Abstract
Numerous studies have highlighted the prognostic significance of hyperglycemia in the outcomes of SARS-CoV-2 infection. A number of mechanisms have been proposed as potential drivers of this association, which were, however, up until recently based rather on speculation than on investigational evidence. It has been recently come to light that the development of insulin resistance in the frame of COVID-19 is likely the driving force behind the development of overt hyperglycemia. This results through the infectious insult of the adipose tissue, and is observed in conjunction with aberrant adipokine secretion by host adipocytes, such as decreased adiponectin, as well as a switch towards an antiviral immune secretory profile. These data could have a considerable relevance not only for the management of hyperglycemia in the course of the infection but also for the overall understanding of the pathogenesis of severe COVID-19.
Keywords: Adipokine, Adiponectin, Adipose tissue, COVID-19, Hyperglycemia, Insulin resistance, SARS-CoV-2
The novel coronavirus disease 2019 (COVID-19) caused by the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) constitutes an unprecedented public health challenge with important implications in morbidity and mortality globally [1,2]. Worldwide, as of January 7, 2022, there have been 298, 915,721 confirmed cases of COVID-19, including 5,469,303 confirmed deaths reported to WHO, and these numbers continue to rise every day [3]. While SARS-CoV-2 infection is related with substantial pulmonary disease, including pneumonia and acute respiratory distress syndrome (ARDS), COVID-19 may present with a plethora of extrapulmonary manifestations [4].
Meta-analyses of population-based studies have reported that obesity and diabetes mellitus are significant and independent risk factors for COVID-19 severity, hospitalization, ICU admission, and death in subjects with COVID-19 [5,6]. However, independently from pre-existing DM, hyperglycemia is a biomarker of unfavorable prognosis, being associated with nosocomial adverse outcomes, risk of developing acute respiratory distress syndrome (ARDS) and a 7-fold increased mortality in comparison to well-controlled glycemia [[7], [8], [9], [10]].
Altered glucose homeostasis, considered a tailored response for an effective immune response in infection, is attributed to the following physiological mechanisms: 1) the hypersecretion of inflammatory cytokines, particularly interleukin (IL)-6, IL-1b, and tumor necrosis factor (TNF)-α; 2) the enhanced hypothalamo-pituitary axis resulting in cortisol production; 3) the increase in the release of catecholamines, glucagon and growth hormone; 4) decreased blood flow in muscles resulting in lower glucose uptake by muscles; 5) liver gluconeogenesis fueling the increased metabolic state [[8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]]. The underlying pathophysiology linking hyperglycemia to COVID-19 adverse outcomes is poorly understood, including hypothetical mechanisms such as systemic inflammation resulting in insulin resistance and pancreatic beta cell infection by SARS-CoV-2 and dysfunction.
Two reports recently published in Cell Metabolism have offered novel evidence to elucidate the underlying link between hyperglycemia and SARS-CoV-2 infection [22,23]. Reiterer et al. ascertained the adverse prognostic significance of increased plasma glucose among 3854 individuals hospitalized with COVID-19, whereby the presence of hyperglycemia (defined somewhat arbitrarily as blood glucose >170 mg/dl) was associated with a 15.6-, 9.8- and 3.3-fold elevated risk for intubation, ARDS and death, respectively [22]. Similar rates of hyperglycemia were noted across a subgroup of patients with COVID-19 complicated by ARDS and two control groups of patients with ARDS of other etiologies as well as intensively treated individuals without either COVID-19 nor ARDS. In contrast to the sum of the cohort, among patients with ARDS a detrimental impact of hyperglycemia on patient survival was noted in the two non-COVID-19 groups, which was not the case within the COVID-19/ΑRDS collective. Further circulating metabolic profiling in a representative subset of the three parental cohorts revealed a considerably higher prevalence of insulin resistant COVID-19/ΑRDS patients on the basis of elevated C-peptide and C-peptide/glucose ratios with a three- and six-fold higher prevalence compared with the two respective control groups. These rates rose to 8.6- and 15.1-fold after adjustment for diabetes status and glucocorticoid treatment, and were coupled with an increase in circulating amylin levels as well as a pronounced decrease in circulating adiponectin concentrations and adiponectin/leptin ratio compared with the control groups. This metabolic profile is consistent with an insulin resistant systemic environment and a beta cell hypersecretory profile, likely on the grounds of a dysfunctional and insulin resistant adipose tissue. In order to further examine this hypothesis, the investigators studied a murine model of SARS-CoV-2 infection in Syrian hamsters and ascertained detectable viral mRNA in the adipose tissue of infected animals, together with reduced adiponectin secretion (in visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT)) and gene expression (SAT only) as well as a switch towards an antiviral immune secretory profile which included the induction of interleukin 1-beta, interleukin 18 as well as major chemokine receptor ligands. As a last step, the infectivity of SARS-CoV-2 in human and murine adipocytes in vitro was determined, likely ensuing through the viral membrane entry factors known to date [22]. Limitations of the study include the lack of determination of adiponectin levels prior to the development of ARDS/COVID-19 and the non-performance of fat biopsies from critically ill patients.
To further strengthen these findings, Zickler et al. demonstrated SARS-CoV-2 infectivity in differentiated and lipid-laden adipocytes but not preadipocytes or immature precursors [23]. This results presumably due to an augmented ACE2 membrane expression in the course of adipocyte differentiation, although the authors also ascertained that inhibition of lipid catabolism in mature adipocytes reduced viral replication by a factor of 100-fold and acted so synergistically to atorvastatin, being unclear whether all these effects are mediated via an altered ACE2 expression. In support of the importance of aberrant lipid metabolism in the pathogenesis of COVID-19, viral mRNA was detectable in 10 and 4 of 18 adipose tissue and liver samples from deceased patients, all of which were overweight or obese. In line with the findings by Reiterer et al. [22], it was further shown that adipocyte infection with SARS-CoV-2 results in the suppression of genes related to de novo lipogenesis and the induction of immune response-related genes. This is paralleled by a notable reduction of triglycerides containing de novo synthesized fatty acids and a concomitant increase of those with esterified exogenous polyunsaturated fatty acids, hence signifying an impaired dietary fatty acid (FA) metabolism or effectively, an impaired postprandial triglyceridemia, further adding to the frame of a systemic dysmetabolic milieu [23].
The presented findings are affirmative of previous reports on elevated levels of circulating free FAs in COVID-19 infection, consistent with a failure of insulin to suppress lipolysis and impaired insulin action at the adipocyte level [24]. The insulin resistant metabolic profile manifesting profoundly as hyperglycemia was associated with an increased risk of adverse outcomes in the initial cohort of 3854 patients studied by Reiterer et al. [22]. Nonetheless, hyperglycemia among those hospitalized with COVID-19-related ARDS did not confer an additional mortality risk, which came in stark contrast to the increased corresponding risks observed in the two control groups. This finding gives rise to the notion that it may not be the detrimental effects of hyperglycemia per se and/or insulin resistance that mediate the worse prognosis among these patients. Based on the translational findings of both studies, it could rather be argued that the infectious involvement of the adipose tissue in a subset of patients, which induces the insulin resistant phenotype and hyperglycemia is an indicator of a diffuse, multi-system COVID-19 illness. This would in other words signify overt hyperglycemia as a surrogate of worse prognosis, rather than a deleterious component of SARS-CoV-2 infection. This could harbor important implications for the clinical management of patients with diabetes mellitus or hyperglycemia at risk for or already hospitalized with COVID-19, since it would render glycemic management a matter of less crucial significance with respect to infection outcomes. Insulin is the universally accepted therapeutic option for hyperglycemia in hospitalized patients, especially in critical care settings [25]. Reiterer et al. raise the question whether insulin sensitizing agents such as metformin or thiazolidinediones may be used for the glycemic management among patients with hyperglycemia and severe COVID-19 who display an IR phenotype, as add-ons that may spare insulin usage. While the use of oral antidiabetics, especially metformin, is a questionable practice in acute illness of any sort, it does not seem urgent to approach this likely not pivotal issue with such a pathophysiology-tailored approach. Based on the presented results, it would rather seem justified to intensify the level of in-hospital care and/or escalate therapy on the basis of new-onset or worsening of hyperglycemia.
Another implication concerns the potential tropism of SARS-CoV-2 for lipid-laden cells and tissues, which was highlighted in the study by Zickler et al. [23]. Based on their findings, this may be at least partly due to the upregulated ACE2 membrane expression. Ectopic lipid deposition is a feature of metabolic burden in obesity, and is associated with systemic insulin resistance and overt hyperglycemia [15,[26], [27], [28], [29], [30], [31]]. It can be speculated that this may render tissues which are not principal targets of SARS-CoV-2 (apart from adipose tissue also liver among obese patients as demonstrated in the study) prone to infection, predisposing to a more diffuse organ involvement and higher rates of replication and virion production. In turn, this could partly account for the considerably worse outcome among patients with obesity and diabetes mellitus, and could advocate for a preventive role of antidiabetic medications with known ameliorating effects of fat distribution (e.g. thiazolidinediones).
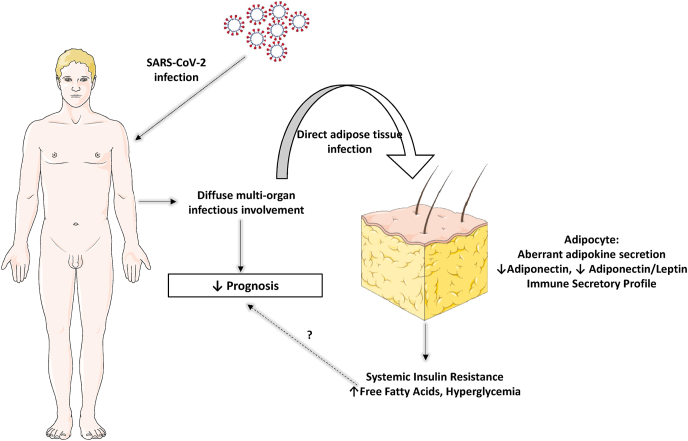
In any case, these recent findings have revealed a rather unexpected mechanism behind COVID-19-related hyperglycemia contributing to the understanding of the pathogenetic mechanisms of SARS- CoV-2 infection (Fig. 1). Whether this new evidence will suffice to drastically change the common practice of hyperglycemia management in the frame of SARS-CoV-2 remains to be elucidated.
Fig. 1.
Adipose tissue involvement in the frame of severe COVID-19. All images are originated from the free medical website http://smart.servier.com/ by Servier licensed under a Creative Commons Attribution 3.0 Unported License.
Conflict of interest
None.
Funding information
None.
Contributor Information
Dimitrios Tsilingiris, Email: tsilingirisd@gmail.com.
Maria Dalamaga, Email: madalamaga@med.uoa.gr.
Junli Liu, Email: liujunli@sjtu.edu.cn.
References
- 1.Tsilingiris D., Vallianou N.G., Karampela I., Dalamaga M. Vaccine induced thrombotic thrombocytopenia: the shady chapter of a success story. Metabolism open. 2021;11:100101. doi: 10.1016/j.metop.2021.100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vallianou N.G., Tsilingiris D., Christodoulatos G.S., Karampela I., Dalamaga M. Anti-viral treatment for SARS-CoV-2 infection: a race against time amidst the ongoing pandemic. Metabol Open. 2021;10:100096. doi: 10.1016/j.metop.2021.100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.https://covid19.who.int/
- 4.Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- 5.Dalamaga M., Christodoulatos G.S., Karampela I., Vallianou N., Apovian C.M. Understanding the Co-epidemic of obesity and COVID-19: current evidence, comparison with previous epidemics, mechanisms, and preventive and therapeutic perspectives. Current obesity reports. 2021;10:214–243. doi: 10.1007/s13679-021-00436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vallianou N.G., Evangelopoulos A., Kounatidis D., Stratigou T., Christodoulatos G.S., Karampela I., et al. Diabetes mellitus and SARS-CoV-2 infection: pathophysiologic mechanisms and implications in management. Curr Diabetes Rev. 2021;17 doi: 10.2174/1573399817666210101110253. [DOI] [PubMed] [Google Scholar]
- 7.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metabol. 2020;31:1068–1077. doi: 10.1016/j.cmet.2020.04.021. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karampela I., Chrysanthopoulou E., Skyllas G., Christodoulatos G.S., Kandri E., Antonakos G., et al. Circulating leptin, soluble leptin receptor and free leptin index in critically ill patients with sepsis: a prospective observational study. Minerva Anestesiol. 2021;87:880–890. doi: 10.23736/S0375-9393.21.15368-4. [DOI] [PubMed] [Google Scholar]
- 9.Karampela I., Christodoulatos G.S., Dalamaga M. The role of adipose tissue and adipokines in sepsis: inflammatory and metabolic considerations, and the obesity paradox. Current obesity reports. 2019;8:434–457. doi: 10.1007/s13679-019-00360-2. [DOI] [PubMed] [Google Scholar]
- 10.Karampela I., Chrysanthopoulou E., Christodoulatos G.S., Dalamaga M. Is there an obesity paradox in critical illness? Epidemiologic and metabolic considerations. Current obesity reports. 2020;9:231–244. doi: 10.1007/s13679-020-00394-x. [DOI] [PubMed] [Google Scholar]
- 11.Dalamaga M., Karmaniolas K., Arsenis G., Pantelaki M., Daskalopoulou K., Papadavid E., et al. Cedecea lapagei bacteremia following cement-related chemical burn injury. Burns : journal of the International Society for Burn Injuries. 2008;34:1205–1207. doi: 10.1016/j.burns.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Dalamaga M., Karmaniolas K., Lekka A., Antonakos G., Thrasyvoulides A., Papadavid E., et al. Platelet markers correlate with glycemic indices in diabetic, but not diabetic-myelodysplastic patients with normal platelet count. Dis Markers. 2010;29:55–61. doi: 10.3233/DMA-2010-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalamaga M., Christodoulatos G.S. Adiponectin as a biomarker linking obesity and adiposopathy to hematologic malignancies. Horm Mol Biol Clin Invest. 2015;23:5–20. doi: 10.1515/hmbci-2015-0016. [DOI] [PubMed] [Google Scholar]
- 14.Spyrou N., Avgerinos K.I., Mantzoros C.S., Dalamaga M. Classic and novel Adipocytokines at the intersection of obesity and cancer: diagnostic and therapeutic strategies. Curr Obes Rep. 2018;7:260–275. doi: 10.1007/s13679-018-0318-7. [DOI] [PubMed] [Google Scholar]
- 15.Kassi E., Dalamaga M., Faviou E., Hroussalas G., Kazanis K., Nounopoulos Ch, Dionyssiou-Asteriou A. Circulating oxidized LDL levels, current smoking and obesity in postmenopausal women. Atherosclerosis. 2009;205:279–283. doi: 10.1016/j.atherosclerosis.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Dalamaga M., Karmaniolas K., Matekovits A., Migdalis I., Papadavid E. Cutaneous manifestations in relation to immunologic parameters in a cohort of primary myelodysplastic syndrome patients. J Eur Acad Dermatol Venereol. 2008;22:543–548. doi: 10.1111/j.1468-3083.2007.02520.x. [DOI] [PubMed] [Google Scholar]
- 17.Papadavid E., Gazi S., Dalamaga M., Stavrianeas N., Ntelis V. Palmoplantar and scalp psoriasis occurring during anti-tumour necrosis factor-alpha therapy: a case series of four patients and guidelines for management. J Eur Acad Dermatol Venereol. 2008;22:380–382. doi: 10.1111/j.1468-3083.2007.02335.x. [DOI] [PubMed] [Google Scholar]
- 18.Dalamaga M., Nikolaidou A., Karmaniolas K., Hsi A., Chamberland J., Dionyssiou-Asteriou A., Mantzoros C.S. Circulating adiponectin and leptin in relation to myelodysplastic syndrome: a case-control study. Oncology. 2007;73:26–32. doi: 10.1159/000120995. [DOI] [PubMed] [Google Scholar]
- 19.Marouga A., Dalamaga M., Kastania A.N., Kroupis C., Lagiou M., Saounatsou K., Dimas K., Vlahakos D.V. Circulating resistin is a significant predictor of mortality independently from cardiovascular comorbidities in elderly, non-diabetic subjects with chronic kidney disease. Biomarkers. 2016;21:73–79. doi: 10.3109/1354750X.2015.1118536. [DOI] [PubMed] [Google Scholar]
- 20.Karampela I., Christodoulatos G.S., Kandri E., Antonakos G., Vogiatzakis E., Dimopoulos G., Armaganidis A., Dalamaga M. Circulating eNampt and resistin as a proinflammatory duet predicting independently mortality in critically ill patients with sepsis: a prospective observational study. Cytokine. 2019;119:62–70. doi: 10.1016/j.cyto.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Karampela I., Kandri E., Antonakos G., Vogiatzakis E., Christodoulatos G.S., Nikolaidou A., Dimopoulos G., Armaganidis A., Dalamaga M. Kinetics of circulating fetuin-A may predict mortality independently from adiponectin, high molecular weight adiponectin and prognostic factors in critically ill patients with sepsis: a prospective study. J Crit Care. 2017;41:78–85. doi: 10.1016/j.jcrc.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Reiterer M., Rajan M., Gomez-Banoy N., Lau J.D., Gomez-Escobar L.G., Ma L., et al. Hyperglycemia in acute COVID-19 is characterized by insulin resistance and adipose tissue infectivity by SARS-CoV-2. Cell Metabol. 2021;33:2174–2188. doi: 10.1016/j.cmet.2021.09.009. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zickler M., Stanelle-Bertram S., Ehret S., Heinrich F., Lange P., Schaumburg B., et al. Replication of SARS-CoV-2 in adipose tissue determines organ and systemic lipid metabolism in hamsters and humans. Cell Metabol. 2021 doi: 10.1016/j.cmet.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas T., Stefanoni D., Reisz J.A., Nemkov T., Bertolone L., Francis R.O., et al. COVID-19 infection alters kynurenine and fatty acid metabolism, correlating with IL-6 levels and renal status. JCI insight. 2020:5. doi: 10.1172/jci.insight.140327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pasquel F.J., Lansang M.C., Dhatariya K., Umpierrez G.E. Management of diabetes and hyperglycaemia in the hospital. Lancet Diabetes Endocrinol. 2021;9:174–188. doi: 10.1016/S2213-8587(20)30381-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Trouwborst I., Bowser S.M., Goossens G.H., Blaak E.E. Ectopic fat accumulation in distinct insulin resistant phenotypes; targets for personalized nutritional interventions. Front Nutr. 2018;5:77. doi: 10.3389/fnut.2018.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J., Dalamaga M. Emerging roles for stress kinase p38 and stress hormone fibroblast growth factor 21 in NAFLD development. Metabol Open. 2021;12:100153. doi: 10.1016/j.metop.2021.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Christodoulatos G.S., Antonakos G., Karampela I., Psallida S., Stratigou T., Vallianou N., Lekka A., Marinou I., Vogiatzakis E., Kokoris S., Papavassiliou A.G., Dalamaga M. Circulating omentin-1 as a biomarker at the intersection of postmenopausal breast cancer occurrence and cardiometabolic risk: an observational cross-sectional study. Biomolecules. 2021;11:1609. doi: 10.3390/biom11111609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsilingiris D., Vallianou N.G., Dalamaga M. Prediabetes screening: questionable benefits in the golden years. Metabol Open. 2021;10:100091. doi: 10.1016/j.metop.2021.100091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsilingiris D., Tzeravini E., Koliaki C., Dalamaga M., Kokkinos A. The role of mitochondrial adaptation and metabolic flexibility in the pathophysiology of obesity and insulin resistance: an updated overview. Curr Obes Rep. 2021;10:191–213. doi: 10.1007/s13679-021-00434-0. [DOI] [PubMed] [Google Scholar]
- 31.Koliaki C., Liatis S., Dalamaga M., Kokkinos A. Sarcopenic obesity: epidemiologic evidence, pathophysiology, and therapeutic perspectives. Curr Obes Rep. 2019;8:458–471. doi: 10.1007/s13679-019-00359-9. [DOI] [PubMed] [Google Scholar]