Highlights
-
•
Health workers were among those most affected by nosocomial Ebola virus disease (EVD) in this outbreak.
-
•
Children had a higher case fatality rate compared with other patients with nosocomial EVD.
-
•
Referral health facilities and privately owned health facilities had the highest number of nosocomial infections (NI).
-
•
Clear case definition of NI is required to prompt transmission chain interruption.
Keywords: Viral haemorrhagic fever, Ebola, Cross-infection, Infection prevention and control, Transmission, Nosocomial infection, Healthcare-associated infection, Health worker infection
Abstract
Objectives
To describe the characteristics of nosocomial cases of Ebola virus disease (EVD) in the Democratic Republic of the Congo between July 2018 and May 2020 in order to inform future interventions.
Methods
Nosocomial cases of EVD were identified during outbreak response surveillance, and a retrospective analysis of cases was conducted according to demographic characteristics and type of health facility (HF).
Results
Of 3481 cases of EVD, 579 (16.6%) were nosocomial. Of these, 332 cases occurred in women (57.3%). Patients and visitors accounted for 419 cases (72.4%), of which 79 (18.9%) were aged 6–≤18 years and 108 (25.8%) were aged ≤5 years. Health workers (HWs) accounted for the remaining 160 (27.6%) nosocomial cases. The case fatality rate (CFR) for HWs (66/160, 41.3%) was significantly lower than the CFR for patients and visitors (292/419, 69.7%) (P<0.001). The CFR was higher among cases aged 6–≤18 years (54/79, 68.4%) and ≤5 years (89/108, 82.4%). Referral HFs (>39 beds) had the highest prevalence of nosocomial EVD (148/579, 25.6%). Among HFs with at least one case of nosocomial infection, 50.0% (98/196) were privately owned.
Conclusions
Nurses and traditional healers should be targeted for infection prevention and control training, and supportive supervision should be provided to HFs to mitigate EVD transmission.
Introduction
Ebola virus disease (EVD) has affected countries in Africa since 1976 (Chowell and Nishiura, 2014; Jacob et al., 2020). Recent EVD outbreaks in West Africa (2014–2016) and the Democratic Republic of the Congo (DRC) (2018–2020) have been declared Public Health Emergencies of International Concern by the World Health Organization (WHO) (WHO, 2014a, 2019a). The 2018–2020 DRC EVD outbreak (the largest in the country) involved cases transmitted within health facilities (HFs) [i.e. nosocomial infections (NIs)], resulting in further transmission chains and prolongation of the outbreak. Prior to the EVD outbreak, no established NI surveillance system existed in DRC.
Transmission of EVD mainly occurs through direct contact with blood and bodily fluids, or indirect contact with contaminated surfaces (Chowell and Nishiura, 2014). In HFs, health workers (HWs) frequently handle blood and bodily fluids, increasing the risk of EVD cross-contamination among patients as well as HW exposure (Kaner and Schaack, 2016). During EVD outbreaks, HFs can become amplification epicentres, enabling transmission chains to extend into communities from contact with infected patients, visitors or HWs (Allegranzi et al., 2017; Selvaraj et al., 2018). Understanding the epidemiology of NIs is key in the development of effective infection prevention and control (IPC) interventions during outbreaks, particularly in limited-resource settings. However, establishing a robust NI definition and validating cases in the context of a large EVD outbreak is a major challenge, as boundaries between nosocomial and community acquisition are unclear.
This article describes the characteristics of nosocomial cases of EVD that occurred in the provinces of Ituri, North Kivu and South Kivu in DRC between 2018 and 2020, and aims to: (i) identify priority IPC strategies to strengthen outbreak preparedness, readiness and response; and (ii) facilitate the development of future EVD NI surveillance systems.
Setting and outbreak response
DRC is the second largest country in Africa, with approximately 20% of the total population living in the provinces of Ituri, North Kivu and South Kivu (INS, 2019). DRC has a Human Development Index of 0.459 (UNDP, 2020) and a Healthcare Access and Quality Index of 29.6 (Fullman et al., 2018). Only 52% and 29% of the population have access to clean water and improved sanitation, respectively (UNICEF, 2020). More than 3500 HFs (HDX, 2018) are registered in the three affected provinces, including informal HFs such as traditional practitioners. During the outbreak response, HFs were classified according to capacity (Category 1: >39 beds; Category 2: 20–39 beds; Category 3: 5–19 beds; and Category 4: 0–4 beds) to facilitate the EVD IPC operational response [e.g. number of consumable items, such as personal protective equipment (PPE) required per HF]. Category 1 HFs include general referral hospitals (non-EVD treatment centre) within the local health zone, and Category 4 HFs include those operated by traditional practitioners or healers, whose formal clinical training is highly variable.
Within the DRC health system, each province is subdivided into health zones, and each health zone is subdivided into health areas. For the outbreak response, intermediary and temporary strategic divisions, or ‘sub-coordinations’, were set up across the EVD-affected provinces. Each sub-coordination incorporated one to 12 health zones pertaining to the routine health system [see Table S1 (online supplementary material) for more information]. Response teams (including the IPC pillar) were embedded within each sub-coordination. On identification of an EVD case, an ‘IPC ring’ (Hageman et al., 2016) was activated to prevent further transmission chains by strengthening IPC measures at HF and community levels within the ring perimeters. IPC ring interventions included decontamination of affected HFs and/or households, HW briefing, IPC/WASH kit donation, and a rapid HF assessment using a standardized tool (scorecard) followed by development of an improvement action plan (Ousman et al., 2019). To further strengthen IPC measures and encourage consistent, evidence-based IPC practices across all response organizations, in September 2019, the Ministry of Health (MoH) endorsed a package of over 70 standardized IPC tools, including training modules and standard operational procedures, for affected localities.
Methods
Study design
A descriptive analysis of nosocomial cases of EVD identified between July 2018 and May 2020 in DRC was performed.
Surveillance and data collection
From July 2018 to May 2020, data were collected prospectively by MoH and WHO through the established response surveillance system. EVD cases were classified as ‘confirmed’ or ‘probable’ depending on laboratory availability, using reverse transcriptase polymerase chain reaction, genetic sequencing and epidemiological investigation, and entered into a line-list database. NI identification was conducted jointly by the surveillance and IPC pillars; all EVD cases (patients, visitors, family members and caregivers) that had either visited or worked in a HF prior to symptom onset were assessed to determine whether the NI case definition criteria was met. Procedures for the identification of EVD cases are described in more detail elsewhere (WHO, 2014b; Aruna et al., 2019).
NI case definition
As the outbreak evolved, the inclusion criteria for a NI case were reviewed and revised accordingly. A confirmed or probable EVD case was defined as a NI if it met the following criteria:
-
•
Period 1 (July–December 2018): being a HW or a patient being cared for by a HW identified as having EVD;
-
•
Period 2 (January–August 2019): having any exposure to a HF within 21 days preceding symptom onset; and
-
•
Period 3 (September 2019–May 2020): having any exposure to a HF (admission, visit or work) where a confirmed or probable symptomatic case was located, within 2–21 days preceding symptom onset, and no documented exposure to an EVD case in the community.
Data analysis
The EVD surveillance line-list database was cross-linked to the IPC monitoring database of each HF to include additional information whenever applicable. Relevant variables recorded, such as age, sex, health zone, HF category, individuals affected, professional occupation (if a HW), time between symptom onset and reporting to surveillance, and mortality were analysed retrospectively. Regarding ownership, HFs were classified as public, private or private not-for-profit (i.e. owned by a religious entity).
Analyses were stratified using SPSS and R software by the three periods corresponding to the different inclusion criteria for NI cases. Where applicable, differences in variables were analysed by Chi-squared test for categorical data, or Student's t-test or analysis of variance for quantitative data. The significance level was 0.05.
Role of funding source
Data collection for this study was part of the support to MoH by the WHO Health Emergencies international response to the EVD outbreak in DRC (2018–2020). The US Centers for Disease Control and Prevention cooperative agreement with WHO (award number: 6 NU2GGH002225-01-02) supported data analysis and manuscript compilation.
Results
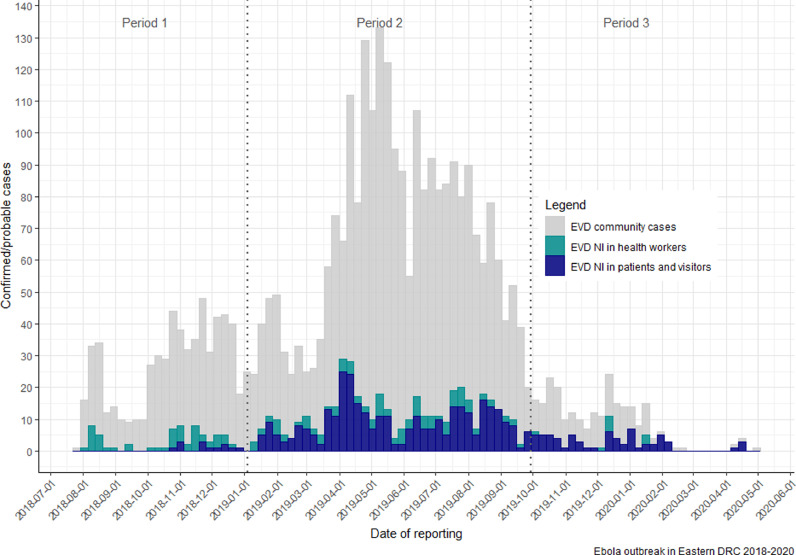
In total, 3481 cases of EVD were identified within the three provinces between July 2018 and May 2020. Of these, 660 (19.1%) were reported as NIs; however, this analysis is of the 579 (87.7%) cases for whom data were available. The distribution of nosocomial EVD during the outbreak mirrored the time distribution of overall EVD cases (Figure 1). The characteristics of the nosocomial cases of EVD are provided in Table 1; the majority of cases were adults (67.7%) and female (57.3%).
Figure 1.
Distribution of weekly confirmed or probable nosocomial and community cases of Ebola virus disease (EVD) during the July 2018––May 2020 outbreak in the Democratic Republic of the Congo (DRC). Note: Periods 1–3 correspond to the different inclusion criteria for nosocomial infection, which was modified as the outbreak progressed.
Table 1.
Characteristics of cases of Ebola virus disease nosocomial infections in the Democratic Republic of the Congo, 2018–2020.
| Characteristics | No. |
|||
|---|---|---|---|---|
| Period 1: Jul–Dec 2018a | Period 2: Jan–Sept 2019a | Period 3: Oct 2019–May 2020a | Total n=579 (%) | |
| Sex (n=579) | ||||
| Female | 40 | 247 | 45 | 332 (57.3) |
| Male | 27 | 190 | 29 | 246 (42.5) |
| No data | 0 | 1 | 0 | 1 (0.2) |
| Age (years) (n=579) | ||||
| ≤5 | 5 | 86 | 17 | 108 (18.7) |
| 6–18 | 2 | 67 | 10 | 79 (13.7) |
| >18 | 60 | 285 | 47 | 392 (67.7) |
| Age of deceased (years) (n=358; 66 health workers and 292 patients/visitors | ||||
| ≤5 | 5 | 74 | 10 | 89 (24.9) |
| 6–18 | 1 | 48 | 5 | 54 (15.1) |
| >18 | 24 | 180 | 11 | 215 (60.1) |
| Health workers | 54 | 98 | 8 | 160 (27.2) |
NA, not applicable.
Periods 1–3 correspond to the different inclusion criteria for nosocomial infection, which was modified as the outbreak progressed.
The overall nosocomial case fatality rate (CFR) was 61.8% (358/579). The CFR was 2.8 times higher among children compared with adults [odds ratio 2.8, 95% confidence interval (CI) 1.7–4.5; P<0.001], impacting those aged ≤5 years most severely (Table 1). Seventeen adults were aged >65 years, and demonstrated the highest group-specific CFR (94.0%).
The average time between the onset and reporting of symptoms was 8.2 days (Table 2) [standard deviation (SD) 12.3, 95% CI 7.0–9.4]. Where information was available, 84.5% (359/419) of NI cases visited at least two HFs (range 2–5) before detection and/or transfer to an EVD transit centre.
Table 2.
Number of days between the onset and reporting of nosocomial Ebola virus disease in the Democratic Republic of the Congo, 2018–2020.
| Statistical measure | Time between onset and reporting of symptoms (days) |
|||
|---|---|---|---|---|
| Period 1: Jul–Dec 2018a (n=66)b | Period 2: Jan–Sept 2019a (n=424)b | Period 3: Oct 2019–May 2020a (n=73)b | Average (n=563)b | |
| Mean | 3.0 | 8.3 | 5.0 | 7.6 |
| Median | 10.4 | 6.0 | 5.0 | 6.0 |
| Standard deviation | 10.4 | 12.2 | 2.9 | 11.3 |
Periods 1–3 correspond to the different inclusion criteria for nosocomial infection, which was modified as the outbreak progressed.
Data were not available for all cases described in Table 1.
Geographic distribution
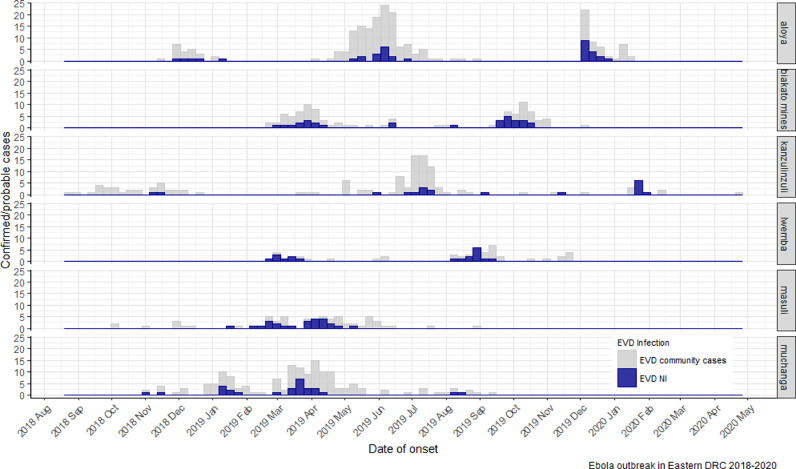
The majority of cases of NI occurred in North Kivu province (484/579, 83.6%), followed by Ituri province (94/579, 16.2%); only one case was reported in South Kivu province (Table S2, see online supplementary material). Cases were concentrated in the sub-coordinations of Butembo (294/579, 50.8%), Beni (115/579, 19.9%) and Mangina (152/579, 26.3%). Within these sub-coordinations, 21 of 29 health zones were affected, with almost half of all cases (402/579, 69.4%) in four health zones: Beni, Katwa, Mabalako (in the North Kivu) and Mandima (in Ituri). Temporal trends in the data within health zones demonstrated that a few localized EVD clusters (Aloya, Biakato mines, Lwemba, Muchanga, Masuli, Kanzulinzuli) were responsible for 162 cases of NI (Figure 2).
Figure 2.
Timeline distribution of clusters of nosocomial cases of Ebola virus disease (EVD) in Aloya, Biakato mines, Lwemba, Masuli, and Muchanga health areas, Democratic Republic of the Congo (DRC), 2018–2020. NI, nosocomial infection.
HF category and ownership
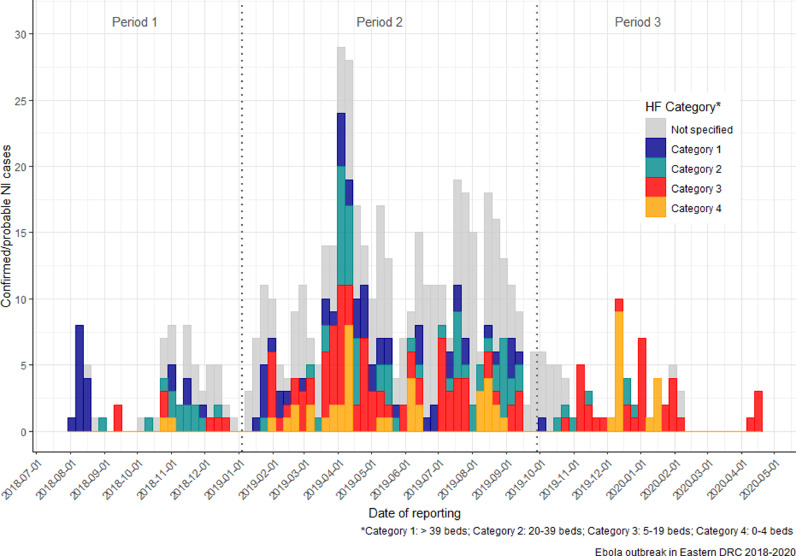
Nosocomial cases of EVD were linked to 196 HFs. Among cases of NI with available category information (403 cases occurring in 141 HFs), the highest proportion of cases occurred in Categories 1 (148/403) and 3 (123/403), accounting for 67.2% of cases. Of note, clusters in Aloya (Mangina sub-coordination), Kanzulinzuli, Masuli and Muchanga (Beni sub-coordination) occurred mainly in Category 3 HFs. The average number of NIs per HF of Categories 1–4 was 4.8, 2.7, 1.8 and 4.5 respectively.
HFs for which category information was available and that had at least one case of NI were mainly Category 3 (48.9%, 69/141), followed by Category 1 (22.0%, 31/141), Category 2 (20.6%, 29/141) and Category 4 (8.5%, 12/141). Category information about the HF in which 176 cases of NI occurred was not available for many HFs (28.1%, 55/196). The distribution of NIs according to HF category is shown in Figure 3, and according to ownership in Figure S2 (see online supplementary material); additional details are provided in Table S3 (see online supplementary material).
Figure 3.
Distribution of weekly confirmed or probable nosocomial and community cases of Ebola virus disease (EVD) according to the category of health facility (HF) during the July 2018–May 2020 outbreak in the Democratic Republic of the Congo (DRC). Note: Periods 1–3 correspond to the different inclusion criteria for nosocomial infection (NI), which was modified as the outbreak progressed.
Among the HFs associated with at least one case of NI and with available information on ownership (information was unavailable for three HFs), privately owned HFs accounted for 69.5% (98/141), public HFs accounted for 19.9% (28/141), and private not-for-profit HFs accounted for 10.6% (15/141). The private sector accounted for the majority of HF ownership across all categories, with the exception of Category 1 (Figure S1, see online supplementary material). Information on whether HFs benefited from the support of non-governmental organizations (NGOs) was not available for 68.4% (134/196) of HFs. Among the HFs with available data, 16.3% (32/196) of HFs received support from at least one NGO, and 15.3% (30/196) of HFs received no NGO support.
NIs among patients and visitors
Patients and visitors accounted for 72.4% (419/579) of cases of NI described in this study. The majority of NIs in patients and visitors occurred in females (264/419; 63.0%), who ranged in age from 0 to 82 years; the mean age (±SD) was 25.9 ± 17.8 years. A total of 34.8% (146/419) of cases among patients and visitors were of reproductive age (15–49 years), and 64.4% (94/146) of these were among women. The difference in age between females (36.2 ± 16.3 years) and males (34.5 ± 13.4 years) was not significant (P=0.445). Children aged ≤18 years represented 43.7% (183/419) of cases of NI among patients and visitors, while children aged ≤5 years represented 25.8% (108/419). The CFR among patients and visitors of all ages with HF-associated EVD was 69.7% (292/419), with CFRs of 72.0% (54/75) among children aged 6–18 years and 82.4% (89/108) among children aged ≤5 years.
NIs among HWs
During this outbreak, 179 cases of EVD among HWs were recorded. However, 19 of these HWs did not meet the criteria for NI because they had community links; these 19 cases were therefore not included in the total for nosocomial cases of EVD among HWs. HWs accounted for 27.6% (160/579) of NIs analysed. The affected HWs were male in 57.7% of cases (92/160). The CFR among HWs was lower (66/160, 41.3%) compared with the CFR among patients or visitors (292/419, 69.7%) (P<0.001). Nurses were the most affected (108/160, 67.5%), followed by physicians (13/160, 8.1%), traditional healers (10/160, 6.3%), laboratory staff (7/160, 4.4%) and hygiene workers (5/160, 3.1%). Seventeen cases (10.6%) had no specified occupation. Seventy-six of 160 (47.5%) HWs worked in Category 1 HFs. Half of the cases among traditional healers (5/10, 50.0%) occurred in Mangina. The proportion of infected HWs was high in some NI clusters. For instance, HWs represented 40.5% (15/37) of nosocomial cases in Aloya, 20.0% (4/20) in Kanzulinzuli, and 13.3% (4/30) in Biakato mines.
Discussion
The occurrence of nosocomial cases during an outbreak of EVD highlights the importance of IPC for global health security (Allegranzi et al., 2017). Although the epidemiology of EVD is better understood now than in the past (Chowell and Nishiura, 2014; Vetter et al., 2016a), this is the first study on NI in a large EVD outbreak. This particularly challenging outbreak affected urban and rural areas, challenged HFs with limited IPC capacity and resources, and occurred within the context of an armed conflict (Aruna et al., 2019). Surveillance of NI is one core component of IPC at national and HF levels (Storr et al., 2017). In countries with weak national programmes, the prevention of NI is particularly challenging; investment in a national IPC programme is required to prevent future EVD and emerging disease outbreaks (Vetter et al., 2016b). Maganga et al. (2014) suggested that the responses in DRC have improved in terms of expertise in managing EVD outbreaks. However, the duration of the 2018–2020 outbreak demonstrated that major gaps still exist, particularly those related to IPC measures within HFs.
These findings highlight the substantial burden of NI in children during an EVD outbreak. The authors did not find other studies on NI in children; however, the CFR observed in this research corroborates a study reporting a high CFR in children admitted to Ebola holding units in Sierra Leone (Fitzgerald et al., 2016). Children are potentially more vulnerable to NI because of their care needs, resulting in frequent contact with HWs or caregivers. High CFRs in children highlight the need for a targeted IPC strategy for this group, including caregivers and families (Dixit et al., 2020). This study also reported high CFRs in adults aged >65 years; however, details on potential comorbidities in this group were unavailable.
The geographic distribution of cases of NI followed a similar trend to cases within the community (Aruna et al., 2019). Clusters of nosocomial cases contributed to outbreak amplification, demonstrating that targeted strategies, such as the IPC ring approach, were appropriate. The existence of clusters also indicates that early case detection is critical to control and mitigate HFs and community transmission chains. A few clusters were responsible for the final trends in the epidemiological curve, which demonstrated a higher proportion of nosocomial cases than case numbers within the community. The non-specific early clinical presentation of EVD can result in its misclassification for other infectious diseases (e.g. malaria, typhoid fever; Ilunga et al., 2019). The resulting delay in diagnosis and implementation of critical IPC measures such as isolation, combined with overcrowded and resource-limited HFs, facilitates rapid EVD dissemination. Another recognized threat in resource-limited countries, potentially contributing to the chain of nosocomial EVD in DRC, is that of unsafe injections (Adewuyi and Auta, 2020). Furthermore, as patient care-seeking behaviour usually involves several visits to different HFs, EVD could be transmitted rapidly between HFs and the community.
Active case-finding within a HF results in early identification and prompt implementation of control measures (Shears, 2007; Dunn et al., 2016; Kunkel et al., 2019). The IPC ring approach targets HFs and homes of affected cases, and includes site decontamination, IPC kit donations, IPC HW briefings, HF assessment, and the development of an improvement action plan, followed by supportive supervision and mentorship (Hageman et al., 2016; Ousman et al., 2019). This short-term response strategy is useful when resources are limited and IPC standards are not established. However, a robust IPC programme is essential to build a sustainable and resilient health system providing quality care (Biedron et al., 2019).
In the context of DRC, Category 3 HFs represented a principal source of NI; these HFs with limited capacity are generally more numerous and accessible, meaning they are often the first point of entry into the health system. The established outbreak referral system, in which a suspected case presenting at a HF should be transferred directly to an EVD treatment centre, meant that a suspected case may have remained at that Category 3 HF for some time until transfer and transport were available; the limited isolation capacity of such HFs would have prolonged the exposure period to staff and other patients. In contrast, Category 1 HFs (public and private) are referral hospitals that cover larger catchment areas and, because of their higher capacity, would have received more IPC support from EVD response teams. It should also be noted that, during the final phase of the outbreak, cases occurred mainly in rural areas, where Category 3 and 4 HFs are more common. These findings demonstrate that HFs of all categories should receive support during future outbreak responses, including training of HWs in IPC and the provision of material resources (e.g. PPE and cleaning products for HWs; posters to inform patients and visitors).
Privately owned HFs accounted for 50% of HFs affected in this outbreak. It is unknown whether this is representative of ownership distribution in these provinces. As well as regulation loopholes in their implementation (DRC, 2016), there is no comprehensive census of private HFs in the country. A relatively high turnover of both HFs and HWs is observed in this sector. For future outbreaks, the private sector should be engaged early in the response to ensure that IPC standards and measures are implemented, and include training programmes, audits and, whenever applicable, supply of PPE.
The lack of HWs trained in IPC was a major obstacle in containing the West African outbreak between 2014 and 2016 (Shoman et al., 2017). According to a study performed by the Social Sciences Analytic Cell (Carter et al., 2020), HWs in DRC reported that a lack of resources and training meant they were unable to prevent transmission within HFs, resulting in an atmosphere of fear, stigma and rejection within communities (Shoman et al., 2017). The present study demonstrated that the majority of HW infections occurred among nurses, the largest proportion of health professionals, highlighting the need to target this group with EVD outbreak preparedness training programmes. Nurses often have the role of HF IPC focal point and community outreach liaison, emphasizing their central role in IPC (Shoman et al., 2017). Traditional healers are also an important type of infected HW; they perform a critical role with respect to health-seeking behaviour in DRC (DRC, 2016), and should therefore be another priority group for future IPC strategies (Shears and O'Dempsey, 2015).
CFRs among HWs were lower compared with patients and visitors; this may be the result of differences in age, health or vaccination status, health-seeking behaviour, time between case recognition and reporting (Maganga et al., 2014; Jacob et al., 2020), and the availability and early implementation of therapeutics for HWs (Fischer et al., 2018). According to a WHO situation report published in June 2020 (WHO, 2020), 99.4% of people identified as eligible received a vaccination. However, vaccination coverage rates among HW are not available.
During outbreaks of highly transmissible diseases, the surveillance and IPC response teams should work in collaboration to identify cases of NI and perform contact tracing, aiming to interrupt transmission chains promptly. A timely investigation of IPC practices within HFs would provide a better understanding of the source of NI, including information on invasive procedures and other risk factors for IPC interventions.
This study has limitations. The evolving definition of cases of NI, necessary in outbreaks of longer duration, limited comprehensive interpretation of the results, with potential underestimation of NI cases. A good balance between sensitivity and specificity for NI definition is of utmost importance. The lack of baseline data on the category or ownership of HFs may have affected associations reported in this study. Other issues that may also have affected the analysis and interpretation include: the unknown number of HWs employed in these provinces; the varying qualifications of nurses and traditional healers (whose details were not collected by the surveillance system); the insecurity making access challenging, especially in rural areas; and the lack of qualified personnel for outbreak surveillance.
Achieving an adequate level of preparedness, readiness, and response to EVD and other highly transmissible diseases in DRC requires a stronger health system (Burki, 2020). A stepwise approach to ensure that at least minimum requirements (WHO, 2019b) are in place, while aiming to achieve the full core components of IPC programmes in a sustainable manner (Storr et al., 2017), includes building capacity for IPC, strengthening IPC advocacy, improving infrastructure and implementing evidence-based recommendations (Allegranzi et al., 2017).
This study highlights the importance of NI surveillance, early case detection for prompt isolation, implementation of IPC measures, and an actionable definition of cases of NI. Going forward, study designs that allow the examination of detailed risk factors for nosocomial EVD are required to understand the role of certain practices (e.g. injections, traditional healing) and to identify potential targeted interventions. HWs and traditional healers should be targeted for IPC training, and supportive supervision of HFs is required to mitigate transmission during EVD outbreaks. Implementation research is key to the identification of efficient strategies to advance and sustain IPC in DRC.
Acknowledgments
Acknowledgements
The authors wish to thank the members of IPC and surveillance teams for their contribution to data collection during the 2018–2020 EVD response in DRC. The authors also wish to thank the Ministry of Health analytic cell team in Goma and the WHO Headquarters Health Information pillar team for their support with data cleaning and analyses, and their contribution to the paper. Finally, the authors wish to thank the WHO Country Office in DRC for their support with the response and IPC activities.
Conflict of interest statement
None declared.
Funding
This study was funded by the WHO Health Emergencies programme and the US Centers for Disease Control and Prevention.
Ethical approval and consent to participate
Not applicable.
Declarations
The authors are staff members of WHO and the US CDC. The findings and conclusions in this article are those of the authors and do not necessarily represent the official position, decisions, policy or views of these institutions.
Data sharing
The data analysed in this paper are those reported in the surveillance system in the outbreak.
Author contributions
AB, MCP and PM designed the study, performed data analysis and compiled the manuscript. All the authors contributed significantly to the activities in the outbreak response and to the critical review of the manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.11.039.
Appendix. Supplementary materials
References
- Adewuyi EO, Auta A. Medical injection and access to sterile injection equipment in low- and middle-income countries: a meta-analysis of Demographic and Health Surveys (2010–2017) Int Health. 2020;12:388–394. doi: 10.1093/inthealth/ihz113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allegranzi B, Kilpatrick C, Storr J, Kelley E, Park BJ, Donaldson L. Global infection prevention and control priorities 2018–22: a call for action. Lancet Glob Health. 2017;5:e1178–e1180. doi: 10.1016/S2214-109X(17)30427-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruna A, Mbala P, Minikulu L, Mukadi D, Bulemfu D, Edidi F, et al. Ebola virus disease outbreak – Democratic Republic of the Congo, August 2018–November 2019. MMWR Morb Mortal Wkly Rep. 2019;68:1162–1165. doi: 10.15585/mmwr.mm6850a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedron C, Lyman M, Stuckey MJ, Homsy J, Lamorde M, Luvsansharav UO, et al. Evaluation of infection prevention and control readiness at frontline health care facilities in high-risk districts bordering Ebola virus disease-affected areas in the Democratic Republic of the Congo – Uganda, 2018. MMWR Morb Mortal Wkly Rep. 2019;68:851–854. doi: 10.15585/mmwr.mm6839a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burki T. Ebola virus disease in DR Congo. Lancet Infect Dis. 2020;20:418–419. doi: 10.1016/S1473-3099(20)30185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SE, Gobat N, Pfaffmann Zambruni J, Bedford J, van Kleef E, Jombart T, et al. What questions we should be asking about COVID-19 in humanitarian settings: perspectives from the Social Sciences Analysis Cell in the Democratic Republic of the Congo. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2020-003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowell G, Nishiura H. Transmission dynamics and control of Ebola virus disease (EVD): a review. BMC Med. 2014;12:196. doi: 10.1186/s12916-014-0196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit D, Masumbuko Claude K, Kjaldgaard L, Hawkes MT. Review of Ebola virus disease in children – how far have we come? Paediatr Int Child Health. 2020;41:12–27. doi: 10.1080/20469047.2020.1805260. [DOI] [PubMed] [Google Scholar]
- DRC. Plan National De Developpement Sanitaire 2016–2020: Vers la couverture sanitaire universelle. Ministry of Health, Democratic Republic of the Congo, Kinshasa; 2016.
- Dunn AC, Walker TA, Redd J, Sugerman D, McFadden J, Singh T, et al. Nosocomial transmission of Ebola virus disease on pediatric and maternity wards: Bombali and Tonkolili, Sierra Leone, 2014. Am J Infect Control. 2016;44:269–272. doi: 10.1016/j.ajic.2015.09.016. [DOI] [PubMed] [Google Scholar]
- Fischer WA, 2nd, Vetter P, Bausch DG, Burgess T, Davey RT, Jr, Fowler R, et al. Ebola virus disease: an update on post-exposure prophylaxis. Lancet Infect Dis. 2018;18:e183–e192. doi: 10.1016/S1473-3099(17)30677-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald F, Naveed A, Wing K, Gbessay M, Ross JCG, Checchi F, et al. Ebola virus disease in children, Sierra Leone, 2014–2015. Emerg Infect Dis. 2016;22:1769–1777. doi: 10.3201/eid2210.160579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullman N, Yearwood J, Abay SM, Abbafati C, Abd-Allah F, Abdela J, et al. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016. Lancet. 2018;391:2236–2271. doi: 10.1016/S0140-6736(18)30994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman JC, Hazim C, Wilson K, Malpiedi P, Gupta N, Bennett S, et al. Infection prevention and control for Ebola in health care settings – West Africa and United States. MMWR Suppl. 2016;65:50–56. doi: 10.15585/mmwr.su6503a8. [DOI] [PubMed] [Google Scholar]
- HDX. DRC Health Data Ebola 2018. Available at: https://data.humdata.org/m/dataset/drc-health-facilities?force_layout=light (accessed 10 November 2021).
- Ilunga KO, Moeti M, Sparrow A, Nguyen V-K, Lucey D, Ghebreyesus TA. The ongoing Ebola epidemic in the Democratic Republic of Congo, 2018–2019. N Engl J Med. 2019;381:373–383. doi: 10.1056/NEJMsr1904253. [DOI] [PubMed] [Google Scholar]
- INS. Enquête par grappes à indicateurs multiples, 2017–2018, rapport de résultats de l'enquête. Rapport final Décembre 2019. Kinshasa, République Démocratique du Congo; 2019. Available at: https://www.unicef.org/drcongo/rapports/resume-mics-palu-2017-2018 (accessed 10 November 2021).
- Jacob ST, Crozier I, Fischer WA, Hewlett A, Kraft CS, Vega MA, et al. Ebola virus disease. Nat Rev Dis Primers. 2020;6:13. doi: 10.1038/s41572-020-0147-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaner J, Schaack S. Understanding Ebola: the 2014 epidemic. Glob Health. 2016;12:53. doi: 10.1186/s12992-016-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel A, Keita M, Diallo B, le Polain de Waroux O, Subissi L, Wague B, et al. Assessment of a health facility based active case finding system for Ebola virus disease in Mbandaka, Democratic Republic of the Congo, June–July 2018. BMC Infect Dis. 2019;19:981. doi: 10.1186/s12879-019-4600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganga GD, Kapetshi J, Berthet N, Kebela Ilunga B, Kabange F, Mbala Kingebeni P, et al. Ebola virus disease in the Democratic Republic of Congo. N Engl J Med. 2014;371:2083–2091. doi: 10.1056/NEJMoa1411099. [DOI] [PubMed] [Google Scholar]
- Ousman K, Kabego L, Talisuna A, Diaz J, Mbuyi J, Houndjo B, et al. The impact of infection prevention and control (IPC) bundle implementation on compliance during the Ebola virus outbreak in Mbandaka/Democratic Republic of the Congo: a before and after design. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-029717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj SA, Lee KE, Harrell M, Ivanov I, Allegranzi B. Infection rates and risk factors for infection among health workers during Ebola and Marburg virus outbreaks: a systematic review. J Infect Dis. 2018;218(Suppl. 5):S679–S689. doi: 10.1093/infdis/jiy435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears P. Poverty and infection in the developing world: healthcare-related infections and infection control in the tropics. J Hosp Infect. 2007;67:217–224. doi: 10.1016/j.jhin.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears P, O'Dempsey TJ. Ebola virus disease in Africa: epidemiology and nosocomial transmission. J Hosp Infect. 2015;90:1–9. doi: 10.1016/j.jhin.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Shoman H, Karafillakis E, Rawaf S. The link between the West African Ebola outbreak and health systems in Guinea, Liberia and Sierra Leone: a systematic review. Glob Health. 2017;13:1. doi: 10.1186/s12992-016-0224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storr J, Twyman A, Zingg W, Damani N, Kilpatrick C, Reilly J, et al. Core components for effective infection prevention and control programmes: new WHO evidence-based recommendations. Antimicrob Resist Infect Control. 2017;6:6. doi: 10.1186/s13756-016-0149-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNDP. Congo (Democratic Republic of the). Human Development Report 2020. United Nations Development Programme; 2020. Available at: http://hdr.undp.org/en/countries/profiles/COD (accessed 10 November 2021).
- UNICEF. Democratic Republic of Congo. Water, sanitation and hygiene. New York: United Nations Children's Fund; 2020. Available at: https://www.unicef.org/drcongo/en/what-we-do/water-sanitation-and-hygiene (accessed 10 November 2021).
- Vetter P, Fischer WA, 2nd, Schibler M, Jacobs M, Bausch DG, Kaiser L. Ebola virus shedding and transmission: review of current evidence. J Infect Dis. 2016;214(Suppl. 3):s177–s184. doi: 10.1093/infdis/jiw254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetter P, Dayer J-A, Schibler M, Allegranzi B, Brown D, Calmy A, et al. The 2014–2015 Ebola outbreak in West Africa: hands on. Antimicrob Resist Infect Control. 2016;5:17. [Google Scholar]
- WHO. Ebola virus disease in West Africa (2014–2015) IHR Emergency Committee. Geneva: World Health Organization; 2014a. Available at: https://www.who.int/groups/ebola-virus-disease-in-west-africa- (2014-2015)-ihr-emergency-committee (last accessed 10 November 2021).
- WHO. Case definition recommendations for Ebola or Marburg virus desease. Geneva: World Health Organization; 2014b. Available at: https://www.who.int/csr/resources/publications/ebola/case-definition/en/ (accessed 10 November 2021).
- WHO. Ebola outbreak in the Democratic Republic of the Congo declared a Public Health Emergency of International Concern. Geneva: World Health Organization; 2019a. Available at: https://www.who.int/news-room/detail/17-07-2019-ebola-outbreak-in-the-democratic-republic-of-the-congo-declared-a-public-health-emergency-of-international-concern (accessed 10 November 2021).
- WHO. Minimum requirements for infection prevention and control. Geneva: World Health Organization; 2019b. Available at: https://www.who.int/publications/i/item/9789241516945 (accessed 10 November 2021).
- WHO. Regional Office for Africa. Ebola virus disease. Democratic Republic of the Congo. External Situation Report 98. Geneva: World Health Organization; 2020. Available at: https://www.who.int/publications/i/item/10665-332654 (accessed 10 November 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.