Abstract
Background:
Body mass index (BMI) change after a lung cancer diagnosis has been associated with non-small cell lung cancer (NSCLC) survival. This study aimed to quantify the association based on a large-scale observational study.
Methods:
Included in the study were 7,547 NSCLC patients with prospectively collected BMI data from Massachusetts General Hospital and Brigham and Women’s Hospital/Dana Faber Cancer Institute. Cox proportional hazards regression with time-dependent covariates was used to estimate effect of time varying post-diagnosis BMI change rate (% per month) on overall survival (OS), stratified by clinical subgroups. Spline analysis was conducted to quantify the non-linear association. A Mendelian Randomization (MR) analysis with a total of 3,495 patients further validated the association.
Results:
There was a J-shape association between post-diagnosis BMI change and OS among NSCLC patients. Specifically, a moderate BMI decrease (0.5-2.0; HR = 2.45, 95% CI = 2.25-2.67) and large BMI decrease (≥ 2.0; HR = 4.65, 95% CI = 4.15-5.20) were strongly associated with worse OS, whereas moderate weight gain (0.5-2.0) reduced the risk for mortality (HR = 0.78, 95% CI = 0.68-0.89) and large weight gain (≥ 2.0) slightly increased the risk of mortality without reaching statistical significance (HR = 1.10, 95% CI = 0.86-1.42). MR analyses supported the potential causal roles of post-diagnosis BMI change in survival.
Conclusions:
This study indicates that BMI change after diagnosis was associated with mortality risk.
Impact:
Our findings, which reinforce the importance of post-diagnosis BMI surveillance, suggesting that weight loss or large weight gain maybe unwarranted.
Keywords: NSCLC, BMI change, overall survival
Introduction
BMI has been found to be associated with lung cancer survival. Being underweight or morbidly obese at the time of diagnosis is associated with worse outcomes, whereas overweight and obese patients had better prognosis.(1-8) Moreover, weight change after a lung cancer diagnosis may impact survival(9-12) by changing patients’ performance status, quality of life, response to treatment as well as other clinical outcomes.(13) Post-diagnosis weight loss is associated with worse survival, as involuntary weight loss may be an indicator of cancer-induced cachexia, contributing to poor survival through progressive depletion of patients’ energy reserves.(14) On the other hand, some studies reported a positive relationship between weight gain after diagnosis and prolonged survival.(15-18)
However, there are some limitations in these association studies. First, most studies only evaluated weight change at a single time point post diagnosis, which may not fully capture the weight change trajectory.(9,11) Secondly, most studies have focused on the amount of weight/BMI change, but not the rate of weight/BMI change which might be more relevant to survival. Thirdly, association results based on observational studies can be biased because of reverse causation and residual confounding and may not have causal interpretations.(19,20) For example, patients with a weight loss may have a higher mortality rate because of illness-induced cachexia.(21,22) To our knowledge, none of these studies have investigated the causal relationship between BMI change and non-small cell lung cancer (NSCLC) survival.
Using electronic medical records (EMRs) data from the Mass General Brigham Healthcare System, we examined systematically how post-diagnosis BMI change impacts overall survival for NSCLC patients. We first examined the effect of the BMI change rate on OS at multiple time points, and then applied Mendelian Randomization (MR) analysis, which used genetic variants associated with exposure of interest as instruments, to validate the potential causal effect.
Materials and Methods
Data source and study population
EMR data with written informed consent obtained are from the Massachusetts General Hospital and Brigham and Women’s Hospital using/Dana Faber Cancer Institute in the Mass General Brigham HealthCare System Research Patient Data Registry. Institutional Review Board of Partners HealthCare (Protocol Number: 1999P004935/PHS) approved this study. Lung cancer patients were identified via a semi-supervised machine algorithm, for which the clinical information (including the histological type) was extracted from structured and unstructured data via natural language processing tools.(23) Patients were included between Dec 1990 and Jan 2018 with histologically and stage confirmed NSCLC to allow for at least 3 years of follow-up. We limited the age range to be between 18 and 90. A total of 12,906 patients were identified with BMI data available in the database.
Data collection
BMI were entered prospectively into EMR system and were extracted from structured data. BMI closest to the diagnosis date served as BMI at-diagnosis within three months; BMI measured at least one month after at-diagnosis BMI measurement was assigned to post-diagnosis BMI, and BMI measured three months to 1 year before diagnosis was used as pre-diagnosis BMI. We grouped repeated post-diagnosis BMI measures into consecutive 1-month time intervals after the measurement of at-diagnosis BMI and selected the BMI measures closest to the end of each month interval. Post-diagnosis BMI change (%) was calculated as: . The monthly rate of BMI change (% per month) was calculated by dividing BMI change by the time difference between post-diagnosis BMI measurement date and at-diagnosis BMI measurement date. Patients with extreme BMI records (BMI below 14 or BMI above 50) or extreme BMI change over a short period (over 20% in one month) were excluded out of data quality concern. BMI at-diagnosis was categorized into six groups: BMI < 18.5 kg/m2 (underweight), 18.5 kg/m2 ≤ BMI < 25 kg/m2 (normal), 25 kg/m2 ≤ BMI < 30 kg/m2 (overweight), 30 kg/m2 ≤ BMI < 35 kg/m2 (obese), 35 kg/m2 ≤ BMI < 40 kg/m2 (severe obese), and BMI ≥ 40 kg/m2 (morbid obese). For patients with multiple post-diagnosis BMI measurements, we calculated the mean rate of BMI change per month for each patient, with positive or negative values indicating average BMI increase or decrease. In this study, we identified 9,327 patients with BMI measured less than three months before or after the initial lung cancer diagnosis and 7,547 of them had at least one post-diagnosis BMI measurement.
Demographic characteristics [i.e., age, sex, race/ethnicity, smoking status (ever-smoker, non-smoker) at the time of diagnosis] and clinical features (i.e., tumor histological type, stage, year of diagnosis, and treatments) were available from EMR. Race/ethnicity was categorized into white and non-white (including Black, Asian, Hispanic). Overall survival, the main outcome, was defined as the time lag between the date of diagnosis and the date of death, which may be censored at the last visit.
Statistical analysis
Cox proportional hazards regression models were used to examine the effect of BMI at diagnosis (both continuous and categorical variable) on OS, adjusting for sex, age at diagnosis, race/ethnicity, and year of diagnosis, smoking history, stage, and histological type. Treatment status was not adjusted given that treatment was given after at-diagnosis BMI measurement. For post-diagnosis BMI change, we used Cox proportional model with the BMI change rate (% per month) included as a time-dependent covariate, adjusting for the aforementioned variables, at-diagnosis BMI, and treatment status. Treating BMI change as a time-dependent variable enables us to study patients who had both a weight gain and weight loss at different times. We further accessed non-linear relationships between BMI change rate and OS using penalized smoothing spline (PSS) curves. We categorized the percent of BMI change per month into five categories: large decrease (≥2.0), moderate decrease (0.5–2.0), stable (decrease/increase < 0.5), moderate increase (0.5–2.0) and large increase (≥2.0). These categories were chosen based on the distribution of average BMI change per month. The 10th percentile of average BMI change was 2.1% (round to 2%), decrease per month and 33rd percentile was 0.48% (round to 0.5%), decrease per month. Furthermore, we considered an interaction between the BMI change and the BMI category at diagnosis to assess the effect of BMI change within the initial BMI categories.
We conducted analyses of post-diagnosis BMI change and OS, stratified by sex, age at diagnosis (< 66 vs. ≥ 66 years[median of study population]), race, stage, histological type, and treatment status. We examined the heterogeneity in associations in stratified analyses via inclusion of cross-product terms of stratified variables and post-diagnosis BMI change in the Cox regression models. We conducted sensitivity analyses by restricting to the population with complete information on pre-diagnosis BMI to test the effect of post-diagnosis BMI change after adjusting pre-diagnosis BMI, and also by excluding patients whose deaths occurred within the first six months to address the possible reverse causality.
MR analysis
DNA was extracted from peripheral white blood cells of a subset of the Boston Lung Cancer Study cohort using standard protocols and was genotyped using the Human610-Quad BeadChip (Illumina, San Diego, CA) and OncoArray platform. Details of sample selection, genotyping, and quality control (including population structure) can be found in our previous studies(24,25) and supplementary materials. A total of 3,495 European ancestry NSCLC patients with complete survival information and genotyping data were retained for genetic association analysis. These patients were part of the MGH study population and can be matched to EMR database. Among them, 1,061 have both at-diagnosis and post-diagnosis BMI measurements available.
We used MR analysis to assess the potential causal relationship between the post-diagnosis BMI change and OS. We partitioned our patients into two subpopulations, with 1,061 patients testing for the association between genetic and exposure (post-diagnosis BMI change) and 2,434 patients for the association between genetic and outcome (NSCLC OS). First, we conducted a post-diagnosis BMI change GWAS containing 1,061 cases via linear regression analysis with adjustments of age at diagnosis, sex, smoking status, stage, tumor type, treatments, and top three principal components (PCs) of population structure; and we then applied the criteria of P < 5 × 10−8 and linkage disequilibrium (LD) of r2 < 0.001 within 10,000 kb to identify independently exposure associated SNPs as genetic instruments. To assess the association between genetic instruments and NSCLC survival, we performed a NSCLC survival GWAS for the 2,434 cases via Cox regression model with adjustments of age at diagnosis, sex, smoking status, stage, tumor type, treatments, and principal components 1-3 of population structure, followed by a fixed meta-analysis via METAL.(26) The causal effect estimates were then calculated via inverse-variance-weighted (IVW) methods.(27) As sensitivity analyses, we used the MR-Egger method and the weighted median method to test for the potential directional pleiotropy as well as outlying variant-specific causal estimates.(28) Additionally, we conducted heterogeneity test and leave one out analysis to evaluate the robustness of our results.(29,30)
Results
Patient and Characteristics
Among 12,906 patients with BMI data available, 7547 of them were included in the analysis, with 3,962 deaths were observed. Characteristics of patients included and excluded in the analysis were summarized in S Table 1. Patients who received treatments were more likely to have BMI collected. Patient characteristics stratified by average BMI increase (3,040) and decrease (4,507) were shown in Table 1. The median number of post-diagnosis BMI measures was 7 (interquartile range [IQR], 11). The median follow up was 2.77 years. Older, male patients were more likely to lose weight than younger, female patients. Patients with adenocarcinoma were more likely to gain weight compared to squamous carcinoma and other NSCLC. Patients diagnosed with later stage (stage 3 or 4) were more likely to lose weight, while stage 1 patients were more likely to gain weight. Consistent with the findings from stage, patients who received surgery were more likely to gain weight than those didn't received surgery, and patients who received chemotherapy or radiation therapy were more likely to lose weight than those didn’t receive chemotherapy or radiation therapy. Underweight and normal-weight patients at diagnosis were more likely to gain weight, while obese patients were more likely to lose weight.
Table 1.
Basic characteristics for patients
| BMI increase | BMI decrease | P | |
|---|---|---|---|
| No. patients | 3040 | 4507 | |
| Age at diagnosis (median [IQR]) | 64.97 [57.33, 71.34] | 66.30 [59.20, 72.71] | <0.001 |
| Sex | 0.005 | ||
| Female | 1727 (56.8) | 2410 (53.5) | |
| Male | 1313 (43.2) | 2097 (46.5) | |
| Race | 0.050 | ||
| White | 2703 (88.9) | 3955 (87.8) | |
| Non-white | 337 (11.1) | 552 (12.2) | |
| Diagnosis year (median [IQR]) | 2011 [2008, 2013] | 2011 [2008, 2013] | 0.060 |
| Smoking | 1.000 | ||
| Ever smokier | 2807 (92.3) | 4157 (92.2) | |
| Non-smoker | 233 (7.7) | 350 (7.8) | |
| Histological type | 0.009 | ||
| Adenocarcinoma | 2277 (74.9) | 3233 (71.7) | |
| Squamous cell | 603 (19.8) | 980 (21.7) | |
| Other NSCLC | 160 (5.3) | 294 (6.5) | |
| Stage | <0.001 | ||
| 1 | 1130 (37.2) | 1292 (28.7) | |
| 2 | 365 (12.0) | 527 (11.7) | |
| 3 | 643 (21.2) | 1114 (24.7) | |
| 4 | 902 (29.7) | 1574 (34.9) | |
| Surgery | 1830 (60.2) | 2458 (54.5) | <0.001 |
| Chemotherapy | 1746 (57.4) | 2771 (61.5) | 0.004 |
| Radiation therapy | 1448 (47.6) | 2545 (56.5) | <0.001 |
| BMI at diagnosis | <0.001 | ||
| Under weight | 133 (4.4) | 94 (2.1) | |
| Normal | 1289 (42.4) | 1547 (34.3) | |
| Overweight | 1035 (34.0) | 1617 (35.9) | |
| Obese | 416 (13.7) | 833 (18.5) | |
| Severe obese | 123 (4.0) | 293 (6.5) | |
| Morbid obese | 44 (1.4) | 123 (2.7) | |
| BMI change (% per month) | <0.001 | ||
| (median [IQR]) | 0.36 [0.14, 0.80] | −0.61 [−1.49, −0.23] |
Effect of BMI on NSCLC OS
At-diagnosis BMI was significantly associated with OS (S Table 2). Compared with normal-weight patients, underweight patients had a higher mortality rate (HR, 1.37; 95% CI, 1.14-1.63), whereas patients who were overweight (HR, 0.90; 95% CI, 0.84-0.97) and obese (HR, 0.87; 95% CI, 0.79-0.95) had a lower risk of death. Patients with server/morbid obese showed no difference compared with normal-weight patients.
As for longitudinal changes in BMI, a decrease in BMI post-diagnosis was significantly associated with a higher risk of death (HR, 2.53; 95% CI, 2.36-2.72; Table 2) than those with increased BMI post-diagnosis. The association between BMI change post-diagnosis and OS appeared to be non-linear (Figure 1a; P nonlinearity< 0.001). Decrease of BMI after diagnosis was strongly associated with worse OS, the risk for mortality increased markedly with increasing weight loss, with HR of 2.45 (95% CI, 2.25-2.67) for moderate BMI decrease and HR of 4.65 (95% CI, 4.15-5.20) for large BMI decrease (Figure 1a and Table 2). Moderate BMI increase reduced the risk for mortality (HR, 0.78; 95% CI, 0.68-0.89); however, large BMI increase led to a trend toward higher risk of mortality, but the association was not significant (HR, 1.10; 95% CI, 0.86-1.42; Figure 1a and Table 2).
Table 2.
Associations between post-diagnosis BMI change (% per month) and NSCLC OS
| BMI change | No. of events | HR | 95% CI | P-value | |
|---|---|---|---|---|---|
| Binary | Increase | 1104 | ref | ||
| Decrease | 2854 | 2.53 | [2.36;2.72] | < 0.001 | |
| Categorical | Stable | ||||
| Within 0.5 | 2038 | Ref | |||
| Decrease | |||||
| Large ≥2.0 | 568 | 4.65 | [4.15;5.20] | < 0.001 | |
| Moderate 0.5–2.0 | 1001 | 2.45 | [2.25;2.67] | < 0.001 | |
| Increase | |||||
| Moderate 0.5–2.0 | 284 | 0.78 | [0.68;0.89] | < 0.001 | |
| Large ≥2.0 | 67 | 1.1 | [0.86;1.42] | 0.439 |
Cox proportional hazards regression models with the BMI change as time-dependent variable adjusted for sex, age at diagnosis, race, year of diagnosis, smoking history, stage, histological type, at-diagnosis BMI, and treatment status.
Figure 1.
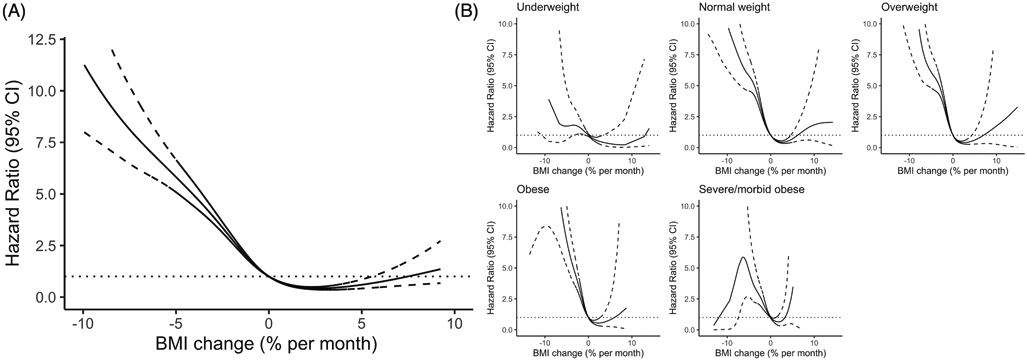
Associations between post-diagnosis BMI change (% per month) and OS based on penalized smoothing spline (A) in 7547 NSCLC patients and (B) stratified by at-diagnosis BMI. The reference value (HR = 1) was set at change equals to 0. Time-dependent Cox proportional model with the change of BMI (% per month) as a time-dependent covariate and adjusted the sex, age at diagnosis, race, year of diagnosis, smoking history, stage, and histological type, at-diagnosis BMI and treatment status.
We tested whether the effect of post-diagnosis BMI change and OS differed by the at-diagnosis BMI status (Figure 1b and S Table 3). The BMI decrease associated risk was more evident in normal weight, overweight and obese patients compared to underweight, severe obese and morbid obese. A moderate increase of BMI reduced risk for mortality for normal weight and overweight patients, but not for underweight, obese patients. Large weight gain was non-significant among all of the BMI strata.
Stratified analysis and sensitivity analysis
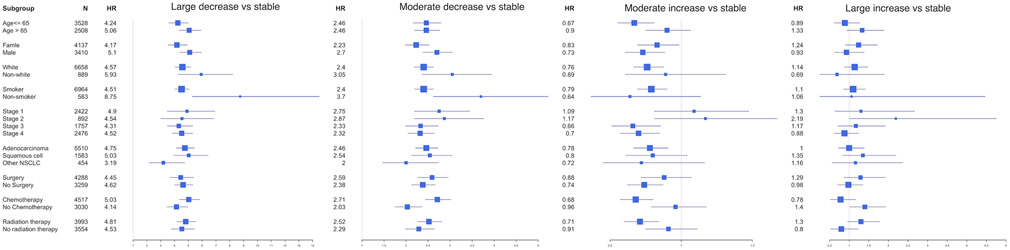
In stratified analyses, a decrease in BMI post-diagnosis was associated with a higher risk of death compared with stable BMI among all subsets of the patients, whereas the effects of BMI increase were more heterogeneous by sex, stage, and treatments (Figure 2). The elevated risks for patients who had a BMI decrease post diagnosis were more evident among patients who were male, and who received chemotherapy (P < 0.001 for interactions). A moderate increase of BMI was associated with better OS among patients with late-stage and who received chemotherapy or radiation therapy but was not significant among those with early-stage and did not receive chemotherapy or radiation therapy. Early-stage patients who gained weight post diagnosis had a trend toward a higher risk of mortality. The results of stratified analysis by treatment are consistent with stage, as early-stage patients usually received surgery and late-stage patients are more likely to receive chemotherapy and radiation therapy. Sensitivity analyses by excluding early deaths (within six months) presented negligible changes in associations for post-diagnosis BMI change and mortality (S Table 4). After adjusting pre-diagnosis BMI, the elevated risks for patients who had a BMI decrease post diagnosis were more evident (S Table 4).
Figure 2.
Associations between post-diagnosis BMI change and OS in subsets of patients. (A) BMI decrease vs. stable (B) BMI increase vs. stable The squares represent the size of each subgroup and are centered on the HR. The whiskers represent the 95% CIs.
MR analysis
Among 3,495 patients used for MR analysis, 2474 death were observed. We observed a significant effect of post-diagnosis BMI change on NSCLC OS (beta = −0.162, P < 0.001; Figure 3) via methods of IVW using 3 genetic instruments. No substantial directional pleiotropy was detected using the MR-Egger test (MR-Egger intercept 0.196, P > 0.05). We did not observe outlying genetic variants from weighted median method results (P = 0.03) as well as the leave-one-out analysis. We did not find any significant heterogeneity when comparing the effect sizes of IVs on exposure to the effect sizes of IVs on NSCLC survival (Pheterogeneity > 0.05). The direction of the MR estimates shows that a decrease in BMI increases the risk of mortality, which is consistent with the observation study.
Figure 3.
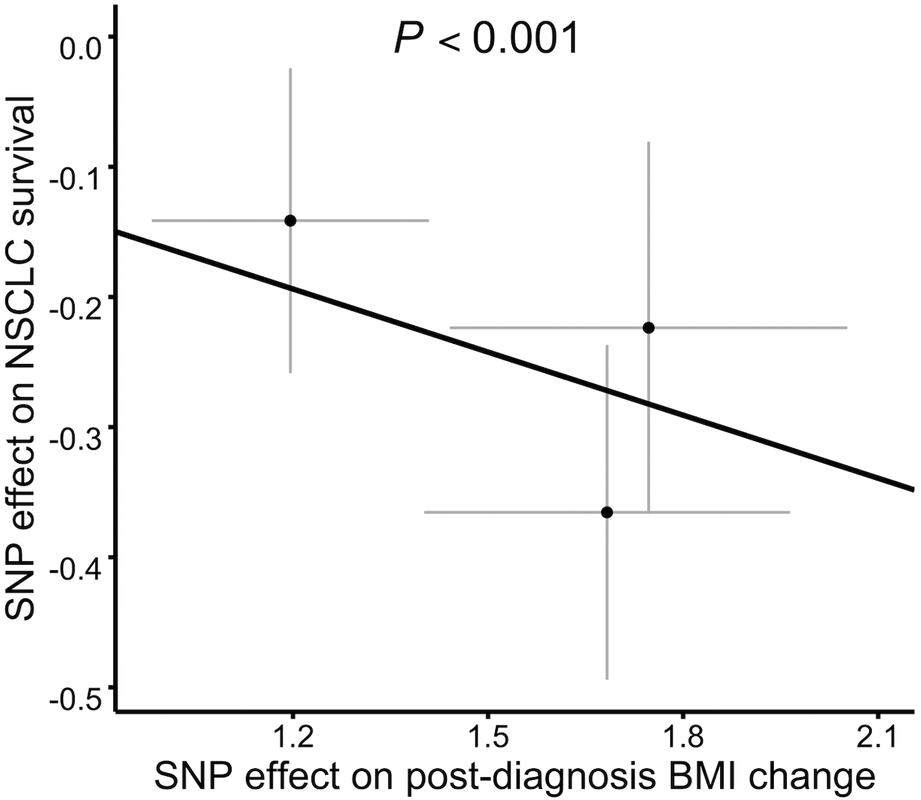
SNP-exposure (post-diagnosis BMI change) and SNP-outcome (NSCLC survival) coefficients used in the MR analysis with 3 SNPs as instrument variables. The line represents the inverse-variance-weighted estimate.
Discussion
Using large-scale data from EMR of 7,547 NSCLC patients, we found a J-shaped relationship between post-diagnosis BMI change and mortality. Specifically, a decrease of BMI post diagnosis was associated with an increased risk of mortality, a moderate increase of BMI was associated with better survival. Similar to our findings, previous studies have generally shown that weight loss is associated with poorer lung cancer outcomes.(9,11,12) Weight loss has been considered as a part of the eligibility criteria for some NSCLC clinical trials where patients with weight loss above a certain threshold (<10%) are excluded.(31) To our knowledge, this study is the rst to further explore results by subgroups of patients. We found the negative effect of weight loss is consistent across all subsets but is more evident among male and patients who received chemotherapy. The positive effect of weight gain on lung cancer outcomes has also been reported in a few previous studies. Two studies evaluated the prognostic significance of weight gain during the course of treatments in 54 and 92 patients.(15,16) Patel et al.’s study analyzed 421 advanced non-squamous NSCLC patients with weight gain during treatment.(18) However, they had a limited sample size and mainly focus on late-stage patients. Our study found that a moderate weight gain was associated with improved OS, but large weight gain may pose an increased risk, especially for early-stage patients. When stratified by at-diagnosis BMI, we found that moderate weight gain did not reduce the risk for mortality for obese patients. Our results may suggest that early-stage or obese patients should not gain an excessive amount of weight post diagnosis.
Gaining weight was not associated with reduced survival among early-stage patients, though we did see a positive association in late-stage patients. One possible reason is that part of the early-stage patients had a chance to recover after surgery and gaining weight or being obese after recovery may impose a negative effect on OS, which has been reported in other cancers.(32,33) The effect of weight gain might be different for cancer patients and cancer survivors, and thus lead to conflicting estimates among early stage patients. Future studies are needed to determine whether weight gaining leads to worse OS among survivors of lung cancer who have had curative treatment. For late-stage patients, an increase or decrease of BMI may be mainly affected by tumor status and treatment-related side effects. Losing weight post diagnosis may be an indicator of tumor progression or treatment toxicity that increased the risk of overall mortality.(14)
We validated further the relationship between post-diagnosis BMI change and OS using causal methods. In MR analysis, we found the potential causal effect of post-diagnosis BMI change on OS were similar to what we found in association analysis with multiple genetic variants as instrument variables that are less biased by reverse causation or unmeasured confounding. In sensitivity analysis, we eliminated early death, which may also indicate that our results were not attributable to reverse causality especially cancer-induced cachexia and sarcopenia. We conducted a sensitivity analysis that adjusted pre-diagnosis BMI, which has been shown to be a strong predictor of lung cancer survival.(34)
One strength of this study is the large sample size with multiple prospectively collected post-diagnosis BMI measurements, enabling us to access the impact of longitudinal BMI changes on survival. Other strengths include having data on germline genetics, pre-diagnosis BMI measurements and treatment.
Our study also has several limitations. First, there may be selection bias as patients included in this study were selected based on the BMI availability in the EMR database. For example, BMI data were more likely to be available for patients who received treatments, because it was often collected before treatment to help determine the overall health status. Thus, the selection bias can arise where treatments influence whether an individual to be a study participant and also the survival outcome. Second, the instrument variable approach may address confounding issue partially, but residual confounding may still exist. For example, the possibility of residual confounding by smoking intensity and durations cannot be ruled out, as smoking was only captured categorically as never and ever. Second, with a small number of underweight and morbidly obese patients, the estimates from these groups might not be precise and need to be interpreted with caution. Third, the sample size for MR analysis was limited, which might cause unstable effect estimates. More powerful genetic instruments may be identified to validate the MR results as GWAS become larger. Finally, the long period of accrual with the heterogeneity of treatments may introduce variance of the effects.
Conclusions
Using observational data from the EMR database with available genetics data, this study supports a possible role of BMI change in lung cancer prognosis. Our results reinforce the importance of postdiagnosis BMI change surveillance, which inform dietary and exercise recommendations for NSCLC patients.
Supplementary Material
Funding
Research reported in this publication was supported by National Cancer Institute of the National Institutes of Health under award number U01CA209414 to David C. Christiani
Footnotes
Conflicts of Interest
The authors declare no potential conflicts of interest
Reference
- 1.Dahlberg SE, Schiller JH, Bonomi PB, Sandler AB, Brahmer JR, Ramalingam SS, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol 2013;8(9):1121–7 doi 10.1097/JTO.0b013e31829cf942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, et al. Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer 2016;102:49–59 doi 10.1016/j.lungcan.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Kimura M, Naito T, Kenmotsu H, Taira T, Wakuda K, Oyakawa T, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer 2015;23(6):1699–708 doi 10.1007/s00520-014-2534-3. [DOI] [PubMed] [Google Scholar]
- 4.Lam VK, Bentzen SM, Mohindra P, Nichols EM, Bhooshan N, Vyfhuis M, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer 2017;104:52–7 doi 10.1016/j.lungcan.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa T, Toyazaki T, Chiba N, Ueda Y, Gotoh M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg 2016;23(4):560–6 doi 10.1093/icvts/ivw175. [DOI] [PubMed] [Google Scholar]
- 6.Shepshelovich D, Xu W, Lu L, Fares A, Yang P, Christiani D, et al. Body Mass Index (BMI), BMI Change, and Overall Survival in Patients With SCLC and NSCLC: A Pooled Analysis of the International Lung Cancer Consortium. J Thorac Oncol 2019;14(9):1594–607 doi 10.1016/j.jtho.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsuoka K, Yamada T, Matsuoka T, Nagai S, Ueda M, Miyamoto Y. Significance of Body Mass Index for Postoperative Outcomes after Lung Cancer Surgery in Elderly Patients. World J Surg 2018;42(1):153–60 doi 10.1007/s00268-017-4142-0. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Liu Y, Shao H, Zheng X. Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J Thorac Oncol 2017;12(10):1478–88 doi 10.1016/j.jtho.2017.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Mytelka DS, Li L, Benoit K. Post-diagnosis weight loss as a prognostic factor in non-small cell lung cancer. J Cachexia Sarcopenia Muscle 2018;9(1):86–92 doi 10.1002/jcsm.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fiorelli A, Vicidomini G, Mazzella A, Messina G, Milione R, Di Crescenzo VG, et al. The influence of body mass index and weight loss on outcome of elderly patients undergoing lung cancer resection. The Thoracic and cardiovascular surgeon 2014;62(07):578–87. [DOI] [PubMed] [Google Scholar]
- 11.Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol 2015;33(1):90–9 doi 10.1200/JCO.2014.56.1894. [DOI] [PubMed] [Google Scholar]
- 12.Le-Rademacher J, Lopez C, Wolfe E, Foster NR, Mandrekar SJ, Wang X, et al. Weight loss over time and survival: a landmark analysis of 1000+ prospectively treated and monitored lung cancer patients. J Cachexia Sarcopenia Muscle 2020;11(6):1501–8 doi 10.1002/jcsm.12625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonomi P, Batus M, Fidler MJ, Borgia JA. Practical and theoretical implications of weight gain in advanced non-small cell lung cancer patients. Ann Transl Med 2017;5(6):152 doi 10.21037/atm.2017.03.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aoyagi T, Terracina KP, Raza A, Matsubara H, Takabe K. Cancer cachexia, mechanism and treatment. World J Gastrointest Oncol 2015;7(4):17–29 doi 10.4251/wjgo.v7.i4.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gielda BT, Mehta P, Khan A, Marsh JC, Zusag TW, Warren WH, et al. Weight gain in advanced non-small-cell lung cancer patients during treatment with split-course concurrent chemoradiotherapy is associated with superior survival. Int J Radiat Oncol Biol Phys 2011;81(4):985–91 doi 10.1016/j.ijrobp.2010.06.059. [DOI] [PubMed] [Google Scholar]
- 16.Sher DJ, Gielda BT, Liptay MJ, Warren WH, Batus M, Fidler MJ, et al. Prognostic significance of weight gain during definitive chemoradiotherapy for locally advanced non-small-cell lung cancer. Clin Lung Cancer 2013;14(4):370–5 doi 10.1016/j.cllc.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 17.Topkan E Weight gain as a surrogate marker of longer survival in advanced non-small cell lung cancer patients. Ann Transl Med 2016;4(19):381 doi 10.21037/atm.2016.09.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel JD, Pereira JR, Chen J, Liu J, Guba SC, John WJ, et al. Relationship between efficacy outcomes and weight gain during treatment of advanced, non-squamous, non-small-cell lung cancer patients. Ann Oncol 2016;27(8):1612–9 doi 10.1093/annonc/mdw211. [DOI] [PubMed] [Google Scholar]
- 19.Hernán MA, Robins JM. Causal inference. CRC Boca Raton, FL;; 2010. [Google Scholar]
- 20.Kroenke CH, Neugebauer R, Meyerhardt J, Prado CM, Weltzien E, Kwan ML, et al. Analysis of Body Mass Index and Mortality in Patients With Colorectal Cancer Using Causal Diagrams. JAMA Oncol 2016;2(9):1137–45 doi 10.1001/jamaoncol.2016.0732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flegal KM, Graubard BI, Williamson DF, Cooper RS. Reverse causation and illness-related weight loss in observational studies of body weight and mortality. Am J Epidemiol 2011;173(1):1–9 doi 10.1093/aje/kwq341. [DOI] [PubMed] [Google Scholar]
- 22.Banack HR, Kaufman JS. The obesity paradox: understanding the effect of obesity on mortality among individuals with cardiovascular disease. Prev Med 2014;62:96–102 doi 10.1016/j.ypmed.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Yuan Q, Cai T, Hong C, Du M, Johnson BE, Lanuti M, et al. Performance of a Machine Learning Algorithm Using Electronic Health Record Data to Identify and Estimate Survival in a Longitudinal Cohort of Patients With Lung Cancer. JAMA Netw Open 2021;4(7):e2114723 doi 10.1001/jamanetworkopen.2021.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKay JD, Hung RJ, Han Y, Zong X, Carreras-Torres R, Christiani DC, et al. Large-scale association analysis identifies new lung cancer susceptibility loci and heterogeneity in genetic susceptibility across histological subtypes. Nat Genet 2017;49(7):1126–32 doi 10.1038/ng.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, Wei Y, Zhang R, Su L, Gogarten SM, Liu G, et al. Multi-Omics Analysis Reveals a HIF Network and Hub Gene EPAS1 Associated with Lung Adenocarcinoma. EBioMedicine 2018;32:93–101 doi 10.1016/j.ebiom.2018.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics 2010;26(17):2190–1 doi 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7 doi 10.7554/eLife.34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent Estimation in Mendelian Randomization with Some Invalid Instruments Using a Weighted Median Estimator. Genet Epidemiol 2016;40(4):304–14 doi 10.1002/gepi.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bowden J, Del Greco MF, Minelli C, Zhao Q, Lawlor DA, Sheehan NA, et al. Improving the accuracy of two-sample summary-data Mendelian randomization: moving beyond the NOME assumption. Int J Epidemiol 2019;48(3):728–42 doi 10.1093/ije/dyy258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol 2015;44(2):512–25 doi 10.1093/ije/dyv080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia S, Bisen A, Yan J, Xie XJ, Ramalingam S, Schiller JH, et al. Thoracic Oncology Clinical trial Eligibility Criteria and Requirements Continue to Increase in Number and Complexity. J Thorac Oncol 2017;12(10):1489–95 doi 10.1016/j.jtho.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caan BJ, Kwan ML, Shu XO, Pierce JP, Patterson RE, Nechuta SJ, et al. Weight change and survival after breast cancer in the after breast cancer pooling project. Cancer Epidemiol Biomarkers Prev 2012;21(8):1260–71 doi 10.1158/1055-9965.EPI-12-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Troeschel AN, Hartman TJ, Jacobs EJ, Stevens VL, Gansler T, Flanders WD, et al. Postdiagnosis Body Mass Index, Weight Change, and Mortality From Prostate Cancer, Cardiovascular Disease, and All Causes Among Survivors of Nonmetastatic Prostate Cancer. J Clin Oncol 2020;38(18):2018–27 doi 10.1200/JCO.19.02185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morel H, Raynard B, d'Arlhac M, Hauss PA, Lecuyer E, Oliviero G, et al. Prediagnosis weight loss, a stronger factor than BMI, to predict survival in patients with lung cancer. Lung Cancer 2018;126:55–63 doi 10.1016/j.lungcan.2018.07.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.