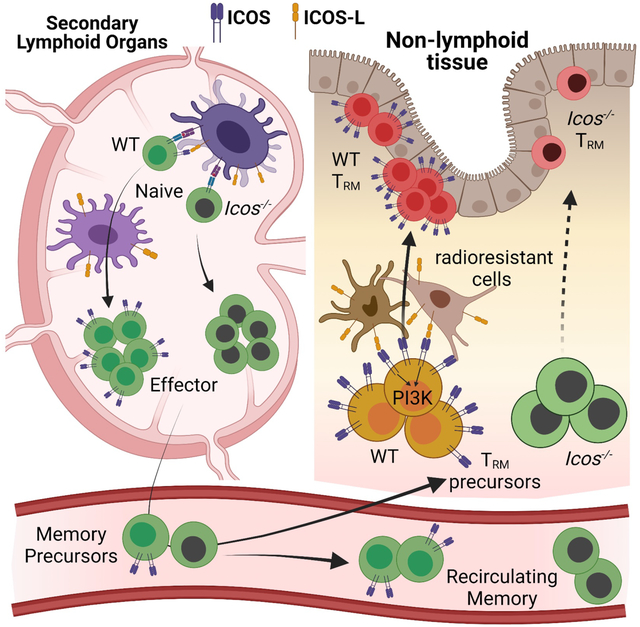
Summary
Elevated gene expression of the costimulatory receptor Icos is a hallmark of CD8+ tissue-resident memory (Trm) T cells. Here we examined the contribution of ICOS in Trm cell differentiation. Upon transfer into WT mice, Icos−/− CD8+ T cells exhibited defective Trm generation but produced recirculating memory populations normally. ICOS-deficiency or ICOS-L blockade compromised establishment of CD8+ Trm cells but not their maintenance. ICOS ligation during CD8+ T cell priming did not determine Trm induction; rather effector CD8+ T cells showed reduced Trm differentiation after seeding into Icosl−/− mice. IcosYF/YF CD8+ T cells were compromised in Trm generation, indicating a critical role for PI3K signaling. Modest transcriptional changes in the few Icos−/− Trm cells suggest that ICOS-PI3K signaling primarily enhances the efficiency of CD8+ T cell tissue residency. Thus, local ICOS signaling promotes production of Trm cells, providing insight into the contribution of costimulatory signals in the generation of tissue-resident populations.
Keywords: ICOS, resident memory T cells, PI3K
Graphical Abstract
eTOC blurb
Elevated gene expression of the costimulatory receptor Icos is a hallmark of CD8+ tissue-resident memory (Trm) T cells. Peng et al. examine the contribution of ICOS to Trm cell differentiation and find that local ICOS signaling is required for efficient induction of Trm cells, involving encounter with ICOS-ligand during settling of cells in non-lymphoid tissue sites.
Introduction
The T cell memory population is comprised of distinct cell subsets with characteristic patterns of trafficking, function and longevity (Jameson and Masopust, 2018). Based on their trafficking properties, memory T cells can be divided into a recirculating pool (including central and effector memory cells - Tcm and Tem respectively), which traffics through the blood and lymphoid tissues, and a tissue-resident population that is prominent in nonlymphoid tissues and, during normal homeostasis, does not exchange with the circulating population (Masopust and Soerens, 2019; Mueller and Mackay, 2016; Szabo et al., 2019). Since CD8+ tissue-resident memory T cells (Trm) mediate rapid barrier protective functions against infections and contribute to tumor control (Ariotti et al., 2014; Malik et al., 2017; Park et al., 2019; Schenkel et al., 2013), the factors that regulate their differentiation and maintenance are of considerable interest. Specific cytokines and transcription factors have been shown to preferentially promote Trm generation (Mackay et al., 2015, 2016; Milner et al., 2017; Skon et al., 2013), but whether cell-cell interactions occurring within tissues dictate the differentiation of tissue-resident populations is unknown.
Costimulatory molecules such as CD28 are expressed on naïve T cells and serve to enhance and diversify signals induced by initial TCR stimulation. Other costimulatory molecules such as ICOS and 4–1BB are induced by T cell activation and can shape subsequent T cell differentiation and survival (Attanasio and Wherry, 2016; Chen and Flies, 2013; Esensten et al., 2016). Therefore, we considered whether a costimulatory signal might be selectively required for differentiation of Trm. The costimulatory molecules CD40L, OX40, GITR and 4–1BB contribute preferentially to generation of effector-phase and memory CD8+ T cells in non-lymphoid sites including the small intestine (Lefrançois et al., 1999; Pope et al., 2001) and lung (Chu et al., 2020, 2019; Salek-Ardakani et al., 2011; Zhou et al., 2017, 2019), yet deficiency and/or blockade of these factors also limits generation of “conventional” memory cells in lymphoid tissues, suggesting these factors have broad roles in directing memory CD8+ T cell differentiation (Chu et al., 2019; Lefrançois et al., 1999; Salek-Ardakani et al., 2011; Zhou et al., 2019). Furthermore, the distinct kinetics in the generation and prominence of tissue-resident versus recirculating memory populations complicates interpretation of whether these costimulatory signals are preferentially involved in production of Trm, and whether these interactions occur locally during recruitment of cells to non-lymphoid tissues. Also, some costimulatory molecules function by promoting CD4+ T cells that “help” the CD8+ T cell response, rather than playing a CD8+ T cell-intrinsic role (as may apply to the role of CD40-CD40L interactions during acute LCMV infection, for example) (Durlanik et al., 2016). Hence, it is currently unclear whether any costimulatory molecules expressed by CD8+ T cells are selectively involved in driving the differentiation of CD8+ Trm and the spatiotemporal dynamics of these co-stimulatory interactions.
The inducible costimulatory molecule ICOS has a well-defined role in CD4+ T cell differentiation, especially with regard to its ability to promote generation of follicular helper cells (Tfh) (Crotty, 2019; Nurieva et al., 2003). The upregulation of ICOS occurs rapidly after initial T cell priming, such that ICOS expressed on antigen-specific activated CD4+ T cells interacts with ICOS ligand (ICOS-L, also known as B7h) expressed on B cells or dendritic cells (DC) and induces Tfh differentiation in a pathway that requires signals through both PI3K and TBK1 and inhibition of the transcription factors FOXO1 and KLF2 (Lee et al., 2015; Pedros et al., 2016; Stone et al., 2015; Weber et al., 2015; Xu et al., 2013). ICOS also contributes to survival and localization of CD4+ regulatory T cells (Treg) (Mittelsteadt et al., 2021; Smigiel et al., 2014) and differentiation and maintenance of CD4+ Tem and Tcm (Burmeister et al., 2008; Marriott et al., 2015; Moore et al., 2011a). In addition to T cells, ICOS is expressed on type 2 innate lymphoid cells (ILC2) and is essential for their survival and function (Maazi et al., 2015)
The role of ICOS in CD8+ T cell differentiation is less clear. Interestingly, elevated Icos gene expression is a hallmark of CD8+ Trm relative to Tem and Tcm in both mouse and human studies (Kumar et al., 2017; Milner et al., 2017). Furthermore, forced ICOS expression promotes preferential homing of effector phase CD8+ T cells to non-lymphoid tissues (Liu et al., 2016). On the other hand, studies in mice (using Icos/Icosl deficient mice or antibody blockade) and in ICOS-deficient patients suggest reduced frequencies of CD8+ effector and/or memory cells in the circulation and lymphoid tissues (Bertram et al., 2002; Takahashi et al., 2009), while no defects were reported for CD8+ T cells in the small intestine intraepithelial (IEL) and lamina propria (LP) at effector timepoints (Mittrücker et al., 2002). Interpretation of these studies are complicated, however, by the potential contribution of ICOS expression by other cell types, such as CD4+ T cells and ILCs.
Here we examined the cell-intrinsic role of ICOS in CD8+ T cell memory, specifically its function in promoting recirculating versus resident memory. Icos−/− CD8+ T cells failed to generate a Trm population as efficiently as WT cells, but circulating Tcm or Tem populations were exempt. PI3K signaling through ICOS was essential for Trm generation, in keeping with the finding that KLF2 downregulation was compromised in Icos−/− CD8+ T cells auditioning for tissue-residency. We also provide evidence that the key phase of ICOS/ICOS-L interactions occurs following recruitment to non-lymphoid tissues (NLTs), not during activation in lymphoid sites or during Trm homeostasis. Expression of ICOS-L by radioresistant cells rather than radiosensitive populations in NLTs provided optimal ICOS stimulation. Thus, the ICOS-ICOSL axis helps to determine the choice between resident and recirculating memory CD8+ T cell generation.
Results
Icos deficient CD8+ T cells exhibit impaired generation of Trm but not recirculating populations
To evaluate the role of ICOS in generating CD8+ memory cells after acute infection, we co-adoptively transferred equal number of wild-type (WT) and Icos−/− TCR transgenic P14 CD8+ T cells into mice followed by lymphocytic choriomeningitis virus Armstrong strain (LCMV-Arm) infection. At memory time points after infection (>40 days), we observed that Icos−/− P14 T cells were present at similar (or slightly greater) frequencies relative to WT P14 T cells in the peripheral blood and lymphoid tissues (spleen and lymph nodes) (Figure 1A). We further examined different subsets of these recirculating memory cells in the spleen and found that the ratio of Icos−/− to WT P14 T cells was similar in recirculating Tcm, Tem and long-lived effector (LLEC) populations (Figure 1B). In contrast to these findings, we observed a significant defect (~3-10-fold disadvantage) among Icos−/− P14 T cells in all nonlymphoid tissues (NLTs) studied, including small intestine intraepithelial lymphocytes (SI-IEL), small intestine lamina propria (SI-LP), kidney and salivary gland (SG), when gating on cells in the parenchyma (defined by lack of intravascular labeling; “iv-“) (Figure 1C). Despite the substantial disadvantage in their representation, Icos−/− P14 T cells acquired a typical Trm phenotype in most NLTs, indicated by similar expression of CD69 and CD103 compared to WT P14 T cells in the same site (Figure 1D). An exception was the kidney, in which we noticed a significant reduction of the CD69+ Trm population among iv- Icos−/− P14 T cells. Icos−/− P14 T cells showed minimal defect in the vascular-associated (“iv+”) circulating memory population identified in NLTs, in contrast to the resident population in the parenchyma from the same tissues (Figure 1C). These findings echoed the elevated ICOS transcript levels detected in CD8+ T cells from non-lymphoid tissues compared to the spleen (Figure S1A) (Kurd et al., 2020).
Figure 1. ICOS is critical for generation of resident but not circulating memory CD8+ T cells.
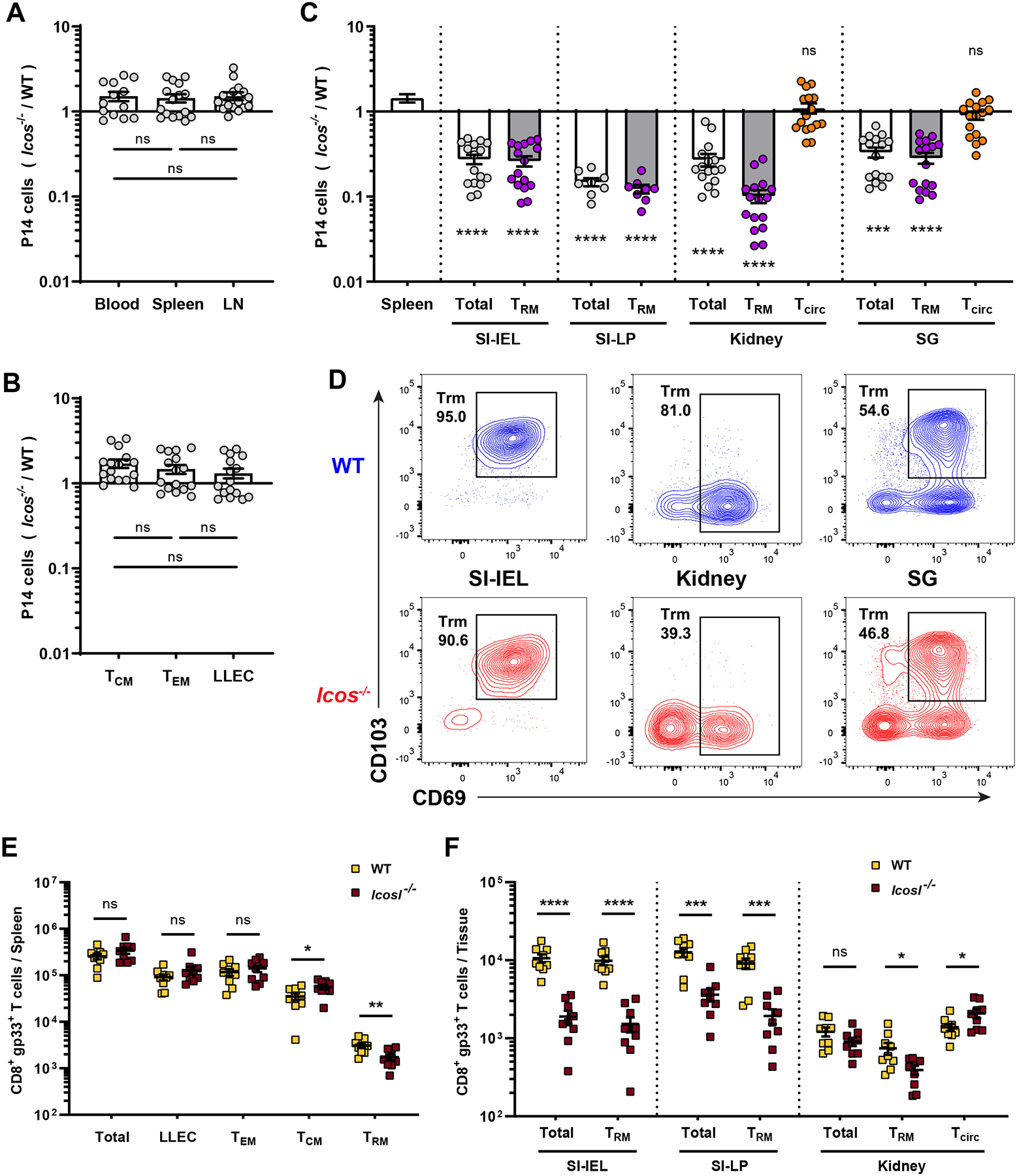
(A–D) Equal numbers of congenically distinct WT and Icos−/− P14 T cells were co-transferred into WT recipient mice, followed by LCMV-Arm infection. At least 40 days later, cells were isolated and the compiled relative frequencies of donor WT and Icos−/− P14 T cells from indicated tissues were determined. (A) shows the ratio among cells in blood and lymphoid tissues, while (B) distinct splenic circulating memory subsets, defined as Tcm (CD62L+), Tem (CD62L− KLRG1− CD127Hi) and LLEC (CD62L− KLRG1+ CD127Lo). In (C),Trm defined as CD8αiv−CD69+CD103+ (kidney Trm as CD8αiv−CD69+) while vascular contaminants (“Tcirc”) were defined as CD8αiv+CD69−. (D) shows representative phenotypic analysis for the indicated tissues. (E–F) WT and Icosl−/− mice were infect with LCMV-Arm. At least 35 days post infection, the indicated tissues were analyzed for the number of gp33/Db-specific CD8+ T cells. Data are from 2–5 independent experiments with a total 8–16 mice. Error bars represent mean ± SEM. Statistical significance was calculated with: One-way ANOVA with multiple comparisons in (A and B), Kruskal-Wallis test with multiple comparisons relative to spleen in (C), and unpaired t-test in (E and F): ns, not significant (p>0.05); * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Trm population can also be generated in secondary lymphoid organs (SLO) (Schenkel et al., 2014). Consistent with our data on NLT Trm, we found Icos−/− P14 T cells were underrepresented among SLO-Trm population in the spleen (Figure S1B). It was possible that the observed role for ICOS in generating CD8+ Trm was specific to the LCMV model – to test this, we evaluated the response to recombinant Listeria monocytogenes (LM-gp33) and influenza (PR8-gp33), using similar approaches, and again found a profound defect of Icos−/− P14 Trm while circulating P14 T cells were minimally affected (Figure S1C, D). These data suggest a critical role for ICOS in CD8+ Trm (but not recirculating memory) formation in distinct infection models.
To exclude the possibility that the P14 TCR transgenic system was especially dependent on ICOS, we generated mixed bone marrow chimeras from non-TCR-transgenic WT and Icos−/− donors, comparing the responses of polyclonal antigen-specific CD8+ T cells. LCMV-primed polyclonal Icos−/− gp33/Db-specific CD8+ T cells showed minimal defects (or a slight advantage) in circulating memory populations in the lymphoid sites (Figure S1E) but were significantly under-represented among Trm from diverse NLTs (Figure S1F). The mild advantage of Icos−/− gp33/Db-specific CD8+ T cells in lymphoid tissues (especially Tcm subsets: Fig. S1E) matched with previous reports on Icos−/− CD4+ T cells (Moore et al., 2011b). However, the defective generation of Icos−/− Trm has not, to our knowledge, been reported previously.
It was also possible that the role for ICOS/ICOS-L interactions was exaggerated by placing Icos−/− and WT CD8+ T cells in competition with each other. As a complementary approach, we examined the CD8+ T cell response to LCMV in mice lacking ICOS-L, the binding partner for ICOS. Compared to WT controls, the numbers of gp33/Db-specific CD8+ Trm were significantly reduced in both lymphoid and non-lymphoid sites, but similar numbers (or slightly more in Tcm) were observed in the recirculating populations in lymphoid tissues (Figure 1E, F). While these results could be influenced by dysregulated ICOS/ICOS-L interactions between other cell populations, these findings illustrate that the requirement for ICOS stimulation to generate CD8+ Trm can be observed even in the absence of competition between WT and Icos−/− CD8+ T cells.
In summary, we found that Icos−/− CD8+ T cells showed no defects (and, in some sites, a slight advantage) in generating recirculating memory populations while ICOS deficiency significantly and substantially compromised the establishment of CD8+ Trm cells in diverse non-lymphoid tissues, using various models of acute infection.
Icos−/− CD8+ T cells showed impaired initial establishment of tissue residency
Trm cells are thought to be derived from memory precursors that seed NLTs soon after priming in lymphoid organs (Kurd et al., 2020; Masopust et al., 2010). To identify the time point at which the defect of Icos−/− CD8+ T cells was detectable in NLTs and to define the role of ICOS in CD8+ Trm development, we tracked co-transferred WT and Icos−/− P14 T cells from the early effector phase until memory phase following LCMV-Arm infection. Minimal defects were observed for Icos−/− P14 T cells in secondary lymphoid organs (SLOs) at early effector (D4~D5), late contraction (D14) or memory (D40+) phases (Figure S2A). This data suggested that ICOS deficiency does not affect the clonal expansion and contraction of CD8+ T cells in SLOs.
Icos−/− and WT P14 T cells showed similar initial access to NLTs at the early effector phase (D4~D5), but Icos−/− P14 T cells showed severe impairment at subsequent times, correlating with their numerical disadvantage in the Trm population (Figure 2A, B). Upregulation of CD69 and CD103 are key features for Trm generation in IEL (Casey et al., 2012; Masopust et al., 2006), and ICOS-deficiency resulted in a substantial loss of the CD69hiCD103hi population as early as D7 post infection (Figure 2A, B). As a result, subdividing IEL P14 T cells based on their CD69 and CD103 expression at day 7~8 post infection revealed a more substantial defect in Icos−/− CD69hiCD103hi/lo Trm-phenotype cells, whereas ‘immature’ CD69loCD103hi/lo precursors were equally proportioned between WT and Icos−/− cells (Figure 2C). By later time points, such as D14 and D40, Icos−/− and WT P14 T cells exhibited similar Trm phenotypes in the SI-IEL, but by that stage the Icos−/− population was at a substantial numerical disadvantage (Fig. 2B).
Figure 2. ICOS signaling affects initial establishment of CD8+ T-cell tissue residency.
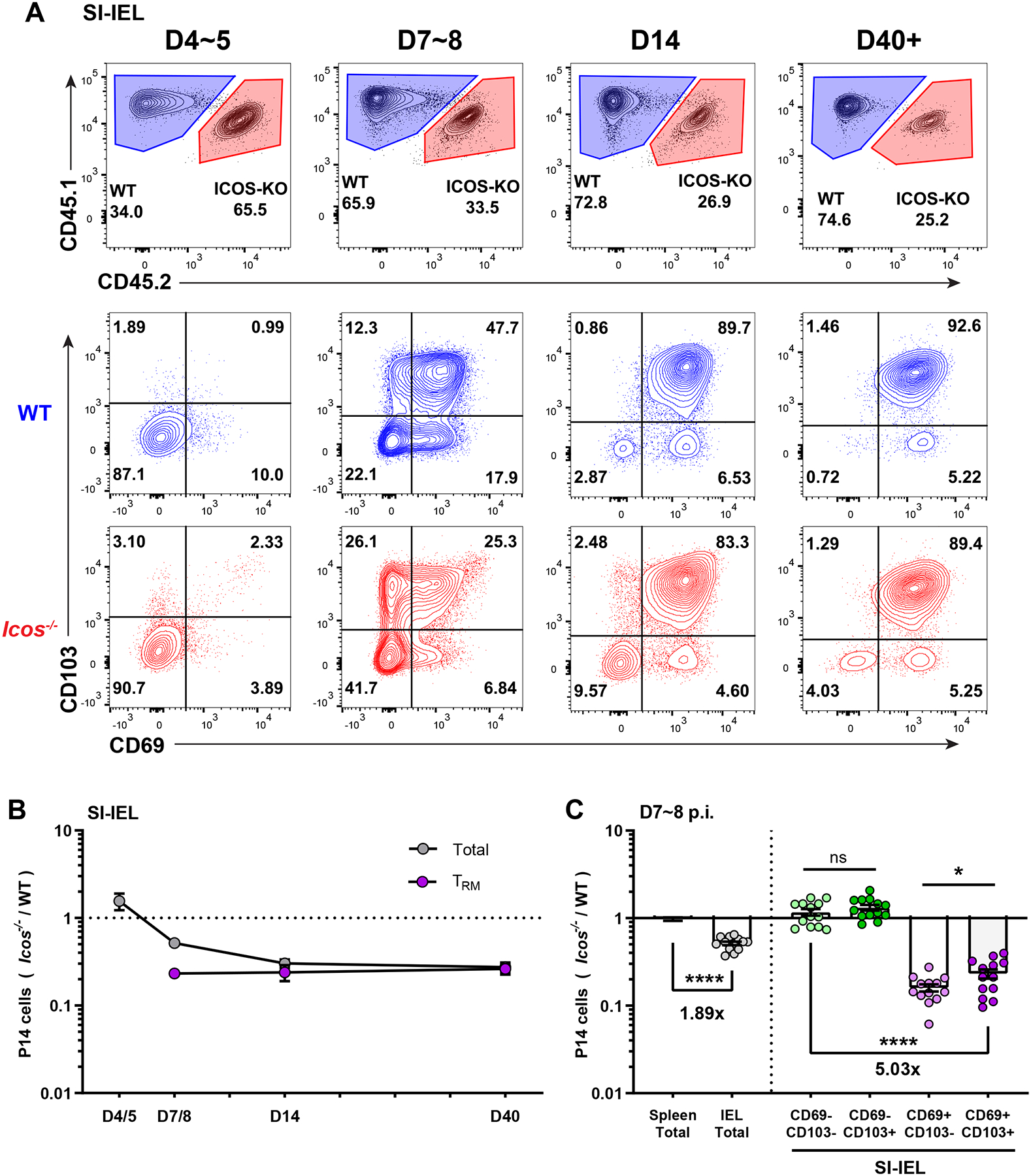
(A and B) WT mice receiving co-transferred WT and Icos−/− P14 T cells and LCMV infection were sacrificed at sequential time points as indicated, and P14 donor cells in the SI-IEL recovered and assessed for relative frequency and CD69 and CD103 expression. Representative data is shown in (A) and changes in ratio with time tracked in (B). Trm are defined as CD8αiv−CD69+CD103+. In (C), the compiled ratio of Icos−/− to WT P14 T cells is assessed for the indicated phenotypic subsets in spleen and SI-IEL from 7~8 days post infection. Data are from 2–5 independent experiments with a total 6–13 recipient mice. Error bars represent mean ± SEM. Statistical significance was determined with unpaired t-test: ns, not significant (p>0.05); * p<0.05; **** p<0.0001.
Interestingly, we noticed that the magnitude of the defect in Trm-phenotype populations was maintained from effector peak (D7) until late memory (D40+), whereas the defect of total P14 T cells gradually increased until it was of the same magnitude as Trm in both IEL, kidney and SG (Figure 2B and S2B, C). These data indicated that Icos−/− P14 T cells show impaired generation of Trm soon after seeding the non-lymphoid tissues, and this defect does not increase in magnitude as the response reaches memory phase.
Previous studies identified ~D4.5 after LCMV infection as being the time when antigen specific CD8+ T cells most efficiently migrate from SLOs to NLTs like the SI-IEL (Masopust et al., 2010), at which point CD8+ T cells need to further differentiate into Trm populations within the local environment. Our data indicated that ICOS deficiency does not affect initial entry of CD8+ T cells into non-lymphoid tissues after priming but rather impairs the efficiency of their subsequent differentiation into tissue resident memory cells.
ICOS/ICOS-L interaction is essential for optimal CD8+ Trm establishment
If ICOS interactions with ICOS-L were required to mediate downstream signal transduction, as occurs for CD4+ T cell differentiation (Nurieva et al., 2003), we would anticipate that the competitive advantage observed for WT CD8+ T cells in generation of Trm would be lost if the responses of WT and Icos−/− donor P14 T cells were assayed in an ICOS-L-deficient host. As for WT hosts, we found roughly equivalent representation of WT and Icos−/− donor P14 T cells in the blood and lymphoid tissues in ICOS-L deficient hosts at both effector and memory time points (Figure 3A, S3A). In contrast to WT hosts, however, in the NLTs of Icos−/− hosts, the frequency and phenotype of donor WT and Icos−/− P14 T cells were similar (Figure 3A, S3A, B), indicating that the competitive superiority of WT cells depended on access to ICOS-L. In support of that interpretation, we observed reduced numbers of WT P14 T cells residing in NLT SI-IEL in Icosl−/− relative to WT hosts, but comparable numbers in the spleen (Figure 3B). Thus, we concluded that both WT and Icos−/− P14 T cells failed to efficiently establish Trm in an ICOS-L deficient environment.
Figure 3. ICOS/ICOS-L interaction is essential for the optimal CD8+ Trm establishment.
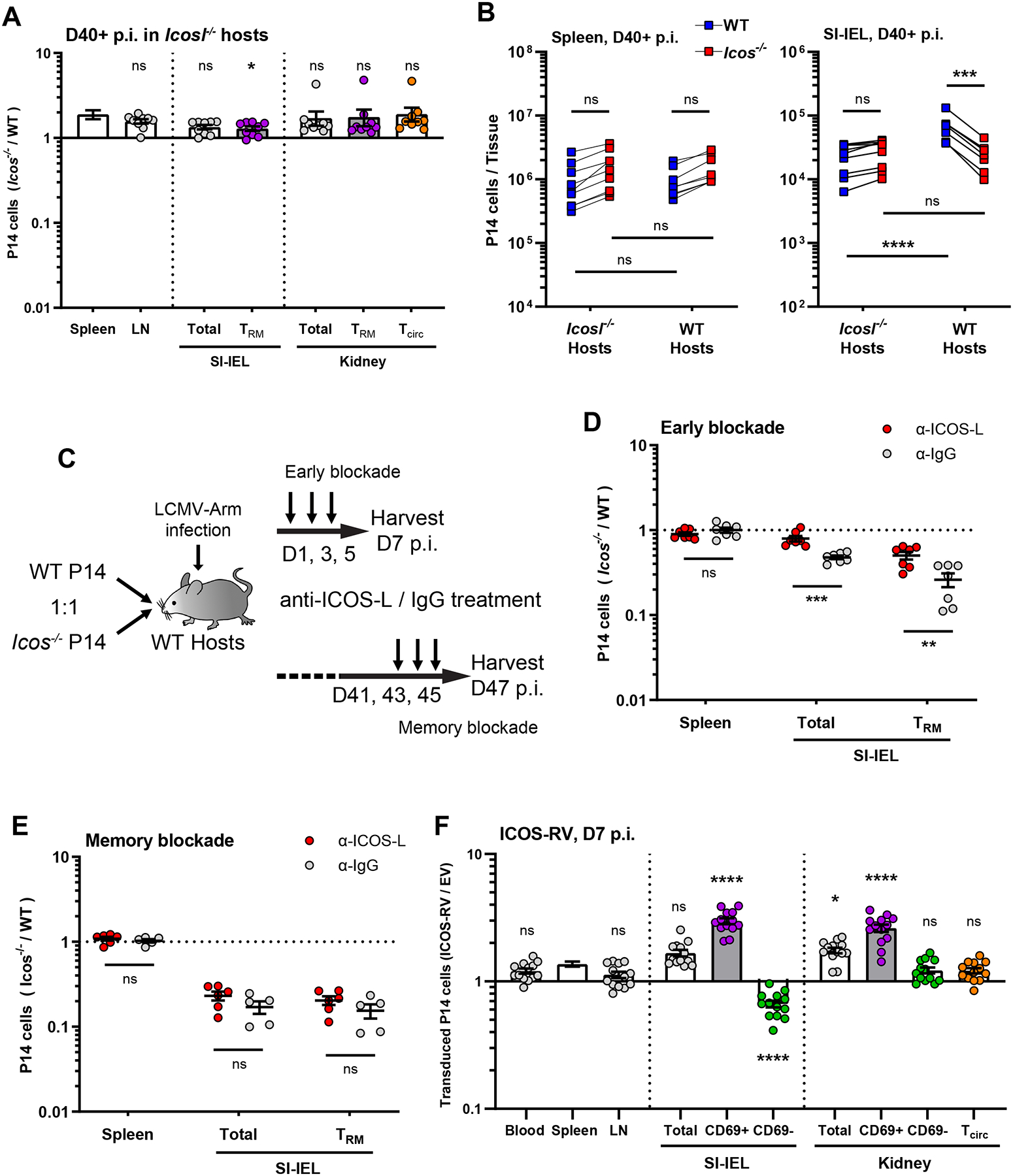
(A) WT and Icos−/− P14 T cells were co-adoptively transferred into Icosl−/− recipient mice followed by LCMV infection. The ratio of transferred P14 T cells was determined in the indicated tissues ≥40 days later; Data are from 3 independent experiments with a total 9 recipient mice. (B) shows the absolute numbers of WT or Icos−/− donor P14 T cells in either WT or Icosl−/− recipient mice at 40+ days following LCMV infection, for the spleen and SI-IEL. Data are from 3 independent experiments with a total 8–9 recipient mice. (C–E) WT host mice received equal number of WT and Icos−/− P14 T cells were treated with ICOS-L blocking antibody (or isotype control) either early (D1–5) or late (D41–45) following LCMV infection, as illustrated in (C), and the ratio of donor populations determined at the indicated time points (D, E). Data are from 2 independent experiments with a total 5–7 recipient mice.
(F) Congenically distinct WT P14 T cells were transduced with retroviruses encoding ICOS (ICOS-RV) or an empty vector (EV) and co-transferred into WT hosts that were then infected with LCMV. At 7 days post infection, cells were isolated from the indicated tissues and the ratio between transduced cell populations was assessed. Data are from 3 independent experiments with a total 13 recipient mice.
Error bars represent mean ± SEM. Statistical significance was calculated with: Kruskal-Wallis test with multiple comparisons relative to spleen in (A), One-way ANOVA with multiple comparisons in (B), unpaired t-test in (D and E), and One-way ANOVA with multiple comparisons relative to spleen in (F): ns, not significant (p>0.05); * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
To extend these studies, we tested the impact of a blocking antibody against ICOS-L as a distinct way to interrupt ICOS signaling. This approach had the additional advantage of allowing us to block ICOS-L at different time points relative to infection, permitting an initial assessment of when ICOS/ICOS-L interactions dictate generation or maintenance of Trm (Figure 3C). ICOS-L blockade at early times points (D1~5) led to results resembling those observed for Icosl−/− hosts, with minimal advantage for WT P14 T cells to form Trm in NTLs (Figure 3D, S3C). In contrast, ICOS-L blockade after memory establishment (D41~45) showed a negligible impact on already differentiated Trm cells (Figure 3E, S3D). These experiments suggested that the requirement for ICOS/ICOS-L interactions in optimal Trm establishment takes place during generation of Trm, rather than being involved in Trm maintenance.
We also considered whether increased ICOS expression would enhance the generation of Trm. Hence, we transduced P14 CD8+ T cells with a retrovirus encoding ICOS (versus an empty vector control) and tested the ability of these cells to seed tissue sites following adoptive transfer and LCMV infection. Analysis was limited to day 7 post-infection because occasional transduced donor cell rejection, detected at day 14, compromised analysis of later time points. Indeed, while forced expression of ICOS did not materially alter the frequency of P14 T cells in the circulation or lymphoid sites, it did enhance induction of early resident-phenotype (i.e., the CD69+ populations) CD8+ T cells in the IEL and kidney (Figure 3F, S3E), suggesting that production of tissue-resident cells may be enhanced by ectopic ICOS expression.
ICOS/ICOS-L interactions with radioresistant populations are required during settling of CD8+ T cells in NLTs
The peak of ICOS expression is induced after CD8+ T cell activation and can be sustained for many days (Fig. S1A), and ICOS-L is expressed by diverse antigen-presenting cells (B cells, DCs, macrophages), innate lymphoid cells (ILCs) and some non-hematopoietic cells, offering numerous possibilities as to when and where ICOS co-stimulation might act to promote efficient Trm generation (Hutloff et al., 1999; Roussel et al., 2018; Swallow et al., 1999; Teichmann et al., 2015). To investigate this, we employed a secondary cross-transfer model, in which P14 T cells were initially primed in either WT (Icosl+/+) or Icosl−/− primary hosts, but at day 4.5 post infection (a time point at which effector CD8+ T cells are poised for migration to NLTs), they were harvested from spleen and peripheral lymph nodes and retransferred into secondary hosts with the opposite Icosl genotype (Figure 4A, D). With this approach, we sought to separate the establishment of CD8+ Trm into two steps - initial activation in SLOs (D0 to D4.5) and subsequent seeding to NLTs (D4.5 to D14) - and evaluate the requirement for ICOS-L engagement at each step.
Figure 4. ICOS-L engagement after initial priming is required for CD8+ T cell retention in NLTs.

(A–C) Equal number of WT and Icos−/− P14 T cells were first transferred into WT host which were infected with LCMV. At D4.5 post-infection, total CD8+ T cells from SLO were isolated and transferred into infection matched Icosl−/− secondary hosts as illustrated in (A). (B) Fourteen days post infection, secondary hosts were sacrificed and relative frequencies of donor P14 T cells assessed in the indicated tissues. (C) shows representative data of relative frequencies and phenotype of transferred cell from kidney. Data are from 3 independent experiments with a total 11 recipient mice.
(D–F) The same scheme was followed as in A-C, except that the first recipient mouse was Icosl−/− and the secondary recipient WT. Data are from 3 independent experiments with a total 10 recipient mice.
Error bars represent mean ± SEM. Statistical significance was determined by One-way ANOVA with multiple comparisons relative to spleen in (B and E): ns, not significant (p>0.05); * p<0.05; *** p<0.001; **** p<0.0001.
In the first group, co-transferred WT and Icos−/− P14 T cells were primed in WT hosts then transferred to infection matched secondary Icosl−/− hosts (Figure 4A). Despite the opportunity to receive ICOS signaling during the initial 4.5 days of priming, the WT P14 T cell population showed no advantage over Icos−/− P14 T cells in forming Trm when transferred into Icosl−/− secondary hosts (Fig 4B, C). This outcome suggested that ICOS co-stimulation during initial priming is not sufficient to enhance Trm generation. In the opposite scenario, WT P14 T cells were unable to encounter ICOS-L during initial activation, but ICOS-L was available after cross-transfer (Figure 4D). In this situation, WT P14 T cells exhibited a significant advantage over Icos−/− P14 T cells in generating Trm-phenotype cells in NLTs, while both donor populations were similarly represented in SLOs and the blood (Figure 4E, F). Of note, at the time of the cross-transfer, Icos−/− P14 T cells did not exhibit a defect in numeric representation or the expression of the tissue-homing molecule LPAM-1 in either primary host (Fig S4A), consistent with our previous observation of equal expansion of WT and Icos−/− P14 T cells and their similar abilities to enter NLTs. These findings indicated that ICOS-L encounter solely after initial priming was sufficient to promote Trm establishment.
These cross-transfer studies suggested that ICOS-L encounter after D4.5 was necessary and sufficient to mediate the beneficial effects of ICOS co-stimulation in Trm generation. However, it was unclear whether ICOS/ICOS-L interactions needed to occur in the context of inflammatory factors (some of which can upregulate ICOS-L expression (Swallow et al., 1999) and/or residual antigen presentation. Hence, we also employed the cross-transferring approach using naïve instead of infection-matched secondary hosts. Fourteen days after initial LCMV infection (~10 days after cross-transfer), transferred P14 T cells could be recovered from NLTs of the naïve secondary hosts with a typical Trm phenotype (i.e. the expression of CD69 and CD103). As in infection-matched secondary hosts, WT P14 T cells initially primed with ICOS-L competent host lost their advantage over Icos−/− P14 T cells in naïve secondary Icosl−/− hosts (Figure S4B, S4C), while naïve secondary hosts with homeostatic ICOS-L expression were sufficient to promote tissue residency through ICOS co-stimulation (Figure S4D, S4E).
In another set of studies, we queried which cell populations must express ICOS-L in order to promote generation of CD8+ Trm. While ICOS-L expression by hematopoietic cells such as B cells and DC is well characterized, single-cell mouse cell atlas analyses indicate some non-hematopoietic cells in non-lymphoid tissues show robust Icosl transcription (Figure S5A, B) (Han et al., 2018) and we further verified ICOS-L at the protein level by flow cytometry (Figure S5C). To further investigate, we generated reciprocal sets of bone marrow chimeras in which WT and Icosl−/− mice were lethally irradiated and each group reconstituted with either Icosl−/− or WT bone marrow cells (Figure 5A). Once chimerism was established and verified (Figure S5D, E), we adoptively transferred WT and Icos−/− P14 T cells and infected the mice with LCMV. The key question was whether the competitive advantage of WT P14 in generating Trm would be lost when ICOS-L expression was restricted to radiosensitive cells (including most hematopoietic cells) or radioresistant cells (organspecific and stromal populations). As anticipated, in all four sets of chimeras, recirculating cells in the periphery were unimpacted (Figure 5B). Responses in control chimeras - WT → WT and Icosl−/− → Icosl−/− also followed the expected pattern, with the advantage of WT P14 T cells being lost in reconstituted Icosl−/− animals. Unexpectedly, reconstitution of Icosl−/− mice with WT bone marrow cells, capable of expressing ICOS-L in diverse hematopoietic lineages, did not efficiently restore the competitive advantage of WT P14 T cells (as seen in WT → WT chimeras). In contrast, Icosl−/− → WT chimeras, in which ICOS-L is expressed on radioresistant cells, phenocopied WT → WT chimeras in their efficient generation of WT CD8+ Trm in both lymphoid and nonlymphoid sites (Figure 5B, C). Our data indicate that ICOS-L expression by bone marrow-derived cells is insufficient to meet the requirements of Trm. However, which cell type(s) must express ICOS-L among the highly diverse (and tissue-specific) radioresistant populations remains to be delineated in future studies.
Figure 5. ICOS-L on radioresistant cells alone is sufficient for the optimal CD8+ Trm induction.
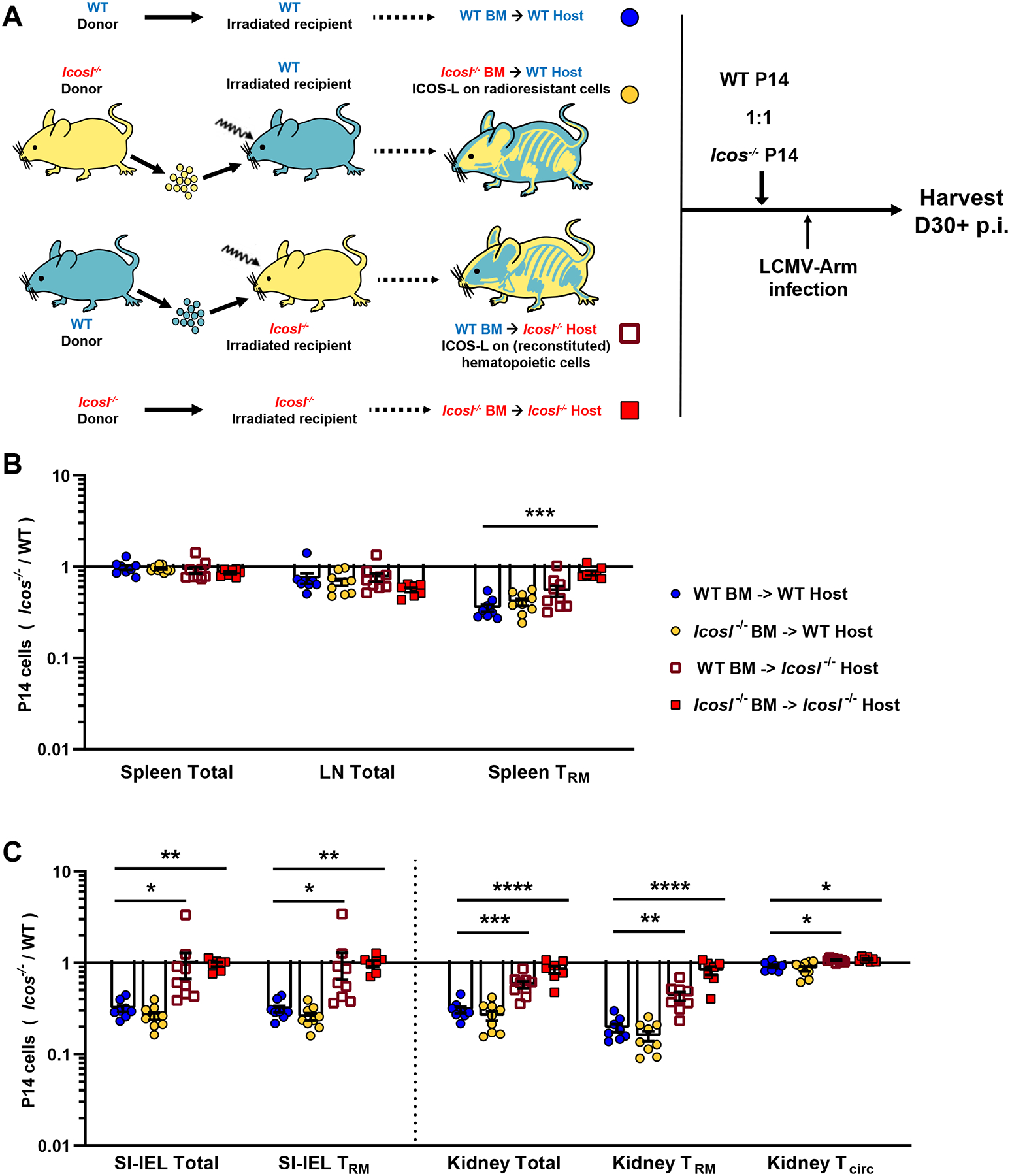
(A) Four types of mixed bone marrow chimeras were generated as illustrated in (A). At least 8 weeks after reconstitution, congenically distinct WT and Icos−/− P14 T cells were then co-transferred at equal numbers into the chimeras, followed by LCMV infection. Mice were sacrificed at least 30 days post infection, and the ratio of donor P14 T cells was analyzed in lymphoid tissues (B) and non-lymphoid tissues (C).
Data are from 3 independent experiments with a total 7–11 recipient mice in each chimera group. Error bars represent mean ± SEM. Statistical significance was determined by Kruskal-Wallis test multiple comparisons relative to the “WT BM ->WT Host” group as control: statistical significance is not significant (p>0.05) unless indicated; * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
Collectively, these results suggest that, rather than pre-programming their tissue residency during early effector stage in lymphoid organs, CD8+ Trm precursors need to be conditioned in local niches with ICOS co-stimulation in an antigen-independent manner for the optimal retention in NLTs, and that the cells expressing ICOS-L for this interaction are local radioresistant populations.
Limited differences in gene expression profiles of Icos−/− resident CD8+ T cells
To broaden our understanding of how ICOS contributes to Trm generation, we performed single-cell RNA sequencing (scRNA-seq) on co-transferred WT and Icos−/− P14 T cells at D5.5 and D32 post LCMV infection. Donor cells were sorted from the spleen, SI-IEL and kidney and sequenced as a single library at each time point. CITE-seq antibodies were used to distinguish different tissues and WT/Icos−/− donor cells to avoid any potential bias during sorting or sequencing. Gene expression profiles from both donor populations and all three tissues were characterized by unsupervised uniform manifold approximation and projection (UMAP) analysis at each time point (Figure 6A, F). Cells from all conditions were clustered together and their tissue and P14 donor cell origins were subsequently labeled (Figure 6B, G). While donor cells from different tissue compartments clustered distinctly from each other, WT and Icos−/− P14 populations were largely superimposable in the UMAP projection for all tissues, indicating broadly similar gene expression profiles of donor cells in both lymphoid and non-lymphoid sites (Figure 6B, G).
Figure 6. scRNA-seq analysis reveals limited differences between WT and Icos−/− CD8+ T cells.
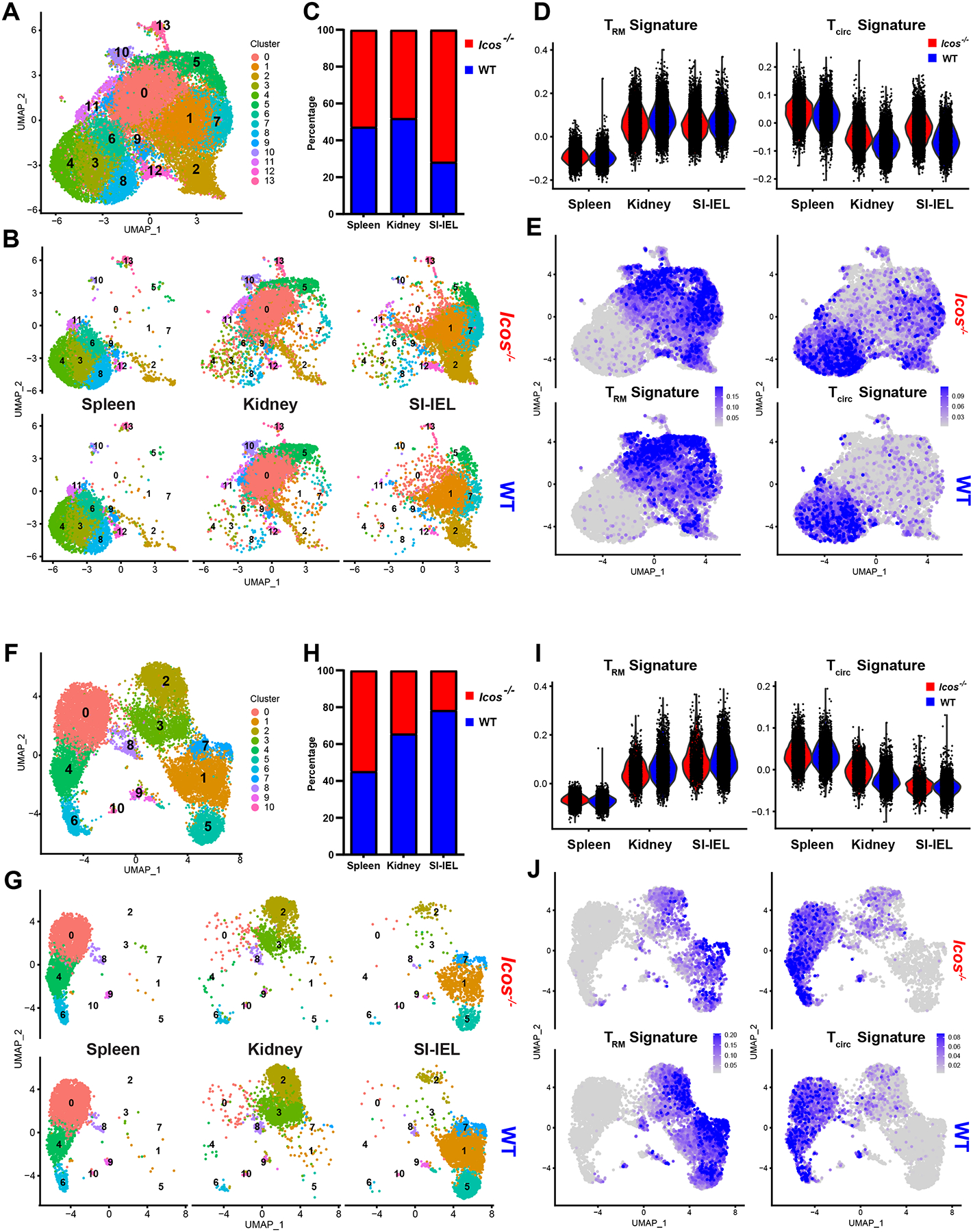
Equal number of WT and Icos−/− P14 T cells were co-transferred into WT recipients followed by LCMV infection. Donor cells were isolated on day 5.5 (A–E) or day 32 (F–J) from spleen, kidney or SI-IEL for scRNA-seq using the 10x Genomics platform.
In (A) and (F), UMAP plots were generated from both WT and Icos−/− P14 T cells in all tissues for each time point. These data were then annotated to show the clustering of WT and Icos−/− donor cells derived from each tissue in (B) or (G). (C) and (H) show the ratio of WT and Icos−/− donor cells captured by scRNA-seq from each tissue. Throughout, WT and Icos−/− cells were identified by CITE-seq antibodies against congenic marker (CD45.2) and ICOS. In (D-E) and (I–J), “Trm” or “Tcirc” signature scores were calculated based on the average expression levels of the correspondent core signature gene sets (Milner et al., 2017)on a single cell level. Violin plots (D and I) and feature plots (E and J) were further generated to visualize the “Trm” and “Tcirc” signature scores indicating the degree of match with residency/recirculating core signatures of donor cells in each tissue or cluster.
As expected, we found similar representation of both donor populations (or a slight advantage for Icos−/− cells) in all tissues at early effector time points, while proportionally fewer Icos−/− P14 T cells were recovered from kidney and SI-IEL at a memory timepoint (Figure 6C, H). Nevertheless, both donor populations were represented in each cluster at both time points (Figure S6A, E). Further assessment of cell cycle phases through transcriptional analysis suggested no significant differences between WT and Icos−/− P14 populations (Figure S6B, F).
Using published core signatures for CD8+ Trm and circulating memory T cells (“Tcirc”) (Milner et al., 2017), we were able to estimate the residency/circulating state of each donor cell inferred from its transcriptome profile (Figure 6D, E, I, J). This analysis confirmed that both WT and Icos−/− P14 T cells adopted similar residency signatures in non-lymphoid tissues as early as 5.5 days post infection, although we noted a modest increase of Tcirc characteristics in the Icos−/− P14 population in both SI-IEL and kidney at this time point (Figure 6D, E). Upon further analysis, we observed only modest differences in gene expression between co-clustered WT and Icos−/− P14 T cells in SI-IEL and kidney (Figure S6C, D), including increased expression of Klf2 and S1pr1 in the Icos−/− populations, suggested a potential defect in downregulating circulating signatures after seeding NLTs. Consistent with our prior assessment of surface markers by flow cytometry (Figure 1D), transcriptional analysis at the memory time point showed comparable Trm and Tcirc signatures and no significant differentially expressed genes between WT and Icos−/− P14 T cells in both spleen and SI-IEL (Figure 6I, J and S6G)(Table S1). In the kidney, we found higher expression of some genes associated with the Tcirc signature (Klf2, S1pr1 etc) and lower expression of some Trm signature factors (Xcl1, Rgs1 etc) in the Icos−/− population (Figure 6I, J and S6H). This result echoes the reduction of CD69+ Trm seen by flow cytometry, but these limited gene expression differences were quite modest.
In summary, our transcriptome analysis indicated that ICOS-deficiency does not lead to substantial transcriptional reprogramming of tissue-resident CD8+ T cells, but primarily compromises the efficiency of their residency establishment during tissue adaptation.
Impaired downregulation of KLF2 in Icos−/− CD8+ T cells during Trm establishment
Our scRNA sequencing indicated that Klf2 transcripts were elevated in Icos−/− P14 T cells during Trm establishment in NLTs. Since downregulation of KLF2 and its target gene S1pr1 is important during Trm establishment (Skon et al., 2013) and studies on CD4+ Tfh cells show that ICOS signaling can suppress the KLF2 via FOXO1 (Stone et al., 2015). we sought to verify whether ICOS influences KLF2 protein expression after effector CD8+ T cells enter NLTs.
Using the previously described KLF2-GFP reporter model (Weinreich et al., 2009), we were able to track the kinetics of Klf2 expression in co-transferred WT and Icos−/− P14 T cells during the response to LCMV. Although D4~5 Icos−/− P14 T cells showed no defect (and even a slight advantage) in their representation among cells entering the SI-IEL and kidney (Figure 2B, S2B), the frequency of KLF2Hi cells in this population was modestly but significantly increased compared to WT cells, and this effect was also observed at days 7–14 (Figure 7A, B, S7A, B). In contrast to these findings for P14 T cells in the NLT parenchyma, KLF2 expression was similar among WT and Icos−/− P14 in the vasculature (iv+ cells) and in lymphoid sites (Figure 7B, S7B), suggesting a selective defect in KLF2 regulation among Icos−/− CD8+ T cells preparing for tissue-residency. Further analysis showed that the numbers of WT and Icos−/− KLF2Hi cells were comparable throughout the effector phase, but there were significantly fewer KLF2Lo Icos−/− P14 T cells in the NLTs after D7 (Figure 7C, S7C), indicating that some Icos−/− CD8+ T cells failed to acquire the KLF2Lo phenotype associated with tissue residency during the late effector phase.
Figure 7. Downregulation of KLF2 and PI3K pathway play roles in ICOS affecting CD8+ Trm establishment.
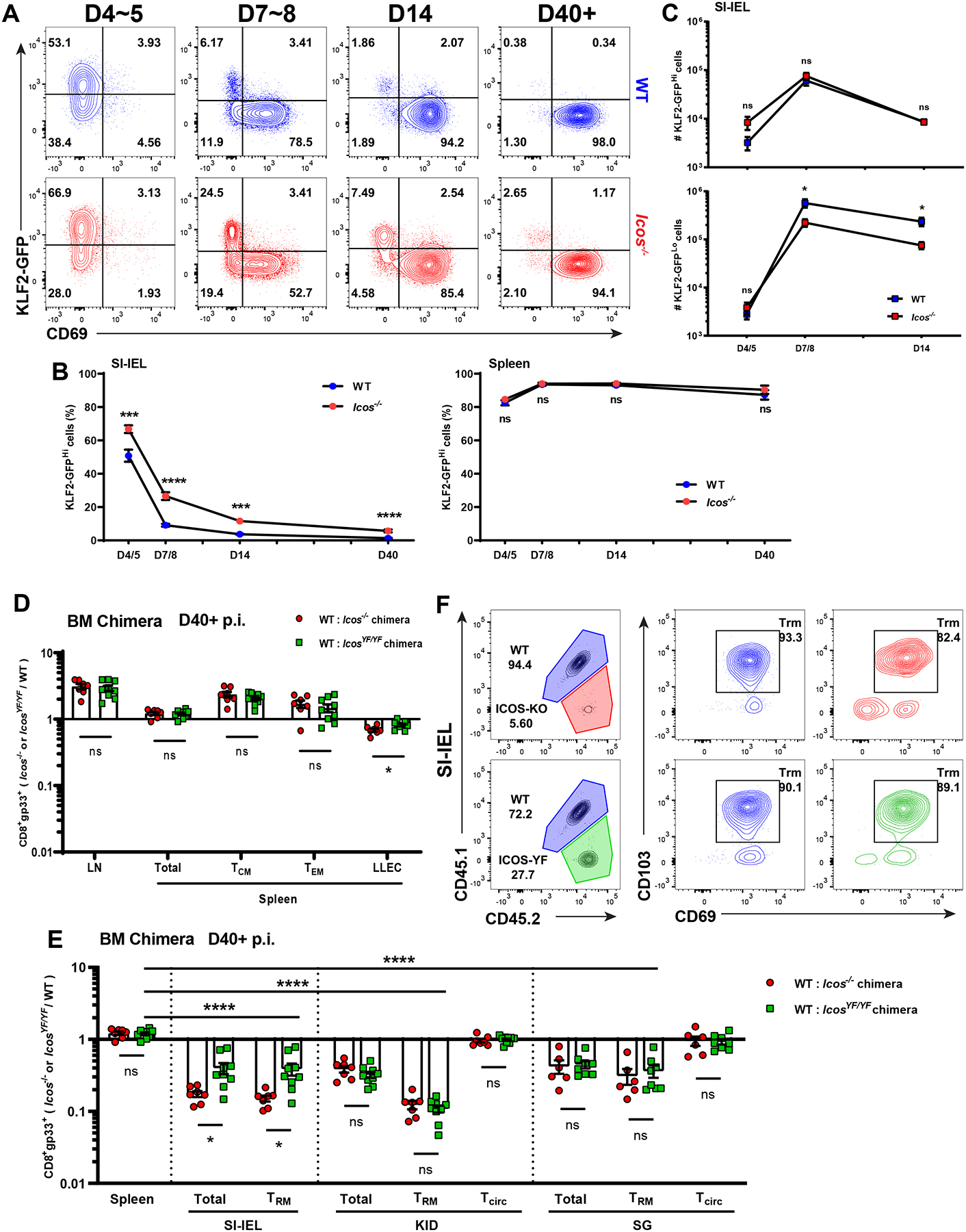
(A–C) Equal number of WT and Icos−/− Klf2-gfp P14 T cells were co-adoptively transferred into WT recipients which were then infected with LCMV and sacrificed at the indicated time points. (A) shows representative flow cytometry data for KLF2-GFP and CD69 expression on donor cells isolated from SI-IEL. (B) shows the frequency of KLF2-GFPHi population among transferred cells in the SI-IEL and spleen. (C) indicates the absolute numbers of KLF2-GFPHi and KLF2-GFPLo cells among transferred WT or Icos−/− P14 T cells in the SI-IEL. Data are from 2 independent experiments with a total 4–12 recipient mice.
In (D–F), mixed bone marrow chimeras of WT:Icos−/− or WT:IcosYF/YF were generated and infected with LCMV. At least 40 days post infection, indicated tissues were harvested and LCMV gp33/Db-specific CD8+ T cells identified by tetramer staining. (D–E) shows the compiled ratio of CD8+ gp33+ cells from each bone marrow donor population in memory subsets from lymphoid (D) or non-lymphoid tissues (E). (F) shows representative frequencies as well as the CD69 and CD103 phenotype of CD8+ gp33+ cells from each donor population recovered from the SI-IEL. Ratio of Icos−/−/WT or IcosYF/YF/WT CD8+ gp33+ cells in different tissues are normalized to the ratio in peripheral blood from the same host. Data are from 2 independent experiments with a total 7–9 mice.
Error bars represent mean ± SEM. Statistical significance was determined with unpaired t-test: ns, not significant (p>0.05); * p<0.05; ** p<0.01; *** p<0.001; **** p<0.0001.
These findings are consistent with previous data suggesting that downregulation of KLF2 is a necessary step in the efficient generation of CD8+ Trm (Skon et al., 2013), but that does not necessarily mean that loss of KLF2 expression would be sufficient for driving Trm differentiation. Studies using KLF2 ablation or knockdown are complicated by the requirement for KLF2 to permit T cell trafficking into the blood, which is a prerequisite for trafficking to NLTs. Hence determining the primacy of KLF2 downregulation in the generation of ICOS-expressing CD8+ Trm will require additional study.
IcosYF/YF mutation affects CD8+ Trm establishment through PI3K pathway
ICOS can mediate signals through both the phosphoinositide 3-kinase (PI3K) and TBK1 pathways, which can contribute to different steps of Tfh maturation. (Pedros et al., 2016). Previous studies suggested that induction of the PI3K pathway supports differentiation of CD8+ Trm and CD4+ Tfh cells, and PI3K drives loss of KLF2 expression (Stone et al., 2015). To investigate if PI3K activation is required for the effect of ICOS on CD8+ Trm establishment, we employed IcosYF/YF mice, which carry a tyrosine-to-phenylalanine mutation of the ICOS cytoplasmic tail that selectively abrogates ICOS-mediated PI3K signaling (Gigoux et al., 2009). By generating WT:Icos−/− and WT:IcosYF/YF mixed bone marrow chimeras in parallel, we were able to compare the effect of complete ICOS deficiency and a selective defect in downstream PI3K signaling on antigen-specific CD8+ T cells side by side. At memory timepoints (D40+) after LCMV infection, neither the ICOS-YF mutation nor ICOS deficiency impaired generation of CD8+ circulating memory cells in the periphery – indeed, both Icos−/− and IcosYF/YF gp33+ CD8+ T cells were modestly overrepresented relative to WT cells in spleen and lymph nodes (Figure 7D). In contrast, despite normal surface expression levels of the ICOS-YF mutant protein, gp33+ CD8+ T cells with the PI3K-defective ICOS exhibited a substantial defect in representation of Trm compared to WT cells in all NLTs examined (Figure 7E, S7D). While in the kidney and salivary gland we observed defects of similar magnitude when comparing Icos−/− and IcosYF/YF donor-derived antigen specific CD8+ T cells, IcosYF/YF donor cells were slightly less compromised in their contribution to SI-IEL Trm, indicating that ICOS-dependent PI3K signaling is partially dispensable for generation of some Trm populations. As for Icos−/− donor cells, Trm populations derived from the IcosYF/YF donor were phenotypically similar to their WT-derived counterparts (Figure 7F). Taken together, these data highlighted the importance of ICOS-driven PI3K activity in promoting optimal CD8+ Trm establishment.
Discussion
Costimulatory signals play key roles not only in efficient T cell activation, but also to provide proper contextual regulation of T cell differentiation, function, and memory generation (Attanasio and Wherry, 2016; Chen and Flies, 2013). Among costimulatory molecules reported to have a role in CD8+ T cell memory generation, lack of GITR or 4–1BB severely reduces the frequency of memory CD8+ T cells in the lung following influenza infection, although defects in memory generation in lymphoid sites were also noted (Chu et al., 2020, 2019; Zhou et al., 2019). CD40L deficiency or blockade preferentially impacts generation of CD8+ T cell memory in the small intestine, although some approaches also cause reduced circulating CD8+ T cell memory (Lefrançois et al., 1999; Masopust et al., 2001). This interaction is also important in CD4+ T cell activation and the cell-intrinsic role for CD40L expression by CD8+ T cells in generation of Trm awaits clarification. In contrast, we found that cell-intrinsic ICOS deficiency substantially impaired CD8+ Trm establishment in a variety of model systems, while circulating memory in the blood and lymphoid tissues were exempt from an ICOS requirement. Indeed, in some competitive settings we observed a modest advantage for Icos−/− CD8+ T cell memory in lymphoid sites, especially notable for Tcm. Hence, these findings suggest a selective role for ICOS in promoting the generation of resident, but not recirculating, memory CD8+ T cells.
Studies on the function of ICOS have mainly focused on CD4+ T cell responses, where it plays a critical role in regulating Tfh and a subset of regulatory T cells (Smigiel et al., 2014; Weber et al., 2015). Previous studies of Icos−/− mice or ICOS-deficient patients finds intact CD8+ effector responses but impaired memory generation, although the impact of ICOS deficiency on other population, including CD4+ T cells, may affects the CD8+ T cell population (Bertram et al., 2002; Kopf et al., 2000; Takahashi et al., 2009). Another study using retrogenic ICOS over-expression showed enhanced CD8+ T cell effector responses, but its physiological role was less clear (Liu et al., 2016). Our approach primarily focused on adoptive transfer of Icos−/− antigen-specific CD8+ T cells, allowing us to characterize the cell-intrinsic role of ICOS in CD8+ T cell differentiation and memory generation during acute infection, while keeping CD4+ T cell responses intact.
This previously unappreciated role for ICOS in specifically supporting CD8+ Trm generation is consistent with its higher expression level in resident versus circulating memory CD8+ T cells(Kumar et al., 2017; Mackay et al., 2013; Milner et al., 2017). However, it is important to note that we did not observe an absolute defect in Icos−/− CD8+ Trm generation, and it has been shown previously that various cytokines can promote induction and maintenance of Trm phenotype (Casey et al., 2012; Skon et al., 2013). Hence these and other signals may play the dominant role during Trm establishment and may partially compensate for ICOS-deficiency. Our data using ICOS-L blockade suggested ICOS interactions were needed for induction but not for maintenance of CD8+ Trm in the tissue sites studied. This contrasts with the reported requirement for ICOS in maintenance of long-lived CD4+ Tfh (Künzli et al., 2020). Whether this reflects a decreased requirement of ICOS signaling only in Trm or redundancy of different signals in the NLTs is still unclear.
The generation of CD8+ Trm normally involves distinct spatiotemporal phases: priming in lymphoid tissues, trafficking to NLTs, and establishing residency locally. While foundational studies indicate that local microenvironmental cues such as TGF-β promote Trm generation (Casey et al., 2012; Mackay et al., 2015), recent evidence reveals that TGF-β can mediate preconditioning of naïve CD8+ T cells to favor Trm generation and that crosspriming by specific dendritic cells in the draining lymph nodes can also enhance Trm differentiation (Iborra et al., 2016; Mani et al., 2019). Hence, it was important to address when and where the key ICOS/ICOS-L interactions took place for efficient CD8+ Trm induction. During formation of germinal centers, ICOS/ICOS-L interactions drive repetitive transient contacts between specific CD4+ T cells and B cells (termed entanglement), promoting differentiation of both populations (Qi, 2016), and ICOS-L is also expressed by myeloid populations. Hence, we initially expected that ICOS-L on hematopoietic cells would dictate ICOS-dependent production of CD8+ Trm. However, our studies suggested that interactions with ICOS-L bearing cells during initial priming were neither necessary nor sufficient to promote generation of CD8+ Trm, and that ICOS-L expression by radiosensitive hematopoietic cells was unable to provide the needed signal. Instead, we found that provision of ICOS-L signals after D4.5 was critical and that ICOS-L expression by radioresistant cells alone was sufficient to drive ICOS-dependent Trm induction. Although it is certainly possible that radioresistant hematopoietic cells expressing ICOS-L contribute to differentiation of CD8+ Trm, our data also supports the interpretation that non-hematopoietic cell populations in non-lymphoid sites mediate this interaction during initial settling of Trm precursors. Studies on CD4+ Tfh differentiation suggests a significant role of ICOSL expressed by bystander B cells in the follicle (Xu et al., 2013). Our data on sequential adoptive transfer of CD8+ T cells into uninfected hosts indicated that basal expression of ICOS-L in NLTs in the absence of foreign antigen was sufficient to engage ICOS and promote CD8+ T cell tissue residency, which echoes those findings for Tfh cells. Together, our findings support the hypothesis that ICOS/ICOS-L interactions occur locally, within NLTs, to reinforce the acquisition of CD8+ T cell residency.
Through scRNA sequencing, we found the few Icos−/− CD8+ T cells that did acquire residency characteristics in NLTs were broadly similar to WT cells in their gene expression. This indicates that ICOS stimulation is not essential for the major transcriptional changes associated with Trm differentiation. Nevertheless, modest changes in gene expression were observed, even among some cells at memory phase, suggesting that regulation of a subset of genes is dysregulated among cells that did not receive ICOS engagement. Among these was Klf2, which was increased at the transcriptional and protein levels among Icos−/− cells. We reported previously that the loss of KLF2 (and subsequent S1pr1 downregulation) promotes tissue-residency of CD8+ T cells, and that forced KLF2 expression significantly impaired Trm generation (Skon et al., 2013). Hence, the impaired downregulation of KLF2 among Icos−/− CD8+ T cells aligns with their inefficient commitment to long-term residency in NLTs and with their slight advantage in forming recirculating memory populations in SLOs. It is worth mentioning that while reduced KLF2 expression may be important, it is presumably just one of the effects of ICOS stimulation in promoting efficient Trm generation. Indeed, we found that the ability of ICOS to stimulate PI3K was essential for its role in promoting CD8+ Trm generation. Given the panoply of pathways regulated by PI3K, including functional inactivation of FOXO1 (a transcription factor that positively regulates expression of numerous genes, including Klf2 (Hedrick et al., 2012)), it is likely that a constellation of factors mediates the impact of PI3K in efficiently generating CD8+ Trm.
Recent studies noted high ICOS expression on CXCR5+ CD8+ T cells produced during chronic viral infection, a population that is especially responsive to PD-1 blockade as a way to transiently reverse CD8+ T cell exhaustion (Beltra et al., 2020; Im et al., 2016). Whether ICOS plays a key role in generation of these cells has not yet been addressed, but it is notable that these ICOSHi cells are thought to be resident in lymphoid tissues (Beltra et al., 2020; Im et al., 2020) and our data indicated that ICOS is also critical to generate SLO-Trm in an acute infection model. Like the populations elicited by infection, CD8+ T cells in tumor-infiltrating lymphocyte populations can exhibit many characteristics of Trm. Previous studies show that ICOS-L expression on tumor cells or ectopic ICOS-L expression in a tumor could increase tumor infiltrating CD8+ T cells and enhance tumor rejection (Liu et al., 2001; Wallin et al., 2001; Zamarin et al., 2016), while we found that forced ICOS expression on activated CD8+ T cells enhanced their recruitment to NLT and acquisition of residency markers, suggesting physiological ICOS levels on activated CD8+ T cells may be limiting for Trm induction. On the other hand, blocking ICOS/ICOS-L signaling can reduce pathological CD8+ T cells infiltrating into target organs in graft-versus-host disease or lupus models (Taylor et al., 2005; Teichmann et al., 2015). Hence, targeting the ICOS/ICOS-L pathway may present a therapeutic opportunity to regulate the generation of Trm.
Given the extensive studies of ICOS in regulating differentiation and maintenance of CD4+ T helper subsets, it is informative to explore parallels in its roles in these populations and in CD8+ T cells. It has been reported that ICOS is highly expressed and required for the maintenance of “effector” Treg – a population with limited recirculation capacity that is predominantly found in NLTs (Smigiel et al., 2014). To promote Tfh generation, ICOS induces the expression of key transcription factor Bcl6 (Choi et al., 2011), but also induces the inactivation of FOXO1 (Stone et al., 2015) and decreased KLF2 expression (Lee et al., 2015), both of which are well-known for their roles in T cell trafficking. Furthermore, recent studies demonstrated a population of CD4+ T cells in the lung with merged Tfh/Trm properties and high ICOS expression compared to splenic Tfh cells (Son et al., 2021; Swarnalekha et al., 2021) – it will be interesting to determine whether generation of this population is also ICOS-dependent. Going further, ICOS has also been shown to play a key role in homeostasis of lung ILC2 (Maazi et al., 2015): hence, ICOS signaling may induce differentiation programs, including core characteristics of tissue residency, among varied lymphocyte populations (Peng and Jameson, 2020).
Limitations of Study:
While our findings indicate a key role for ICOS-L expression on radioresistant cells in supporting efficient generation of CD8+ Trm, the identity of those ICOS-L+ populations is unclear and it will be important to determine whether shared or different cell types fulfill this role in distinct NLTs. Furthermore, while largely similar, we observed some differences in transcriptional profiles of WT and Icos−/− CD8+ Trm: It will be interesting to test whether Trm that do or do not receive ICOS stimulation display distinct properties during recall responses. Future work will be needed to develop ways to harness the ICOS stimulation pathway to favor Trm generation following vaccination, in order to induce protective immunity more effectively at barrier tissues.
STAR METHODS
RESOURCE AVAILABILITY
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Stephen Jameson (james024@umn.edu).
Materials availability
Retroviral vector plasmids and mouse strains generated in this study are available pending approval of a simple MTA.
Data and code availability
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed under “Bioinformatics analysis” and in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD8a BUV395 (53-6.7) | BD Biosciences | Cat# 563786, RRID: AB_2732919 |
| Anti-mouse CD8a PerCP-Cy5.5 (53-6.7) | Tonbo Biosciences | Cat# 65-0081, RRID:AB_2621882 |
| Anti-mouse CD8a PE (53-6.7) | Tonbo Biosciences | Cat# 50-0081, RRID:AB_2621741 |
| Anti-Mouse CD45.1 violetFluor 450 (A20) | Tonbo Biosciences | Cat# 75-0453, RRID:AB_2621949 |
| Anti-Mouse CD45.1 PE-Cyanine7 (A20) | Tonbo Biosciences | Cat# 60-0453, RRID:AB_2621850 |
| Anti-Mouse CD45.2 violetFluor 450 (104) | Tonbo Biosciences | Cat# 75-0454, RRID:AB_2621950 |
| Anti-Mouse CD45.2 PE-Cyanine7 (104) | Tonbo Biosciences | Cat# 60-0454, RRID:AB_2621851 |
| Anti-Mouse CD45.2 FITC (104) | Tonbo Biosciences | Cat# 35-0454, RRID:AB_2621692 |
| Anti-human/mouse/rat CD278 (ICOS) PE (C398.4A) | BioLegend | Cat# 313508, RRID:AB_416332 |
| Anti-human/mouse/rat CD278 (ICOS) APC (C398.4A) | BioLegend | Cat# 313510, RRID:AB_416334 |
| Anti-mouse CD44 BV786 (IM7) | BD Biosciences | Cat# 563736, RRID:AB_2738395 |
| Anti-mouse CD44 BV605 (IM7) | BD Biosciences | Cat# 563058, RRID:AB_2737979 |
| Anti-mouse CD62L BV786 (MEL-14) | BD Biosciences | Cat# 564109, RRID:AB_2738598 |
| Anti-mouse CD62L BV605 (MEL-14) | BioLegend | Cat# 104438, RRID:AB_2563058 |
| Anti-mouse KLRG1 PE-Cyanine7 (2F1) | Thermo Fisher Scientific | Cat# 25-5893-82, RRID:AB_1518768 |
| Anti-mouse KLRG1 BV711 (2F1) | BD Biosciences | Cat# 564014, RRID:AB_2738542 |
| Anti-Mouse CD127 BUV737 (SB/199) | BD Biosciences | Cat# 612841, RRID:AB_2870163 |
| Anti-Mouse CD69 PE (H1.2F3) | Thermo Fisher Scientific | Cat# 12-0691-83, RRID:AB_465733 |
| Anti-Mouse CD69 PE-CF594 (H1.2F3) | BD Biosciences | Cat# 562455, RRID:AB_11154217 |
| Anti-mouse CD103 BV510 (M290) | BD Biosciences | Cat# 563087, RRID:AB_2721775 |
| Anti-mouse CD103 APC (2E7) | BioLegend | Cat# 121414, RRID:AB_1227502 |
| Anti-mouse CD275 (B7-RP1, ICOSL, B7H2) PE (HK5.3) | BioLegend | Cat# 107405, RRID:AB_2248797 |
| Anti-mouse LPAM-1 (Integrin a4b7) PE (DATK32) | BioLegend | Cat# 120606, RRID:AB_493267 |
| Anti-Mouse CD45 APC (30-F11) | Tonbo Biosciences | Cat# 20-0451, RRID:AB_2621573 |
| Anti-Mouse CD19 PE-Cyanine7 (1D3) | Tonbo Biosciences | Cat# 60-0193, RRID:AB_2621840 |
| Anti-Mouse CD45R/B220 BUV395 (RA3-6B2) | BD Biosciences | Cat# 563793, RRID:AB_2738427 |
| Anti-Mouse Va2 TCR APC (B20.1) | BD Biosciences | Cat# 560622, RRID:AB_1727582 |
| Ghost Dye Red 780 Viability Dye | Tonbo Biosciences | Cat # 13-0865-T100 |
| InVivoMAb anti-mouse CD3ε (145-2C11) | BioXCell | Cat# BE0001-1, RRID:AB_1107634 |
| InVivoMAb anti-mouse CD28 (37.51) | BioXCell | Cat# BE0015-1, RRID:AB_1107624 |
| InVivoMAb anti-mouse ICOSL (CD275) (HK5.3) | BioXCell | Cat# BE0028, RRID:AB_1107566 |
| InVivoMAb rat IgG2a isotype control (2A3) | BioXCell | Cat# BE0089, RRID:AB_1107769 |
| TotalSeq™-A0157 anti-mouse CD45.2 (104) | BioLegend | Cat# 109853, RRID:AB_2783051 |
| TotalSeq™-A0171 anti-human/mouse/rat CD278 (ICOS) (C398.4A) | BioLegend | Cat# 313555, RRID:AB_2800824 |
| TotalSeq™-A0301 anti-mouse Hashtag 1 (M1/42; 30-F11) | BioLegend | Cat# 155801, RRID:AB_2750032 |
| TotalSeq™-A0302 anti-mouse Hashtag 2 (M1/42; 30-F11) | BioLegend | Cat# 155803, RRID:AB_2750033 |
| TotalSeq™-A0303 anti-mouse Hashtag 3 (M1/42; 30-F11) | BioLegend | Cat# 155805, RRID:AB_2750034 |
| TotalSeq™-A0309 anti-mouse Hashtag 9 (M1/42; 30-F11) | BioLegend | Cat# 155817, RRID:AB_2750042 |
| TotalSeq™-A0310 anti-mouse Hashtag 10 (M1/42; 30-F11) | BioLegend | Cat# 155819, RRID:AB_2750043 |
| TotalSeq™-A0311 anti-mouse Hashtag 11 (M1/42; 30-F11) | BioLegend | Cat# 155821, RRID:AB_2750136 |
| Bacterial and virus strains | ||
| Lymphocytic choriomeningitis virus (LCMV) Armstrong strain | Dr. R. Ahmed, Emory University | N/A |
| Listeria monocytogenes-GP33 (LM-gp33) Generated by Dr. Hao Shen (UPenn) first reported PMID: 11323695 | Dr. R. Ahmed, Emory University | N/A |
| Influenza A-PR8-gp33. Generated by Dr. Ryan Langois (UMN), first reported in PMID: 31235953. | Dr. D. Masopust, (UMN) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Collagenase, Type 1 | Worthington | Cat# LS004197 |
| Percoll | GE Healthcare | Cat# 17-0891-09 |
| Dithioerythritol | EMD Millipore | Cat# 233152-5GM |
| RPMI 1640 | Corning | Cat# 10-040-CV |
| HBSS 10x | Corning | Cat# 20-021-CV |
| Fetal Bovine Serum | Atlas Biologicals | Cat# FS-0500-AD |
| L-Glutamine, 100x, Liquid | Corning | Cat# 25-005-CI |
| Penicillin/Streptomycin | Gibco | Cat# 15070063 |
| DMEM | Gibco | Cat# 11995065 |
| Critical commercial assays | ||
| CD8a+ T cell Isolation Kit, Mouse | Miltenyi Biotec | Cat# 130-104-075 |
| BD Cytofix/Cytoperm Solution Kit | BD Biosciences | Cat#554714 |
| Deposited data | ||
| scRNA-seq of co-transferred WT and Icos−/− P14 T cells | this paper | GEO: GSE185342 |
| TRM single-cell time course study | Kurd et al., 2020 | GEO: GSE131847 |
| mouse cell atlas study | Han et al., 2018 | GEO: GSE108097 |
| Experimental models: Cell lines | ||
| Plat-E | Cell Biolabs | Cat#RV-101, RRID: CVCL_B488 |
| Experimental models: Organisms/strains | ||
| Mouse: B6.SJL-PtprcaPepcb/BoyCrCrl (B6-CD45.1) | NCI Charles River | Cat# CRL:564, RRID:IMSR_CRL:564 |
| Mouse: C57BL/6NCrl (B6-CD45.2) | NCI Charles River | Cat# CRL:027, RRID:IMSR_CRL:027 |
| Mouse: P14 | Dr. R. Ahmed, Emory University | N/A |
| Mouse: B6.129P2-Icostm1Mak/J (ICOS-KO) | The Jackson Laboratory | Cat# JAX:004859, RRID:IMSR_JAX:004859 |
| Mouse: B6.129P2-Icosltm1Mak/J (ICOSL-KO) | The Jackson Laboratory | Cat# JAX:004657, RRID:IMSR_JAX:004657 |
| Mouse: B6(C)-Klf2tm1.1Khog/JmsnJ (KLF2-GFP) | Generated in lab (Weinreich et al., 2009) | RRID:IMSR_JAX:036331 |
| Mouse: ICOS-YF | Dr. D. Campbell (Mittelsteadt et al., 2021) | N/A |
| Oligonucleotides | ||
| N/A | ||
| Recombinant DNA | ||
| MIGR1 (pMIGR-EV) | Dr. W. Pear (Addgene) | Cat# 27490 |
| pMIGR-mICOS | in-house | N/A |
| Software and algorithms | ||
| GraphPad Prism v9.2.0 | GraphPad Software | RRID:SCR_002798 |
| FlowJo v10.8 | BD Biosciences | RRID:SCR_008520 |
| BD FACSDiva | BD Biosciences | RRID:SCR_001456 |
| Cell Ranger v6.0.1 | 10xGenomics | RRID:SCR_017344 |
| Seurat v 3.0.3.9039 | https://satijalab.org/seurat/get_started.html | RRID:SCR_016341 |
| ggplot2 | https://cran.r-project.org/web/packages/ggplot2/index.html | RRID:SCR_014601 |
| MAGIC | van Dijk et al., 2018 | N/A |
| Other | ||
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
EXPERIMENTAL MODEL DETAILS
Mice
C57BL/6 (B6) and B6.SJL mice were purchased from the National Cancer Institute (via Charles River). Icos−/− and Icosl−/− mice were obtained from Jackson Laboratories and were both fully backcrossed onto C57BL/6 background. LCMV-DbGP33-specific TCR transgenic P14 mice were fully backcrossed to B6 and Icos−/− mice, with introduction of CD45.1 and CD45.2 congenic markers for identification. Klf2-gfp mice have been previously described (Weinreich et al., 2009) and were crossed to Icos−/− P14 mice. Animals were maintained under specific-pathogen-free conditions at the University of Minnesota. In all experiments, mice were randomly assigned to experimental groups. All experimental procedures were approved by the Institutional Animal Care and Use Committee at the University of Minnesota. B6.IcosYF/YF mice were crossed to Foxp3mRFP mice and were maintained at Benaroya Research Institute.
Pathogens and Infections
When applicable, mice were infected with LCMV Armstrong strain (2 × 105 PFU) by intraperitoneal (i.p.) injection; or L. monocytogenes expressing gp33 (LM-gp33) (3 × 103 CFU) by intravascular (i.v.) injection; or recombinant influenza virus PR8 expressing gp33 (PR8-gp33) (500 PFU) by intranasal (i.n.) infection following anesthesia with ketamine and xylazine.
METHOD DETAILS
Adoptive cell transfer
CD8+ P14 T cells were negatively enriched from WT and Icos−/− P14 mice with distinct congenic markers. The cell populations were mixed at a ratio of 1:1 and a total of 5 × 104 P14 T cells were co-adoptively transferred into recipient mice, which were infected the next day with the indicated pathogens. At the indicated times after infection, mice were sacrificed for analysis. The exact ratio of WT and Icos−/− P14 T cells were verified by flow cytometry immediately after each transfer, and these values were used to normalize the final ratios. For cross-transfers, primary recipient mice were co-transferred with WT and Icos−/− P14 T cells as above. Four-and-a-half days post LCMV infection, total CD8+ T cells were negatively enriched from spleen and peripheral LNs (including inguinal, cervical and mesenteric LNs) from each primary host and then transferred to the corresponding secondary host.
Bone-marrow chimera
Bone marrow cells were collected from femurs, tibias and humeri of indicated donor mice. Red blood cells were lysed in ACK lysis buffer and T cells were further depleted from bone marrow cells through anti-CD3 and anti-CD8α in a column-based separation. Recipient mice were lethally irradiated (2 × 500 rad separated by 4 hr). Single bone marrow chimeras were generated by i.v. injection of 107 bone marrow cells from WT or Icosl−/− donors into recipient mice at 2hr after the last irradiation. Similarly, mixed bone marrow chimeras were generated by i.v. injecting of a 1:1 mixture (5 ×106 cells each) of WT and Icos−/− (or IcosYF) bone marrow cells into recipient mice. Chimeric animals were rested for 8–10 weeks before infection.
Blocking antibody treatment
To block ICOS/ICOS-L interaction, anti-ICOS-L (rat IgG2a, clone HK5.3) and isotype control (anti-trinitrophenol, rat IgG2a, clone 2A3) monoclonal Abs (mAbs) were given to recipient mice three times in total at the described time points. Specifically, the first treatment was two injections via the i.v. and i.p. routes of 100 μg mAbs separately, and the following two treatments were 100 μg mAbs through i.p. injection only.
Retroviral transduction experiments
Congenic distinct WT P14 T cells were activated in vitro with plate-bounded anti-CD3 and anti-CD28 for 20hr at 37°C. Retroviruses encoding ICOS (pMIGR-ICOS) or empty vector control (pMIGR-EV) with GFP as transduction maker were then used to transduce those cell cultures through spin-infection at 2000 rpm for 90 min at 37°C. Cells were then rested overnight (~12hr) and counted. A 1:1 mix of cells (5 ×104 cells each) transduced with pMIGR-ICOS or pMIGR-EV were transferred into WT recipient mice that were infected with LCMV 2hr later.
Isolation of lymphocytes from tissues
Lymphocytes were isolated from tissues including spleen, inguinal lymph nodes, cervical lymph nodes, small intestine epithelium (SI-IEL), small intestine lamina propria (SI-LP), kidney, salivary glands (SG) and peripheral blood as previously described (Skon et al., 2013; Steinert et al., 2015). To differentiate vascular-associated circulating lymphocytes in NLTs, in vivo i.v. injection of PerCP-Cy5.5-conjugated CD8α antibody was performed as previously described (Anderson et al., 2014).
Flow cytometry
To identify LCMV-gp33 antigen-specific CD8+ T cells, H-2Db gp33 tetramers were prepared and stained as described previously (Daniels and Jameson, 2000). Direct ex vivo staining was performed after lymphocyte isolation as previously described (Borges-da-Silva et al., 2020; Skon et al., 2013). Flow cytometric analysis was performed on an LSRFortessa (BD Biosciences) and data were analyzed using FlowJo software (BD Biosciences).
Single cell RNA-seq
Post cell transfer and LCMV infection (as described above), donor cells were isolated from spleen, SI-IEL, and kidney on Day 5.5 or Day 32 post infection and collected by FACS (Aria II, BD Bioscience) using CD8α+TCR-Vα2+CD45.1+ (spleen) or CD8α-iv− (SI-IEL and Kidney) markers. Cells were stained with antibody-oligo derived tags (ADTs) against CD45.2 (BioLegend, A0157) and ICOS (BioLegend, A0171) to distinguish WT from Icos−/− cells and hashtag oligos (HTOs) to label cells by their tissue of origin (BioLegend, A0301, 0302, 0303, 0309, 0310, 0311). After sorting, cells from different tissues were mixed at equal proportion and captured in the same library using the 10x Genomics Chromium Single Cell 3’ (v 3.1) reagent kits, generating independent gene expression and feature barcode libraries for each time point (D5.5 and D32). Libraries were sequenced with Illumina NovaSeq 6000 (2×150bp PE) at the University of Minnesota Genomics Center.
Bioinformatics analysis
Raw sequencing data were processed using Cell Ranger (v 6.0.1; 10x Genomics) against the mouse genome (mm10, provided by 10x Genomics, ver 2020-A) to generate the mRNA transcript count table. Feature barcode (ADT and HTO) count tables were also generated by Cell Ranger count. Raw gene expression and feature barcode count data were analyzed with the Seurat package (v 3.0.3.9039) (Butler et al., 2018; Stuart et al., 2019) using R (ver 4.0.3). Each scRNA dataset (D5.5 or D32) were independently filtered to include only cells expressing >300 genes and genes expressed in more than 3 cells. The proportion of mitochondrial RNA in each cell was calculated and cells with extreme levels were removed from the analysis. Genes with extreme expression levels were removed. Contaminating cells expressing non-CD8+ T cell lineage marker genes were removed prior to downstream analysis. Each time point dataset was analyzed similarly in parallel and not integrated. Raw RNA counts were normalized and transformed using the Seurat SCTransform (Hafemeister and Satija, 2019) including the percent of mitochondria expression as regression factors. Each cell was labeled its cell cycle status according to the expression of canonical cell cycle genes using the Seurat CellCycleScoring function (S-phase and G2/M-phase gene sets from Tirosh et al. (Tirosh et al., 2016)). Raw RNA counts were normalized and transformed using Seurat SCTransform (Hafemeister and Satija, 2019) including the percent of mitochondria expression and cell cycle score as regression factors. Principal components analysis (PCA) was performed using the normalized, mean-centered, and scaled SCT dataset (RunPCA function). Two-dimensional projections were generated using the top 30 PCA vectors as input to RunUMAP function in Seurat. Cells from the same library (including two different genotypic populations from three different tissues) were clustered using the FindNeighbors (top 30 PCA vectors) and FindClusters functions (testing a range of possible resolutions: 0.1, 0.3, 0.4, 0.5, 0.7). A final resolution of 0.7 (D5.5) or 0.4 (D32) best represented the biological processes within each dataset.
After basic transcriptome analysis, the HTO feature barcode count table was analyzed using GMM-Demux (Xin et al., 2020) to classify each Gel Bead-in-Emulsion (GEM) by tissue of origin (spleen, SI-IEL or kidney). HTO singlet GEMs were retained and GMM-Demux was repeated using the ADT feature barcode count table to classify GEMs by strain/genotype (only WT cells should be CD45.2+ and ICOS+). GEMs with a GMM-Demux classification confidence value >= 0.7 were retained. Pairwise DE testing (Wilcox rank-sum) using the FindMarkers function was performed between all clusters (including WT and Icos−/− cells) or between WT and Icos−/− cells within each cluster. DE genes were significant based on log2-fold-change (≥0.25) and BH adjusted p value (≤0.01). Figures were generated using the ggplot2 R package (Wickham, 2016). To evaluate the residency features of each cell in our datasets, AddModuleScore function from Seurat was used to calculate the average expression levels of “Core Trm signature” or “Core Circulating signature” described in previous study (Milner et al., 2017) on single cell level.
Raw and processed data have been deposited at the Gene Expression Omnibus (GEO) database (accession number: GSE185342).
Icos and Icosl single-cell expression pattern were extracted from the previous Trm single-cell time course study (Kurd et al., 2020) or mouse cell atlas study (Han et al., 2018). Cell-type annotation and the filtered gene-cell UMI count tables were obtained directly from the geo deposit GSE131847 or GSE108097. Sequence depth were normalized, and square root transformed prior to imputation. We choose to use MAGIC (Dijk et al., 2018) to impute the scRNA-seq data. Icos and Icosl expression profile were extracted from the imputed data and transformed back into TPM (Transcripts Per Million) values. Violin plot was generated to visualize the Icos time-course expression pattern and top 15 Icosl expressing cell types in either adult small intestine or kidney, respectively.
STATISTICAL ANALYSIS
Details on statistics used can be found in figure legends. Data were firstly subjected to either D’Agostino-Pearson test, Kolmogorov-Smirnov test, or Shapiro-Wilk test to assess normality distribution of each sample. Unpaired two-tailed Student’s t-test (for parametric test) or Mann-Whitney test (for nonparametric test) was used as indicated when comparing two groups. For comparisons with more than two groups, statistical differences were calculated by Ordinary one-way ANOVA following with either Tukey’s multiple comparisons test (for comparisons between each other) or Dunnett’s multiple comparisons test (for comparisons to one “control”. For any situations where at least one group was not normally distributed, Kruskal-Wallis one-way ANOVA following with Dunn’s multiple comparisons test was used. All experiments were analyzed using Prism 9 (GraphPad Software). Samples are shown with medians with error bars showing the SEM. P values of >0.05 (not significant), <0.05 (*), <0.01 (**), <0.001 (***) or <0.0001 (****) indicated significant differences between groups.
Supplementary Material
Supplemental Table 1 (Related to Figure 6 A,B): Genes that were differentially expressed (Log2-fold-change ≥0.25, p-value ≤0.01 and detected in at least 10% of cells) between WT and Icos−/− cells at day 5.5 following LCMV infection are listed by the clusters shown in Figure 6 A,B.
Supplemental Table 2 (Related to Figure 6 F,G): Genes that were differentially expressed (Log2-fold-change ≥0.25, p-value ≤0.01 and detected in at least 10% of cells) between WT and Icos−/− cells at day 32 following LCMV infection are listed by the clusters shown in Figure 6 F,G.
Highlights.
Optimal generation of CD8+ resident- but not circulating-memory pools require ICOS.
ICOS stimulation after tissue seeding is critical for generation of CD8+ Trm cells.
ICOS-L expression by radioresistant populations drives CD8+ Trm induction.
ICOS deficiency limits Trm cell production with minimal effects on their transcriptome.
Acknowledgments
We thank the members of the Jamequist lab for intellectual input. The authors acknowledge the Minnesota Supercomputing Institute (MSI) at the University of Minnesota for providing resources that contributed to the research results reported. This work was funded by NIH awards to S.C.J. (R01 AI038903, AI145147). The graphical abstract was created with BioRender.com.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest
The authors declare no competing interests.
References
- Anderson KG, Mayer-Barber K, Sung H, Beura L, James BR, Taylor JJ, Qunaj L, Griffith TS, Vezys V, Barber DL, et al. (2014). Intravascular staining for discrimination of vascular and tissue leukocytes. Nat Protoc 9, nprot.2014.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariotti S, Hogenbirk MA, Dijkgraaf FE, Visser LL, Hoekstra ME, Song J-Y, Jacobs H, Haanen JB, and Schumacher TN (2014). Skin-resident memory CD8+ T cells trigger a state of tissue-wide pathogen alert. Science 346, 101–105. [DOI] [PubMed] [Google Scholar]
- Attanasio J, and Wherry EJ (2016). Costimulatory and Coinhibitory Receptor Pathways in Infectious Disease. Immunity 44, 1052–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltra J-C, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ, et al. (2020). Developmental Relationships of Four Exhausted CD8+ T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 52, 825–841.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram EM, Tafuri A, Shahinian A, Chan VSF, Hunziker L, Recher M, Ohashi PS, Mak TW, and Watts TH (2002). Role of ICOS versus CD28 in antiviral immunity. Eur J Immunol 32, 3376–3385. [DOI] [PubMed] [Google Scholar]
- Borges-da-Silva H, Peng C, Wang H, Wanhainen KM, Ma C, Lopez S, Khoruts A, Zhang N, and Jameson SC (2020). Sensing of ATP via the Purinergic Receptor P2RX7 Promotes CD8+ Trm Cell Generation by Enhancing Their Sensitivity to the Cytokine TGF-β. Immunity 53, 158–171.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmeister Y, Lischke T, Dahler AC, Mages HW, Lam K-P, Coyle AJ, Kroczek RA, and Hutloff A (2008). ICOS Controls the Pool Size of Effector-Memory and Regulatory T Cells. J Immunol 180, 774–782. [DOI] [PubMed] [Google Scholar]
- Butler A, Hoffman P, Smibert P, Papalexi E, and Satija R (2018). Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol 36, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, et al. (2012). Antigen-Independent Differentiation and Maintenance of Effector-like Resident Memory T Cells in Tissues. J Immunol 188, 4866–4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, and Flies DB (2013). Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol 13, 227–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YS, Kageyama R, Eto D, Escobar TC, Johnston RJ, Monticelli L, Lao C, and Crotty S (2011). ICOS Receptor Instructs T Follicular Helper Cell versus Effector Cell Differentiation via Induction of the Transcriptional Repressor Bcl6. Immunity 34, 932–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K, Batista NV, Girard M, Law JC, and Watts TH (2020). GITR differentially affects lung effector T cell subpopulations during influenza virus infection. J Leukocyte Biol 107, 953–970. [DOI] [PubMed] [Google Scholar]
- Chu K-L, Batista NV, Wang KC, Zhou AC, and Watts TH (2019). GITRL on inflammatory antigen presenting cells in the lung parenchyma provides signal 4 for T-cell accumulation and tissue-resident memory T-cell formation. Mucosal Immunol 12, 363–377. [DOI] [PubMed] [Google Scholar]
- Crotty S (2019). T Follicular Helper Cell Biology: A Decade of Discovery and Diseases. Immunity 50, 1132–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MA, and Jameson SC (2000). Critical Role for Cd8 in T Cell Receptor Binding and Activation by Peptide/Major Histocompatibility Complex Multimers. J Exp Medicine 191, 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk D, Sharma R, Nainys J, Yim K, Kathail P, Carr AJ, Burdziak C, Moon KR, Chaffer CL, Pattabiraman D, et al. (2018). Recovering Gene Interactions from Single-Cell Data Using Data Diffusion. Cell 174, 716–729.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durlanik S, Loyal L, Stark R, Alp ÖS, Hartung A, Radbruch A, von Herrath M, Matzmohr N, Frentsch M, and Thiel A (2016). CD40L expression by CD4 + but not CD8 + T cells regulates antiviral immune responses in acute LCMV infection in mice: CD40L expression by CD4+ but not CD8+ T cell regulates. Eur J Immunol 46, 2566–2573. [DOI] [PubMed] [Google Scholar]
- Esensten JH, Helou YA, Chopra G, Weiss A, and Bluestone JA (2016). CD28 Costimulation: From Mechanism to Therapy. Immunity 44, 973–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigoux M, Shang J, Pak Y, Xu M, Choe J, Mak TW, and Suh W-K (2009). Inducible costimulator promotes helper T-cell differentiation through phosphoinositide 3-kinase. Proceedings of the National Academy of Sciences 106, 20371–20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafemeister C, and Satija R (2019). Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol 20, 296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Wang R, Zhou Y, Fei L, Sun H, Lai S, Saadatpour A, Zhou Z, Chen H, Ye F, et al. (2018). Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 172, 1091–1107.e17. [DOI] [PubMed] [Google Scholar]
- Hedrick SM, Michelini RH, Doedens AL, Goldrath AW, and Stone EL (2012). FOXO transcription factors throughout T cell biology. Nat Rev Immunol 12, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutloff A, Dittrich AM, Beier KC, Eljaschewitsch B, Kraft R, Anagnostopoulos I, and Kroczek RA (1999). ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature 397, 263–266. [DOI] [PubMed] [Google Scholar]
- Iborra S, Martínez-López M, Khouili SC, Enamorado M, Cueto FJ, Conde-Garrosa R, del Fresno C, and Sancho D (2016). Optimal Generation of Tissue-Resident but Not Circulating Memory T Cells during Viral Infection Requires Crosspriming by DNGR-1+ Dendritic Cells. Immunity 45, 847–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Hashimoto M, Gerner MY, Lee J, Kissick HT, Burger MC, Shan Q, Hale JS, Lee J, Nasti TH, et al. (2016). Defining CD8+ T cells that provide the proliferative burst after PD-1 therapy. Nature 537, 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im SJ, Konieczny BT, Hudson WH, Masopust D, and Ahmed R (2020). PD-1+ stemlike CD8 T cells are resident in lymphoid tissues during persistent LCMV infection. Proc National Acad Sci 201917298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson SC, and Masopust D (2018). Understanding Subset Diversity in T Cell Memory. Immunity 48, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopf M, Coyle AJ, Schmitz N, Barner M, Oxenius A, Gallimore A, Gutierrez-Ramos J-C, and Bachmann MF (2000). Inducible Costimulator Protein (Icos) Controls T Helper Cell Subset Polarization after Virus and Parasite Infection. J Exp Medicine 192, 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BV, Ma W, Miron M, Granot T, Guyer RS, Carpenter DJ, Senda T, Sun X, Ho S-H, Lerner H, et al. (2017). Human Tissue-Resident Memory T Cells Are Defined by Core Transcriptional and Functional Signatures in Lymphoid and Mucosal Sites. Cell Reports 20, 2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künzli M, Schreiner D, Pereboom TC, Swarnalekha N, Litzler LC, Lötscher J, Ertuna YI, Roux J, Geier F, Jakob RP, et al. (2020). Long-lived T follicular helper cells retain plasticity and help sustain humoral immunity. Sci Immunol 5, eaay5552. [DOI] [PubMed] [Google Scholar]
- Kurd NS, He Z, Louis TL, Milner JJ, Omilusik KD, Jin W, Tsai MS, Widjaja CE, Kanbar JN, Olvera JG, et al. (2020). Early precursors and molecular determinants of tissue-resident memory CD8+ T lymphocytes revealed by single-cell RNA sequencing. Sci Immunol 5, eaaz6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Skon CN, Lee YJ, Oh S, Taylor JJ, Malhotra D, Jenkins MK, Rosenfeld MG, Hogquist KA, and Jameson SC (2015). The Transcription Factor KLF2 Restrains CD4+ T Follicular Helper Cell Differentiation. Immunity 42, 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois L, Olson S, and Masopust D (1999). A Critical Role for Cd40–Cd40 Ligand Interactions in Amplification of the Mucosal Cd8 T Cell Response. J Exp Medicine 190, 1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Burd EM, Coopersmith CM, and Ford ML (2016). Retrogenic ICOS Expression Increases Differentiation of KLRG-1hiCD127loCD8+ T Cells during Listeria Infection and Diminishes Recall Responses. J Immunol 196, 1000–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bai X-F, Wen J, Gao J-X, Liu J, Lu P, Wang Y, Zheng P, and Liu Y (2001). B7H Costimulates Clonal Expansion of, and Cognate Destruction of Tumor Cells by, CD8+ T Lymphocytes In Vivo. J Exp Medicine 194, 1339–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maazi H, Patel N, Sankaranarayanan I, Suzuki Y, Rigas D, Soroosh P, Freeman GJ, Sharpe AH, and Akbari O (2015). ICOS:ICOS-Ligand Interaction Is Required for Type 2 Innate Lymphoid Cell Function, Homeostasis, and Induction of Airway Hyperreactivity. Immunity 42, 538–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, et al. (2013). The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Immunol 14, 1294–1301. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Wynne-Jones E, Freestone D, Pellicci DG, Mielke LA, Newman DM, Braun A, Masson F, Kallies A, Belz GT, et al. (2015). T-box Transcription Factors Combine with the Cytokines TGF-β and IL-15 to Control Tissue-Resident Memory T Cell Fate. Immunity 43, 1101–1111. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Minnich M, Kragten NA, Liao Y, Nota B, Seillet C, Zaid A, Man K, Preston S, Freestone D, et al. (2016). Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science 352, 459–463. [DOI] [PubMed] [Google Scholar]
- Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, Molodtsov AK, Bowers JS, Angeles CV, Paulos CM, et al. (2017). Resident memory T cells in the skin mediate durable immunity to melanoma. Science Immunology 2, eaam6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani V, Bromley SK, Äijö T, Mora-Buch R, Carrizosa E, Warner RD, Hamze M, Sen DR, Chasse AY, Lorant A, et al. (2019). Migratory DCs activate TGF-β to precondition naïve CD8+ T cells for tissue-resident memory fate. Science 366, eaav5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott CL, Carlesso G, Herbst R, and Withers DR (2015). ICOS is required for the generation of both central and effector CD4(+) memory T-cell populations following acute bacterial infection. Eur J Immunol 45, 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, and Soerens AG (2019). Tissue-Resident T Cells and Other Resident Leukocytes. Annu Rev Immunol 37, 521–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Jiang J, Shen H, and Lefrançois L (2001). Direct Analysis of the Dynamics of the Intestinal Mucosa CD8 T Cell Response to Systemic Virus Infection. J Immunol 166, 2348–2356. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, and Ahmed R (2006). Cutting Edge: Gut Microenvironment Promotes Differentiation of a Unique Memory CD8 T Cell Population. J Immunol 176, 2079–2083. [DOI] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, et al. (2010). Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Medicine 207, 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JJ, Toma C, Yu B, Zhang K, Omilusik K, Phan AT, Wang D, Getzler AJ, Nguyen T, Crotty S, et al. (2017). Runx3 programs CD8+ T cell residency in non-lymphoid tissues and tumours. Nature 552, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittelsteadt KL, Hayes ET, and Campbell DJ (2021). ICOS signaling limits regulatory T cell accumulation and function in visceral adipose tissue. J Exp Med 218, e20201142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittrücker H-W, Kursar M, Köhler A, Yanagihara D, Yoshinaga SK, and Kaufmann SHE (2002). Inducible Costimulator Protein Controls the Protective T Cell Response Against Listeria monocytogenes. J Immunol 169, 5813–5817. [DOI] [PubMed] [Google Scholar]
- Moore TV, Clay BS, Ferreira CM, Williams JW, Rogozinska M, Cannon JL, Shilling RA, Marzo AL, and Sperling AI (2011a). Protective Effector Memory CD4 T Cells Depend on ICOS for Survival. Plos One 6, e16529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TV, Clay BS, Cannon JL, Histed A, Shilling RA, and Sperling AI (2011b). Inducible Costimulator Controls Migration of T Cells to the Lungs via Down-Regulation of CCR7 and CD62L. American Journal of Respiratory Cell and Molecular Biology 45, 843–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SN, and Mackay LK (2016). Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol 16, 79–89. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Mai XM, Forbush K, Bevan MJ, and Dong C (2003). B7h is required for T cell activation, differentiation, and effector function. Proc National Acad Sci 100, 14163–14168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SL, Buzzai A, Rautela J, Hor JL, Hochheiser K, Effern M, McBain N, Wagner T, Edwards J, McConville R, et al. (2019). Tissue-resident memory CD8+ T cells promote melanoma–immune equilibrium in skin. Nature 565, 366–371. [DOI] [PubMed] [Google Scholar]
- Pedros C, Zhang Y, Hu JK, Choi YS, Canonigo-Balancio AJ III, J. RY, Altman A, Crotty S, and Kong K-F (2016). A TRAF-like motif of the inducible costimulator ICOS controls development of germinal center TFH cells via the kinase TBK1. Nat Immunol 17, 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C, and Jameson SC (2020). The relationship between CD4+ follicular helper T cells and CD8+ resident memory T cells: sisters or distant cousins? Int Immunol 32, dxaa045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C, Kim S-K, Marzo A, Williams K, Jiang J, Shen H, and Lefrançois L (2001). Organ-Specific Regulation of the CD8 T Cell Response to Listeria monocytogenes Infection. J Immunol 166, 3402–3409. [DOI] [PubMed] [Google Scholar]
- Qi H (2016). T follicular helper cells in space-time. Nat Rev Immunol 16, 612–625. [DOI] [PubMed] [Google Scholar]
- Roussel L, Landekic M, Golizeh M, Gavino C, Zhong M-C, Chen J, Faubert D, Blanchet-Cohen A, Dansereau L, Parent M-A, et al. (2018). Loss of human ICOSL results in combined immunodeficiency. J Exp Med 215, 3151–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salek-Ardakani S, Moutaftsi M, Sette A, and Croft M (2011). Targeting OX40 Promotes Lung-Resident Memory CD8 T Cell Populations That Protect against Respiratory Poxvirus Infection. J Virol 85, 9051–9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, and Masopust D (2013). Sensing and alarm function of resident memory CD8+ T cells. Nat Immunol 14, 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, and Masopust D (2014). Cutting Edge: Resident Memory CD8 T Cells Occupy Frontline Niches in Secondary Lymphoid Organs. J Immunol 192, 2961–2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon CN, Lee J-Y, Anderson KG, Masopust D, Hogquist KA, and Jameson SC (2013). Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8+ T cells. Nat Immunol 14, 1285–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smigiel KS, Richards E, Srivastava S, Thomas KR, Dudda JC, Klonowski KD, and Campbell DJ (2014). CCR7 provides localized access to IL-2 and defines homeostatically distinct regulatory T cell subsets. J Exp Med 211, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son YM, Cheon IS, Wu Y, Li C, Wang Z, Gao X, Chen Y, Takahashi Y, Fu Y-X, Dent AL, et al. (2021). Tissue-resident CD4+ T helper cells assist the development of protective respiratory B and CD8+ T cell memory responses. Sci Immunol 6, eabb6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, Southern PJ, and Masopust D (2015). Quantifying Memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell 161, 737–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EL, Pepper M, Katayama CD, Kerdiles YM, Lai C-Y, Emslie E, Lin YC, Yang E, Goldrath AW, Li MO, et al. (2015). ICOS Coreceptor Signaling Inactivates the Transcription Factor FOXO1 to Promote Tfh Cell Differentiation. Immunity 42, 239–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM, Hao Y, Stoeckius M, Smibert P, and Satija R (2019). Comprehensive Integration of Single-Cell Data. Cell 177, 1888–1902.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow MM, Wallin JJ, and Sha WC (1999). B7h, a Novel Costimulatory Homolog of B7.1 and B7.2, Is Induced by TNFα. Immunity 11, 423–432. [DOI] [PubMed] [Google Scholar]
- Swarnalekha N, Schreiner D, Litzler LC, Iftikhar S, Kirchmeier D, Künzli M, Son YM, Sun J, Moreira EA, and King CG (2021). T resident helper cells promote humoral responses in the lung. Sci Immunol 6, eabb6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo PA, Miron M, and Farber DL (2019). Location, location, location: Tissue resident memory T cells in mice and humans. Sci Immunol 4, eaas9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N, Matsumoto K, Saito H, Nanki T, Miyasaka N, Kobata T, Azuma M, Lee S-K, Mizutani S, and Morio T (2009). Impaired CD4 and CD8 Effector Function and Decreased Memory T Cell Populations in ICOS-Deficient Patients. J Immunol 182, 5515–5527. [DOI] [PubMed] [Google Scholar]
- Taylor PA, Panoskaltsis-Mortari A, Freeman GJ, Sharpe AH, Noelle RJ, Rudensky AY, Mak TW, Serody JS, and Blazar BR (2005). Targeting of inducible costimulator (ICOS) expressed on alloreactive T cells down-regulates graftversus-host disease (GVHD) and facilitates engraftment of allogeneic bone marrow (BM). Blood 105, 3372–3380. [DOI] [PubMed] [Google Scholar]
- Teichmann LL, Cullen JL, Kashgarian M, Dong C, Craft J, and Shlomchik MJ (2015). Local Triggering of the ICOS Coreceptor by CD11c+ Myeloid Cells Drives Organ Inflammation in Lupus. Immunity 42, 552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, Rotem A, Rodman C, Lian C, Murphy G, et al. (2016). Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallin JJ, Liang L, Bakardjiev A, and Sha WC (2001). Enhancement of CD8+ T Cell Responses by ICOS/B7h Costimulation. J Immunol 167, 132–139. [DOI] [PubMed] [Google Scholar]
- Weber JP, Fuhrmann F, Feist RK, Lahmann A, Baz MSA, Gentz L-J, Van DV, Mages HW, Haftmann C, Riedel R, et al. (2015). ICOS maintains the T follicular helper cell phenotype by down-regulating Krüppel-like factor 2. J Exp Med 212, 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich MA, Takada K, Skon C, Reiner SL, Jameson SC, and Hogquist KA (2009). KLF2 transcription-factor deficiency in T cells results in unrestrained cytokine production and upregulation of bystander chemokine receptors. Immunity 31, 122–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016). ggplot2: Elegant Graphics for Data Analysis (Springer-Verlag; New York: ). [Google Scholar]
- Xin H, Lian Q, Jiang Y, Luo J, Wang X, Erb C, Xu Z, Zhang X, HeidrichO’Hare E, Yan Q, et al. (2020). GMM-Demux: sample demultiplexing, multiplet detection, experiment planning, and novel cell-type verification in single cell sequencing. Genome Biol 21, 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Li X, Liu D, Li J, Zhang X, Chen X, Hou S, Peng L, Xu C, Liu W, et al. (2013). Follicular T-helper cell recruitment governed by bystander B cells and ICOS-driven motility. Nature 496, 523–527. [DOI] [PubMed] [Google Scholar]
- Zamarin D, Holmgaard RB, Ricca J, Plitt T, Palese P, Sharma P, Merghoub T, Wolchok JD, and Allison JP (2016). Intratumoral modulation of the inducible co-stimulator ICOS by recombinant oncolytic virus promotes systemic anti-tumour immunity. Nature Communications 8, ncomms14340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou AC, Wagar LE, Wortzman ME, and Watts TH (2017). Intrinsic 4–1BB signals are indispensable for the establishment of an influenza-specific tissue-resident memory CD8 T-cell population in the lung. Mucosal Immunol 10, 1294–1309. [DOI] [PubMed] [Google Scholar]
- Zhou AC, Batista NV, and Watts TH (2019). 4–1BB Regulates Effector CD8 T Cell Accumulation in the Lung Tissue through a TRAF1-, mTOR-, and Antigen-Dependent Mechanism to Enhance Tissue-Resident Memory T Cell Formation during Respiratory Influenza Infection. J Immunol 202, 2482–2492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1 (Related to Figure 6 A,B): Genes that were differentially expressed (Log2-fold-change ≥0.25, p-value ≤0.01 and detected in at least 10% of cells) between WT and Icos−/− cells at day 5.5 following LCMV infection are listed by the clusters shown in Figure 6 A,B.
Supplemental Table 2 (Related to Figure 6 F,G): Genes that were differentially expressed (Log2-fold-change ≥0.25, p-value ≤0.01 and detected in at least 10% of cells) between WT and Icos−/− cells at day 32 following LCMV infection are listed by the clusters shown in Figure 6 F,G.
Data Availability Statement
Single-cell RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. Accession numbers are listed under “Bioinformatics analysis” and in the key resources table.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-mouse CD8a BUV395 (53-6.7) | BD Biosciences | Cat# 563786, RRID: AB_2732919 |
| Anti-mouse CD8a PerCP-Cy5.5 (53-6.7) | Tonbo Biosciences | Cat# 65-0081, RRID:AB_2621882 |
| Anti-mouse CD8a PE (53-6.7) | Tonbo Biosciences | Cat# 50-0081, RRID:AB_2621741 |
| Anti-Mouse CD45.1 violetFluor 450 (A20) | Tonbo Biosciences | Cat# 75-0453, RRID:AB_2621949 |
| Anti-Mouse CD45.1 PE-Cyanine7 (A20) | Tonbo Biosciences | Cat# 60-0453, RRID:AB_2621850 |
| Anti-Mouse CD45.2 violetFluor 450 (104) | Tonbo Biosciences | Cat# 75-0454, RRID:AB_2621950 |
| Anti-Mouse CD45.2 PE-Cyanine7 (104) | Tonbo Biosciences | Cat# 60-0454, RRID:AB_2621851 |
| Anti-Mouse CD45.2 FITC (104) | Tonbo Biosciences | Cat# 35-0454, RRID:AB_2621692 |
| Anti-human/mouse/rat CD278 (ICOS) PE (C398.4A) | BioLegend | Cat# 313508, RRID:AB_416332 |
| Anti-human/mouse/rat CD278 (ICOS) APC (C398.4A) | BioLegend | Cat# 313510, RRID:AB_416334 |
| Anti-mouse CD44 BV786 (IM7) | BD Biosciences | Cat# 563736, RRID:AB_2738395 |
| Anti-mouse CD44 BV605 (IM7) | BD Biosciences | Cat# 563058, RRID:AB_2737979 |
| Anti-mouse CD62L BV786 (MEL-14) | BD Biosciences | Cat# 564109, RRID:AB_2738598 |
| Anti-mouse CD62L BV605 (MEL-14) | BioLegend | Cat# 104438, RRID:AB_2563058 |
| Anti-mouse KLRG1 PE-Cyanine7 (2F1) | Thermo Fisher Scientific | Cat# 25-5893-82, RRID:AB_1518768 |
| Anti-mouse KLRG1 BV711 (2F1) | BD Biosciences | Cat# 564014, RRID:AB_2738542 |
| Anti-Mouse CD127 BUV737 (SB/199) | BD Biosciences | Cat# 612841, RRID:AB_2870163 |
| Anti-Mouse CD69 PE (H1.2F3) | Thermo Fisher Scientific | Cat# 12-0691-83, RRID:AB_465733 |
| Anti-Mouse CD69 PE-CF594 (H1.2F3) | BD Biosciences | Cat# 562455, RRID:AB_11154217 |
| Anti-mouse CD103 BV510 (M290) | BD Biosciences | Cat# 563087, RRID:AB_2721775 |
| Anti-mouse CD103 APC (2E7) | BioLegend | Cat# 121414, RRID:AB_1227502 |
| Anti-mouse CD275 (B7-RP1, ICOSL, B7H2) PE (HK5.3) | BioLegend | Cat# 107405, RRID:AB_2248797 |
| Anti-mouse LPAM-1 (Integrin a4b7) PE (DATK32) | BioLegend | Cat# 120606, RRID:AB_493267 |
| Anti-Mouse CD45 APC (30-F11) | Tonbo Biosciences | Cat# 20-0451, RRID:AB_2621573 |
| Anti-Mouse CD19 PE-Cyanine7 (1D3) | Tonbo Biosciences | Cat# 60-0193, RRID:AB_2621840 |
| Anti-Mouse CD45R/B220 BUV395 (RA3-6B2) | BD Biosciences | Cat# 563793, RRID:AB_2738427 |
| Anti-Mouse Va2 TCR APC (B20.1) | BD Biosciences | Cat# 560622, RRID:AB_1727582 |
| Ghost Dye Red 780 Viability Dye | Tonbo Biosciences | Cat # 13-0865-T100 |
| InVivoMAb anti-mouse CD3ε (145-2C11) | BioXCell | Cat# BE0001-1, RRID:AB_1107634 |
| InVivoMAb anti-mouse CD28 (37.51) | BioXCell | Cat# BE0015-1, RRID:AB_1107624 |
| InVivoMAb anti-mouse ICOSL (CD275) (HK5.3) | BioXCell | Cat# BE0028, RRID:AB_1107566 |
| InVivoMAb rat IgG2a isotype control (2A3) | BioXCell | Cat# BE0089, RRID:AB_1107769 |
| TotalSeq™-A0157 anti-mouse CD45.2 (104) | BioLegend | Cat# 109853, RRID:AB_2783051 |
| TotalSeq™-A0171 anti-human/mouse/rat CD278 (ICOS) (C398.4A) | BioLegend | Cat# 313555, RRID:AB_2800824 |
| TotalSeq™-A0301 anti-mouse Hashtag 1 (M1/42; 30-F11) | BioLegend | Cat# 155801, RRID:AB_2750032 |
| TotalSeq™-A0302 anti-mouse Hashtag 2 (M1/42; 30-F11) | BioLegend | Cat# 155803, RRID:AB_2750033 |
| TotalSeq™-A0303 anti-mouse Hashtag 3 (M1/42; 30-F11) | BioLegend | Cat# 155805, RRID:AB_2750034 |
| TotalSeq™-A0309 anti-mouse Hashtag 9 (M1/42; 30-F11) | BioLegend | Cat# 155817, RRID:AB_2750042 |
| TotalSeq™-A0310 anti-mouse Hashtag 10 (M1/42; 30-F11) | BioLegend | Cat# 155819, RRID:AB_2750043 |
| TotalSeq™-A0311 anti-mouse Hashtag 11 (M1/42; 30-F11) | BioLegend | Cat# 155821, RRID:AB_2750136 |
| Bacterial and virus strains | ||
| Lymphocytic choriomeningitis virus (LCMV) Armstrong strain | Dr. R. Ahmed, Emory University | N/A |
| Listeria monocytogenes-GP33 (LM-gp33) Generated by Dr. Hao Shen (UPenn) first reported PMID: 11323695 | Dr. R. Ahmed, Emory University | N/A |
| Influenza A-PR8-gp33. Generated by Dr. Ryan Langois (UMN), first reported in PMID: 31235953. | Dr. D. Masopust, (UMN) | N/A |
| Chemicals, peptides, and recombinant proteins | ||
| Collagenase, Type 1 | Worthington | Cat# LS004197 |
| Percoll | GE Healthcare | Cat# 17-0891-09 |
| Dithioerythritol | EMD Millipore | Cat# 233152-5GM |
| RPMI 1640 | Corning | Cat# 10-040-CV |
| HBSS 10x | Corning | Cat# 20-021-CV |
| Fetal Bovine Serum | Atlas Biologicals | Cat# FS-0500-AD |
| L-Glutamine, 100x, Liquid | Corning | Cat# 25-005-CI |
| Penicillin/Streptomycin | Gibco | Cat# 15070063 |
| DMEM | Gibco | Cat# 11995065 |
| Critical commercial assays | ||
| CD8a+ T cell Isolation Kit, Mouse | Miltenyi Biotec | Cat# 130-104-075 |
| BD Cytofix/Cytoperm Solution Kit | BD Biosciences | Cat#554714 |
| Deposited data | ||
| scRNA-seq of co-transferred WT and Icos−/− P14 T cells | this paper | GEO: GSE185342 |
| TRM single-cell time course study | Kurd et al., 2020 | GEO: GSE131847 |
| mouse cell atlas study | Han et al., 2018 | GEO: GSE108097 |
| Experimental models: Cell lines | ||
| Plat-E | Cell Biolabs | Cat#RV-101, RRID: CVCL_B488 |
| Experimental models: Organisms/strains | ||
| Mouse: B6.SJL-PtprcaPepcb/BoyCrCrl (B6-CD45.1) | NCI Charles River | Cat# CRL:564, RRID:IMSR_CRL:564 |
| Mouse: C57BL/6NCrl (B6-CD45.2) | NCI Charles River | Cat# CRL:027, RRID:IMSR_CRL:027 |
| Mouse: P14 | Dr. R. Ahmed, Emory University | N/A |
| Mouse: B6.129P2-Icostm1Mak/J (ICOS-KO) | The Jackson Laboratory | Cat# JAX:004859, RRID:IMSR_JAX:004859 |
| Mouse: B6.129P2-Icosltm1Mak/J (ICOSL-KO) | The Jackson Laboratory | Cat# JAX:004657, RRID:IMSR_JAX:004657 |
| Mouse: B6(C)-Klf2tm1.1Khog/JmsnJ (KLF2-GFP) | Generated in lab (Weinreich et al., 2009) | RRID:IMSR_JAX:036331 |
| Mouse: ICOS-YF | Dr. D. Campbell (Mittelsteadt et al., 2021) | N/A |
| Oligonucleotides | ||
| N/A | ||
| Recombinant DNA | ||
| MIGR1 (pMIGR-EV) | Dr. W. Pear (Addgene) | Cat# 27490 |
| pMIGR-mICOS | in-house | N/A |
| Software and algorithms | ||
| GraphPad Prism v9.2.0 | GraphPad Software | RRID:SCR_002798 |
| FlowJo v10.8 | BD Biosciences | RRID:SCR_008520 |
| BD FACSDiva | BD Biosciences | RRID:SCR_001456 |
| Cell Ranger v6.0.1 | 10xGenomics | RRID:SCR_017344 |
| Seurat v 3.0.3.9039 | https://satijalab.org/seurat/get_started.html | RRID:SCR_016341 |
| ggplot2 | https://cran.r-project.org/web/packages/ggplot2/index.html | RRID:SCR_014601 |
| MAGIC | van Dijk et al., 2018 | N/A |
| Other | ||
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.


