family history of colorectal cancer in any first-degree relative (yes or no), physical activity (continuous with a ceiling at 50 metabolic equivalent task score-h/week), regular use of aspirin or nonsteroidal antiinflammatory drugs (yes or no), alcohol consumption (continuous with a ceiling at 30 g/d), red and processed meat intake (continuous with a ceiling at 14 servings/week), and folate intake (continuous with a ceiling at 1000 μg/d).
Statistically significant at the stringent α level of .005.
In secondary analyses, we found a differential association of smoking status with the incidence of colorectal cancer subclassified by tumor stromal macrophage densities (Pheterogeneity = .001; Table 3). Compared with never smokers, current smokers were associated with higher incidence of colorectal cancer having low stromal macrophage densities (multivariable-adjusted HR = 1.80, 95% CI = 1.23 to 2.61), whereas there was no such association with cancer having intermediate or high stromal macrophage densities (Table 3).
Table 3.
Smoking status and colorectal cancer incidence, overall and by stromal macrophage density
| Colorectal cancer subtype | Smoking status |
P trend a | P heterogeneity b | ||
|---|---|---|---|---|---|
| Never | Former | Current | |||
| Person-years | 1 695 634 | 1 473 952 | 443 913 | ||
| All colorectal cancer (N = 868) | |||||
| No. | 361 | 424 | 83 | ||
| Age-adjusted | 1 (Referent) | 1.20 (1.07 to 1.34) | 1.16 (0.96 to 1.40) | .004 | — |
| Multivariable-adjusted | 1 (Referent) | 1.17 (1.05 to 1.31) | 1.07 (0.88 to 1.30) | .06 | — |
| Macrophage density | .001d | ||||
| Low (n = 287) | |||||
| No. | 106 | 139 | 42 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.42 (1.10 to 1.83) | 1.95 (1.34 to 2.84) | <.001 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.39 (1.07 to 1.79) | 1.80 (1.23 to 2.61) | .001 | — |
| Intermediate (n = 292) | |||||
| No. | 125 | 141 | 26 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.09 (0.85 to 1.38) | 1.13 (0.73 to 1.75) | .47 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.06 (0.83 to 1.35) | 1.04 (0.67 to 1.61) | .71 | — |
| High (n = 289) | |||||
| No. | 130 | 144 | 15 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.14 (0.90 to 1.45) | 0.59 (0.35 to 1.01) | .47 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.12 (0.88 to 1.42) | 0.54 (0.32 to 0.93) | .27 | — |
| M1-like macrophage density | .40 | ||||
| Low (n = 287) | |||||
| No. | 126 | 125 | 36 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.01 (0.79 to 1.29) | 1.35 (0.92 to 1.99) | .28 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.99 (0.77 to 1.27) | 1.25 (0.85 to 1.84) | .47 | — |
| Intermediate (n = 289) | |||||
| No. | 112 | 145 | 32 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.35 (1.05 to 1.73) | 1.51 (1.00 to 2.26) | .008 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.32 (1.03 to 1.70) | 1.38 (0.92 to 2.07) | .03 | — |
| High (n = 292) | |||||
| No. | 123 | 154 | 15 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.25 (0.99 to 1.58) | 0.66 (0.38 to 1.13) | .92 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.22 (0.96 to 1.55) | 0.60 (0.35 to 1.04) | .76 | — |
| M2-like macrophage density | .10 | ||||
| Low (n = 290) | |||||
| No. | 113 | 148 | 29 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.44 (1.13 to 1.84) | 1.33 (0.87 to 2.03) | .01 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.40 (1.09 to 1.79) | 1.22 (0.80 to 1.87) | .03 | — |
| Intermediate (n = 290) | |||||
| No. | 122 | 136 | 32 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.07 (0.84 to 1.37) | 1.31 (0.88 to 1.95) | .23 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.06 (0.83 to 1.35) | 1.21 (0.82 to 1.81) | .38 | — |
| High (n = 288) | |||||
| No. | 126 | 140 | 22 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.11 (0.87 to 1.42) | 0.88 (0.55 to 1.41) | .90 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.09 (0.85 to 1.39) | 0.81 (0.51 to 1.28) | .78 | — |
P trend was calculated using ordinal categories of smoking status (never, former, and current). CI = confidence interval; HR = hazard ratio.
P heterogeneity was calculated using the meta-regression method with a subtype-specific random effect term.
Inverse probability weighting was applied in the same manner as Table 2. The Cox models were stratified by age, calendar year of questionnaire cycle, and sex (ie, cohort). Multivariable-adjusted models are adjusted for the same set of covariates as Table 2.
Statistically significant at the stringent α level of .005.
In analyses of smoking cessation, no statistically significant heterogeneity between tumor subgroups by macrophage densities was observed (Supplementary Table 4, available online).
To investigate the potential influence of MSI status and T-cell density on our findings, we performed additional stratified analyses according to MSI status and CD3+ cell densities. We conducted analyses limited to non–MSI-high tumors, which yielded similar differential associations of pack-years with colorectal cancer incidence by stromal macrophage densities (Pheterogeneity < .001; Table 4). We also conducted analyses using MSI-high tumors, but the small event count of MSI-high tumors precluded a robust assessment (Supplementary Table 5, available online). Although statistical significance was not reached at the stringent 2-sided α level of .005, the association of pack-years smoked with colorectal cancer incidence differed by stromal M2 macrophage densities in non–MSI-high tumors (Pheterogeneity = .04). In analyses stratified by stromal CD3+ cell densities, the differential association according to stromal macrophage densities appeared consistent regardless of CD3+ cell densities in tumor stromal regions (Supplementary Table 6, available online).
Table 4.
Cumulative pack-years smoked and colorectal cancer incidence by stromal macrophage density in non-microsatellite instability-high tumors
| Colorectal cancer subtype | Cumulative pack-years smoked |
P trend a | P heterogeneity b | |||
|---|---|---|---|---|---|---|
| 0 | 1-19 | 20–39 | ≥40 | |||
| Macrophage density | <.001d | |||||
| Low (n = 253) | ||||||
| No. | 93 | 61 | 45 | 54 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.25 (0.90 to 1.74) | 1.37 (0.95 to 1.97) | 1.89 (1.35 to 2.66) | <.001 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.24 (0.89 to 1.72) | 1.30 (0.90 to 1.87) | 1.71 (1.21 to 2.42) | .006 | — |
| Intermediate (n = 234) | ||||||
| No. | 100 | 49 | 45 | 40 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 0.87 (0.62 to 1.23) | 1.17 (0.82 to 1.66) | 1.33 (0.92 to 1.90) | .02 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.86 (0.61 to 1.21) | 1.10 (0.77 to 1.56) | 1.19 (0.83 to 1.72) | .91 | — |
| High (n = 211) | ||||||
| No. | 98 | 65 | 28 | 20 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.22 (0.89 to 1.66) | 0.79 (0.52 to 1.21) | 0.68 (0.42 to 1.11) | .04 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.20 (0.88 to 1.63) | 0.76 (0.50 to 1.16) | 0.62 (0.38 to 1.01) | .01 | — |
| M1-like macrophage density | .25 | |||||
| Low (n = 258) | ||||||
| No. | 111 | 55 | 48 | 44 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 0.91 (0.66 to 1.26) | 1.12 (0.79 to 1.57) | 1.28 (0.90 to 1.82) | .09 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.90 (0.65 to 1.25) | 1.06 (0.76 to 1.50) | 1.16 (0.81 to 1.67) | .26 | — |
| Intermediate (n = 226) | ||||||
| No. | 91 | 56 | 36 | 43 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.14 (0.82 to 1.60) | 1.09 (0.74 to 1.61) | 1.59 (1.11 to 2.29) | .03 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.12 (0.80 to 1.58) | 1.02 (0.69 to 1.51) | 1.43 (1.00 to 2.06) | .13 | — |
| High (n = 214) | ||||||
| No. | 89 | 64 | 34 | 27 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.30 (0.95 to 1.79) | 1.10 (0.74 to 1.63) | 0.99 (0.64 to 1.51) | .97 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.29 (0.93 to 1.77) | 1.04 (0.70 to 1.56) | 0.89 (0.58 to 1.37) | .59 | — |
| M2-like macrophage density | .04 | |||||
| Low (n = 235) | ||||||
| No. | 88 | 55 | 46 | 46 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.29 (0.92 to 1.81) | 1.44 (1.00 to 2.06) | 1.70 (1.19 to 2.43) | .003 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.26 (0.90 to 1.77) | 1.35 (0.94 to 1.95) | 1.54 (1.07 to 2.20) | .02 | — |
| Intermediate (n = 292) | ||||||
| No. | 103 | 57 | 37 | 37 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 0.91 (0.65 to 1.25) | 0.99 (0.68 to 1.44) | 1.23 (0.85 to 1.78) | .21 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 0.90 (0.65 to 1.25) | 0.94 (0.64 to 1.37) | 1.11 (0.76 to 1.62) | .50 | — |
| High (n = 288) | ||||||
| No. | 100 | 63 | 35 | 31 | ||
| Age-adjusted HR (95% CI)c | 1 (Referent) | 1.16 (0.84 to 1.58) | 0.93 (0.63 to 1.37) | 0.99 (0.66 to 1.49) | .82 | — |
| Multivariable-adjusted HR (95% CI)c | 1 (Referent) | 1.14 (0.83 to 1.57) | 0.89 (0.60 to 1.30) | 0.89 (0.59 to 1.35) | .44 | — |
P trend was calculated using ordinal categories of smoking status (never, former, and current). CI = confidence interval; HR = hazard ratio.
P heterogeneity was calculated using the meta-regression method with a subtype-specific random effect term.
In verse probability weighting was applied in the same manner as Table 2. The Cox models were stratified by age, calendar year of questionnaire cycle, and sex (ie, cohort). Multivariable-adjusted models are adjusted for the same set of covariates as Table 2.
Statistically significant at the stringent α level of .005.
We also conducted stratified analyses by sex (ie, cohort) and observed similar findings in both men and women (Supplementary Table 7, available online). Last, we conducted sensitivity analyses excluding early-onset colorectal cancer diagnosed before 50 years of age (N = 19). We confirmed that the differential association according to stromal macrophage densities was observed in later-onset colorectal cancer (Supplementary Table 8, available online).
Discussion
Colorectal cancer is a heterogeneous group of tumors influenced by tumor-immune interactions as well as various lifestyle factors such as smoking (4,36-38). Considering experimental evidence for the immunosuppressive effect of smoking (39,40), we hypothesized that the tumor-promoting effect of smoking might be stronger for tumors having particular immune features. Using the large prospective cohort studies, we found that the association of pack-years smoked with colorectal cancer incidence was stronger for tumors having lower stromal macrophage densities. Our data provide evidence for influences of smoking on tumor-associated macrophages.
Tumor-associated macrophages constitute an essential component of the tumor microenvironment (18,41). M1-like macrophages play pivotal roles in phagocytosis, antigen presentation, and antitumor immune response, whereas M2-like macrophages typically exhibit immunosuppressive functions (41). Considering their phenotypic heterogeneity, there is an increasing need to better characterize tumor-associated macrophages. However, there is no single specific marker for M1-like or M2-like macrophages. We therefore used a multiplex immunofluorescence assay that incorporates a pan-macrophage marker (CD68), 2 markers generally expressed in M1-like macrophages (CD86, IRF5), 2 markers generally expressed in M2-like macrophages (MAF, MRC1), and a tumor epithelial cell marker (KRT). Our method enabled us to generate more granular data that could not be obtained by traditional single-color immunohistochemistry.
Smoking has been reported to promote tumorigenesis in various organs (42), possibly through various mechanisms such as epigenetic alterations (43) and suppression of antitumor immunity (40,44). Previous studies have shown that smoking impairs the phagocytic function of macrophages (24,40) and augments the function of M2-like macrophages (45,46). Evidence also indicates that immunosuppressive macrophages may promote tumorigenesis (47) and that macrophage densities in colorectal cancer tissue are associated with clinical outcomes (22,23,48). Specifically, higher densities of overall and M1-like macrophages were associated with lower mortality, but higher densities of M2-like macrophages were associated with higher mortality (22,23,48). Considering these lines of evidence, our finding may suggest that, without the immunosuppressive effect of smoking, a portion of tumors may be eliminated by the phagocytic function or the antitumor immune response mediated by macrophages, but because of suppressive effects of smoking, those tumors may progress to become clinically detectable carcinomas.
Evidence suggests differential tumor characteristics between early-onset and later-onset colorectal cancers (49-52). Although we observed the differential association of smoking with the incidence of later-onset colorectal cancer by macrophage densities, analyses for early-onset colorectal cancer were underpowered. Further studies are needed to examine immune features of early-onset colorectal cancer in relation to lifestyle exposures.
We acknowledge some limitations of this study. First, measurement errors may exist in both questionnaire-based and tissue-based data. We conducted a careful validation in our multiplexed assay to measure tumor-associated macrophages (29). Second, we used the multiplex immunofluorescence assay for evaluating macrophage polarization, and defined M1-like and M2-like macrophages as the highest and lowest 30 percentiles, respectively, of the M1:M2 index values using the 4 markers (CD86, IRF5, MAF, and MRC1). There is no established standardized method to characterize macrophage polarization in archival tissue (53), and the estimates of the occurrence of M1-like and M2-like macrophages have varied between studies (16,22,23). Nevertheless, both M1-like and M2-like macrophage densities determined by our method demonstrated prognostic significance in our previous study (29). Third, macrophage data were not available for all colorectal cancer cases within the cohorts. However, we applied the IPW method (35) using all of the 3092 incident colorectal cancers to adjust for the selection bias because of tissue availability. Fourth, there was multiple hypothesis testing using multiple macrophage measures. However, we adopted the stringent α level of .005 (33), which should decrease the possibility of false-positive findings. Fifth, to maximize statistical power, our analyses were based on the mixed datasets consisting of the 2 prospective cohort studies (NHS and HPFS), which might affect generalizability. Although there was between-study heterogeneity, we conducted tests of heterogeneity using the Q statistic and observed no statistically significant heterogeneity between the 2 cohorts (Pheterogeneity > .31) in the analyses of pack-years smoked in relation to the incidence of colorectal cancer subclassified by macrophages densities. Lastly, our study participants were non-Hispanic White health professionals, and therefore, our findings should be validated in other populations.
This study has several strengths. First, in our prospective cohort design, information on smoking and other factors was collected before the subsequent diagnosis of colorectal cancer, which avoided differential recall bias between those who developed cancers and those who did not. Second, more than 131 000 study participants provided updated information on smoking and potential confounders at each questionnaire cycle. Therefore, we were able to evaluate the long-term effect of smoking on incidence of colorectal cancer subtype while adjusting for potential confounders. In addition, our repeated collection of smoking and lifestyle information yielded more accurate cumulative exposure data than 1-time recall of past lifestyle behaviors. Third, our prospective design also allowed us to obtain data on all of the 3092 incident colorectal cancer cases and adjust for selection bias in the 871 cases with tissue macrophage data availability by use of the IPW method. Fourth, our integrated molecular pathological epidemiological approach helped us to evaluate the differential effect of smoking on incidence of tumor subtypes. Subgrouping colorectal cancer cases by relevant biomarkers (such as macrophages and MSI status) is considerably important in cancer incidence analyses. As we have shown using the molecular pathological epidemiology approach, the association of smoking with colorectal cancer incidence was stronger for tumors with fewer macrophages. In this manner, we could observe a refined stronger association for the specific tumor subgroup, which could provide novel pathogenic insight and help establish causality. Fifth, we phenotyped each tumor-associated macrophage and measured macrophage densities by means of multiplex immunofluorescence. In addition, we integrated the multiplex immunofluorescence assay with pathologist-supervised image analysis and machine learning algorithms to phenotype (and count) each individual macrophage in tumor epithelial and stromal regions separately and to evaluate their M1:M2 polarization spectrum (29). This is a powerful tool to simultaneously detect multiple epitopes relevant in the context of macrophage biology.
In conclusion, the current study suggests that the association of smoking with incidence of colorectal cancer is stronger for tumors containing lower stromal macrophage counts. Our findings suggest an interplay of smoking and macrophages in colorectal carcinogenesis.
Funding
This work was supported by U.S. National Institutes of Health (NIH) grants (P01 CA87969 to M.J. Stampfer; UM1 CA186107 to M.J. Stampfer; P01 CA55075 to W.C. Willett; UM1 CA167552 to W.C. Willett; U01 CA167552 to W.C. Willett and L.A. Mucci; P50 CA127003 to C.S.F.; R01 CA118553 to C.S.F.; R01 CA169141 to C.S.F.; R01 CA137178 to A.T.C.; K24 DK098311 to A.T.C.; R35 CA197735 to S.O.; R01 CA151993 to S.O.; R01 CA248857 to S.O., U. Peters, and A.I. Phipps; R00 CA215314 to M.S.; and K07 CA188126 to X.Z.); by Nodal Award (2016–02) from the Dana-Farber Harvard Cancer Center (to S.O.); by the Stand Up to Cancer Colorectal Cancer Dream Team Translational Research Grant (SU2C-AACR-DT22-17 to C.S.F. and M.G.), administered by the American Association for Cancer Research, a scientific partner of SU2C; by the American Cancer Society Mentored Research Scholar Grant (MRSG-17–220-01 - NEC to M.S.); and by grants from the Project P Fund, The Friends of the Dana-Farber Cancer Institute, Bennett Family Fund, and the Entertainment Industry Foundation through National Colorectal Cancer Research Alliance. T.U., K.H., and K.F. were supported by fellowship grants from the Uehara Memorial Foundation. K.H. was supported by the Mitsukoshi Health and Welfare Foundation. T.U. was supported by Yasuda Medical Foundation. S.A.V. was supported by grants from the Finnish Cultural Foundation and Orion Research Foundation sr. J.B. was supported by a grant from the Australia Awards-Endeavour Scholarships and Fellowships Program. K.A. and T.U. were supported by a grant from Overseas Research Fellowship (201860083 to K.A.; 201960541 to T.U.) from Japan Society for the Promotion of Science. M.G. was supported by a Conquer Cancer Foundation of ASCO Career Development Award. J.A.M. research is supported by the Douglas Gray Woodruff Chair fund, the Guo Shu Shi Fund, Anonymous Family Fund for Innovations in Colorectal Cancer, Project P fund, and the George Stone Family Foundation.
Notes
Role of the funder: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosures: C.S.F. is currently employed by Genentech, a subsidiary of Roche, and previously served as a consultant for Agios, Bain Capital, Bayer, Celgene, Dicerna, Five Prime Therapeutics, Gilead Sciences, Eli Lilly, Entrinsic Health, Genentech, KEW, Merck, Merrimack Pharmaceuticals, Pfizer Inc, Sanofi, Taiho, and Unum Therapeutics; C.S.F. also serves as a Director for CytomX Therapeutics and owns unexercised stock options for CytomX and Entrinsic Health. M.G. receives research funding from Bristol-Myers Squibb and Merck. J.A.M. received institutional research funding from Boston Biomedical. J.A.M. has also served as an advisor/consultant to Ignyta, Array Pharmaceutical, and Cota. This study was not funded by any of these commercial entities. No other conflicts of interest exist. The other authors declare that they have no conflicts of interest.
Author contributions: T.U., J.A.M., J.A.N., and S.O. developed the main concept and designed the study. M.S., J.A.M., J.A.N., and S.O. wrote grant applications. T.U., J.P.V., R.Z., K.H., M.G., J.A.M., J.A.N., and S.O. were responsible for the collection of tumor tissue, and acquisition of epidemiologic, clinical and tumor tissue data. T.U. performed data analysis, interpreted the results, and drafted the manuscript. All authors contributed to editing and critical revision for important intellectual contents.
Acknowledgements: We would like to thank the participants and staff of the Nurses’ Health Study and the Health Professionals Follow-up Study for their valuable contributions as well as the following state cancer registries for their help: Alaska, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Missouri, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington, and Wyoming. The authors assume full responsibility for analyses and interpretation of these data.
Supplementary Material
Data Availability
Drs Ugai and Ogino had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Further information including the procedures to obtain and access data from the Nurses’ Health Studies and the Health Professionals Follow-up Study are described at https://www.nurseshealthstudy.org/researchers/ and https://sites.sph.harvard.edu/hpfs/for-collaborators/.
References
- 1. Botteri E, Iodice S, Bagnardi V, et al. Smoking and colorectal cancer: a meta-analysis. JAMA. 2008;300(23):2765–2778. [DOI] [PubMed] [Google Scholar]
- 2. Phipps AI, Alwers E, Harrison T, et al. Association between molecular subtypes of colorectal tumors and patient survival, based on pooled analysis of 7 international studies. Gastroenterology. 2020;158(8):2158–2168.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ogino S, Nowak JA, Hamada T, et al. Insights into pathogenic interactions among environment, host, and tumor at the crossroads of molecular pathology and epidemiology. Annu Rev Pathol. 2019;14:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ogino S, Nowak JA, Hamada T, et al. Integrative analysis of exogenous, endogenous, tumour and immune factors for precision medicine. Gut. 2018;67(6):1168–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zitvogel L, Pietrocola F, Kroemer G.. Nutrition, inflammation and cancer. Nat Immunol. 2017;18(8):843–850. [DOI] [PubMed] [Google Scholar]
- 6. Botteri E, Borroni E, Sloan EK, et al. Smoking and colorectal cancer risk, overall and by molecular subtypes: a meta-analysis. Am J Gastroenterol. 2020;115(12):1940–1949. [DOI] [PubMed] [Google Scholar]
- 7. Amitay EL, Carr PR, Jansen L, et al. Smoking, alcohol consumption and colorectal cancer risk by molecular pathological subtypes and pathways. Br J Cancer. 2020;122(11):1604–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamada T, Nowak JA, Masugi Y, et al. Smoking and risk of colorectal cancer sub-classified by tumor-infiltrating T cells. J Natl Cancer Inst. 2019;111(1):42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jayasekara H, English DR, Haydon A, et al. Associations of alcohol intake, smoking, physical activity and obesity with survival following colorectal cancer diagnosis by stage, anatomic site and tumor molecular subtype. Int J Cancer. 2018;142(2):238–250. [DOI] [PubMed] [Google Scholar]
- 10. Carr PR, Alwers E, Bienert S, et al. Lifestyle factors and risk of sporadic colorectal cancer by microsatellite instability status: a systematic review and meta-analyses. Ann Oncol. 2018;29(4):825–834. [DOI] [PubMed] [Google Scholar]
- 11. Tillmans LS, Vierkant RA, Wang AH, et al. Associations between cigarette smoking, hormone therapy, and folate intake with incident colorectal cancer by TP53 protein expression level in a population-based cohort of older women. Cancer Epidemiol Biomarkers Prev. 2014;23(2):350–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nishihara R, Morikawa T, Kuchiba A, et al. A prospective study of duration of smoking cessation and colorectal cancer risk by epigenetics-related tumor classification. Am J Epidemiol. 2013;178(1):84–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ogino S, Giannakis M.. Immunoscore for (colorectal) cancer precision medicine. Lancet. 2018;391(10135):2084–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pinto ML, Rios E, Duraes C, et al. The two faces of tumor-associated macrophages and their clinical significance in colorectal cancer. Front Immunol. 2019;10:1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Edin S, Kaprio T, Hagstrom J, et al. The prognostic importance of CD20(+) B lymphocytes in colorectal cancer and the relation to other immune cell subsets. Sci Rep. 2019;9(1):19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Koelzer VH, Canonica K, Dawson H, et al. Phenotyping of tumor-associated macrophages in colorectal cancer: impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2016;5(4):e1106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edin S, Wikberg ML, Oldenborg PA, et al. Macrophages: good guys in colorectal cancer. Oncoimmunology. 2013;2(2):e23038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murray PJ. Macrophage polarization. Annu Rev Physiol. 2017;79:541–566. [DOI] [PubMed] [Google Scholar]
- 19. Ginhoux F, Schultze JL, Murray PJ, et al. New insights into the multidimensional concept of macrophage ontogeny, activation and function. Nat Immunol. 2016;17(1):34–40. [DOI] [PubMed] [Google Scholar]
- 20. Lundholm M, Hagglof C, Wikberg ML, et al. Secreted factors from colorectal and prostate cancer cells skew the immune response in opposite directions. Sci Rep. 2015;5:15651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edin S, Wikberg ML, Rutegard J, et al. Phenotypic skewing of macrophages in vitro by secreted factors from colorectal cancer cells. PLoS One. 2013;8(9):e74982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Edin S, Wikberg ML, Dahlin AM, et al. The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One. 2012;7(10):e47045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Algars A, Irjala H, Vaittinen S, et al. Type and location of tumor-infiltrating macrophages and lymphatic vessels predict survival of colorectal cancer patients. Int J Cancer. 2012;131(4):864–873. [DOI] [PubMed] [Google Scholar]
- 24. Yang DC, Chen CH.. Cigarette smoking-mediated macrophage reprogramming: mechanistic insights and therapeutic implications. J Nat Sci. 2018;4(11):e539. [PMC free article] [PubMed] [Google Scholar]
- 25. Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369(12):1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mehta RS, Nishihara R, Cao Y, et al. Association of dietary patterns with risk of colorectal cancer subtypes classified by fusobacterium nucleatum in tumor tissue. JAMA Oncol. 2017;3(7):921–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61(6):847–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61(6):794–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vayrynen JP, Haruki K, Lau MC, et al. The prognostic role of macrophage polarization in the colorectal cancer microenvironment. Cancer Immunol Res. 2021;9(1):8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan AT, Ogino S, Fuchs CS.. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N Engl J Med. 2007;356(21):2131–2142. [DOI] [PubMed] [Google Scholar]
- 31. Fujiyoshi K, Bruford EA, Mroz P, et al. Opinion: standardizing gene product nomenclature-a call to action. Proc Natl Acad Sci USA. 2021;118(3):e2025207118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haruki K, Kosumi K, Li P, et al. An integrated analysis of lymphocytic reaction, tumour molecular characteristics and patient survival in colorectal cancer. Br J Cancer. 2020;122(9):1367–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benjamin DJ, Berger JO, Johannesson M, et al. Redefine statistical significance. Nat Hum Behav. 2018;2(1):6–10. [DOI] [PubMed] [Google Scholar]
- 34. Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu L, Nevo D, Nishihara R, et al. Utility of inverse probability weighting in molecular pathological epidemiology. Eur J Epidemiol. 2018;33(4):381–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bai J, Chen H, Bai X.. Relationship between microsatellite status and immune microenvironment of colorectal cancer and its application to diagnosis and treatment. J Clin Lab Anal. 2021;35(6):e23810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang ST, Cui WQ, Pan D, et al. Tea polyphenols and their chemopreventive and therapeutic effects on colorectal cancer. World J Gastroenterol. 2020;26(6):562–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giannakis M, Mu XJ, Shukla SA, et al. Genomic correlates of immune-cell infiltrates in colorectal carcinoma. Cell Rep. 2016;15(4):857–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yamaguchi NH. Smoking, immunity, and DNA damage. Transl Lung Cancer Res. 2019;8(suppl 1):S3–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Qiu F, Liang CL, Liu H, et al. Impacts of cigarette smoking on immune responsiveness: up and down or upside down? Oncotarget. 2017;8(1):268–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang M, McKay D, Pollard JW, et al. Diverse functions of macrophages in different tumor microenvironments. Cancer Res. 2018;78(19):5492–5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking-50 Years of Progress: A Report of the Surgeon General. Atlanta, GA: Centers for Disease Control and Prevention (US); 2014. [PubMed]
- 43. McCartney DL, Stevenson AJ, Hillary RF, et al. Epigenetic signatures of starting and stopping smoking. EBioMedicine. 2018;37:214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez CP, Morrow K, Velasco C, et al. Effects of cigarette smoke extract on primary activated T cells. Cell Immunol. 2013;282(1):38–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shaykhiev R, Krause A, Salit J, et al. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol. 2009;183(4):2867–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Eapen MS, Hansbro PM, McAlinden K, et al. Abnormal M1/M2 macrophage phenotype profiles in the small airway wall and lumen in smokers and chronic obstructive pulmonary disease (COPD). Sci Rep. 2017;7(1):13392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Noy R, Pollard JW.. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Forssell J, Oberg A, Henriksson ML, et al. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13(5):1472–1479. [DOI] [PubMed] [Google Scholar]
- 49. Akimoto N, Zhao M, Ugai T, et al. Tumor Long Interspersed Nucleotide Element-1 (LINE-1) hypomethylation in relation to age of colorectal cancer diagnosis and prognosis. Cancers (Basel). 2021;13(9):2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Akimoto N, Ugai T, Zhong R, et al. Rising incidence of early-onset colorectal cancer - a call to action. Nat Rev Clin Oncol. 2021;18(4):230–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Willauer AN, Liu Y, Pereira AAL, et al. Clinical and molecular characterization of early-onset colorectal cancer. Cancer. 2019;125(12):2002–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lieu CH, Golemis EA, Serebriiskii IG, et al. Comprehensive genomic landscapes in early and later onset colorectal cancer. Clin Cancer Res. 2019;25(19):5852–5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Murray PJ, Allen JE, Biswas SK, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Drs Ugai and Ogino had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Further information including the procedures to obtain and access data from the Nurses’ Health Studies and the Health Professionals Follow-up Study are described at https://www.nurseshealthstudy.org/researchers/ and https://sites.sph.harvard.edu/hpfs/for-collaborators/.