Abstract
Osteoarthritis (OA) is no longer regarded as a simple wear-and-tear problem of articular cartilage. Instead, OA is a whole joint disorder involving both cartilaginous and non-cartilaginous tissues such as subchondral bone and synovium. Among them, subchondral bone undergoes constant remodeling in response to the changes of mechanical environment. Current understanding of subchondral bone disturbance in OA is limited to its link with an altered local mechanical loading as a result of ligament or meniscus injury. Very recently, hypertension, the most common vascular morbidity, has been emerged as an independent risk factor of OA. It might suggest a plausible role of systemic hemodynamic mechanical stress in subchondral bone remodeling and the pathogenesis of OA. However, their relationship remains not fully understood. Based on our preliminary clinical observation on the association of hemodynamic parameters with subchondral bone mass and microstructure in late-stage knee OA patients, we formulate a vascular etiology hypothesis of OA from a mechanobiology perspective. Noteworthily, hemodynamic stress associated with subchondral bone mineral density; yet compressive mechanical loading does not. Furthermore, hemodynamic parameters positively correlated with subchondral plate-like trabecular bone volume but negatively associated with rod-like trabecular bone volume. In contrast, compressive mechanical loading tends to increase both plate-like and rod-like trabecular bone volume. Taken together, it warrants further investigations into the distinct role of hemodynamic or compressive stress in shaping subchondral bone in the pathophysiology of OA.
The Translational potential of this article
This work provides a new insight, from the angle of biomechanics, into the emerging role of vascular pathologies, such as hypertension, in the pathogenesis of OA. It might open up a new avenue for the development of a mechanism-based discovery of novel diagnostics and therapeutics.
Keywords: Osteoarthritis, Subchondral trabecular bone, Pulse pressure, Heart rate
Abbreviations: OA, osteoarthritis; sBMD, subchondral bone mineral density; BV/TV, bone volume fraction; pBV/TV, plate bone volume fraction; rBV/TV, rod bone volume fraction; PP, pulse pressure; HR, heart rate; MAP, mean arterial pressure; ITS, individual trabeculae segmentation; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; micro-CT, microcomputed tomography; MRI, magnetic resonance imaging; OARSI, Osteoarthritis Research Society International; AChE, acetylcholinesterase
1. Multi-etiology of osteoarthritis
Osteoarthritis (OA) is the most common form of arthritis. It is a leading cause of chronic pain and impaired mobility in elderly people. The etiology of OA is multifactorial as a result of the interplay between local (ligament or meniscal injury) and systemic risk factors (age, gender, BMI). Previously, OA was conceived as a disease initiated by mechanical overuse or injury of the joint [1,2]. An imbalanced loading distribution post overuse or injury triggers joint tissue remodeling and the onset of OA. Meanwhile, the comorbid obesity, metabolic syndrome and low-grade systemic inflammation further impair joint tissue homeostasis and aggravate the deterioration of OA. In addition to these local and systemic risk factors, vascular etiology of OA has been proposed since mid-20 century [3]. A variety of vascular pathologies such as hypertension, atherosclerosis etc. have been associated with OA in different anatomic locations such as hand and knee [[4], [5], [6]]. Among them, hypertension, the commonest vascular comorbidity in the end-stage knee OA [7], is our research interest. It has been well established that reduction of skeletal blood flow leads to decreased angiogenesis and osteogenesis [8]. Yet it remains unclear how high blood pressure modulate subchondral bone blood supply, and ultimately contributes to the development of OA [4,9].
2. Pivotal role of subchondral bone in OA
Subchondral bone plays an important role in OA pathogenesis. Bone mineral density (BMD), a key index of bone mass, is closely associated with OA progression. High BMD in femoral neck and lumbar spine, has been proved as an indicator for hip or spinal OA in both cross-sectional and longitudinal studies [10]. High bone mass was associated with radiographic and clinical changes of knee OA progression [11]. Tibial subchondral BMD predicted joint space narrowing and knee OA progression [12].
In addition to bone mass, subchondral bone microarchitecture such as the rod-like and plate-like trabeculae serves different roles in load bearing and play a part in OA pathogenesis [13]. Plate is the principal load-bearing part while rod serves as transverse connections [14]. To better elucidate their distinct functions, a novel segmentation technique called individual trabeculae segmentation (ITS) has been developed in order to quantitatively measure the volume of rod-like and plate-like trabecular bone separately [15]. This ITS approach enables us to further investigate how the plate-rod ratio could play a role in OA progression. As recently shown, trabecular rod loss and plate thickening could precede the cartilage degeneration in the early stage of OA. As a result, such micro-structural change, i.e., the rod-to-plate conversion, would increase the stiffness of subchondral bone, contributing to wear-and tear of the overlying cartilage and ultimately OA progression [13].
The relationship between BMD and blood pressure has been extensively studied [[16], [17], [18]]. Femoral neck BMD showed a strong correlation with systemic blood pressure. For instance, McFarlane and colleagues reported a negative correlation between femoral neck BMD and PP [16]. Also, an increase of vascular stiffness and heart rate were associated with decreased BMD [19,20].
To further test vascular etiology hypothesis of OA, we conducted a preliminary observation on total twenty-one female patients (52–82 years old) who underwent total knee replacement surgery in Queen Mary Hospital in Hong Kong. The ethic committee approved all the experimental procedures in this study (HKU/HA HKW IRB, no.: UW13–251). Tibia plateaus were harvested and processed for microcomputed tomography (micro-CT) analysis of subchondral bone. The trabecular bone mass indicator, sBMD, was then measured accordingly. Subchondral bone microstructure was further analyzed by ITS. The following parameters of subchondral trabecular bone were generated: bone volume fraction (BV/TV) and plate/rod (p/r) BV/TV via ITS.
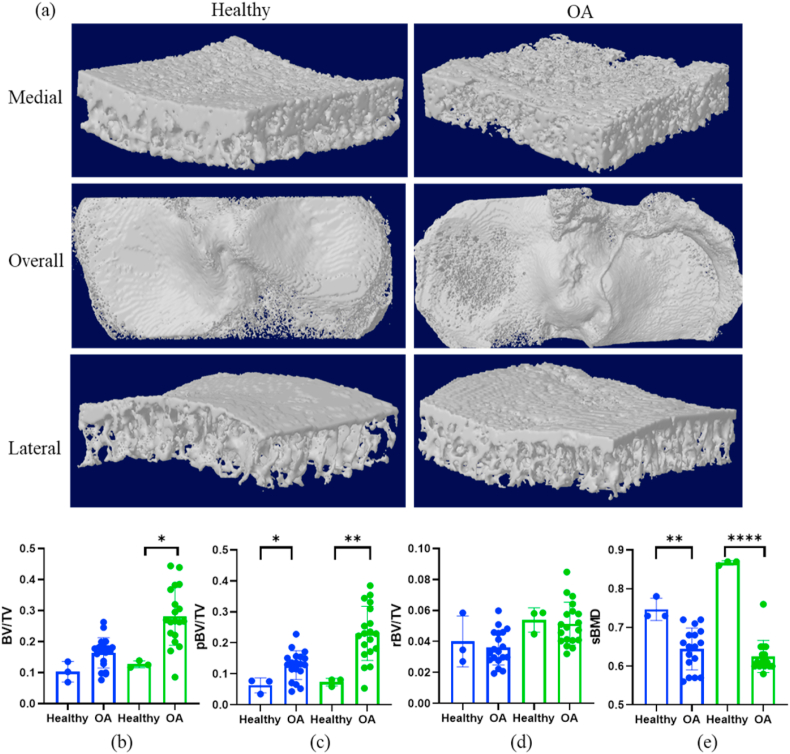
We compared subchondral bone changes in knee OA patients with three intact knee caderic samples from Department of Anatomy, the University of Hong Kong. As shown in Fig. 1, knee OA samples exhibited significantly higher BV/TV on the medial side, notably pBV/TV on both medial and lateral sides, as well as decreased sBMD on both sides, compared to the intact knee samples.
Figure 1.
Subchondral bone changes in healthy and OA subjects. (a) The representative 3-D subchondral bone image of these two groups. (b–e) Blue columns indicate lateral parts and green columns are the medial parts. Compared to the healthy subjects, the OA subjects had significantly higher BV/TV (b, medial side), significantly higher pBV/TV (c, lateral and medial sides) and significantly lower sBMD (e, lateral and medial sides). ∗Statistical significance (p < 0.05); ∗p < 0.01; ∗∗∗∗p < 0.0001.
As shown in Table 1, no significant correlation could be observed between the three metabolic factors (age, body weight and BMI) and the subchondral bone parameters. However, the p-value between age and sBMD was around 0.07, which is only slightly higher than 0.05. Therefore, a significant relationship between age and sBMD could be expected if the sample size was further increased.
Table 1.
Relationship between metabolic factors and subchondral trabecular bone.
| Trabecular bone mass |
Trabecular bone volume |
|||
|---|---|---|---|---|
| sBMD | BV/TV | pBV/TV | rBV/TV | |
| Age | −0.400 | −0.011 | 0.016 | 0.170 |
| Body weight | −0.242 | −0.125 | −0.207 | −0.101 |
| Body mass index (BMI) | −0.148 | −0.061 | −0.090 | −0.227 |
The relationship was tested via Spearman's rank-order correlation and Spearman's coefficient (rho) were listed in this table. ∗: Significant level p < 0.05.
Local compressive mechanical loading re-distribution triggers subchondral bone remodeling but does not change bone mineral density and plate-rod ratio.
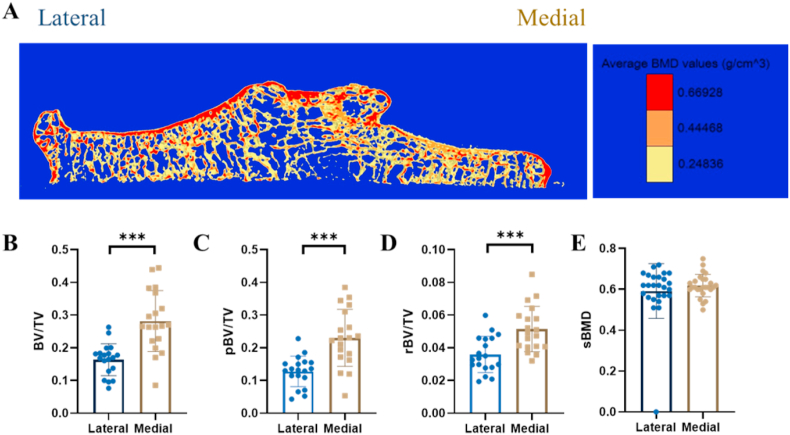
The bone microstructural changes between lateral and medial side were measured via ITS [14]. A significant difference between the lateral and medial side was found, i.e. the lateral side contained a larger bone volume (p < 0.0001 for BV/TV, p < 0.001 for pBV/TV, p < 0.001 for rBV/TV) (Fig. 2B–D). This showed that the lateral side was less affected by the compressive force than the medial side. However, no significant difference was found between the two sides for sBMD, indicating mechanical loading had minor influence on trabeculae mass changes (Fig. 2A, E). Obviously, altered compressive mechanical loading alone cannot explain the change in plate/rod ratio and decreased sBMD in knee OA samples. It prompts further investigations into the other pathogenic factors such as vascular pathology contributing to OA subchondral bone disturbance.
Figure 2.
Subchondral bone disturbance in response to mechanical loading in knee osteoarthritis (KOA). (A–E) Comparisons of subchondral trabecular bone mass and microstructure between lateral and medial tibial plateau subject to differential mechanical loading in KOA patients. (A) The representative color-coded micro-CT image showed the distribution of subchondral trabecular bone mineral density (sBMD) and bone volume fraction (BV/TV). (B–D) Compared to the lateral tibial plateau, subchondral bone on the medial tibial plateau exhibited significantly higher BV/TV (B, p < 0.001), for both plate-like (C, p < 0.001) and rod-like trabeculae (D, p < 0.001). (E) Yet no significance was found in sBMD between lateral and medial side using paired t-test. Data are presented as mean ± standard deviation (SD). ∗∗∗Statistical significance (p < 0.001).
3. Systemic vascular pathology in OA
The exact role of hemodynamic index including systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure (PP), heart rate (HR) and mean arterial pressure (MAP) in OA remains largely unknown. In addition to SBP and DBP, PP was chosen as an indicator of arterial circulation [21], HR was deployed to reflect cardiac function [22], and MAP represented the perfusion for vital organs [23].
Mounting evidence points in high blood pressure as an independent risk factor for both symptomatic and radiographic knee OA [24,25]. High SBP and DBP conferred high risk of symptomatic OA [26]. Similar associations were also observed between high SBP/PP and radiographic knee OA [27]. The causal association of hypertension with knee OA appeared to be stronger in women than in men. Several lines of evidence have demonstrated joint structural changes in response to increased blood pressure. For instance, a significant relationship between higher baseline diastolic blood pressure (DBP) and increased cartilage matrix degeneration has been illustrated in longitudinal magnetic resonance imaging (MRI) studies [28]. In addition, significant bone loss at the subchondral bone plate has been shown in knee OA patients with hypertension from the Hong Kong local knee OA initiative [7]. However, few studies investigated whether blood pressure affected knee OA progression in subchondral trabecular bone.
Furthermore, organ perfusion is another vital function of blood flow and the relationship between perfusion and knee OA progression has been established by previous studies. Abnormalities in subchondral bone marrow perfusion were associated with cartilage degeneration in experimental osteoarthritis [29]. Recent findings from human studies have suggested that brain perfusion is affected by chronic knee pain based on pattern analysis of cerebral blood flow [30].
The causal association between blood pressure and OA makes a strong case to revisit the role of the vascular system in joint disease. Whereas the concept of OA as a whole joint disorder has gained much popularity in the last decade, the role of the vascular system and particularly angiogenesis has not been explored in depth [31]. Importantly, experimental studies in postnatal long bones have conclusively demonstrated that a drug-induced reduction of blood flow leads to loss of mineralized bone and treatment with bisphosphonates enhanced blood flow and vessel growth in bone [8]. However, the majority of findings have been obtained in metaphyseal and diaphyseal bone under non-inflammatory and non-degenerative conditions. Furthermore, three-dimensional visualization and analysis of the vascular system in subchondral bone tissues of mice and humans has until recently remained technically very challenging. Microangiography of osteoarthritic subchondral bone tissue in mice revealed an increase in vascular volume and number of blood vessels in established disease [32]. Optical clearing of bone tissues enabled advanced light and fluorescent microscopy of murine and human bone tissues and led to the identification of a previously unknown blood vessel type in cortical bone [33]. Further exploiting these techniques in human clinical specimens and experimental OA models is warranted to unravel insight into the vascular mechanisms underpinning development or progression of disease.
In our previous study, we once reported the association of hypertension, as well as diabetes, with subchondral bone plate loss in the advanced stage of knee OA [7]. To our best knowledge, there remains a lack of information on the link between subchondral trabecular bone and hemodynamic changes/shear stress in the context of OA pathophysiology.
Hypothesis: Hemodynamic stress increases subchondral bone mineral density and promotes rod-to-plate conversion.
Our hypothesis is that hemodynamic stress is an emerging risk factor of knee OA by modulating subchondral trabecular BMD and microarchitectural changes. As shown in Fig. 2 and Table 1, local compressive mechanical loading and other systemic risk factors such as age, gender and BMI, appeared not to be the dominant factors in subchondral trabecular bone disturbance.
In order to adjust the potential confounding factor-local compressive mechanical loading, we purposely examined the relationship between hemodynamic and trabecular bone parameters on the lateral tibial plateau. The hemodynamic parameters were calculated as follows. First, the supine brachial blood pressure was monitored continuously, after 5 min rest, using an automated oscillometric device (Omron HEM 907). Then the PP was calculated by subtracting DBP from SBP, followed by the MAP which was calculated by dividing PP by 3 and adding DBP. Three randomly selected SBP and DBP were used to calculated PP and MAP, and the averaged PP and MAP from the three measurements were used for the analysis. HR was automatic recorded by cardiac monitor.
These results suggest that there is a rod-to-plate conversion in subchondral bone with increased PP and HR (Table 2). The results showed that there existed a weak to moderate, positive correlation between PP and pBV/TV (rho = 0.445, p = 0.043). Additionally, a weak to moderate, negative correlation was identified between HR and rBV/TV (rho = −0.486, p = 0.026). On the other hand, there was no correlation between SBP, DBP, MAP and any trabecular microstructural changes (Table 2) (Table 2). As show in previous study, the rod-to-plate trabeculae conversion preceded cartilage degeneration and contributed to OA progression [13]. It would be of our interest to know whether high PP and HR would be one of the indicators for disease deterioration.
Table 2.
Relationship between blood flow dynamic indices and subchondral trabecular bone.
| Trabecular bone mass |
Trabecular bone volume |
|||
|---|---|---|---|---|
| sBMD | BV/TV | pBV/TV | rBV/TV | |
| Systolic blood pressure | 0.461∗ | 0.192 | 0.224 | −0.258 |
| Diastolic blood pressure | −0.007 | −0.183 | −0.238 | −0.110 |
| Mean arterial pressure | 0.240 | 0.007 | −0.004 | −0.113 |
| Pulse pressure | 0.453∗ | 0.389 | 0.445∗ | −0.153 |
| Heart rate | 0.507∗ | 0.038 | 0.049 | −0.486∗ |
The relationship was tested via Spearman's rank-order correlation and Spearman's coefficient (rho) were listed in this table. ∗Statistical significance (p < 0.05).
Notably, SBP, PP and HR all positively correlated with subchondral bone mineral density (Table 2). A weak and positive correlation was found between SBP and sBMD (rho = 0.461, p = 0.041). Similarly, there were weak to moderate positive correlations between PP, HR and sBMD (PP: rho = 0.453, p = 0.045; HR: rho = 0.507, p = 0.023) (Table 2). sBMD had no significant association with DBP and MAP. As high BMD is also a predictor of OA progression, SBP, PP and HR emerges as a panel of hemodynamic indicators we should bear in mind in physical examination for knee OA patients.
4. Perspective
To further validate our findings, we will systemically investigate the contributions of mechanical, metabolic, hemodynamic risk factors to subchondral trabecular bone disturbance in a large-scale knee OA patient cohort. As aforementioned, mechanical loading was associated with subchondral bone volume augmentation while the age is the deterministic factor for bone mineral density. Moreover, hemodynamic factors were in a relation to both bone mass and microarchitecture. Specifically, there is the rod-to-plate conversion and elevated BMD in response to an increase of PP and HR. We anticipated a distinct role of the compressive and hemodynamic stress in subchondral trabecular bone disturbance with knee OA progression.
Our study will help understand how subchondral trabecular bone changes in response to blood flow dynamics. We have identified SBP, PP and HR, in association with subchondral BMD and microstructural changes in advanced knee OA. Comparatively, MAP, as well as DBP, contributed to affect subchondral bone in a lesser extent. In other words, the blood circulation (SBP, PP) and cardiac function (HR) appeared closer link with OA pathology than joint tissue perfusion (DBP, MAP).
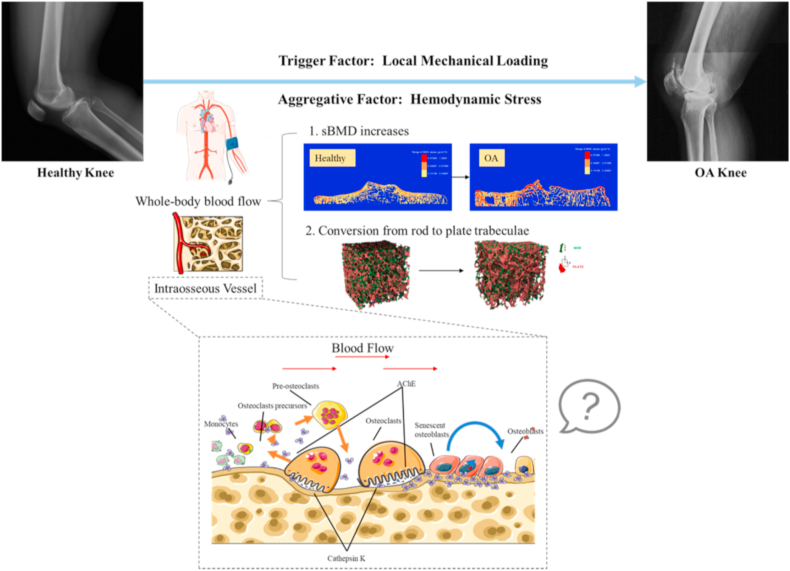
In summary, we propose the differential roles of compressive and hemodynamic stress in subchondral bone disturbance with knee OA progression (Fig. 3). Subchondral trabecular bone volume augmented, regardless of rod-like or plate-like trabeculae in medial compartment of knee joint with re-distribution of mechanical loading in OA knee. Interestingly, subchondral BMD increased, and rod-like trabeculae converted to plate-like trabeculae in response to elevated SBP, PP and HR. Given the importance of subchondral bone mineral density and rod-to-plate morphological conversion in the development of OA, haemodynamic shear stress plays a pivotal role in the disease deterioration whereas mechanical compressive loading triggers the onset of disease.
Figure 3.
Schematic of vascular etiology hypothesis of hypertension-associated OA. Local mechanical loading is the trigger factor of knee OA whereas hemodynamic stress, including the whole-body blood flow and blood flow in intraosseous vessels, is served as an aggregative factor during knee OA progression. We hypothesize and test that the hemodynamic stress generated by the whole-body blood flow increases sBMD and promotes rod-to-plate conversion in trabecular bone. How hemodynamic stress affects subchondral bone remodeling at cellular and molecular levels needs further investigations.
This finding will facilitate the in-depth understanding on the role of blood flow dynamics in subchondral bone remodeling in the context of OA from tissue level down to cellular and molecular levels. More importantly, it will open up a new avenue for us to subtype knee OA based on their distinct pathophysiological fingerprint, i.e. subchondral bone mass and microarchitecture, using high-resolution CT.
The unfilled research gap is the molecular machinery to regulate haemodynamic stress in OA subchondral bone remodelling. A variety of growth factors, e.g. TGF-beta1 [32], IGF-1 [34], PDGF-BB [35] have been recently identified to contribute to subchondral bone disturbance in OA. Among them, TGF-beta1 [32], IGF-1 and PDGF-BB [35] could be released and activated in response to altered compressive mechanical loading. Recently, bone matrix protein, acetylcholinesterase (AChE), arouse our interest in the context of OA pathophysiology [36]. Under physiological condition, fluid shear stress will increase the concentration of acetylcholine for vasodilation [37]. In the situation of hypertension, AChE would be expected to increase to hydrolyze acetylcholine for vasoconstriction [38]. AChE is a multi-functional protein. AChE can also be secreted by osteoblasts as adherent proteins and deposited in bone matrix during bone mineralization to regulate mesenchymal stem cell osteoblast differentiation or macrophage activation towards osteoclasts [39]. It warrants further investigations to elucidate the molecular mechanism underlying the interplay between compressive loading and fluid shear stress in regulation of subchondral bone homeostasis and disorder in OA.
Author statement
RYN and CYW designed the study. RYN performed experiments, XEG, CHY and CYW analyzed data. CHY and CYW contributed to data interpretation from clinical perspective. RYN prepared the draft of the manuscript, which was revised CYW. All authors have read and approved the final version of the manuscript.
Acknowledgments
This work was supported by Health and Medical Research Fund Scheme (01150087#, 16172691#), Research Grants Council of Hong Kong ECS (PolyU 251008/18M), GRF (PolyU 151061/20M, PolyU15100821M), NFSC/RGC schemes (N_PolyU 520/20), ITF MHKJFS (MHP/011/20) and the Hong Kong Polytechnic University Project of Strategic Importance (ZE2C). The authors thank for the support from Research Institute of Smart Ageing, the Hong Kong Polytechnic University.
References
- 1.Wen C., Lu W.W., Chiu K. Importance of subchondral bone in the pathogenesis and management of osteoarthritis from bench to bed. Journal of Orthopaedic Translation. 2014;2(1):16–25. [Google Scholar]
- 2.Boris Chan P.M., Zhu L., Wen C.Y., Chiu K.Y. Subchondral bone proteomics in osteoarthritis: current status and perspectives. J Orthop Translat. 2015;3(2):71–77. doi: 10.1016/j.jot.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stecher R.M. Heberden's nodes; the association of hypertension and obesity to degenerative joint disease of the fingers. J Lab Clin Med. 1946;31:687–693. [PubMed] [Google Scholar]
- 4.Ching K., Houard X., Berenbaum F., Wen C. Hypertension meets osteoarthritis - revisiting the vascular aetiology hypothesis. Nat Rev Rheumatol. 2021;19(9):533–549. doi: 10.1038/s41584-021-00650-x. [DOI] [PubMed] [Google Scholar]
- 5.Hussain S.M., Dawson C., Wang Y., Tonkin A.M., Chou L., Wluka A.E., et al. Vascular pathology and osteoarthritis: a systematic review. J Rheumatol. 2020;47(5):748–760. doi: 10.3899/jrheum.181236. [DOI] [PubMed] [Google Scholar]
- 6.Findlay D.M. Vascular pathology and osteoarthritis. Rheumatology. 2007;46(12):1763–1768. doi: 10.1093/rheumatology/kem191. [DOI] [PubMed] [Google Scholar]
- 7.Wen C.Y., Chen Y., Tang H.L., Yan C.H., Lu W.W., Chiu K.Y. Bone loss at subchondral plate in knee osteoarthritis patients with hypertension and type 2 diabetes mellitus. Osteoarthritis Cartilage. 2013;21(11):1716–1723. doi: 10.1016/j.joca.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 8.Ramasamy S.K., Kusumbe A.P., Schiller M., Zeuschner D., Bixel M.G., Milia C., et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun. 2016;7:13601. doi: 10.1038/ncomms13601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan P.M.B., Wen C.Y., Yang W.C., Yan C.H., Chiu K. Is subchondral bone cyst formation in non-load-bearing region of osteoarthritic knee a vascular problem? Med Hypotheses. 2017;109:80–83. doi: 10.1016/j.mehy.2017.09.027. [DOI] [PubMed] [Google Scholar]
- 10.Hardcastle S.A., Dieppe P., Gregson C.L., Davey Smith G., Tobias J.H. Osteoarthritis and bone mineral density: are strong bones bad for joints? BoneKEy Rep. 2015;4:624. doi: 10.1038/bonekey.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartley A., Hardcastle S.A., Paternoster L., McCloskey E., Poole K.E., Javaid M.K., et al. Individuals with High Bone Mass have increased progression of radiographic and clinical features of knee osteoarthritis. Osteoarthritis and Cartilage. 2020;28(9):1180–1190. doi: 10.1016/j.joca.2020.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Bruyere O., Dardenne C., Lejeune E., Zegels B., Pahaut A., Richy F., et al. Subchondral tibial bone mineral density predicts future joint space narrowing at the medial femoro-tibial compartment in patients with knee osteoarthritis. Bone. 2003;32(5):541–545. doi: 10.1016/s8756-3282(03)00059-0. [DOI] [PubMed] [Google Scholar]
- 13.Chen Y., Hu Y., Yu Y.E., Zhang X., Watts T., Zhou B., et al. Subchondral trabecular rod loss and plate thickening in the development of osteoarthritis. J Bone Miner Res. 2018;33(2):316–327. doi: 10.1002/jbmr.3313. [DOI] [PubMed] [Google Scholar]
- 14.Liu X., Saha P., Wehrli F., Sajda P., Guo X. A 3D morphological analysis of trabecular bone based on individual trabeculae segmentation. Trans Orthop Res Soc. 2006;31:1783. [Google Scholar]
- 15.Liu X.S., Sajda P., Saha P.K., Wehrli F.W., Bevill G., Keaveny T.M., et al. Complete volumetric decomposition of individual trabecular plates and rods and its morphological correlations with anisotropic elastic moduli in human trabecular bone. J Bone Miner Res. 2008;23(2):223–235. doi: 10.1359/JBMR.071009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFarlane I.M., Shin T.H., Bhamra M., Alvarez M.R., Leon S.Z., Ozeri D.J., et al. The relationship of pulse pressure and bone mineral density in adult USA population: analysis of the national health and nutritional examination survey. Rheumatology. 2018;8(2) [Google Scholar]
- 17.Yazici S., Yazici M., Korkmaz U., Erkan M.E., Baki A.E., Erden I., et al. Relationship between blood pressure levels and bone mineral density in postmenopausal Turkish women. Arch Med Sci. 2011;7(2):264–270. doi: 10.5114/aoms.2011.22077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cappuccio F.P., Meilahn E., Zmuda J.M., Cauley J.A. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354(9183):971–975. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 19.Jung M.H., Youn H.J., Ihm S.H., Jung H.O., Hong K.S. Heart rate and bone mineral density in older women with hypertension: results from the korea national health and nutritional examination survey. J Am Geriatr Soc. 2018;66(6):1144–1150. doi: 10.1111/jgs.15359. [DOI] [PubMed] [Google Scholar]
- 20.Lazarieva K., Amosova K., Nishkumay O., Mostbauer G., Lazariev P., Rudenko Y., et al. Relationship between indicators of pulse wave and bone mineral density in elderly aged women with uncomplicated hypertension. J Hypertens. 2019;37 E236-E236. [Google Scholar]
- 21.Dart A.M., Kingwell B.A. Pulse pressure--a review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 22.Safar M.E. Peripheral pulse pressure, large arteries, and microvessels. Hypertension. 2004;44(2):121–122. doi: 10.1161/01.HYP.0000135448.73199.75. [DOI] [PubMed] [Google Scholar]
- 23.Wehrwein E.A., Joyner M.J. Regulation of blood pressure by the arterial baroreflex and autonomic nervous system. Handb Clin Neurol. 2013;117:89–102. doi: 10.1016/B978-0-444-53491-0.00008-0. [DOI] [PubMed] [Google Scholar]
- 24.Lo K., Au M., Ni J., Wen C. Association between hypertension and osteoarthritis: a systematic review and meta-analysis of observational studies. Journal of Orthopaedic Translation. 2022;32(1):12–20. doi: 10.1016/j.jot.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y.M., Wang J., Liu X.G. Association between hypertension and risk of knee osteoarthritis A meta-analysis of observational studies. Medicine. 2017;96(32) doi: 10.1097/MD.0000000000007584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niu J., Clancy M., Aliabadi P., Vasan R., Felson D.T. Metabolic syndrome, its components, and knee osteoarthritis: the framingham osteoarthritis study. Arthritis Rheum. 2017;69(6):1194–1203. doi: 10.1002/art.40087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lo G.H., McAlindon T.E., Katz J.N., Driban J.B., Price L.L., Eaton C.B., et al. Systolic and pulse pressure associate with incident knee osteoarthritis: data from the Osteoarthritis Initiative. Clin Rheumatol. 2017;36(9):2121–2128. doi: 10.1007/s10067-017-3656-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashmeik W., Joseph G.B., Nevitt M.C., Lane N.E., McCulloch C.E., Link T.M. Association of blood pressure with knee cartilage composition and structural knee abnormalities: data from the osteoarthritis initiative. Skeletal Radiol. 2020;49(9):1359–1368. doi: 10.1007/s00256-020-03409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai P.H., Lee H.S., Siow T.Y., Wang C.Y., Chang Y.C., Lin M.H., et al. Abnormal perfusion in patellofemoral subchondral bone marrow in the rat anterior cruciate ligament transection model of post-traumatic osteoarthritis: a dynamic contrast-enhanced magnetic resonance imaging study. Osteoarthritis Cartilage. 2016;24(1):129–133. doi: 10.1016/j.joca.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 30.Iwabuchi S.J., Xing Y., Cottam W.J., Drabek M.M., Tadjibaev A., Fernandes G.S., et al. Brain perfusion patterns are altered in chronic knee pain: a spatial covariance analysis of arterial spin labelling MRI. Pain. 2020;161(6):1255–1263. doi: 10.1097/j.pain.0000000000001829. [DOI] [PubMed] [Google Scholar]
- 31.Mapp P.I., Walsh D.A. Mechanisms and targets of angiogenesis and nerve growth in osteoarthritis. Nat Rev Rheumatol. 2012;8(7):390–398. doi: 10.1038/nrrheum.2012.80. [DOI] [PubMed] [Google Scholar]
- 32.Zhen G., Wen C., Jia X., Li Y., Crane J.L., Mears S.C., et al. Inhibition of TGF-beta signaling in mesenchymal stem cells of subchondral bone attenuates osteoarthritis. Nat Med. 2013;19(6):704–712. doi: 10.1038/nm.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grüneboom A., Hawwari I., Weidner D., Culemann S., Müller S., Henneberg S., et al. A network of trans-cortical capillaries as mainstay for blood circulation in long bones. Nature Metabolism. 2019;1(2):236–250. doi: 10.1038/s42255-018-0016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xian L., Wu X., Pang L., Lou M., Rosen C.J., Qiu T., et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18(7):1095–1101. doi: 10.1038/nm.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su W., Liu G., Liu X., Zhou Y., Sun Q., Zhen G., et al. Angiogenesis stimulated by elevated PDGF-BB in subchondral bone contributes to osteoarthritis development. JCI Insight. 2020;5(8) doi: 10.1172/jci.insight.135446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauwers M., Courties A., Sellam J., Wen C. The cholinergic system in joint health and osteoarthritis: a narrative-review. Osteoarthritis Cartilage. 2021;29(5):643–653. doi: 10.1016/j.joca.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 37.Wilson C., Lee M.D., McCarron J.G. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J Physiol. 2016;594(24):7267–7307. doi: 10.1113/JP272927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lataro R.M., Silva C.A., Tefe-Silva C., Prado C.M., Salgado H.C. Acetylcholinesterase inhibition attenuates the development of hypertension and inflammation in spontaneously hypertensive rats. Am J Hypertens. 2015;28(10):1201–1208. doi: 10.1093/ajh/hpv017. [DOI] [PubMed] [Google Scholar]
- 39.Luo X., Lauwers M., Layer P.G., Wen C. Non-neuronal role of acetylcholinesterase in bone development and degeneration. Front Cell Dev Biol. 2020;8:620543. doi: 10.3389/fcell.2020.620543. [DOI] [PMC free article] [PubMed] [Google Scholar]