Abstract
Purpose
Therapeutic drug monitoring (TDM) supports personalized treatment. For successful implementation, TDM must have a turnaround time suited to the clinical needs of patients and their health care settings. Here, the authors share their views of how a TDM strategy can be tailored to specific settings and patient groups.
Methods:
The authors selected distinct scenarios for TDM: high-risk, complex, and/or critically ill patient population; outpatients; and settings with limited laboratory resources. In addition to the TDM scenario approach, they explored potential issues with the legal framework governing dose escalation.
Results:
The most important issues identified in the different scenarios are that critically ill patients require rapid turnaround time, outpatients require an easy sampling procedure with respect to the sample matrix as well as sample collection times, settings with limited laboratory resources necessitate setting-specific analytical techniques, and all scenarios warrant a legal framework to capture the use of escalated dosages, ideally with the use of trackable dosing software.
Conclusion
To benefit patients, TDM strategies need to be tailored to the intended population. Strategies can be adapted for rapid turnaround time for critically ill patients, convenient sampling for outpatients, and feasibility for those in settings with limited laboratory resources.
Keywords: Therapeutic drug monitoring, limited sampling, dosing software, saliva
Introduction
Dose selection of anti-infective drugs is of critical importance as it increases therapeutic success rate. Moreover, appropriate dose selection will prevent both acquired resistance and toxicity by avoiding underdosing (a concentration below the target range) and overdosing (a concentration above the target range), respectively.1 In other words, appropriate dose selection will most likely lead to a drug concentration within the target concentration range by considering factors known to influence drug pharmacokinetics. Unfortunately, for a proportion of anti-infective drugs, the target concentration is not achieved despite appropriate dose selection, or the target concentration cannot be maintained due to intra- or interpatient pharmacokinetic variability.2 Voriconazole is an excellent example of high intraindividual variability in drug exposure due to drug-drug interactions,3 variations in hepatic function, and inflammation,4 while interindividual variability is related to polymorphisms of the CYP450 enzymes.5 Further, techniques to replace organ function, such as various forms of dialysis, including slow extended daily dialysis, extracorporeal membrane oxygenation, or molecular adsorbent recirculating system therapy, may impact drug exposure.6–9
Therapeutic drug monitoring (TDM) optimizes the dose in an individual patient by dose adjustment based on one or multiple measured drug concentrations to achieve a drug concentration or exposure in the target range or to reach a certain pharmacokinetic/pharmacodynamic (PK/PD) target. Repeated TDM is required for drugs with high intraindividual variability, such as voriconazole, while less frequent TDM may be sufficient for a drug like gentamicin in a patient with stable renal function showing low or no intraindividual variability.10 Traditionally, drug doses are increased or decreased incrementally, based on the measured steady-state drug concentration. Dose changes are followed by re-assessment of the drug concentration when the new steady-state for the adjusted dose has been reached. This is often a cumbersome and lengthy procedure; therefore, the benefit for the patient is not maximized. To overcome this problem, model-informed precision dosing has been introduced, which uses a patient population-specific pharmacokinetic model with Bayesian simulations to allow a more precise dose adjustment.11 This approach uses information on the typical PK in a population (e.g., neonates), factors that may influence pharmacokinetic parameters (e.g., renal function and drug clearance), and a mathematical approach (Bayesian) to assess the drug exposure in an individual based on the measured drug concentrations and patient characteristics. Using this more sophisticated approach enhances the efficiency of the TDM procedure, resulting in a higher proportion of patients achieving a drug concentration or exposure in the target range when highly variable pharmacokinetic profiles are expected, such as in critically ill patients2 or for drugs with complex pharmacokinetics.4 When the susceptibility and minimal inhibitory concentration (MIC) of the causative pathogen is known, a PK/PD target, rather than a population-based threshold efficacy concentration, can be used to guide dose adjustments. The advantage of this approach is that it prevents unnecessary dose increases when a pathogen has a low MIC.
Although TDM implementation is apparently a rational approach for treatment optimization, very few randomized controlled studies have been performed, and most data supporting TDM are observational in nature.12,13 Observational data also revealed flawed study designs, showing selection bias by performing TDM only after clinical failure or toxicity was observed; the actual TDM procedure was not suitable for the situation based on too long turnaround times, wrong sample collection,14 or not considering pathogen susceptibility.12 Indeed, the turnaround time for drug concentration measurement is one of the most important factors to establish an adequate TDM strategy, followed by an appropriate dose adjustment and follow-up. The TDM strategy should be tailored to the specific situation, such that a more rapid turnaround time would be more important for a population with critical illness than for one with a chronic condition requiring months or even lifelong therapy.2,15,16 Here, we share our view of how a TDM strategy can be tailored to specific situations. We have selected three distinct scenarios (critically ill patients, outpatients, and remote settings with limited laboratory resources) for which we propose optimal TDM strategies based on clinical but also logistical, financial, and legal considerations. Rather than providing in-depth insights for TDM experts, the proposed strategies can be of help to those interested in setting up or further expanding their TDM service.
TDM in Critically Ill Patients
TDM is most often performed in the intensive care unit, as these patients are vulnerable and suffer from severe infections requiring treatment optimization. Critically ill patients may experience significant changes in liver function and renal clearance related to their illness. This results in poorly predictable pharmacokinetic profiles further aggravated by fluid supplementation therapy, systemic inflammation, dialysis, and/or extracorporeal membrane oxygenation.17 Thus, changes in protein binding, volume of distribution, and clearance can be observed, leading to variable drug exposure.17 A relative advantage is that sampling in critically ill patients is facilitated by the presence of intravenous catheters. When it comes to certain anti-infectives such as vancomycin and gentamicin, TDM is performed for every patient as the benefits of TDM for efficacy and toxicity have been established.2,18 However, for drugs that are measured less frequently (e.g., voriconazole), TDM can be suggested for patients who show a slow therapeutic response, or when development of resistance or toxicity is suspected. A comprehensive overview of specific suggestions for individual anti-infectives has recently been published.2
In many hospitals, a TDM service is in place, with a set number of measured anti-infectives. The use of software packages (e.g., BestDose, ID-ODS, InsightRx, MWPharm++, and TDMx) to support model-informed precision dosing is increasing.19 These programs perform well and are user-friendly, but integration in health records remains a problem.19 Depending on the specific drug, immunoassays or chromatography-mass spectrometry are used. The latter requires highly skilled lab scientists and significantly greater investments in laboratory equipment and infrastructure and is therefore more common in larger referral/teaching hospitals. In most clinical settings, total drug concentrations are measured because of the complexity of measuring unbound concentrations and the lack of specific PK/PD targets.20 However, unbound concentrations are more informative, as they represent drug exposure that is active against the pathogen. The frequency of TDM service in the clinical setting is often adapted to clinical needs and is reflected in the turnaround time. Ideally, TDM results should be available on the same day to be beneficial for critically ill patients.
TDM in Outpatient Settings — Long-term Care
Management of chronic infectious diseases in outpatient settings has become increasingly common in patients requiring prolonged anti-infective therapy for conditions such as human immunodeficiency virus (HIV) infection, fungal and mycobacterial diseases, and recalcitrant infections in patients with underlying immunodeficiency or structural anatomic predisposition, such as those with cystic fibrosis.
For people living with HIV, antiviral therapy is lifelong. As such, efforts are made to prescribe a one-pill-once per day combination of antiretroviral therapy with well-studied bioavailability and predictable PK/PD relationships.21 However, TDM is often of value when drugs for other diseases are co-administered. For example, rifamycins or proton-pump inhibitors can cause potential drug-drug interactions when added to antiretroviral therapy.22 In these cases, TDM is often performed to assess the effect on the trough concentrations of antiretroviral drugs and can be used to assure minimal exposure to antiretroviral therapy or guide dose modifications. For other common coexisting conditions, such as hepatitis B or C, pre-emptive TDM for antiretroviral drugs may aid in dose optimization while minimizing hepatotoxicity.23
In contrast to TDM for antiretroviral drugs, TDM may be required for drugs used to treat complicated fungal infections. For example, a meta-analysis of voriconazole, which has significant exposure-related toxicities and pharmacokinetic variability, reported that patients with therapeutic concentrations were twice as likely to respond to treatment, while those with supratherapeutic concentrations were four times more likely to experience toxicity.24 This suggests that TDM is required for all patients. TDM is important for posaconazole suspension, in contrast with the modified release tablets, because of poor drug absorption.25
Unlike most fungal and bacterial diseases, infections with nontuberculous mycobacteria (NTM) require multidrug therapy. The high treatment failure rate and risk of acquired resistance26 make TDM appealing as a tool to optimize drug exposure. However, for NTM, the lack of randomized controlled trials makes it difficult to identify therapeutic cut-offs indicative of treatment success, thereby relying on in vitro and in vivo studies, such as hollow-fiber models and animal models to determine PK/PD targets.27 In practice, for critical drugs in an NTM regimen, such as azithromycin or rifampicin for Mycobacterium avium complex, clinicians rely on TDM to ensure that estimates of peak or area under the concentration time curve (AUC) are within the expected range reported in larger case series, even though specific exposure targets have not been determined.27
For drugs such as aminoglycosides and vancomycin, which may be necessary for bacterial infections of weeks duration, including endocarditis or osteomyelitis, but have narrow therapeutic indices and irreversible toxicities, TDM is performed at the initiation of therapy and then weekly thereafter.28 Outpatient parenteral antibiotic therapy teams are increasingly employed for this purpose and are often composed of infectious disease clinicians and/or pharmacists affiliated with hospitals or large outpatient clinics.29
With advances in the use of pharmacometrics to optimize dosing, the need to collect multiple samples to obtain a full pharmacokinetic profile has been minimized. For example, adopting limited sampling strategies to collect only one or two well-timed samples to capture peak or trough levels or provide an estimate of AUC has significantly improved access to TDM for outpatient care.30 In most settings, the collected serum (or plasma) can then be timed for a certain number of hours after dose administration in the home of the patient or at first arrival in the clinic, which facilitates the total amount of clinical time. Following sample collection, serum samples were transferred to a referral laboratory with high-performance liquid chromatography, liquid chromatography-mass spectrometry, or immunoassay capabilities. Even with these advances, the requisites for personnel trained in sample collection, cold storage and transport to referral laboratories and the associated costs can be considerable. Although specialty referral laboratories may provide expert consultation for the interpretation of results and dose recommendations, clinicians often rely more heavily on monitoring the clinical response. In these situations, pharmacists and pharmacologists have the opportunity to provide TDM support, taking into consideration the PK/PD indices of the anti-infective drugs.
TDM in Remote Settings — Settings with Limited Laboratory Resources
In settings with limited laboratory capacity, such as in sub-Saharan Africa where there is a considerable burden of infectious diseases,31 the implementation of TDM for anti-infective drugs to optimize the management of major endemic infectious diseases is uncommon.32 However, in these settings, people with endemic infectious diseases, including tuberculosis (TB), HIV, and malaria, usually have multiple infections, such as TB/HIV, or comorbidities, such as malnutrition and/or diabetes mellitus (DM).33,34 Frequently, they also harbor drug-resistant pathogens requiring modifications of anti-infective drug therapies with unknown drug-drug interactions and pharmacokinetic variability.35 The emergence of drug-resistant TB, for example, prompted the adoption of new or repurposed drugs with even more limited evidence and uncertain safety profiles. Thus, in 2018, the World Health Organization changed the second-line anti-TB regimen for multidrug-resistant TB treatment to include linezolid, which ideally requires TDM for dose adjustments.36,37 Similarly, there are many examples of patients with multiple comorbidities, such as TB, DM, and HIV coinfection, requiring TDM when they have been prescribed drugs with considerable class variations, such as metformin, dolutegravir, and rifampicin.38
Despite the need for TDM in settings with limited laboratory resources, several health-system challenges impede the provision of TDM to people with the highest need. One of the challenges is the lack of sufficient skilled human workforce for the operation of clinics and affiliated TDM laboratories.39 Patients with the greatest need for TDM, such as those with malnutrition, may dwell in areas with poor infrastructure and unreliable electricity, hindering the support of a cold chain to collect, store, and analyze serum samples, one of the most common matrices for TDM.40 Furthermore, there are no guidelines on the application of TDM that can be adapted programmatically, such as for many other externally funded disease programs such as those for the care of people with TB/HIV.41,42 However, opportunities exist to implement TDM, for example, for key drugs in multi-drug resistant TB43 To address the challenges of cold chains, the dried blood spot (DBS) technique presents a viable solution, as these paper spots allow for storage and transport at unconditioned temperatures.44,45 DBS has been examined for TDM in rifampicin, pyrazinamide, and ethambutol, and second-line drugs, such as moxifloxacin and linezolid, and has been successfully used in resource-limited-remote settings.44,46–48 Prior to the implementation of DBS, clinical validation must be performed to determine the conversion factor to establish DBS target concentrations that represent traditional serum-based reference values. However, as DBS requires LC-MS/MS analysis, only central laboratories are likely equipped to provide such a service.41 Similarly, alternative matrices, including oral fluid (saliva) and urine, have been explored.49,50 In 2018, a simple, low-cost, and robust assay using mobile spectrophotometry quantified levofloxacin in human saliva in a TB endemic setting.51 The assay was performed in a routine clinical setting in Tanzania and was accurate and reliable for measuring levofloxacin and performing TDM.52 The main advantage of this assay is that it can be performed as a point-of-care test as a drop of saliva can be applied to the detector without sample pretreatment. Similar to DBS, a clinical validation study must be performed to compare drug concentrations in saliva and in a paired plasma or serum sample. The predictive value of the drug concentration in saliva samples for the concentration in plasma or serum samples can then be calculated.52 More recently, a similar assay has been developed for linezolid.53 These studies demonstrated that the implementation of TDM can be integrated in the programmatic management of major infectious diseases to subsequently contribute to improved patient outcomes. Adopting novel communication approaches is required to more swiftly facilitate individual dose adjustments.54
Regulatory Aspects of TDM (Guidelines and Laws)
TDM-guided dose optimization may result in the use of a dose exceeding approved doses and may be challenging due to regulatory restrictions or the lack of regulatory guidance. Many countries lack definitive policies to guide clinicians in making decisions regarding the use of off-label doses. Over 50% (11/21) of the countries of the European Union reported no specific policy tools for off-label drug use at the regulatory or healthcare system level.55 In such cases, the decision to use an unapproved dose depends on the professional responsibility of the prescriber and patient consent. France, Germany, Greece, Hungary, Italy, Lithuania, the Netherlands, Spain, Sweden, and the United Kingdom have policies outlining off-label use of medicinal products; however, they are more directed toward off-label indications rather than unapproved doses based on TDM.55 Often, clinicians are still required to request permission from the regulatory authority and obtain informed consent from the patient to prescribe off-label doses, which will delay dose adjustments.
In Australia, the Council of Australian Therapeutic Advisory Groups provided an algorithm clinicians should follow when they need to use a drug off-label, defined as use for an unapproved indication, at a different dose, via an alternative administration route, or for unapproved patient populations.56 The suggested algorithm guides clinicians step-by-step to consider the quality of evidence regarding efficacy and safety; category of use (e.g., routine, exceptional, conditional, or investigational); required approvals (e.g., drug and therapeutic committee or human research ethics committee); patient consent; and subsequent monitoring.56 Unfortunately, high-quality evidence, such as coming from randomized clinical trials initially conducted for marketing approvals, are not likely available for new doses given the lack of incentives driving pharmaceutical companies and may not necessarily contain thorough PK/PD assessments.56 Decision algorithms specific to TDM-related dosing should consider alternative, well-designed studies demonstrating the required PK/PD evidence for TDM. A well-designed study would include relevant elements to describe the patient population, drug use, PK/PD assessment, infection, and treatment outcomes. A stepwise approach for developing a well-designed trial has been described in detail earlier.12 Furthermore, specific liability and legal protection guidance should be made available to clinicians working through the recommended decision algorithm.
Dosing software regulations are other aspects to be considered, as model-informed precision dosing is a growing part of TDM.11 Regulatory approval status and policies regarding software as medical devices could restrict their use in a country-dependent manner. For example, software used in making treatment-related decisions is defined as a medical device by the Australian Register of Therapeutic Goods and the European Commission.57,58 In contrast, software that provides dose recommendations aligned with the United States Food and Drug Administration drug label is considered “non-device clinical decision support software.59 However, there is no regulation for software that provides unapproved dose recommendations.19
Accountability and training should also be considered, as various healthcare professionals could be involved in dose calculations and recommendations. Initial and ongoing user training is required under the EU medical device regulation.58 Specific accountability and role divisions can be outlined by hospitals at the local level. The involvement of different local/national stakeholders, such as clinicians, researchers, pharmaceutical companies, and policy regulators, in identifying barriers and working through specific policy development represents important steps to secure the widespread uptake of any future TDM strategy.
Discussion, Gap Analysis, and Outlook
We shared our view of how a TDM strategy can be tailored to different clinical settings, including among hospitalized critically ill patients, for people with chronic illnesses in outpatient clinics, and in remote settings with limited laboratory resources (Figure 1). To support informed decision making in establishing a TDM service, we have included a flow chart to guide the selection of drugs, assays, turnaround time, and costs (Figure 2). For complex patients, such as critically ill patients, several strategies have been discussed, including performing TDM for drugs with a narrow therapeutic window and highly variable pharmacokinetics and in patients with a lack of response or suspected toxicity. The use of different software for model-informed precision dosing and sample analysis by immunoassays or LC-MS represents areas that enable the development of an individualized, context-specific TDM strategy. Depending on the strategic tools, effective implementation may require integrating information into electronic health records or educating or hiring staff. In the outpatient setting, a TDM strategy that incorporates limited sampling with model-informed precision dosing is likely to be most conducive to uptake, although logistics and costs around sample transport and storage remain a challenge. The emerging use of alternative sampling matrices, such as DBS collection with LC-MS analyses or saliva or urine collection with colorimetric analyses, could provide more opportunities for TDM in both outpatient settings and remote areas with limited access to laboratories capable of conventional serum-based assays. Lastly, despite the available strategies to support dose optimization by TDM, the use of drug doses exceeding the maximum licensed dose could be a barrier for appropriate TDM performance by clinicians. Countries may have various regulations, and often there is a lack of guidance on off-label drug use.
Figure 1.
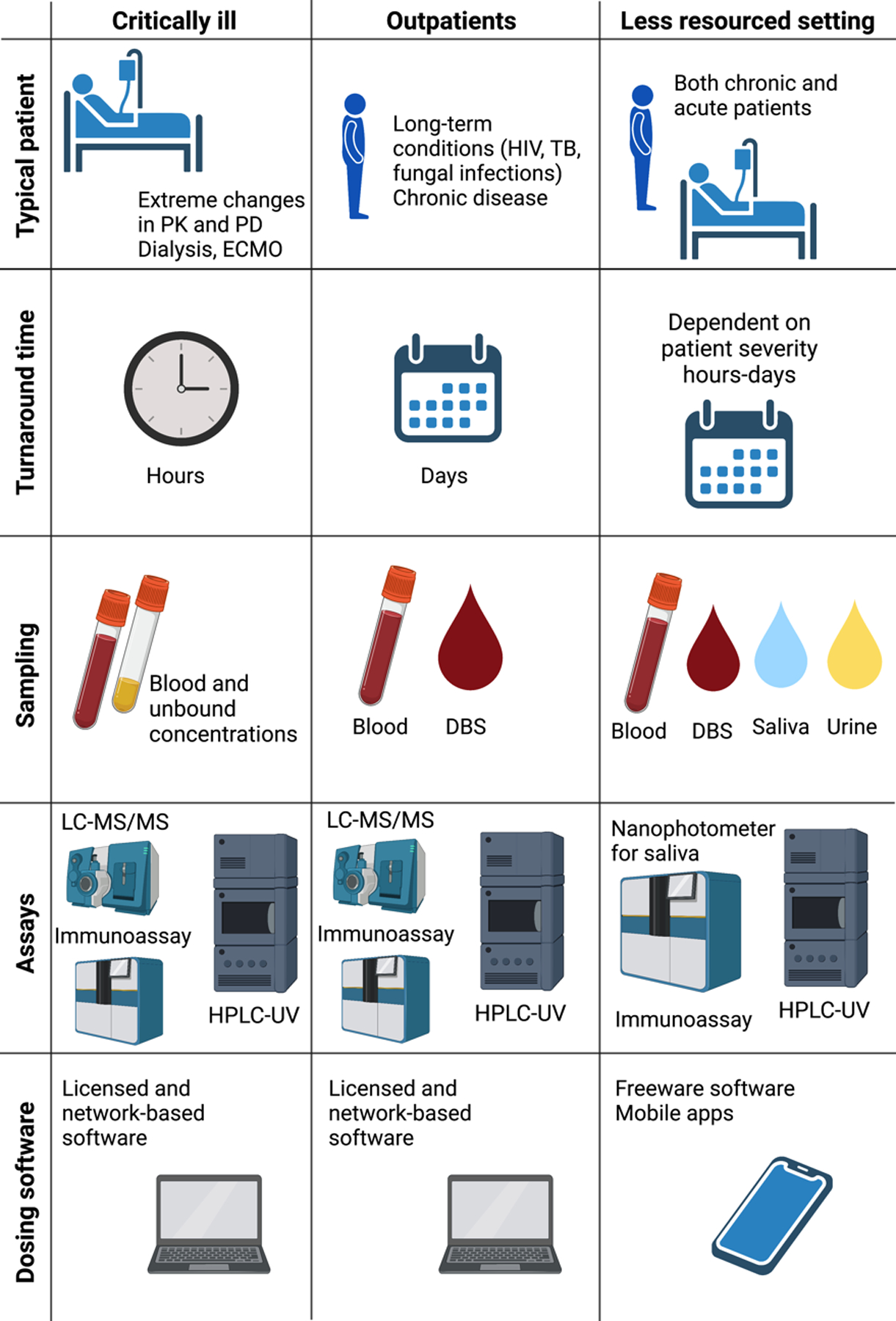
TDM strategy tailored to different clinical settings
PK, pharmacokinetics; PD, pharmacodynamics; ECMO, extracorporeal membrane oxygenation; HIV, human immunodeficiency virus; TB, tuberculosis; DBS, dried blood spot; LC-MS/MS, liquid chromatography-tandem mass spectrometry; HPLC-UV, high-performance liquid chromatography-UV/visible spectrometry.
Conducting TDM is different in various settings and mostly depends on patient factors, access to a laboratory and skilled medical personnel. In a critical care setting, extreme pharmacokinetic and pharmacodynamic variability can be expected, which requires fast turnaround time (hours) using full blood as well as a special methodology to measure potential unbound drug concentrations. In an outpatient setting, the turnaround time does not need to be as fast as in the ICU, as patients do not expect to have such large and rapid changes in drug exposure. In this setting, chronically ill patients with long-term conditions are monitored, which means alternative matrices, such as dried blood spot (DBS), could be used. In this case, patients can send in their samples via mail without coming to the clinic. In a less resourced setting, both chronic and acute patients must be monitored; however, an on-site laboratory is often not available. In this setting, DBS, urine, and saliva could be used to estimate potential under- or overexposure and concordance with therapy. In the ICU and outpatient settings, licensed and network-based software with trained staff can be utilized to provide TDM advice. In a less resourced setting, freeware mobile apps are apparently more appropriate as less funding and training would be needed.
Figure 2.
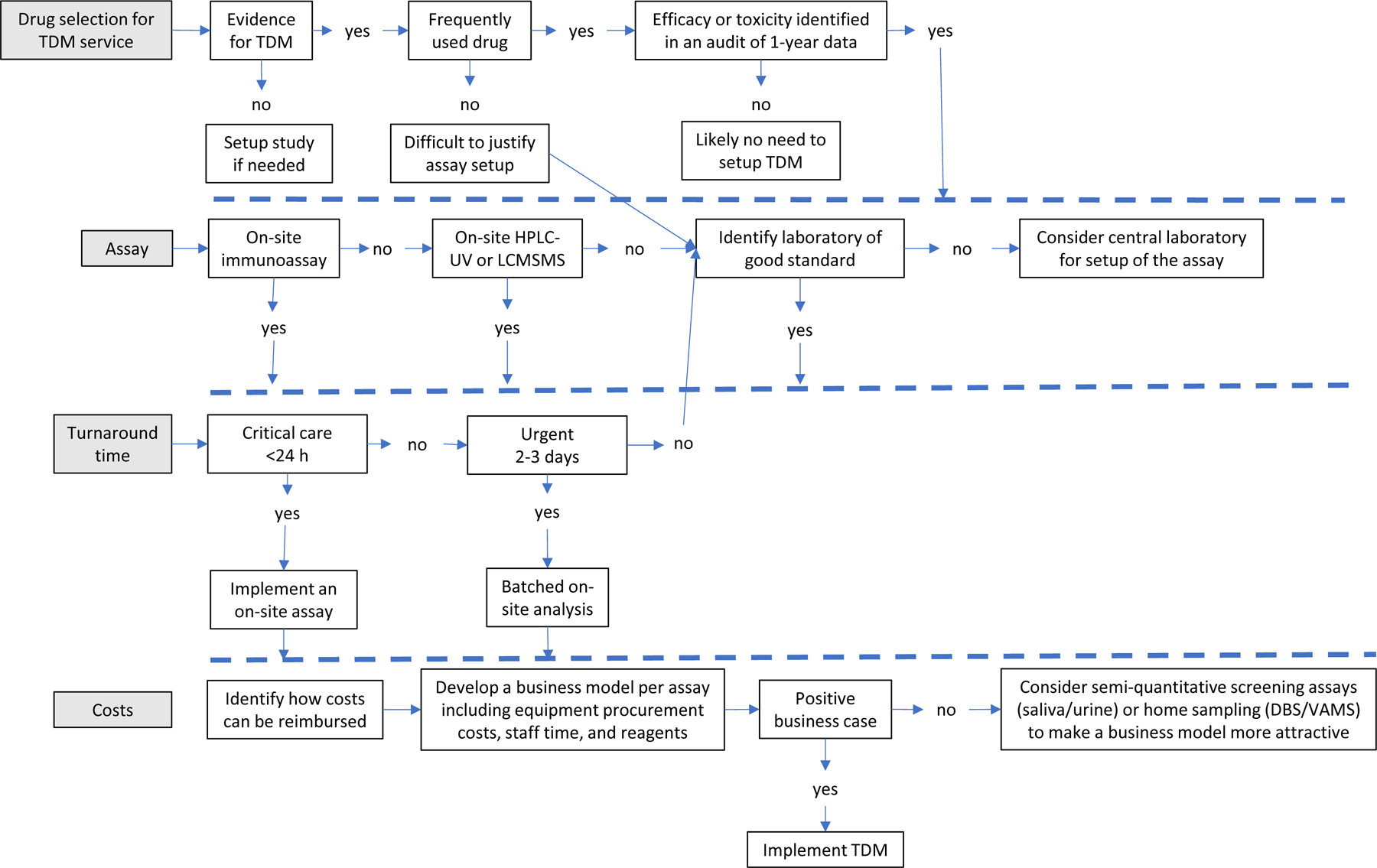
Setting up a TDM program; from drug selection to a business case
When setting up a TDM program, the first step is to select the drugs for which TDM will be supported. This selection needs to be evidence-based, which means that TDM for a specific drug is supported by clinical studies. An additional important consideration is the actual use of the drug and the clinical need for TDM services. A review of dispensing data and a clinical audit can provide relevant data to make an informed decision demonstrating that drug use results in either suboptimal efficacy or toxicity. The next step is to decide on the most appropriate assay, location, and turnaround time of TDM. Automatically, a rapid turnaround time will require the availability of an assay on-site, while in the case of less urgent TDM, batch analysis or off-site analysis can be sufficient. After identifying how costs for the TDM service can be reimbursed, a business model can be developed to justify setup and implementation. Taking the above into account, a business model will be clearly unique per site but also per drug and cannot automatically be assumed as positive based on an economic standpoint.
Future studies should focus on generating clinical PK/PD evidence from more appropriately designed TDM studies.12 PK/PD data generated from well-designed TDM studies could allow accurate validation of more PK models and more precise drug exposure estimation and dose recommendation. An important part of PK model validation is related to differences between patient populations. Pharmacokinetic parameters, such as clearance or volume of distribution, may differ among neonates, obese patients, and critically ill patients. This would require a population-specific PK model that includes relevant covariates to allow appropriate model-informed precision dosing.11 When using appropriate population pharmacokinetic models, individualized starting doses can be employed to achieve earlier target attainment.11 This may be highly relevant in light of adequate dosing to prevent early mortality due to sepsis.60 Moreover, TDM of patients with decreasing renal function can be better managed using model-informed precision dosing if covariates are included.
TDM implementation would be facilitated by the development and validation of assays for other drugs using alternative biological matrices, point-of-care assays, and methodologies detecting unbound drug concentrations at the site of action. Microdialysis, a novel methodology measuring drug concentrations in interstitial space fluid, is of interest for TDM in critically ill patients, as it better reflects tissue concentrations.61–63 In a study measuring glucose and lactase with microdialysis, anti-infectives (e.g., vancomycin) could also be determined.64 Full utilization of the technique remains to be determined given the need to compare drug concentrations in the dialysate and at the infection site, which may be less relevant, for example, for urinary tract infections. At the regulatory level, there is a need for legal or policy structures on dose optimization outside the licensed maximum and for the use of dosing software. Further understanding of regulatory policies regarding TDM guidelines is required. Together with such approaches, local and national TDM frameworks should be sought to guide clinicians and increase the uptake of TDM.
Conclusion
To benefit patients, TDM strategies need to be tailored to the intended population. Strategies will likely differ for critically ill patients, where rapid turnaround time must be prioritized; for outpatients that require convenient sampling methods; and for those with limited access to laboratories capable of performing conventional assays, where feasible alternatives must be developed and trailed.
Conflicts of Interest and Source of Funding:
SKH and PR were supported by the National Institutes of Health, grant R01 AI137080. Anne-Grete Märtson was funded by Marie Skłodowska-Curie Actions (grant number 713660-PRONKJEWAIL-H2020-MSCA-COFUND-2015). KCB and SGM were funded by the Danish Ministry of Foreign Affairs (DFC File No. 17-03-KU). SGM received financial support from the EDCTP2 program supported by a European Union project (grant number TMA2016SF-1463-REMODELTZ). Other authors none declared.
References
- 1.de Velde F, Mouton JW, de Winter BCM, et al. Clinical applications of population pharmacokinetic models of antibiotics: challenges and perspectives. Pharmacol Res. 2018; 134:280–288. [DOI] [PubMed] [Google Scholar]
- 2.Abdul-Aziz MH, Alffenaar JWC, Bassetti M, et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: a position paper. Intensive Care Med. 2020; 46:1127–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brüggemann RJM, Alffenaar J-WC, Blijlevens NMA, et al. Clinical relevance of the pharmacokinetic interactions of azole antifungal drugs with other coadministered agents. Clin Infect Dis. 2009; 48:1441–1458. [DOI] [PubMed] [Google Scholar]
- 4.Veringa A, ter Avest M, Span LFR, et al. Voriconazole metabolism is influenced by severe inflammation: a prospective study. J Antimicrob Chemother. 2017; 72:261–267. [DOI] [PubMed] [Google Scholar]
- 5.Bolcato L, Khouri C, Veringa A, et al. Combined impact of inflammation and pharmacogenomic variants on voriconazole trough concentrations: a meta-analysis of individual data. J Clin Med. 2021; 10:2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jamal JA, Economou CJP, Lipman J, et al. Improving antibiotic dosing in special situations in the ICU: burns, renal replacement therapy and extracorporeal membrane oxygenation. Curr Opin Crit Care. 2012; 18:460–471. [DOI] [PubMed] [Google Scholar]
- 7.Jager NGL, Zandvliet AS, Touw DJ, et al. Optimization of anti-infective dosing regimens during online haemodiafiltration. Clin Kidney J. 2017; 10:282–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Personett HA, Larson SL, Frazee EN, et al. Extracorporeal elimination of piperacillin/tazobactam during molecular adsorbent recirculating system therapy. Pharmacotherapy. 2015; 35:e136–e139. [DOI] [PubMed] [Google Scholar]
- 9.Mushatt DM, Mihm LB, Dreisbach AW, et al. Antibiotic dosing in slow extended daily dialysis. Clin Infect Dis. 2009; 49:433–437. [DOI] [PubMed] [Google Scholar]
- 10.Hodiamont CJ, Janssen JM, De Jong MD, et al. Therapeutic drug monitoring of gentamicin peak concentrations in critically ill patients. Ther Drug Monit. 2017; 39:522–530. [DOI] [PubMed] [Google Scholar]
- 11.Wicha SG, Märtson AG, Nielsen EI, et al. From therapeutic drug monitoring to model-informed precision dosing for antibiotics. Clin Pharmacol Ther. 2021; 109:928–941. [DOI] [PubMed] [Google Scholar]
- 12.Märtson AG, Sturkenboom MGG, Stojanova J, et al. How to design a study to evaluate therapeutic drug monitoring in infectious diseases? Clin Microbiol Infect. 2020; 26:1008–1016. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wang T, Zhang D, et al. Therapeutic drug monitoring coupled with bayesian forecasting could prevent vancomycin-associated nephrotoxicity in renal insufficiency patients: a prospective study and pharmacoeconomic analysis. Ther Drug Monit. 2020; 42:600–609. [DOI] [PubMed] [Google Scholar]
- 14.Sturkenboom M, Bolhuis M, Akkerman O, et al. Therapeutic drug monitoring of first-line anti-tuberculosis drugs comprises more than C2H measurements. Int J Tuberc Lung Dis. 2016; 20:1695–1696. [DOI] [PubMed] [Google Scholar]
- 15.Cattaneo D, Baldelli S, Cozzi V, et al. Impact of therapeutic drug monitoring of antiretroviral drugs in routine clinical management of people living with HIV: a narrative review. Ther Drug Monit. 2020; 42:64–74. [DOI] [PubMed] [Google Scholar]
- 16.Bos JC, Van Hest RM, Prins JM. Pharmacokinetics of antibiotics in sub-Saharan African patient populations: a systematic review. Ther Drug Monit. 2017; 39:387–398. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JA, Abdul-Aziz MH, Lipman J, et al. Individualised antibiotic dosing for patients who are critically ill: challenges and potential solutions. Lancet Infect Dis. 2014; 14:498–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rybak MJ, Le J, Lodise TP, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020; 77:835–864. [DOI] [PubMed] [Google Scholar]
- 19.Kantasiripitak W, Van Daele R, Gijsen M, et al. Software tools for model-informed precision dosing: how well do they satisfy the needs? Front Pharmacol. 2020; 11:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kees MG, Wicha SG, Seefeld A, et al. Unbound fraction of vancomycin in intensive care unit patients. J Clin Pharmacol. 2014; 54:318–323. [DOI] [PubMed] [Google Scholar]
- 21.Goldschmidt R, Chu C. HIV infection in adults: initial management. Am Fam Physician. 2021; 103:407–416. [PubMed] [Google Scholar]
- 22.Klis S, Daskapan A, Akkerman OW, et al. Raltegravir and rifampicin in patients with HIV and tuberculosis. Lancet Infect Dis. 2014; 14:1046–1047. [DOI] [PubMed] [Google Scholar]
- 23.Back DJ, Khoo SH, Gibbons SE, et al. The role of therapeutic drug monitoring in treatment of HIV infection. Br J Clin Pharmacol. 2001; 51:301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luong ML, Al-Dabbagh M, Groll AH, et al. Utility of voriconazole therapeutic drug monitoring: a meta-analysis. J Antimicrob Chemother. 2016; 71:1786–1799. [DOI] [PubMed] [Google Scholar]
- 25.Ullmann AJ, Aguado JM, Arikan-Akdagli S, et al. Diagnosis and management of Aspergillus diseases: executive summary of the 2017 ESCMID-ECMM-ERS guideline. Clin Microbiol Infect. 2018; 24:e1–e38. [DOI] [PubMed] [Google Scholar]
- 26.Van Ingen J, Aksamit T, Andrejak C, et al. Treatment outcome definitions in nontuberculous mycobacterial pulmonary disease: an NTM-NET consensus statement. Eur. Respir. J 2018; 51:1800170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alffenaar JW, Märtson AG, Heysell SK, et al. Therapeutic drug monitoring in non-tuberculosis Mycobacteria infections. Clin Pharmacokinet. 2021; 60:711–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamada T, Fujii S, Shigemi A, et al. A meta-analysis of the target trough concentration of gentamicin and amikacin for reducing the risk of nephrotoxicity. J Infect Chemother. 2021; 27:256–261. [DOI] [PubMed] [Google Scholar]
- 29.Quintens C, Steffens E, Jacobs K, et al. Efficacy and safety of a Belgian tertiary care outpatient parenteral antimicrobial therapy (OPAT) program. Infection. 2020; 48:357–366. [DOI] [PubMed] [Google Scholar]
- 30.Van den Elsen SHJ, Sturkenboom MGG, Van’t Boveneind-Vrubleuskaya N, et al. Population pharmacokinetic model and limited sampling strategies for personalized dosing of levofloxacin in tuberculosis patients. Antimicrob Agents Chemother. 2018; 62:e01092–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.The World Bank. Rural population. 2018. [Google Scholar]
- 32.Mabilat C, Gros MF, Nicolau D, et al. Diagnostic and medical needs for therapeutic drug monitoring of antibiotics. Eur J Clin Microbiol Infect Dis. 2020; 39:791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nilson EAF, Metlzer AB, Labonté ME, et al. Modelling the effect of compliance with WHO salt recommendations on cardiovascular disease mortality and costs in Brazil. PLoS One. 2020; 15:e0235514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kagaruki GB, Mayige MT, Ngadaya ES, et al. Magnitude and risk factors of non-communicable diseases among people living with HIV in Tanzania: A cross sectional study from Mbeya and Dar es Salaam regions. BMC Public Health. 2014; 14:904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song WM, Shao Y, Liu JY, et al. Primary drug resistance among tuberculosis patients with diabetes mellitus: a retrospective study among 7223 cases in China. Infect Drug Resist. 2019; 12:2397–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization. Rapid communication: key changes to the treatment of drug-resistant tuberculosis. 2019.
- 37.Bolhuis MS, Akkerman OW, Sturkenboom MGG, et al. Linezolid-based regimens for multidrug-resistant tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis. 2018; 67:S327–S335. [DOI] [PubMed] [Google Scholar]
- 38.Faurholt-Jepsen D, Range N, PrayGod G, et al. Diabetes is a risk factor for pulmonary tuberculosis: A case-control study from Mwanza, Tanzania. PLoS One. 2011; 6:e24215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gile PP, Buljac-Samardzic M, Van De Klundert J. The effect of human resource management on performance in hospitals in sub-Saharan Africa: a systematic literature review. Hum Resour Health. 2018; 16:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geiling J, Burkle FM, Amundson D, et al. Resource-poor settings: infrastructure and capacity building: care of the critically ill and injured during pandemics and disasters: CHEST consensus statement. Chest. 2014; 146:e156S–e167S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alffenaar JWC, Heysell SK, Mpagama SG. Therapeutic drug monitoring: the need for practical guidance. Clin Infect Dis. 2019; 68:1065–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alffenaar JWC, Tiberi S, Verbeeck RK, et al. Therapeutic drug monitoring in tuberculosis: practical application for physicians. Clin Infect Dis. 2017; 64:104–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghimire S, Bolhuis MS, Sturkenboom MGG, et al. Incorporating therapeutic drug monitoring into the World Health Organization hierarchy of tuberculosis diagnostics. Eur Respir J. 2016; 47:1867–1869. [DOI] [PubMed] [Google Scholar]
- 44.Martial LC, Kerkhoff J, Martinez N, et al. Evaluation of dried blood spot sampling for pharmacokinetic research and therapeutic drug monitoring of anti-tuberculosis drugs in children. Int J Antimicrob Agents. 2018; 52:109–113. [DOI] [PubMed] [Google Scholar]
- 45.Vu DH, Alffenaar JWC, Edelbroek PM, et al. Dried blood spots: a new tool for tuberculosis treatment optimization. Curr Pharm Des. 2011; 17:2931–2939. [DOI] [PubMed] [Google Scholar]
- 46.Vu DH, Koster RA, Wessels AMA, et al. Troubleshooting carry-over of LC-MS/MS method for rifampicin, clarithromycin and metabolites in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2013; 917–918:1–4. [DOI] [PubMed] [Google Scholar]
- 47.Vu DH, Bolhuis MS, Koster RA, et al. Dried blood spot analysis for therapeutic drug monitoring of linezolid in patients with multidrug-resistant tuberculosis. Antimicrob Agents Chemother. 2012; 56:5758–5763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vu DH, Koster RA, Alffenaar JWC, et al. Determination of moxifloxacin in dried blood spots using LC-MS/MS and the impact of the hematocrit and blood volume. J Chromatogr B Analyt Technol Biomed Life Sci. 2011; 879:1063–1070. [DOI] [PubMed] [Google Scholar]
- 49.Szipszky C, Van Aartsen D, Criddle S, et al. Determination of rifampin concentrations by urine colorimetry and mobile phone readout for personalized dosing in tuberculosis treatment. J Pediatric Infect Dis Soc. 2021; 10:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Den Elsen SHJ, Oostenbrink LM, Heysell SK, et al. Systematic review of salivary versus blood concentrations of antituberculosis drugs and their potential for salivary therapeutic drug monitoring. Ther Drug Monit. 2018; 40:17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alffenaar JWC, Jongedijk EM, Van Winkel CAJ, et al. A mobile microvolume UV/visible light spectrophotometer for the measurement of levofloxacin in saliva. J Antimicrob Chemother. 2021; 76:423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mohamed S, Mvungi HC, Sariko M, et al. Levofloxacin pharmacokinetics in saliva as measured by a mobile microvolume UV spectrophotometer among people treated for rifampicin-resistant TB in Tanzania. J Antimicrob Chemother. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HY, Ruiter E, Jongedijk EM, et al. Saliva-based linezolid monitoring on a mobile UV spectrophotometer. J Antimicrob Chemother. 2021; 76:1786–1792. [DOI] [PubMed] [Google Scholar]
- 54.Mpagama SG, Ramaiya K, Lillebæk T, et al. Protocol for establishing an Adaptive Diseases control Expert Programme in Tanzania (ADEPT) for integrating care of communicable and non-communicable diseases using tuberculosis and diabetes as a case study. BMJ Open. 2021; 11:e041521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.European Union. European Commission. Study on off-label use of medicinal products in the European Union. 2017.
- 56.Council of Australian Therapeutic Advisory Groups. Rethinking medicines decision-making in Australian hospitals. 2013.
- 57.Australian Government Department of Health: Therapeutic Goods Administration. Consultation: regulation of software, including software as a medical device (SaMD). 2019.
- 58.European Union: European Commission. Regulation (EU) 2017/745 of the European Parliament and of the Council on medical devices. 2017.
- 59.U.S. Food and Drug Administration (FDA). Clinical decision support software. Draft guidance for industry and food and drug administration staff. 2019.
- 60.De Backer D, Dorman T. Surviving sepsis guidelines: A continuous move toward better care of patients with sepsis. JAMA. 2017; 317:807–808. [DOI] [PubMed] [Google Scholar]
- 61.Kiang TKL, Ranamukhaarachchi SA, Ensom MHH. Revolutionizing therapeutic drug monitoring with the use of interstitial fluid and microneedles technology. Pharmaceutics. 2017; 9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rawson TM, O’Hare D, Herrero P, et al. Delivering precision antimicrobial therapy through closed-loop control systems. J Antimicrob Chemother. 2018; 73:835–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deitchman AN, Heinrichs MT, Khaowroongrueng V, et al. Utility of microdialysis in infectious disease drug development and dose optimization. AAPS J. 2017; 19:334–342. [DOI] [PubMed] [Google Scholar]
- 64.van der Mast JE, Nijsten MW, Alffenaar JWC, et al. In vitro evaluation of an intravenous microdialysis catheter for therapeutic drug monitoring of gentamicin and vancomycin. Pharmacol Res Perspect. 2019; 7:e00483. [DOI] [PMC free article] [PubMed] [Google Scholar]


