Abstract
Background and aims
Micronutrient supplements such as vitamin D, vitamin C, and zinc have been used in managing viral illnesses. However, the clinical significance of these individual micronutrients in patients with Coronavirus disease 2019 (COVID-19) remains unclear. We conducted this meta-analysis to provide a quantitative assessment of the clinical significance of these individual micronutrients in COVID-19.
Methods
We performed a comprehensive literature search using MEDLINE, Embase, and Cochrane databases through December 5th, 2021. All individual micronutrients reported by ≥ 3 studies and compared with standard-of-care (SOC) were included. The primary outcome was mortality. The secondary outcomes were intubation rate and length of hospital stay (LOS). Pooled risk ratios (RR) and mean difference (MD) with corresponding 95% confidence intervals (CI) were calculated using the random-effects model.
Results
We identified 26 studies (10 randomized controlled trials and 16 observational studies) involving 5633 COVID-19 patients that compared three individual micronutrient supplements (vitamin C, vitamin D, and zinc) with SOC. Nine studies evaluated vitamin C in 1488 patients (605 in vitamin C and 883 in SOC). Vitamin C supplementation had no significant effect on mortality (RR 1.00, 95% CI 0.62–1.62, P = 1.00), intubation rate (RR 1.77, 95% CI 0.56–5.56, P = 0.33), or LOS (MD 0.64; 95% CI -1.70, 2.99; P = 0.59). Fourteen studies assessed the impact of vitamin D on mortality among 3497 patients (927 in vitamin D and 2570 in SOC). Vitamin D did not reduce mortality (RR 0.75, 95% CI 0.49–1.17, P = 0.21) but reduced intubation rate (RR 0.55, 95% CI 0.32–0.97, P = 0.04) and LOS (MD -1.26; 95% CI -2.27, −0.25; P = 0.01). Subgroup analysis showed that vitamin D supplementation was not associated with a mortality benefit in patients receiving vitamin D pre or post COVID-19 diagnosis. Five studies, including 738 patients, compared zinc intake with SOC (447 in zinc and 291 in SOC). Zinc supplementation was not associated with a significant reduction of mortality (RR 0.79, 95% CI 0.60–1.03, P = 0.08).
Conclusions
Individual micronutrient supplementations, including vitamin C, vitamin D, and zinc, were not associated with a mortality benefit in COVID-19. Vitamin D may be associated with lower intubation rate and shorter LOS, but vitamin C did not reduce intubation rate or LOS. Further research is needed to validate our findings.
Keywords: Micronutrient supplements, Vitamin D, Vitamin C, Zinc, Mortality, COVID-19
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection has become a worldwide pandemic [1]. The mortality rate of patients with COVID-19 is estimated to be 5% and can reach up to 32.5% in severe cases [1]. No specific antiviral treatment for COVID-19 infection has been available until now due to the rapid spread of SARS-CoV-2 [2]. Cost-effective, globally available, safe, and effective therapeutic interventions are warranted to ameliorate morbidity and mortality during this pandemic [2].
Inflammatory over-reaction and cytokine storm are hypothesized to play a role in COVID-19, and host immune response against SARS-CoV-2 can influence disease outcome [3]. Previous studies have demonstrated low serum levels of micronutrients such as vitamin C, vitamin D, and zinc in critically ill patients with COVID-19 [[4], [5], [6]]. Furthermore, few studies showed that low serum levels of some micronutrients such as vitamin D and zinc are associated with poor outcomes in patients with COVID-19 [6,7].
Some studies suggested a clinical benefit of some of the micronutrients in the management of COVID-19 due to their potential antimicrobial and immunomodulatory properties [[8], [9], [10]]. However, other studies suggested no clinical benefits in mortality in these patients [[11], [12], [13], [14], [15]]. The clinical significance of micronutrient supplements in patients with COVID-19 remains unclear and conflicting. Therefore, we conducted this comprehensive systematic review and meta-analysis to provide a quantitative assessment of the clinical significance of micronutrient supplements in patients with COVID-19.
2. Methods
2.1. Data sources and search strategy
We performed a comprehensive search of PUBMED/MEDLINE, Embase, and Cochrane databases from inception through December 5th, 2021. Medical subject headings representing micronutrient supplements (vitamins A, B, C, D, E, magnesium, selenium, or zinc) and (COVID-19 or SARS-CoV-2) were applied. We used the guideline for the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) and devised research questions using the PICO format for systematic review: for COVID-19 patients (Participants), does individual micronutrients supplementation (Intervention) impact the clinical outcomes (Outcomes) for intervention group compared to the control group (Comparison) in observational or randomized controlled trials (RCTs) (Type of studies)?. Supplementary Table 1 describes the full search term used in each database searched. The search was not limited by language, study design, or country of origin. We also performed a manual search for additional relevant studies using references of the included articles. Two investigators (HA and OS) performed the literature search and screened the studies independently. Discrepancies were resolved consensus.
2.2. Inclusion and exclusion criteria
The titles and abstracts of identified studies were screened, and full texts were examined. We included all published RCTs and observational studies (prospective and retrospective) that all compared individual micronutrients versus standard-of-care (SOC) in adult patients with COVID-19 and examined the impact of individual micronutrient supplementation on clinical outcomes of COVID-19. Only individual micronutrients reported in ≥3 studies were included in the final analysis. We excluded single-arm studies, case reports, reviews, commentaries, abstracts, studies with no clear outcomes, and preprint studies. We also excluded studies that examined micronutrient supplements as a combination. A study by Carlucci et al. [16] that evaluated zinc supplements' role in patients with COVID-19 was excluded because the mortality rate was reported as a composite outcome as death/hospice.
2.3. Data extraction
Data on study design, patients’ demographics, study characteristics, follow-up duration, route of administration and dosage, and outcome measures were extracted from the included studies. Two investigators (AB and MM) finished the data extraction independently. Discrepancies were resolved by a third reviewer (HA).
2.4. Outcomes of interest
The primary outcome of our study was mortality between the micronutrient and SOC groups. The secondary outcomes were intubation rate and length of hospital stay (LOS).
2.5. Statistical analysis
We performed a meta-analysis of the included studies using Review Manager 5.3 (Cochrane Collaboration, Copenhagen, The Nordic Cochrane Centre) and Comprehensive Meta-Analysis (Biostat, Englewood, USA). The median and interquartile range were converted to mean and SD where applicable [17]. The random-effects model was used to calculate the weighted pooled risk ratio (RR) and mean difference (MD) with the corresponding 95% confidence intervals (CI) of our desired outcome. A P-value <0.05 was considered statistically significant. The heterogeneity of the effect size estimates across the studies was quantified using the Q statistic and I2 (P < 0.10 was considered significant). A value of I2 <50% indicates low heterogeneity, and ≥50% indicates substantial heterogeneity [18].
2.6. Subgroup and sensitivity analyses
We performed a subgroup analysis based on the study design (RCTs vs. observational studies) and route of administration (intravenous [IV] or oral) if at least three studies reported the outcome. We also conducted a subgroup analysis of studies reporting the supplementation of vitamin D before the diagnosis of COVID-19 and those reporting the supplementation of vitamin D post-COVID-19 diagnosis. To confirm the robustness of the results in the presence of significant heterogeneity, sensitivity analysis for mortality using leave-one-out meta-analysis was performed to see if it had a significant influence on the meta-analysis result (i.e., jack-knife sensitivity analysis).
2.7. Bias assessment
The Jadad composite scale was used to assess the methodological quality of the clinical trials based on randomization, blinding, and withdrawals. The scale ranged from 0 to 5 points. Studies with total scores of ≥3 were considered to have a low risk of bias. The Newcastle Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the observational studies based on the selection of the study groups, comparability of study groups, and ascertainment of exposure/outcome. Studies with total scores of ≥6 were considered to have a low risk of bias. For outcomes reported by ≥ 10 studies, publication bias was assessed qualitatively by visually assessing the funnel plot and quantitively using Egger's regression analysis. Two authors (AB and MM) independently assessed each study for bias. Discrepancies were resolved by a third reviewer (HA).
3. Results
3.1. Study selection
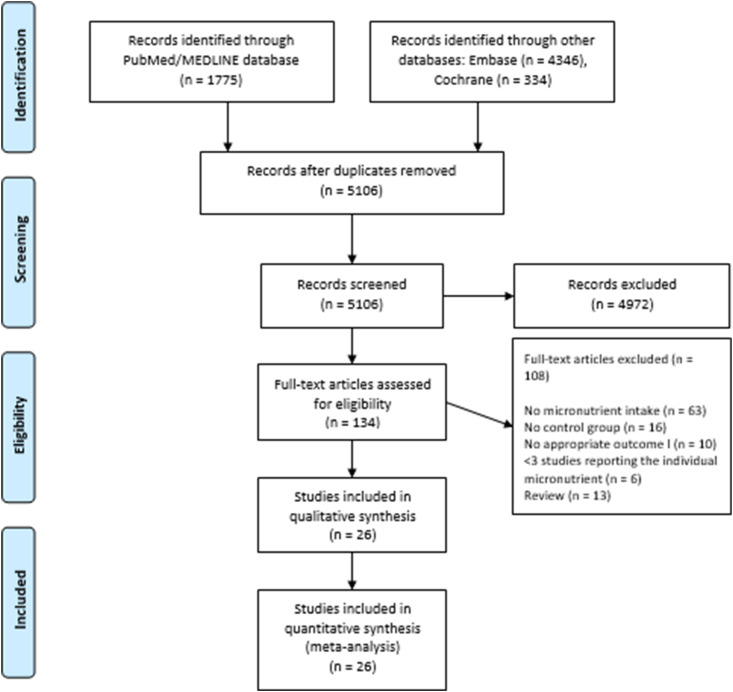
We identified a total of 1034 studies that examined the clinical significance of micronutrient supplements in patients with COVID-19. Based on full texts, we excluded 108 studies that had no control group, no appropriate outcome, or <3 studies reporting the individual micronutrient. Eventually, 26 studies met our inclusion criteria and were included in the meta-analysis. These studies investigated three individual micronutrient supplements: vitamin C, D, and zinc. Figure 1 shows the PRISMA flow chart that illustrates how the final studies were selected.
Fig. 1.
PRISMA flow diagram for the selection of studies.
3.2. Vitamin C supplementation
Characteristics and patient demographics of the included studies for vitamin C supplementation are shown in Table 1 . Nine studies [8,11,13,14,[19], [20], [21], [22], [23]] (four RCTs and five retrospective cohort studies) that examined the effect of vitamin C on clinical outcomes of 1488 patients with COVD-19 (605 patients in the vitamin C group and 883 in SOC) were included. The mean age was 61.0 ± 15.02, and 57.4% were males. These studies were conducted in China [8,14,22], Iran [13], Pakistan [20], Saudi Arabia [24], Turkey [25], and United States [11,21]. The studies provided daily doses alone [11,13,14,[20], [21], [22],24,25] or daily doses after a loading dose [8]. The studies provided IV [8,13,14,[20], [21], [22],25] or oral [11,24] vitamin C supplementations. Daily doses used were oral 1 gm [24] and 8 gm [11] daily, and IV 50 mg/kg/day [20], 2 gm [25], 2–4 gm [22], 6 gm [8,13,21], or 24 gm [14] daily. The loading dose was 6 gm IV infusion for 12 h [8]. The duration of treatment varied from four to 18 days. There was a low risk of bias for seven studies [8,11,14,21,22,24,25], while the risk of bias for two studies was high [13,20], as shown in Supplementary Table 2.
Table 1.
Characteristics and outcomes of the included studies for vitamin C supplementation.
| Al Sulaiman, 2021 [1] | Gao, 2021 | JamaliMoghadam, 2021 | Kumari, 2020 | Li, 2021 | Suna, 2021 | Thomas, 2021 | Zhang, 2020 | Zheng, 2021 | |
|---|---|---|---|---|---|---|---|---|---|
| Country of origin | Saudi Arabia | China | Iran | Pakistan | USA | Turkey | USA | China | China |
| Study design | RC | RC | RCT | RCT | RC | RC | RCT | RCT | RC |
| Patient setting | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Outpatient | Hospitalized | Hospitalized |
| Total patients (ttt/control) | 296 (148/148) | 76 (46/30) | 60 (30/30) | 150 (75/75) | 32 (8/24) | 323 (153/170) | 98 (48/50) | 56 (27/29) | 397 (70/327) |
| Age, mean ± SD, years | 60.6 ± 15.15 | 61 (52–71)a | 59.3 ± 17.1 | 52.5 ± 11.5 | 64.7 ± 10.9 | 62.3 ± 14.2 | 43.8 ± 14.8 | 66.7 ± 12.7 | 67 (61–74)a |
| Male, n (%) | 202 (68) | 30 [39] | 30 (50) | 99 (56.9) | 12 (37.5) | 204 (63.2) | 34 (34.7) | 36 (64.3) | 207 (52.1) |
| Coexisting comorbities | |||||||||
| Smoker, n (%) | NR | 5(10.9)/3(10) | NR | NR | 0/1(4) | NR | 17(35.4)/14(28) | NR | NR |
| Diabetes, n (%) | 90(60.8)/83(56) | 11(24)/4(13.3) | 12(40)/11(36.7) | NR | 4(50)/11(46) | 49(32)/47(27.6) | 2(4.2)/3(6) | 2(7.4)/3(10.3) | 11(15.7)/51(15.6) |
| Hypertension, n (%) | 82(55.4)/83(56) | 16(34.8)/6(20) | 15(50)/10(33.3) | NR | 6(75)/13(54) | 61(39.9)/75(44.1) | 8(16.7)/14(28) | 8(29.6)/14(48.3) | 13(18.6)/70(21.4) |
| CAD, n (%) | 12(8)/11(7.4) | 3(6.5)/2(6.7) | 4(13.3)/7(23.3) | NR | 1(13)/1(4) | 25(16.3)/29(17.1) | NR | 17(63)/14(48.3) | 3(4.3)/22(6.7) |
| Route of administration, dose, and duration of treatment | 1000 mg once daily enterally, median 11 days [[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18]] | LD: 6 g IV infusion for 12 h on 1st day. MD: 6 gm/day for 4 days | 6 g IV for 5 days | 50 mg/kg/day of IV vitamin C | 1.5 g IV every 6 h for a total of 4 days | 2 g IV per day | Oral ascorbic acid (8000 mg daily) | 24 g IV ascorbic acid for 7 days | 2–4 g IV daily, median 8.8 days (5.1–16) |
| Concurrently administered other drugs | NR | Antivirals, antibiotics, steroids, gamma globulins, statins | Steroids, HCQ, and lopinavir/ritonavir | Antipyretics steroids, antibiotics |
Steroids, remdesivir, thiamine, and tocilizumab | Steroids, and favipiravir | NR | Antibiotics and steroids | NR |
| Mortality (ttt/control) | 46/59 | 1/5 | 3/3 | 7/11 | 7/19 | 17/24 | 1/0 | 6/11 | 12/7 |
| Intubation (ttt/control) | NR | 2/1 | 5/4 | 12/15 | NR | NR | NR | NR | 5/2 |
| LOS, days | 8.5 (5–15)/7 (4–12)a | NR | 8.50 (7–12)/6.5(4–12)a | 8.1 ± 1.8/10.7 ± 2.2 | 18 ± 13/16 ± 14 | 8.1 ± 4.2/7.1 ± 4.9 | NR | 35 ± 17/32.8 ± 17 | NR |
| Follow-up duration | NR | 28 days | NR | NR | NR | NR | 28 days | 28 days | 29.3 days (28.5–30.1)a |
Abbreviations: CAD: coronary artery disease, IV: intravenous, LOS: length of hospital stay, NR: not reported, RCT: randomized controlled trial, RC: retrospective cohort, SD: standard deviation, ttt: treatment.
Median (interquartile range).
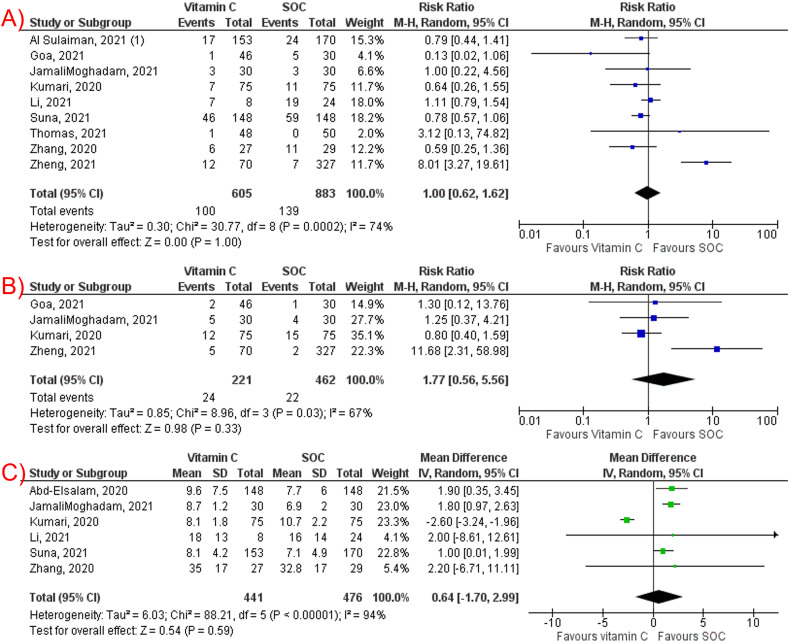
Vitamin C (ascorbic acid) supplementation had no significant effect on mortality among COVID-19 patients (RR 1.00, 95% CI 0.62–1.62, P < 0.001, I2 = 74%, Fig. 2 A), intubation rate (RR 1.77, 95% CI 0.56–5.56, P = 0.33, I2 = 67%, Fig. 2B), and LOS (MD 0.64; 95% CI -1.70, 2.99; P = 0.59, I2 = 94%, Fig. 2C). The subgroup analysis of RCTs for mortality was consistent (RR 0.69, 95% CI 0.39–1.20, P = 0.19, I2 = 0%) (Supplementary Fig. 1A). Consistent results in mortality were demonstrated as well for studies reporting IV vitamin C (RR 1.01, 95% CI 0.57–1.80, P = 0.98, I2 = 80%) (Supplementary Fig. 1B).
Fig. 2.
Forest plots of studies comparing vitamin C supplementation and standard-of-care regarding: (A) mortality, (B) intubation rate, (C) length of hospital stay.
A leave-one-out sensitivity analysis showed consistent results for mortality and intubation rates (Supplementary Fig. 2A,B, respectively). However, on sensitivity analysis for LOS, removal of Kumari et al. [20] moved the overall effect to favor against vitamin C with an MD of 1.54 (95% CI: 0.95–2.12, P < 0.001, I2 = 0%), suggesting that Kumari et al. was partly the reason for the significant between-study heterogeneity (Supplementary Fig. 2C).
3.3. Vitamin D supplementation
Characteristics and patient demographics of the included studies for vitamin D supplementation are shown in Table 2, Table 3 . Thirteen studies [9,10,15,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35]] (four RCTs and nine observational studies) that evaluated the effect of vitamin D on clinical outcomes of 3407 patients with COVID-19 (878 patients in the vitamin D group and 2529 in SOC) were included. Four studies [27,28,33,35] assessed the use of vitamin D before the diagnosis of COVID-19, while eight studies [9,15,26,[29], [30], [31], [32],34] reported the use of vitamin D after the diagnosis of COVID-19, and one study [10] reported the use of vitamin D pre and post COVID-19 diagnosis. The mean age was 65.25 ± 18 years, and 49.9% were males. These studies were conducted in France [9,10], Spain [26,28,32,33], Brazil [15], India [29,31], Italy [27,35], Turkey [34], and United States [30]. All studies provided oral vitamin D supplementations with highly variable dosages and duration of treatment across the included studies. There was a low risk of bias for all studies [9,10,15,[26], [27], [28], [29], [30], [31], [32], [33], [34], [35]], as shown in Supplementary Table 3.
Table 2.
Characteristics and outcomes of the included studies that supplemented vitamin D pre-COVID-19 diagnosis.
| Study, year | Arroyo-Diaz, 2021 | Cangiano, 2021 | Cereda, 2021 | G. Annweiler, 2020 | Hernandez, 2020 |
|---|---|---|---|---|---|
| Country of origin | Spain | Italy | Italy | France | Spain |
| Study design | Cross-sectional | RC | RC | Non-RCT (Quasi experimental) | Case control |
| Patient setting | Hospitalized | Nursing home residents | Outpatient and hospitalized | Hospitalized | Hospitalized |
| Total patients (treatment/control) | 1267 (189/1078) | 98 (20/78) | 324 (38/286) | 61 (29/32) | 216 (19/197) |
| Age, mean ± SD, years | 64.7 ± 16.3 | 89.9 ± 6.5 | 70.3 ± 12.8 | 88.4 ± 5.3 | 59.4 ± 16.8 |
| Male, n (%) | 696 (54.9) | 28 (28.6) | 157 (48.5) | 39 (50.6) | 123 (57) |
| Coexisting comorbidities | |||||
| Smoker, n | 18/111 | NR | NR | NR | 2/14 |
| COPD, n | 40/179 | 25a | 2/27 | NR | 2/15 |
| DM, n | 47/205 | 11a | 6/51 | NR | 0/34 |
| HTN, n | 118/484 | 48a | 20/162 | 28/21 | 12/76 |
| CAD, n | 40/179 | NR | 8/53 | NR | 3/21 |
| Serum 25OHD, ng/mL, mean (SD) (treatment/control) | NR | NR | 32.9 (14.8)/11.3 (8.6) | NR | 21.1 (5.9)/13.8 (7.2) |
| Route of administration, dose, and duration of treatment | NR | Chronic oral cholecalciferol supplements | (∼800 IU/day), 25OHD supplements (mean intake, 58.846 IU/month) | 50,000 IU vitamin D3 monthly, or 80,000 IU or 100,000 IU vitamin D3 every 2–3 months | 11 patients were taking cholecalciferol, 25,000 IU/monthly in 10 cases, and 5600 IU/weekly in 1, and 8 patients were on calcifediol, 0.266 mg/monthly |
| Concurrently administered other drugs | NR | NR | NR | Corticosteroids and antibiotics | Anakinra, azithromycin, corticosteroids, HCQ, lopinavir/ritonavir, tocilizumab |
| Mortality (treatment/control) | 50/167 | 3/39 | ([7/18]/40/152]) | 2/10 | 2/20 |
| Intubation (treatment/control) | 11/113 | NR | NR | NR | 1/43 |
| LOS, days (treatment/control) | 7.86 ± 8.5/8.91 ± 10.7 | NR | NR | NR | NR |
| Duration of symptoms, days | NR | NR | NR | NR | NR |
| Follow-up duration, mean ± SD, days | NR | 60 | NR | 14 | 11.8 ± 6.1 |
Abbreviations: COPD: chronic obstructive pulmonary disease, CAD: coronary artery disease, DM: diabetes mellitus, HCQ: hydroxychloroquine, HTN: hypertension, IU: international unit, LOS: length of hospital stay, NR: not reported, RCT: randomized controlled trial, RC: retrospective cohort, SD: standard deviation.
Total number in both groups (vitamin D and control).
Table 3.
Characteristics and outcomes of the included studies that supplemented vitamin D post-COVID-19 diagnosis.
| Alcala-Diaz, 2021 | Castillo et al., 2020 | C. Annweiler, 2020 | Elamir, 2021 | G. Annweiler, 2020 | Guven, 2021 | Jevalikar, 2021 | Lakkireddy, 2021 | Murai, 2021 | |
|---|---|---|---|---|---|---|---|---|---|
| Country of origin | Spain | Spain | France | USA | France | Turkey | India | India | Brazil |
| Study design | RC | RCT | Non-RCT (Quasi experimental) | RCT | Non-RCT (Quasi experimental) | RC | Cross-sectional | RCT | RCT |
| Patient setting | Hospitalized | Hospitalized | Nursing home residents | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized | Hospitalized |
| Total patients (treatment/control) | 537 (79/458) | 76 (50/26) | 66 (9/57) | 50 (25/25) | 48 (16/32) | 175 (113/62) | 197 (128/69) | 87 (44/43) | 237 (119/118) |
| Age, mean ± SD, years | 67.3 ± 15.9 | 53.01 ± 10.24 | 87.7 ± 9.0 | 66.5 ± 17.04 | 88.4 ± 5.3 | 74 (61–82)a | 46.7 ± 18.8 | 45 ± 13 | 56.2 ± 14.4 |
| Male, n (%) | 113 [21] | 45 (59.2) | 15 (22.7) | 25 (50) | 39 (50.6) | 105 (60) | 134 (68) | 65 (75) | 133 (56.1) |
| Comorbidities | |||||||||
| Smoker, n | 3/5 | NR | NR | NR | NR | NR | NR | NR | NR |
| COPD, n | 3/8 | 4/2 | NR | 5/3 | NR | NR | NR | NR | 7/5 |
| DM, n | 20/20 | 3/5 | NR | 9/11 | 77 | NR | 77 | NR | 49/35 |
| HTN, n | 58/56 | 11/15 | NR | 13/17 | 58 | NR | 58 | NR | 67/58 |
| CAD, n | 9/12 | 2/1 | NR | NR | 16 | NR | 16 | NR | 16/16 |
| Serum 25OHD, ng/mL, mean (SD) (treatment/control) | NR | NR | NR | NR | 9.8 [5] | 6.7 (5.1–9.1)/7.1 (5.2–8.2)a | 9.8 [5] | 16 (6)/17 (6) | 21.2 (10.1)/20.6 (8.1) |
| Route of administration, dose, and duration of treatment | oral calcifediol (0.532 mg), then (0.266 mg) on day 3 & 7, and then weekly until discharge or ICU admission |
oral calcifediol (0.266 mg) on day 3 & 7, and then weekly until discharge or ICU admission | oral bolus of 80,000 IU vitamin D3 | calcitriol 0.5 μg daily for 14 days or hospital discharge | oral supplement of 80,000 IU vitamin D3 within a few hours of the diagnosis of COVID-19 | single dose of 300,000 IU vitamin D3 intramuscularly within the first 24 h of admission | median total dose of 60,000 IU of cholecalciferol | 60,000 IUs of vitamin.D (aqueol nano solution) daily for 8 or 10 days depending on the BMI | oral dose of 200,000 IU of vitamin D3 dissolved in a 10-mL peanut oil solution |
| Concurrently administered other drugs | Coticosteroids | Azithromycin, HCQ | Antibiotics, Corticosteroids, HCQ | Convalescent plasma, remdesivir, and corticosteroids | Corticosteroids and antibiotics | NR | NR | Ivermectin, favipiravir, remdesivir, and corticosteroids | Antibiotics, antivirals, and corticosteroids |
| Mortality (treatment/control) | 4/90 | 0/2 | 5/10 | 0/3 | 3/10 | 43/30 | 1/3 | 2/5 | 9/6 |
| Intubation (treatment/control) | 3/26 | NR | 0/0 | 0/2 | NR | 44/31 | NR | NR | 1/14 |
| LOS, days (treatment/control) | NR | NR | NR | 5.5 ± 3.9/9.24 ± 9.4 | NR | 9 (6–16)/9 (5–17)a | NR | 13 ± 5/14 ± 5 | 7(4–10)/7(5–13)a |
| Duration of symptoms, days | NR | NR | NR | NR | NR | NR | NR | 5/5a | NR |
| Follow-up duration, mean ± SD, days | 30 days | NR | 36 ± 17 | NR | 14 days | NR | NR | NR | 7.7 ± 5.3 |
Abbreviations: COVID-19: coronavirus disease 2019, HCQ: hydroxychloroquine, IU: international unit, LOS: length of hospital stay, NR: not reported, RCT: randomized controlled trials, RC: retrospective cohort, SD: standard deviation.
Median (interquartile range).
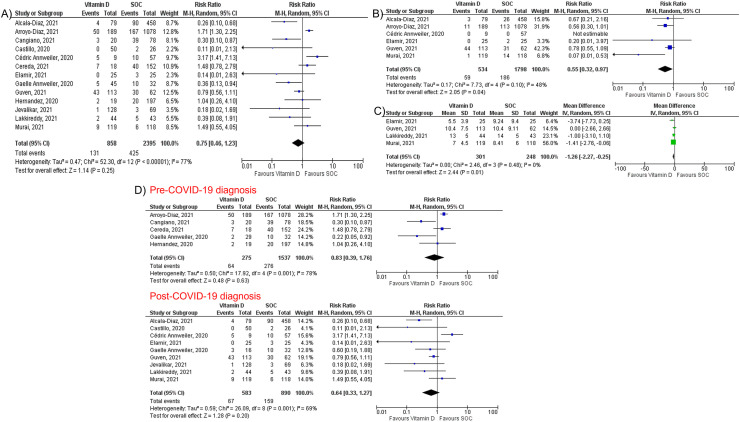
Overall, vitamin D supplementation did not reduce the risk of mortality among COVID-19 patients (RR 0.75, 95% CI 0.46–1.23, P = 0.25, I2 = 77%) (Fig. 3 A). However, vitamin D supplementation was associated with significant reduction in intubation rate (RR 0.55, 95% CI 0.32–0.97, P = 0.04, I2 = 48%, Fig. 3B) and LOS (MD -1.26; 95% CI -2.27, −0.25; P = 0.01, I2 = 0%, Fig. 3C). Subgroup analysis showed that vitamin D supplementation was not associated with a mortality benefit in patients receiving vitamin D pre or post COVID-19 (Fig. 3D). Sensitivity analysis showed consistent results for mortality (Supplementary Fig. 2). There was a visible asymmetry in the funnel plots of the included studies for mortality, which suggests the presence of publication bias (Supplementary Fig. 3). Furthermore, Egger's regression analysis demonstrated a statistically significant publication bias (P = 0.047).
Fig. 3.
Forest plots of studies comparing vitamin D supplementation and standard-of-care regarding: (A) mortality, (B) intubation rate, (C) length of hospital stay. (D) Subgroup analysis of studies that supplemented vitamin D pre and post COVID-19 diagnosis.
3.4. Zinc supplementation
Characteristics and patient demographics of the included studies for zinc supplementation are shown in Table 4 . A total of five studies [2,11,12,24,36] (three randomized controlled trials and two retrospective cohort studies), including 738 patients with COVID-19 (447 patients in zinc group and 291 in SOC group), were included. The mean age was 55 ± 18.8 years, and 58.8% were males. The studies were conducted in Australia [36], Egypt [2], Saudi Arabia [24], and the USA [11,12]. The studies provided IV [36] or oral [2,11,12,24] zinc supplementations in the form of zinc chloride [36], zinc gluconate [11], or zinc sulfate [2,12,24]. The duration of treatment varied from 7 to 15 days. There was a low risk of bias for four studies [11,12,24,36], while the risk of bias of one study [2] was high, as shown in Supplementary Table 4.
Table 4.
Characteristics and outcomes of the included studies for zinc supplementation.
| Abd-Elsalam, 2020 | Al Sulaiman, 2021 [2] | Patel, 2021 | Thomas, 2021 | Yao, 2021 | |
|---|---|---|---|---|---|
| Country of origin | Egypt | Saudi Arabia | Australia | USA | USA |
| Study design | RCT | RC | RCT | RCT | RC |
| Patient setting | Hospitalized | Hospitalized | Hospitalized | Outpatient | Hospitalized |
| Total patients (treatment/control) | 191 (96/95) | 164 (82/82) | 33 (15/18) | 108 (58/50) | 242 (196/46) |
| Age, mean ± SD, years | 43.6 ± 13.9 | 58.1 ± 15.21 | 62 ± 16.7 | 43.1 ± 14.7 | 66.14 ± 18.4 |
| Male, n (%) | 116 (60.7) | 119 (72.6) | 21 (63.6) | 40 [37] | 138 (57) |
| Coexisting comorbities | |||||
| Smoker, n | 42/39 | NR | NR | 16/14 | NR |
| Diabetes, n | 19/7 | 47/42 | 3/3 | 7/3 | 68/18 |
| Hypertension, n | 21/16 | 47/39 | 7/9 | 21/14 | 98/29 |
| CAD, n | NR | 7/6 | 4/3 | NR | 33/6 |
| COPD/asthma, n | NR | NR | NR | 10/7 | 58/7 |
| Route of administration, dose, and duration of treatment | Oral zinc sulfate 220 mg (50 mg of elemental zinc) twice daily for 15 days + HCQ | Oral zinc 220 mg (50 mg of elemental zinc) daily | IV high dose zinc chloride dose (0.5 mg/kg/d (elemental zinc concentration 0.24 mg/kg/d) for a maximum of 7 days, or until hospital discharge or death) | Oral 50 mg of zinc gluconate at bedtime daily for a duration of mean 5.9 ± 4.9 days | Oral zinc sulfate at a total daily dose of 440 mg (100 mg elemental zinc) |
| Concurrently administered other drugs | HCQ | Corticosteroids and tocilizumab | Dexamethasone and Remdesivir | Corticosteroids | Corticosteroids, HCQ, IL-6 inhibitors, lopinavir/ritonavir |
| Mortality (treatment/control) | 5/5 | 23/32 | 2/3 | 0/0 | 73/21 |
| Intubation (treatment/control) | 4/6 | NR | NR | NR | NR |
| LOS, days | 13.51 ± 5.34/14.01 ± 6.26 | 17 (12–29)/16 (10–28)a | NR | NR | NR |
| Follow-up duration, days | 28 days | NR | 28 days | 28 days | NR |
Abbreviation: COPD: chronic obstructive pulmonary disease, CAD: coronary artery disease, LOS: length of hospital stay, NR: not reported, RCT: randomized controlled trials, RC: retrospective cohort, SD: standard deviation.
Median (interquartile range).
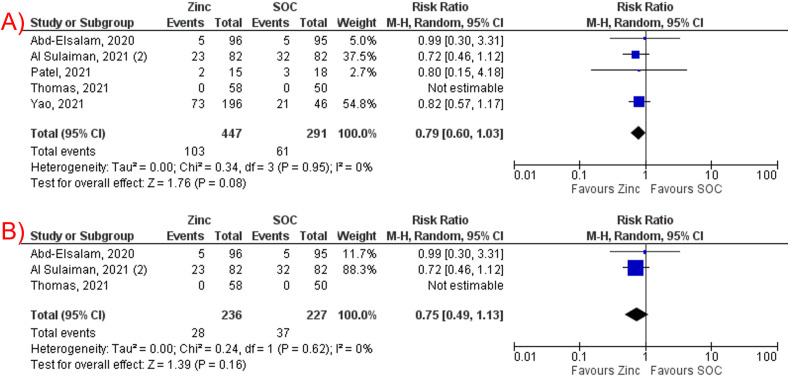
Zinc supplementation was not associated with a significant reduction in mortality among COVID-19 patients (RR 0.79, 95% CI 0.60–1.03, P = 0.08, I2 = 0%, Fig. 4 A). The subgroup analysis for RCTs was consistent (RR 0.75, 95% CI 0.49–1.13, P = 0.16, I2 = 0%, Fig. 4B).
Fig. 4.
(A) Forest plot of studies comparing zinc supplementation and standard-of-care regarding mortality. (B) Subgroup analysis of studies that supplemented intravenous zinc.
4. Discussion
To the best of our knowledge, this is the first comprehensive systematic review and meta-analysis that investigated the clinical significance of individual micronutrient supplements (e.g., vitamin C, vitamin D, and zinc) in patients with COVID-19, with more than 5600 patients from 26 articles. Our study findings indicate that all the three individual micronutrients, including vitamin C, vitamin D, zinc, were not associated with a mortality benefit in patients with COVID-19. While vitamin C was not associated with a reduction in intubation rate and length of hospital stay, vitamin D supplementation showed a lower intubation rate and shorter hospital stay length than SOC.
4.1. Vitamin C supplementation
Vitamin C therapy is usually perceived as harmless, inexpensive, with potential benefits in various respiratory infections, so it is not surprising to be an attractive therapy in the current pandemic. It is thought that vitamin C improves the immune system through various mechanisms, such as increasing lymphocyte and phagocyte activity, enhancing the response of T-cells to infections, and augmenting interferon levels [37]. Some authors suggested that vitamin C could play a role in hastening the pro-inflammatory and pro-oxidant environment (the cytokine storm) that is believed to be the cornerstone in SARS-CoV2 infection and pathogenies [38]. Vitamin C could also mediate the binding of SARS-CoV-2 with the angiotensin-converting enzyme 2 (ACE2) [39]. Interestingly, certain populations (male, African American, older, diabetics, hypertensive, and COPD patients) at a higher risk of severe COVID-19 have also been found to have lower serum vitamin C levels [40]. In a recent study, 94.4% of the 18 patients with COVID-19-induced ARDS had undetectable vitamin C levels, and one patient had low levels [4]. None of the studies included in our study reported the baseline vitamin C status.
The data regarding the value of ascorbic acid in the management of viral acute respiratory infections (ARIs) is inconsistent, with some studies showing that high doses of vitamin C may reduce the mortality among COVID-19 patients [8], while other studies have demonstrated no benefit [11,13,14]. Our meta-analysis demonstrated that vitamin D supplements had no significant effect on mortality, intubation rate, and LOS in COVID-19. Our findings were consistent with a recent meta-analysis by Rawat et al. [41], which showed no significant effect of vitamin C on the mortality of COVID-19 patients. However, the latter meta-analysis included studies that reported combined micronutrients [41]. In our meta-analysis, we included only studies that reported the use of individual micronutrient supplements for a more accurate assessment of their clinical significance.
4.2. Vitamin D supplementation
Vitamin D is postulated to regulate innate and adaptative immune responses, and antigen-presenting cells can synthesize 1,25-dihydroxy vitamin D from 25-hydroxy vitamin D [42]. Since cytokine storm plays an important role in COVID-19, vitamin D may also inhibit pro-inflammatory cytokine production in monocytes/macrophages [43]. A recent meta-analysis by Pereira et al. [7] showed a positive association between vitamin D deficiency and the severity of COVID-19. However, there is conflicting data regarding the value of vitamin D supplements in the management of COVID-19. Some studies [9,10] showed that vitamin D supplements might reduce the severity and mortality of COVID-19 patients, while other studies have shown no differences in mortality [15,28]. Our meta-analysis demonstrated no significant reduction in mortality with vitamin D supplementation in COVID-19 patients, with consistent results on subgroup analysis of studies supplemented vitamin D pre or post COVID-19 diagnosis. Our study findings align with the most recent trial's results which showed no significant difference between the vitamin D and placebo groups in mortality risk [15]. The RCT showed no significant difference in terms of intubation rate or LOS between vitamin D and placebo groups [15]. Elamir et al. [30] demonstrated shorter LOS in the vitamin D group compared to SOC group (mean 5.5 vs. 9.2 days, respectively), but it did not reach statistical significance (P = 0.14). However, our study showed a significant reduction in intubation rate and LOS in patients who received vitamin D supplementation after COVID-19 diagnosis. This discrepancy could be explained by the small sample size of these studies as they were likely underpowered to detect the difference.
4.3. Zinc supplementation
Zinc is a trace element that has been shown to play an important role in both innate and adaptive immune systems and cytokine production [44]. Zinc also has antioxidant effect, and it inhibits viral replication because it is hypothesized that zinc has shown an inhibitory effect on the RNA-dependent RNA polymerase of SARS-CoV in cell culture studies [45]. Zinc deficiency appears to be common in COVID-19 patients, with an incidence ranging from 31% up to 57% [5,6]. Although some studies have shown that zinc deficiency was associated with more risks of complications, hospital stay, and increased mortality [6], there is no clear data yet about the effect of zinc supplements on the clinical outcomes of COVID-19 patients. A report on four patients suggested that oral zinc (not exceeding 200 mg) was well tolerated and was associated with better recovery from COVID-19 infections [46]. However, Yao et al. [12] showed that the use of zinc sulfate was not significantly associated with a change in the risk of in-hospital mortality (adjusted hazard ratio 0.66; 95% CI, 0.41–1.07; P = 0.09). Our meta-analysis showed no mortality benefit from adding zinc supplements to COVID-19 patients. Our findings were consistent with the recently published clinical trial by Thomas et al. [11], which demonstrated no significant reduction in mortality risk between the patients who received zinc and those who did not.
4.4. Future studies of micronutrient supplements for COVID-19
There are several registered clinical trials still in the recruitment stage evaluating the effect of different micronutrient supplements on the clinical outcomes of COVID-19, including selenium (NCT04869579), vitamin C (NCT04357782 and NCT04264533), vitamin D, and zinc (NCT04641195). The pending results of these trials are expected to provide more solid evidence regarding the role of micronutrients in the management of COVID-19 patients.
4.5. Limitations and strengths
Our study has certain limitations. First, this meta-analysis is limited by the small number of studies with a small sample size and low event rate for zinc supplementation. Therefore, our findings should be interpreted with caution. Second, observational studies were included, which could be affected by confounding variables. In addition, some included trials were open-label trials. Therefore, double-blinded RCTs are needed to evaluate the clinical effectiveness of these supplements. Third, significant heterogeneity was found in the measurement of vitamin C and D. This could be explained by variation in the patient population, disease severity, co-administered medications used for COVID-19, and lack of standardization across the studies in medication dosage, duration of treatment. Fourth, lack of information on micronutrient deficiencies of individuals in both the treated and control groups. The patient's nutritional status and oxidative scavenging capacity could provide fundamental data to predict the severity of disease following SARS-CoV2 infection [47]. Fifth, we could not assess the impact of these supplements on the duration of symptoms and recovery time for the micronutrients and the effect of zinc on intubation rate and LOS due to limited studies that reported these outcomes. Lastly, we could not assess the effect of other micronutrients such as magnesium and selenium on COVID-19 due to the limited number of studies.
Despite these limitations, our study has significant strengths. First, we included a total of 26 studies that evaluated three different micronutrients (vitamin C, D, and zinc) with over 5600 patients with COVID-19. To our knowledge, this is the first comprehensive meta-analysis to investigate the clinical significance of individual micronutrient supplements in patients with COVID-19. Although substantial heterogeneity was noted in the measurement of mortality for vitamin C and D, we performed sensitivity analysis and subgroup analysis to evaluate the robustness of our results. Consistent results were observed on sensitivity analysis and subgroup analysis of RCTs and studies that reported the use of vitamin D pre and post COVID-19 diagnosis. Lastly, most of the studies in our meta-analysis had a low risk of bias based on quality assessment.
4.6. Conclusions
Micronutrient supplementations, including vitamin C, D, and zinc, did not reduce mortality in patients with COVID-19. While vitamin C did not reduce intubation rate or length of hospital stay, vitamin D supplementation may be associated with a lower intubation rate and shorter length of hospital stay. However, further research is needed to better understand the clinical significance of these individual micronutrient supplements in patients with SARS-CoV-2 infection.
Author contributions
AB, MM, and OS conceived and designed the study. AB, HA, WK, ASM, and DG collected, analyzed, and interpreted the data, and drafted the manuscript. YK, WS, JMS, and MH collected the data and reviewed the literature. AB and RA critically revised the manuscript. All authors read and approved the final manuscript.
Grants and funding
None.
Declaration of competing interest
All authors declare no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2021.12.033.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li X., Xu S., Yu M., Wang k., Tao Y., Zhou Y., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abd-Elsalam S., Soliman S., Esmail E.S., Khalaf M., Mostafa E.F., Medhat M.A., et al. Do zinc supplements enhance the clinical efficacy of hydroxychloroquine?: a randomized, multicenter trial. Biol Trace Elem Res. 2020:1–5. doi: 10.1007/s12011-020-02512-1. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 3.Costela-Ruiz V.J., Illescas-Montes R., Puerta-Puerta J.M., Ruiz C., Melguizo-Rodríguez L. SARS-CoV-2 infection: the role of cytokines in COVID-19 disease. Cytokine Growth Factor Rev. 2020;54:62–75. doi: 10.1016/j.cytogfr.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiscano-Camón L., Ruiz-Rodriguez J.C., Ruiz-Sanmartin A., Roca O., Ferrer R. Vitamin C levels in patients with SARS-CoV-2-associated acute respiratory distress syndrome. Crit Care. 2020;24:522. doi: 10.1186/s13054-020-03249-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yasui Y., Yasui H., Suzuki K., Saitou T., Yamamoto Y., Ishizaka T., et al. Analysis of the predictive factors for a critical illness of COVID-19 during treatment - relationship between serum zinc level and critical illness of COVID-19. Int J Infect Dis. 2020;100:230–236. doi: 10.1016/j.ijid.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jothimani D., Kailasam E., Danielraj S., Nallathambi B., Ramachandran H., Sekar P., et al. COVID-19: poor outcomes in patients with zinc deficiency. Int J Infect Dis. 2020;100:343–349. doi: 10.1016/j.ijid.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pereira M., Dantas Damascena A., Galvão Azevedo L.M., de Almeida Oliveira T., da Mota Santana J. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020:1–9. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 8.Gao D., Xu M., Wang G., Lv J., Ma X., Guo Y., et al. The efficiency and safety of high-dose vitamin C in patients with COVID-19: a retrospective cohort study. Aging (Albany NY) 2021;13:7020–7034. doi: 10.18632/aging.202557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annweiler C., Hanotte B., Grandin de l'Eprevier C., Sabatier J.-M., Lafaie L., Célarier T. Vitamin D and survival in COVID-19 patients: a quasi-experimental study. J Steroid Biochem Mol Biol. 2020;204 doi: 10.1016/j.jsbmb.2020.105771. 105771-105771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Annweiler G., Corvaisier M., Gautier J., Dubée V., Legrand E., Sacco G., et al. Vitamin D supplementation associated to better survival in hospitalized frail elderly COVID-19 patients: the GERIA-COVID quasi-experimental study. Nutrients. 2020;12 doi: 10.3390/nu12113377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas S., Patel D., Bittel B., Wolski K., Wang Q., Kumar A., et al. Effect of high-dose zinc and ascorbic acid supplementation vs usual care on symptom length and reduction among ambulatory patients with SARS-CoV-2 infection: the COVID A to Z randomized clinical trial. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yao J.S., Paguio J.A., Dee E.C., Tan H.C., Moulick A., Milazzo C., et al. The minimal effect of zinc on the survival of hospitalized patients with COVID-19: an observational study. Chest. 2021;159:108–111. doi: 10.1016/j.chest.2020.06.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.JamaliMoghadamSiahkali S., Zarezade B., Koolaji S., SeyedAlinaghi S., Zendehdel A., Tabarestani M., et al. Safety and effectiveness of high-dose vitamin C in patients with COVID-19: a randomized open-label clinical trial. Eur J Med Res. 2021;26:20. doi: 10.1186/s40001-021-00490-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang J., Rao X., Li Y., Zhu Y., Liu F., Guo G., et al. Pilot trial of high-dose vitamin C in critically ill COVID-19 patients. Ann Intensive Care. 2021;11:5. doi: 10.1186/s13613-020-00792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murai I.H., Fernandes A.L., Sales L.P., Pinto A.J., Goessler K.F., Duran C.S.C., et al. Effect of a single high dose of vitamin D3 on hospital length of stay in patients with moderate to severe COVID-19: a randomized clinical trial. JAMA. 2021;325:1053–1060. doi: 10.1001/jama.2020.26848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carlucci P.M., Ahuja T., Petrilli C., Rajagopalan H., Jones S., Rahimian J. Zinc sulfate in combination with a zinc ionophore may improve outcomes in hospitalized COVID-19 patients. J Med Microbiol. 2020;69:1228–1234. doi: 10.1099/jmm.0.001250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27:1785–1805. doi: 10.1177/0962280216669183. [DOI] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. Bmj. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel M., Hong G., Schmidt B., Al-janabi L., Adusumilli R.K., Tusha J., et al. The significance of oral ascorbic acid in patients with COVID-19. Chest. 2020;158 A325-A325. [Google Scholar]
- 20.Kumari P., Dembra S., Dembra P., Bhawna F., Gul A., Ali B., et al. The role of vitamin C as adjuvant therapy in COVID-19. Cureus. 2020;12 doi: 10.7759/cureus.11779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M., Ching T.H., Hipple C., Lopez R., Sahibzada A., Rahman H. Use of intravenous vitamin C in critically ill patients with COVID-19 infection. J Pharm Pract. 2021 doi: 10.1177/08971900211015052. [DOI] [PubMed] [Google Scholar]
- 22.Zheng S., Chen Q., Jiang H., Guo C., Luo J., Li S., et al. No significant benefit of moderate-dose vitamin C on severe COVID-19 cases. Open Med (Wars) 2021;16:1403–1414. doi: 10.1515/med-2021-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Sulaiman K., Aljuhani O., Saleh K.B., Badreldin H.A., Al Harthi A., Alenazi M., et al. Ascorbic acid as an adjunctive therapy in critically ill patients with COVID-19: a propensity score matched study. Sci Rep. 2021;11:17648. doi: 10.1038/s41598-021-96703-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Al Sulaiman K., Aljuhani O., Al Shaya A.I., Kharbosh A., Kensara R., Al Guwairy A., et al. Evaluation of zinc sulfate as an adjunctive therapy in COVID-19 critically ill patients: a two center propensity-score matched study. Crit Care. 2021;25:363. doi: 10.1186/s13054-021-03785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suna K., Melahat U., Murat Y., Figen Ö.E., Ayperi Ö. Effect of high-dose intravenous vitamin C on prognosis in patients with SARS-CoV-2 pneumonia. Med Clin (Barc) 2021 doi: 10.1016/j.medcli.2021.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Entrenas Castillo M., Entrenas Costa L.M., Vaquero Barrios J.M., Alcalá Díaz J.F., Miranda J.L, Bouillon R., et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: a pilot randomized clinical study. J Steroid Biochem Mol Biol. 2020;203 doi: 10.1016/j.jsbmb.2020.105751. 105751-105751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cereda E., Bogliolo L., Lobascio F., Barichella M., Zecchinelli A.L., Pezzoli G., et al. Vitamin D supplementation and outcomes in coronavirus disease 2019 (COVID-19) patients from the outbreak area of Lombardy, Italy. Nutrition. 2021;82:111055. doi: 10.1016/j.nut.2020.111055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M.A., López-Hoyos M., et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metabol. 2021;106:e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 29.Jevalikar G., Mithal A., Singh A., Sharma R., Farooqui K.J., Mahendru S., et al. Lack of association of baseline 25-hydroxyvitamin D levels with disease severity and mortality in Indian patients hospitalized for COVID-19. Sci Rep. 2021;11:6258. doi: 10.1038/s41598-021-85809-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elamir Y.M., Amir H., Lim S., Rana Y.P., Gonzalez Lopez C., Viera Feliciano N., et al. A randomized pilot study using calcitriol in hospitalized COVID-19 patients. Bone. 2022;154:116175. doi: 10.1016/j.bone.2021.116175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakkireddy M., Gadiga S.G., Malathi R.D., Karra M.L., Prasad Murthy Raju I.S.S.V., Ragini, et al. Impact of daily high dose oral vitamin D therapy on the inflammatory markers in patients with COVID 19 disease. Sci Rep. 2021;11:10641. doi: 10.1038/s41598-021-90189-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 32.Alcala-Diaz J.F., Limia-Perez L., Gomez-Huelgas R., Martin-Escalante M.D., Cortes-Rodriguez B., Zambrana-Garcia J.L., et al. Calcifediol treatment and hospital mortality due to COVID-19: a cohort study. Nutrients. 2021;13 doi: 10.3390/nu13061760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arroyo-Díaz J.A., Julve J., Vlacho B., Vlacho B., Corcoy R., Ponte P., et al. Previous vitamin D supplementation and morbidity and mortality outcomes in people hospitalised for COVID19: a cross-sectional study. Front Publ. Health. 2021;9 doi: 10.3389/fpubh.2021.758347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Güven M., Gültekin H. The effect of high-dose parenteral vitamin D3 on COVID-19-related inhospital mortality in critical COVID-19 patients during intensive care unit admission: an observational cohort study. Eur J Clin Nutr. 2021;75:1383–1388. doi: 10.1038/s41430-021-00984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cangiano B., Fatti L.M., Danesi L., Gazzano G., Croci M., Vitale G., et al. Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging (Albany NY) 2020;12:24522–24534. doi: 10.18632/aging.202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patel O., Chinni V., El-Khoury J., Perera M., Neto A.S., McDonald C., et al. A pilot double-blind safety and feasibility randomised controlled trial of high-dose intravenous zinc in hospitalised COVID-19 patients. J Med Virol. 2021;93(5):3261–3267. doi: 10.1002/jmv.26895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carr A.C., Maggini S. Vitamin C and immune function. Nutrients. 2017;9 doi: 10.3390/nu9111211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng R.Z. Can early and high intravenous dose of vitamin C prevent and treat coronavirus disease 2019 (COVID-19)? Med Drug Discov. 2020;5:100028. doi: 10.1016/j.medidd.2020.100028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zabetakis I., Lordan R., Norton C., Tsoupras A. COVID-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients. 2020;12 doi: 10.3390/nu12051466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patterson T., Isales C.M., Fulzele S. Low level of Vitamin C and dysregulation of Vitamin C transporter might be involved in the severity of COVID-19 Infection. Aging Dis. 2021;12:14–26. doi: 10.14336/AD.2020.0918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rawat D., Roy A., Maitra S., Gulati A., Khanna P., Baidya D.K. Vitamin C and COVID-19 treatment: a systematic review and meta-analysis of randomized controlled trials. Diab Metabol Synd: Clin Res Rev. 2021;15:102324. doi: 10.1016/j.dsx.2021.102324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hewison M. Vitamin D and the immune system: new perspectives on an old theme. Endocrinol Metab Clin N Am. 2010;39:365–379. doi: 10.1016/j.ecl.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Leung D.Y., Richers B.N., Liu Y., Remigio L.K., Riches D.W., et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase-1. J Immunol. 2012;188:2127–2135. doi: 10.4049/jimmunol.1102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lai Y.-J., Chang H.-S., Yang Y.-P., Lin T.W., Lai W.Y., Lin Y.Y., et al. The role of micronutrient and immunomodulation effect in the vaccine era of COVID-19. J Chin Med Assoc. 2021:84. doi: 10.1097/JCMA.0000000000000587. [DOI] [PubMed] [Google Scholar]
- 45.te Velthuis A.J., van den Worm S.H., Sims A.C., Baric R.S., Snijder E.J., van Hemert M.J. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finzi E. Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis. 2020;99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gasmi A., Chirumbolo S., Peana M., Noor S., Menzel A., Dadar M., et al. The role of diet and supplementation of natural products in COVID-19 prevention. Biol Trace Elem Res. 2022;200:27–30. doi: 10.1007/s12011-021-02623-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.