Abstract
Berberine is a natural product that has long been used in traditional Chinese medicine due to its antimicrobial, anti-inflammatory and metabolism-regulatory properties. Osimertinib is the first third-generation EGFR-tyrosine kinase inhibitor (TKI) approved for the treatment of non-small cell lung cancer (NSCLC) with activating EGFR mutations and those resistant to earlier generation EGFR-TKIs due to a T790M mutation. However, emergence of acquired resistance to osimertinib limits its long-term efficacy in the clinic. One known mechanism of acquired resistance to osimertinib and other EGFR-TKIs is MET (c-MET) gene amplification. Here, we report that berberine, when combined with osimertinib, synergistically and selectively decreased the survival of several MET-amplified osimertinib-resistant EGFR mutant NSCLC cell lines with enhanced induction of apoptosis likely through Bim elevation and Mcl-1 reduction. Importantly, this combination effectively enhanced suppressive effect on the growth of MET-amplified osimertinib-resistant xenografts in nude mice and was well tolerated. Molecular modeling showed that berberine was able to bind to the kinase domain of non-phosphorylated MET, occupy the front of the binding pocket, and interact with the activation loop, in a similar way as other known MET inhibitors do. MET kinase assay showed clear concentration-dependent inhibitory effects of berberine against MET activity, confirming its kinase inhibitory activity. These findings collectively suggest that berberine can act as a naturally-existing MET inhibitor to synergize with osimertinib in overcoming osimertinib acquired resistance caused by MET amplification.
Keywords: Berberine, EGFR, osimertinib, resistance, MET, lung cancer
Graphical abstract
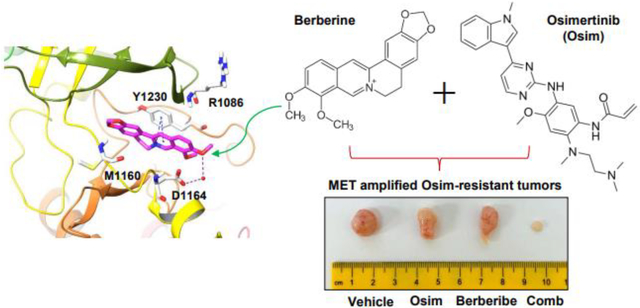
1. Background
Lung cancer remains the leading cause of cancer mortality worldwide. Non-small cell lung cancer (NSCLC) constitutes over 80% of lung cancer cases, and has an improved 5 year survival rate of close to 20% recently due to the advances in early diagnosis and availability of various targeted therapies including immunotherapy (1, 2). A significant advance in lung cancer treatment occurred with the discovery of epidermal growth factor receptor (EGFR) activating mutations as a therapeutic target, which led to the development of EGFR tyrosine kinase inhibitors (EGFR-TKIs) for NSCLC. This novel targeted therapy has greatly benefited patients (3, 4). Unfortunately, patients eventually relapsed to these EGFR-TKIs largely due to development of acquired resistance, limiting its long-term therapeutic efficacy.
Osimertinib (AZD9291 or TAGRISSO®) is the first approved third-generation EGFR-TKI that targets both the common activating EGFR mutations such as 19del and L858R and the acquired T790M mutation that causes resistance to earlier generation EGFR-TKIs. Thus, osimertinib is often used as a second-line treatment when resistance to other EGFR-TKIs has emerged and as a frontline treatment for NSCLC patients with activating EGFR mutations. However, acquired resistance eventually occurs after about 10 and 19 months, respectively, resulting in disease progression and treatment failure (5, 6). Therefore, it is imperative to understand the mechanisms underlying osimertinib acquired resistance and accordingly develop novel strategies to improve clinical outcomes for resistant patients via overcoming the acquired resistance.
One well-characterized major resistant mechanism is MET (or c-MET) gene amplification, which is found in approximately 5–22% of EGFR-mutant (EGFRm) NSCLC patients with acquired resistance to first-generation EGFR-TKIs (7). MET amplification bypasses first generation EGFR-TKIs by activating EGFR-independent phosphorylation of ErbB3 and downstream activation of the PI3K/AKT pathway (7). Beyond, MET amplification is also a critical resistance mechanism to osimertinib and other third-generation EGFR-TKIs, and accounts for 5–30% of resistant cases (7–9). Moreover, patients who became resistant to osimertinib due to MET amplification had worse survival rates compared to patients without MET amplification (9). Accordingly, combination treatment with an EGFR-TKI and a MET inhibitor such as crizotinib generated impressive effects against the growth of osimertinib-resistant tumors with MET amplification in preclinical models (8, 10) and achieved a partial response in patients with acquired osimertinib resistance caused by MET amplification (9).
Berberine is an isoquinoline alkaloid compound extracted mainly from the coptis chinensis plant, which has been extensively used in traditional Chinese medicine (11, 12). This natural product is traditionally used for its antimicrobial, anti-inflammatory and metabolism-modulatory properties. Today, berberine and its derivatives are being studied as possible therapeutics for a myriad of diseases, including diabetes, obesity, polycystic ovary syndrome, coronary artery disease, and cancer (11–13). Berberine showed anti-tumor effects in several types of cancer cell lines and tumors, including lung cancer, liver cancer, breast cancer and leukemia with different proposed mechanisms including induction of cell cycle arrest and/or apoptosis, inhibition of cancer invasion and metastasis, and modulation of tumor microenvironment (14, 15). In this study, we have discovered MET as a novel target for berberine, revealing a new mechanism accounting for the synergistic and selective effects of berberine combined with osimertinib against the growth of MET-amplified, osimertinib-resistant EGFRm NSCLC cells and tumors. Findings in this study strongly suggest the therapeutic potential of berberine as a MET inhibitor, when combined with osimertinib or other third generation EGFR-TKIs, in overcoming acquired resistance to osimertinib or other third generation EGFR-TKIs caused by MET amplification.
2. Material and Methods
2.1. Chemicals and reagents
Berberine was purchased from Sigma (B3251; St. Louis, MO). Osimertinib was purchased from LC laboratories (O-7200; Woburn, MA). The compounds were dissolved in DMSO and aliquots were stored at −80°C. Hepatocyte growth factor (HGF) was purchased from ProteinTech Group, Inc (HZ-1084; Rosemont, IL). Antibodies and other reagents used were the same as described in previous works (8, 16).
2.2. Cell lines and cell culture
The human EGFRm NSCLC cell lines and their derived resistant cell lines used in this study were described previously (8, 10, 16). Bim knockout (KO) and ectopic Mcl-1 expression in HCC827/AR cell lines were established in our pervious study (10). These cell lines have not been authenticated recently. All cell lines were cultured in RPMI 1640 media (Corning; Corning, NY) with 5% fetal bovine serum (Gemini Bio-Products; West Sacramento, CA) in a fully humidified incubator with 5% CO2.
2.3. Detection of MET gene copy
Extraction of DNA and detection of the MET gene copy number were done using the same methods previously described (8).
2.4. Cell survival and colony formation assays
Cells were seeded in 96-well plates and next day exposed to different tested drugs for 72 hours. Cell numbers were determined using the sulforhodamine B (SRB) assay as previously described (17). Cell growth was also determined using a colony formation assay as we previously described (16). Combinational index (CI) for drug interaction was calculated based on cell survival data using CompuSyn software (ComboSyn, Inc; Paramus, NJ).
2.5. Apoptosis assays
Apoptosis was evaluated with the annexin V/7-AAD apoptosis detection kit (BD Biosciences; San Jose, CA) according to the manufacturer’s protocol. Apoptosis was also shown by protein cleavages detected by Western blotting.
2.6. Western blot analysis
Preparation of whole-cell protein lysates and Western blot analysis were performed as previously described (18).
2.7. Molecular docking
The molecular docking study was performed with Small Molecule Drug Discovery Suite (Schrödinger Release 2020–4, Schrödinger, LLC; New York, NY). Crystal structure of non-phosphorylated MET (PDB ID: 3ZXZ) was obtained from RCSB protein database (PDB) bank and preprocessed with Schrödinger Protein Preparation Wizard. A grid box in size of 20 Å on all sides was generated using Glide Grid and was centered on the existing ligand PF-04217903. Berberine 3-D structure was downloaded from PubChem and was prepared with LigPrep to generate a suitable conformation for docking. Ligand docking was performed with Glide using SP mode with default settings. The docked poses were incorporated into Maestro pose viewer for visualization and binding site interaction analysis. The top ranked docked pose was imported into Maestro to overlay with the co-crystal structure of non-phosphorylated MET for a binding mode comparison.
2.8. MET kinase assay
The MET kinase assay was performed using MET Kinase Enzyme System purchased from Promega (V3361; Madison, WI) according to the manufacturer’s instructions.
2.9. Animal xenograft and treatments
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Emory University. HCC827/AR cells suspended in sterile PBS at 3 × 106 per mouse were injected into the flank of 4 week old nu/nu nude mice purchased from The Jackson Laboratory (Bar Harbor, Maine). On day 7, when the average tumor was 75 mm3, the mice were divided into groups with equal average tumor volumes and body weights. The following treatments were administered daily: vehicle, osimertinib (5 mg/kg, og), berberine (25 mg/kg, ip), and the combination of osimertinib and berberine. Tumor volume was measured using calipers every 2 or 3 days and calculated by V = π(length × width2)/6. Body weight was also measured every 2 or 3 days. At the end of the experiment, mice were sacrificed using CO2. The tumors were then removed, weighed and stored in formalin for further analysis.
2.10. Immunohistochemistry (IHC)
Tissue slides were dewaxed with xylene followed by rehydration with a graded alcohol series. Tissue slides were incubated in 3% (v/v) hydrogen peroxide for 10 min and then blocked with 10% BAS in PBS for 1 h. After being washed with PBS 3 times, the slides were incubated with primary antibodies (cleaved PARP 1:50, Bim 1:200 and Mcl-1 1:100) at 4°C in a humidified chamber overnight and then treated with ImmPRESS® Horse Anti-Rabbit IgG Polymer Kit (MP-7401; Vector Laboratories, Burlingame, CA) and DAB Peroxidase Substrate Kit (SK-4100; Vector Laboratories). Cleaved PARP (#5625) and Bim (#2933) antibodies used in IHC were purchased from Cell Signaling Technology, Inc (Danvers, MA). Mcl-1 (sc-12756) was purchased from Santa Cruz Biotechnology (Dallas, TX).
2.11. Statistical analyses
Significant differences between two groups were analyzed using two-sided unpaired Student’s t-tests. Differences among multiple groups were evaluated with one-way ANOVA tests. All statistical analyses were conducted using GraphPad Prism 8 (GraphPad Software, San Diego, CA). Results were considered statistically significant when P values were less than 0.05.
3. Results
3.1. Berberine and osimertinib combination synergistically decreases the survival of osimertinib-resistant cell lines with MET amplification
During our effort to identify agents that may enhance therapeutic efficacy of osimertinib against EGFRm NSCLC cells with acquired resistance to osimertinib, we found that berberine exerted such an activity in some of the osimertinib-resistant cell lines. Among the initial tested cell lines including HCC827/AR (osimertinib-resistant with MET amplification), PC-9/AR (osimertinib-resistant with undefined mechanisms) and PC-9/GR/AR (gefitinib-resistant and then osimertinib-resistant with T790M mutation), HCC827/AR was the only one that showed response to the combination of berberine and osimertinib, i.e., an enhanced decrease in cell survival compared to either single agent treatment (Figs. 1A and B). Considering the possible selectivity of this combination toward MET amplified osimertinib-resistant cell lines, two additional osimertinib-resistant cell lines with MET amplification derived from HCC827, named HCC827/AR0.5 and HCC827/AR2, which were established by repeatedly treating HCC827 cells with the fixed concentrations of 0.5 μM and 2 μM osimertinib, respectively (10), were tested. Consistently, the combination of berberine and osimertinib was more potent than either single agent in decreasing the survival of both HCC827/AR0.5 and HCC827/AR2 cell lines (Fig. 1A). In addition, the combination was also more effective than either single agent in decreasing the survival of HCC827/ER cell line (Fig. 1A), which was initially established to be resistant to erlotinib and showed cross-resistant to osimertinib due to MET amplification (8). In these sensitive cell lines, CIs for many combinations were < 1, suggesting synergistic effects of the combination in decreasing the survival of these osimertinib-resistant cell lines. Moreover, we conducted colony formation assays that allow us to repeat the treatments for a relatively long period of time and generated similar results: i.e., the combination of berberine and osimertinib effectively inhibited the formation and growth of colonies of HCC827/AR, HCC827/AR2 and HCC827/ER cell lines while each single agent had no or minimal effects under the tested conditions (Fig. 1C). In contrast, the combination had no significant effects in decreasing colony numbers of PC-9/AR cells (Fig. 1C). These results together clearly demonstrate that berberine, when combined with osimertinib, synergistically and selectively decreases the survival of osimertinib-resistant EGFRm NSCLC cells with MET amplification.
Fig. 1. Berberine and osimertinib combination synergistically decreases the survival of osimertinib-resistant NSCLC cell lines (A-C) with MET amplification (D-F).
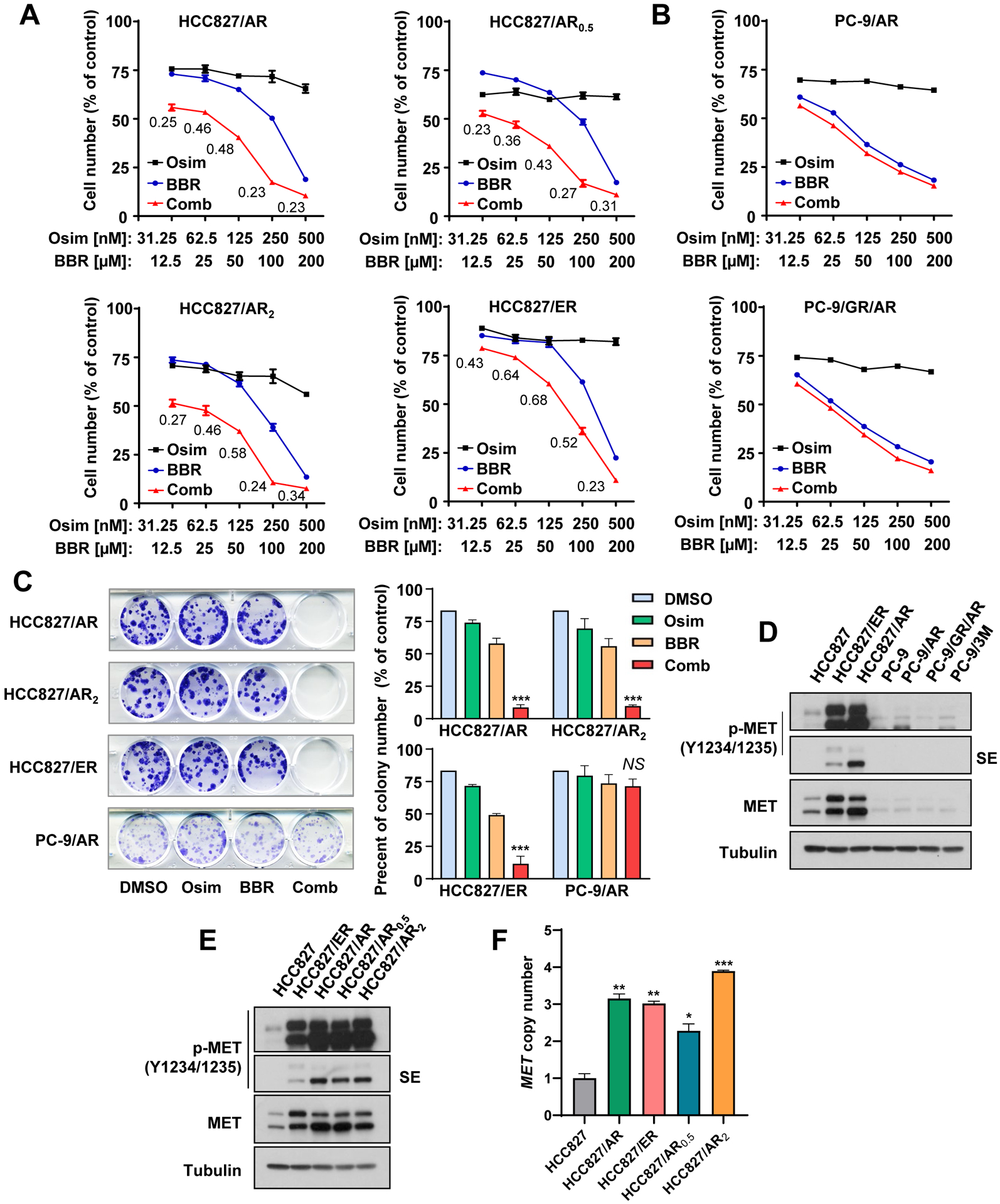
A and B, The indicated cell lines were seeded in 96-well plates and treated on the second day with varied concentrations of osimertinib (Osim) alone, berberine (BBR) alone and their respective combinations. After 3 days, cell numbers were determined with the SRB assay. The data are means ± SDs of four replicate determinations. The numbers inside the graphs are CIs. C, The indicated cell lines seeded in 12-well plates were treated with DMSO, 100 nM osimertinib, 5 μM berberine or the combination of osimertinib and berberine; these treatments were repeated with fresh medium every 3 days. After 10 days, the cells were then fixed and stained with crystal violet dye. Columns are means ± SDs of triplicate determinations. D and E, The indicated cell lines were harvested for preparation of whole-cell protein lysates and then used for Western blotting to detect the proteins of interest. SE, shorter exposure. F, qPCR was used to detect MET gene copy numbers in the given cell lines. The data are means ± SEs of two experiments. *, P < 0.05, **, P < 0.01 and ***, P < 0.001 compared with parental cell line.
In agreement with our previous reports (8, 10), we further validated the status of MET expression and activity (p-MET) in the tested cell lines used in this study and found that elevated levels of both p-MET and MET were detected only in HCC827-derived osimertinib-resistant cell lines, but not in PC-9-derived osimertinib-resistant cell lines (Figs. 1D and 1E). At DNA levels, these HCC827-derived resistant cell lines possessed significantly increased MET gene copy numbers compared with HCC827 parental cells (Fig. 1F), indicating gene amplification.
3.2. The combination of berberine and osimertinib augments induction of apoptosis in MET-amplified osimertinib-resistant cells
Our previous work has demonstrated that targeting MET can overcome acquired resistance to osimertinib though enhancing induction of apoptosis (8, 10). Thus, we examined if the combination of berberine and osimertinib could function in a similar mechanism via enhancing apoptosis in these MET-amplified osimertinib resistant cell lines. Indeed, the combination enhanced cleavage of PARP and caspase-3 (Fig. 2A) and annexin V-positive cell populations (Fig. 2B), whereas each single agent did not or minimally did so, in all HCC827-derived osimertinib-resistant cell lines including HCC827/AR, HCC827/AR0.5, HCC827/AR2 and HCC827/ER, indicating an augmented effect on inducing apoptosis. Again, the combination did not enhance induction of apoptosis in both PC-9/AR and PC-9/GR/AR cells (Fig. 2A). These results clearly demonstrate that the combination of berberine and osimertinib effectively and selectively augments induction of apoptosis in osimertinib-resistant cell lines with MET amplification.
Fig. 2. The combination of berberine and osimertinib augments induction of apoptosis in MET-amplified osimertinib-resistant cells.
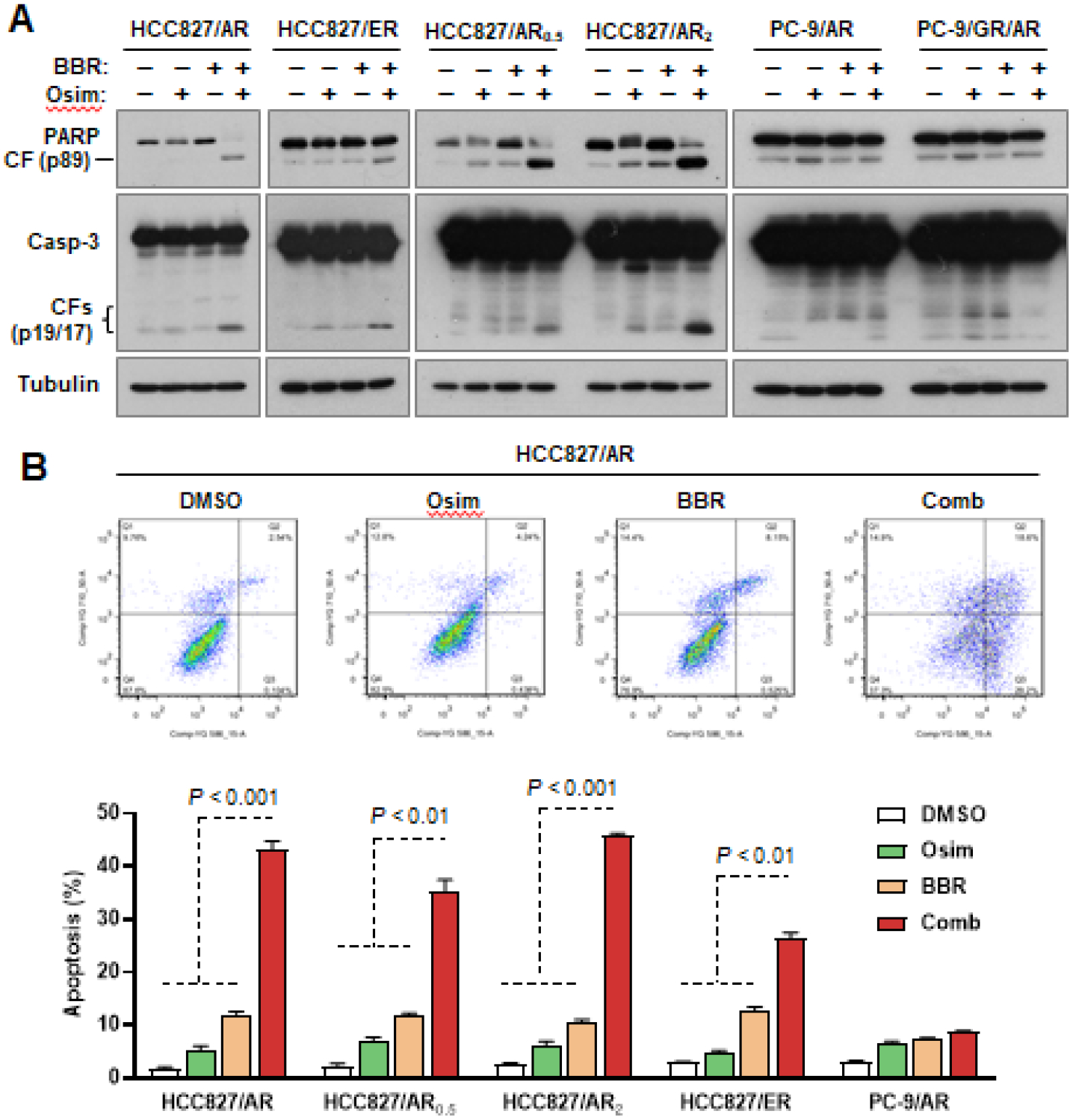
The indicated cell lines were treated with DMSO, 200 nM osimertinib (Osim), 100 μM berberine (BBR) or their combination (Comb) for 48 h. Apoptosis was detected with Western blotting for protein cleavage (A) and with flow cytometry for annexin V-positive cells (B). Data in each group are means ± SDs of duplicate determinations.
3.3. The combination of berberine and osimertinib augments induction of apoptosis through enhanced effects on modulation of Bim and Mcl-1 degradation in MET-amplified osimertinib-resistant cells
In order to understand the mechanisms accounting for the enhanced induction of apoptosis, we tested the effects of berberine and osimertinib combination at different time points on modulation of Bim and Mcl-1 levels, which are critical events in mediating osimertinib-induced apoptosis in EGFRm NSCLC cell lines (16) and enhanced apoptosis by the combination of osimertinib and MET inhibition in MET-amplified osimertinib-resistant cells (8, 10). This combination was much more effective than either of the single agents in decreasing Mcl-1 levels and in increasing Bim levels in HCC827/AR cells, particularly at the time points when enhanced PARP cleavage was detected (Fig. 3A). Our prior studies have shown that osimertinib combined with other MET inhibitors, such as crizotinib and HQP8361, modulated the levels of these proteins by altering their degradation rates (8, 10). Therefore, we conducted a cycloheximide (CHX) chase assay to determine if berberine and osimertinib combination modulates Bim and Mcl-1 levels. Bim protein was degraded much more slowly in cells treated with the combination compared to those in cells exposed to the DMSO control or either single agent treatment (Fig. 3B). In contrast, Mcl-1 degradation was faster in cells exposed to combination treatment than those in cells treated with each single agent alone (Fig. 3C). These results suggest that the combination of berberine and osimertinib enhances Mcl-1 degradation while slowing down Bim degradation in MET-amplified osimertinib-resistance cells.
Fig. 3. The combination of berberine and osimertinib augments Bim elevation and Mcl-1 reduction (A) through modulating their degradation rates (B and C), which critically mediates the enhanced apoptosis by the combination (D-G), in MET-amplified osimertinib-resistant cells.
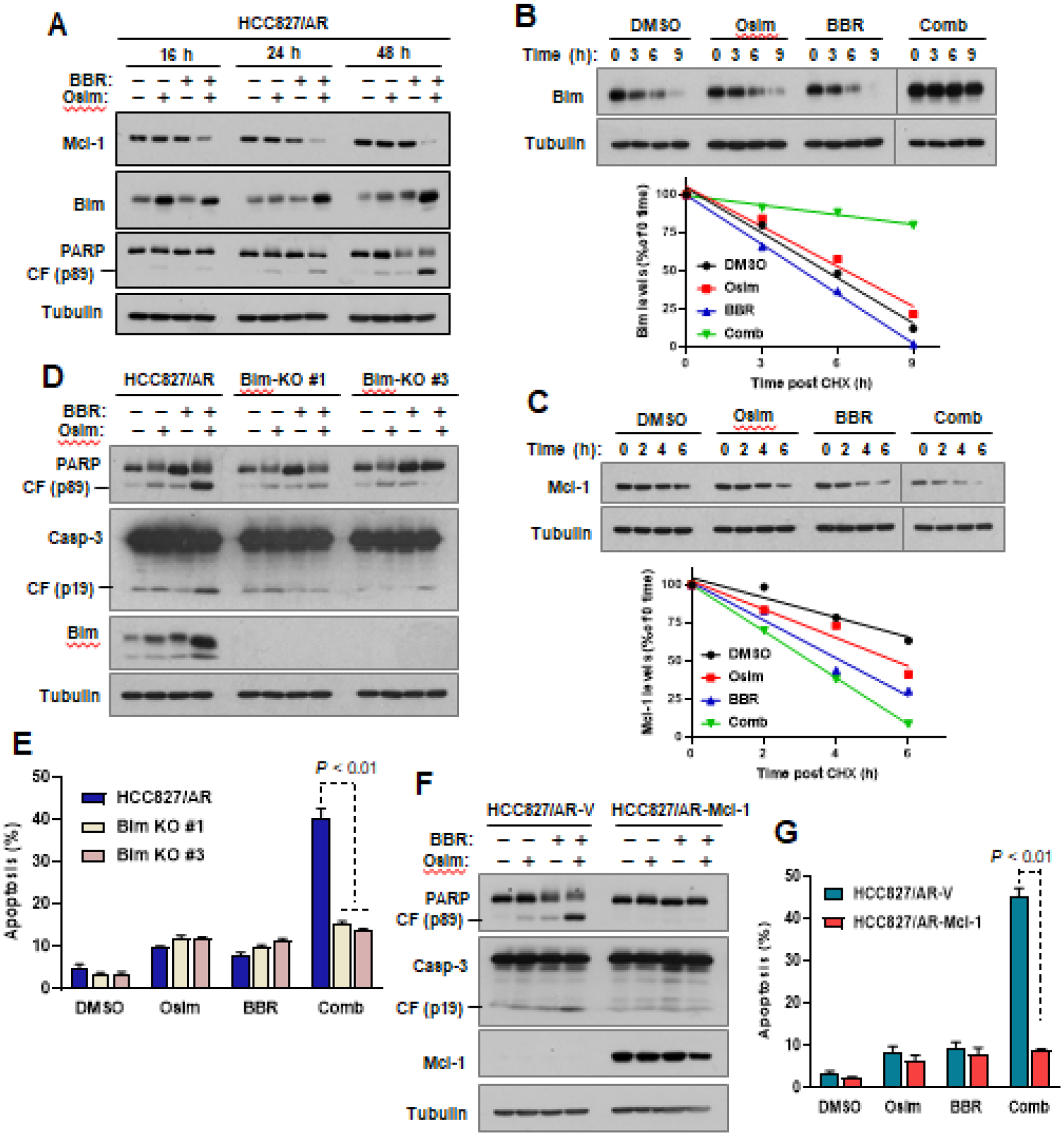
A, HCC827/AR were treated with DMSO, 200 nM osimertinib (Osim), 100 μM berberine (BBR) or their combination (Comb) for varied times as indicated. Western blotting was used to detect the proteins of interest. B and C, HCC827/AR cells were exposed to DMSO, 200 nM Osimertinib, 100 μM berberine or the combination of osimertinib and berberine for 12 h followed by the addition of 10 μg/ml CHX. The cells were then harvested at the indicated times for preparation of whole cell-protein lysates and subsequent detection of the tested proteins by Western blotting. NIH image J software was used to quantify band intensities. Bim and Mcl-1 levels are shown as percentage of levels at 0 time post CHX treatments. D-G, The indicated cell lines with Bim knockout (D and E) and Mcl-1 overexpression (F and G) were exposed to DMSO, 200 nM osimertinib, 100 μM berberine or osimertinib plus berberine for 48 h. The cells were then harvested for preparation of whole-cell lysates and subsequent Western blotting to detect the indicated proteins (D and F) and for detection of apoptotic cells with annexin V/flow cytometry (E and G). The data are means ± SDs of duplicate determinations.
We then determined whether the enhanced Bim elevation and Mcl-1 reduction contribute to enhanced induction of apoptosis by berberine and osimertinib combination in MET-amplified osimertinib-resistance cells. To this end, we compared the effect of berberine and osimertinib combination on induction of apoptosis in the control HCC827/AR cell line with those in its derived cell lines deficient with Bim (Bim-KO) and found that the combination effectively caused cleavage of both PARP and caspase-3 (Fig. 3D) and increased annexin V-positive cells (Fig. 3E) in HCC827/AR cells, but not in both Bim-KO cell lines. We also compared the effects of the combination on induction of apoptosis in HCC827/AR cells with that in its derived cells expressing ectopic Mcl-1 (HCC827/AR-Mcl-1). Like Bim-KO cell lines, HCC827/AR-Mcl-1 cells showed resistance to induction of apoptosis in terms of detecting both PARP and caspase-3 cleavage and annexin V-positive cells compared to the vector control cell line (HCC827/AR-V) when exposed to berberine and osimertinib combination treatment (Fig. 3F and G). Hence, it is clear that both Bim elevation and Mcl-1 reduction contribute to enhanced induction of apoptosis by berberine and osimertinib combination.
3.4. Berberine and osimertinib combination exerts enhanced inhibitory activity against the growth of MET-amplified osimertinib-resistant tumors in vivo
Following these in vitro findings, we then determined the in vivo effect of osimertinib and berberine combination on the growth of MET-amplified osimertinib-resistant tumors. Using HCC827/AR xenografts in nude mice as a model, we found that single agent treatment with either osimertinib or berberine did not significantly reduce tumor sizes and weights; yet, the combination treatment showed a significant inhibitory effect on tumor growth in terms of evaluating both tumor sizes and weights (Figs. 4A–C). In addition, mouse body weight did not alter significantly between groups, showing that this combination is well tolerated in vivo (Fig. 4D). At the end of the experiment, tumors were harvested from the mice and used for IHC to detect alterations of several relevant proteins. The combination group tumors, but not other group tumors, showed an increase in cleaved PARP staining, indicating enhanced apoptosis in vivo. Moreover, the combination group tumors showed the highest levels of Bim and the lowest levels of Mcl-1 compared to tumors in the control or single agent treatment groups (Fig. 4E). These results together clearly demonstrate that the combination of osimertinib and berberine effectively inhibits the growth of MET-amplified osimertinib-resistant tumors with enhanced induction of Bim-mediated apoptosis in vivo and is well tolerated in mice.
Fig. 4. Berberine and osimertinib combination exerts enhanced inhibitory activity against the growth of MET-amplified osimertinib-resistant tumors in vivo (A-C) with good tolerability (D) and altered levels of cleaved PARP, Bim and Mcl-1 (E).
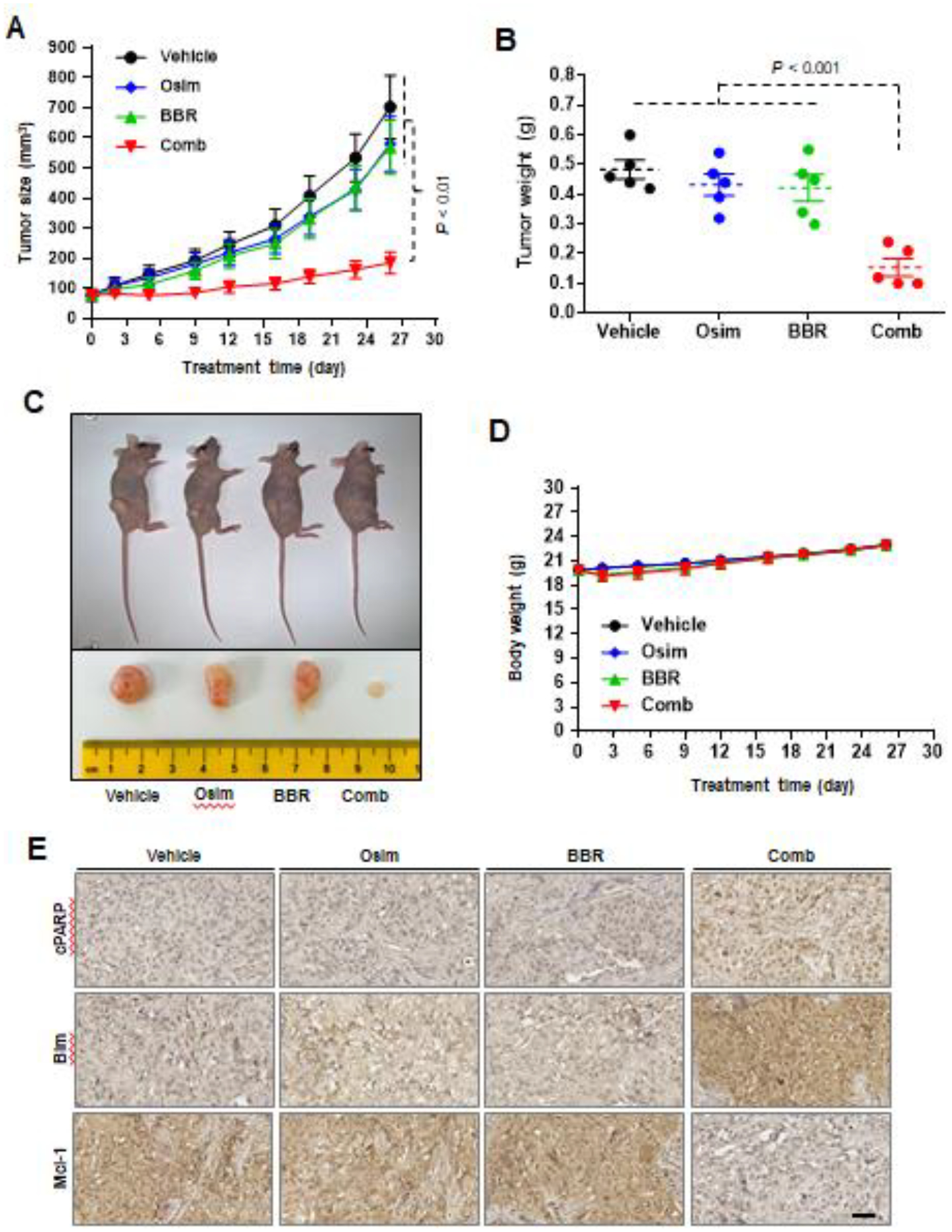
HCC827/AR cells grown in NU/NU mice as xenografted tumors were treated with vehicle, osimertinib (Osim) alone (5 mg/kg/day, og), berberine (BBR) alone (25 mg/kg/day, ip) or the combination (Comb) of osimertinib with berberine. Tumor sizes and mouse body weights were measured at the indicated time points (A and D). At the end of treatments, tumors in each group were also collected, weighed (B) and photographed (C). The data in each group are means ± SEs of 5 tumors from 5 mice. E, IHC staining of Bim, Mcl-1 and cleaved PARP in sections of indicated tumor xenografts. Scale bars: 50 μm.
3.5. Berberine and osimertinib combination enhances suppression of MET and its downstream signaling in MET-amplified osimertinib-resistant cells
Since berberine and osimertinib combination is selectively effective against MET-amplified osimertinib-resistant EGFRm NSCLC cell lines, we then evaluated the effects of berberine and osimertinib combination on MET and its downstream signaling pathways. The combination was much more potent than either agent alone in decreased the levels of p-MET in HCC827/AR cells. In agreement, the combination was also more effective than either single agent in decreasing the levels of p-Akt (S473), p-Akt (T450), p-S6, p-4EBP1 and p-ERK1/2; these effects started at 16 h and were sustained up to 48 h (Fig. 5A), indicating enhanced suppressive effect on the MET/mTOR and MEK/ERK signaling pathways (Fig. 5A).
Fig. 5. Berberine and osimertinib combination enhances suppression of MET and its downstream signaling in MET-amplified osimertinib-resistant cells (A), which are relatively more sensitive to berberine (B).
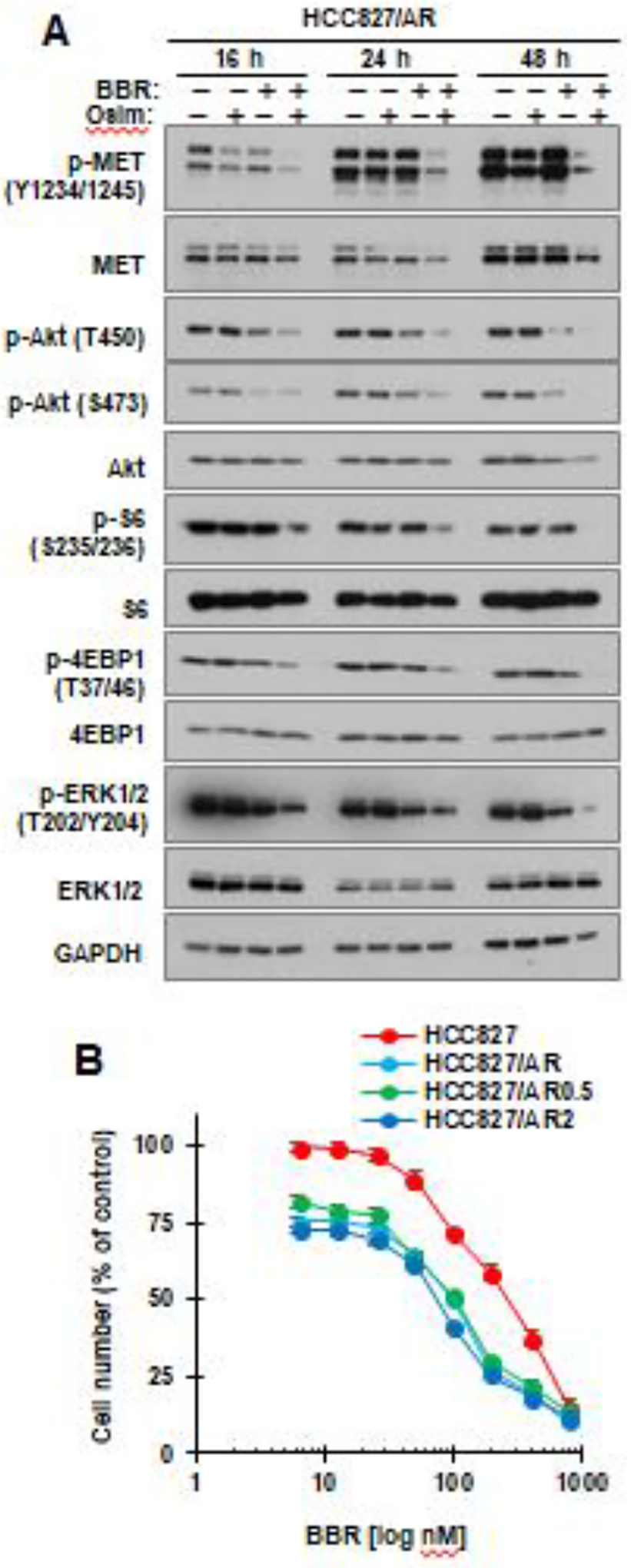
A, HCC827/AR cells were treated with DMSO, 200 nM osimertinib (Osim), 100 μM berberine (BBR) or their combination for varied times as indicated, then harvested for preparation of whole-cell protein lysates and subsequent Western blotting to detect the indicated proteins. B, The indicated cell lines seeded in 96-well plates were exposed to varied concentrations of berberine for 72 h. Cell numbers were determined with the SRB assay. The data are means ± SDs of four replicate determinations.
3.6. Osimertinib-resistant cell lines with MET amplification respond better that its parental cells to berberine
We also determined if berberine preferentially suppresses the growth of osimertinib-resistant cells with MET amplification and protein hyper-activation as other known MET inhibitors do (10, 19) by examining the effects of berberine alone on the growth of HCC827 and its derived osimertinib-resistant cell lines. All three HCC827/AR cell lines were more susceptible than their parental HCC827 cells to berberine, with IC50s of around 100 μM in these HCC827/AR cell lines and of over 270 μM in HCC827, respectively (Fig. 5B). Therefore, berberine preferentially suppresses the growth of cancer cell lines with amplified MET.
3.7. Molecular docking analysis suggests that berberine can potentially bind to MET
The findings presented above suggest that berberine functions in a similar way as other MET kinase inhibitors do, as we previously demonstrated, in overcoming osimertinib resistance caused by MET amplification and protein hyper-activation (8, 10). We were logically wondering whether berberine can function as a MET inhibitor via directly inhibiting MET activity. By comparing chemical structures between berberine and other known MET inhibitors such as crizotinib (PF-02341066) and PF-04217903, we found their similarities in terms of overall structural shapes (Fig. 6A). Thus, we then performed a molecular docking analysis of berberine using the co-crystal structure of non-phosphorylated MET (PDB ID:3ZXZ). The existing ligand PF-04217903 is a known ATP competitive MET inhibitor (20). The docking study demonstrated that berberine binds to the kinase domain of non-phosphorylated MET, occupies the front of the binding pocket, and interacts with the activation loop (Figs. 6B and C). The pyridine ring of berberine interacts with Y1230 through a π-π and a π-cation interaction. The oxygen on one of the methoxy groups of berberine forms a water-mediated H-bond with D1164. Berberine binds at the kinase domain in a similar mode to as PF-04217903 does; however, the conformational restriction of the structure results in a relatively weaker binding (Figs. 6D–G).
Fig. 6. Berberine can potentially bind to MET in a docking analysis.
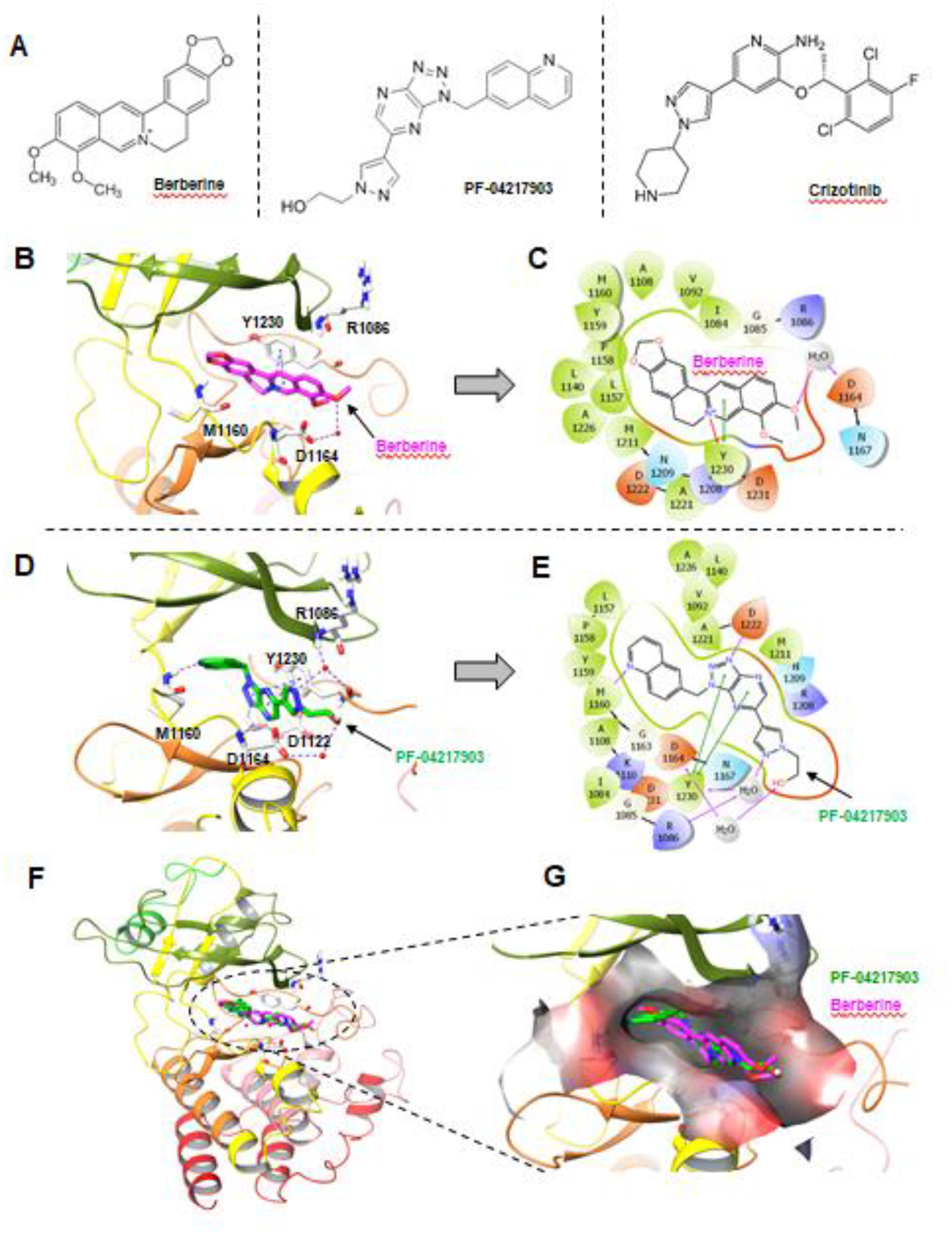
A, Chemical structures of berberine and other known MET inhibitors. B, Berberine docking into MET kinase domain. H-bond: purple dashed line; π-π and π-cation: blue dashed lines; Berberine: magenta sticks; Water molecules: red spheres. C, Interaction diagram of Berberine docking pose. H-bond: purple line; π-π: green line; π-cation: red line. D, Binding site of the MET inhibitor, PF-04217903. H-bond: purple dashed line; π-π: blue dashed lines; PF-04217903: green sticks; Water molecules: red spheres. E, Interaction diagram of PF-04217903. F. Overlay of Berberine docking pose with MET protein co-crystal structures (PDB ID: 3ZXZ). Berberine: magenta; PF-04217903: green. G, Berberine docking pose and PF-04217903 overlay at the binding pocket of MET kinase domain in surface representation. Berberine: magenta; PF-04217903: green.
3.8. Berberine is a MET kinase inhibitor albeit with relatively weak activity
Following the outcome of the molecular docking analysis, we conducted a kinase assay to determine whether berberine has indeed the capacity of inhibiting MET kinase activity. As predicted, berberine inhibited MET kinase activity in a dose-dependent manner, similarly to other known MET inhibitors tested (Fig. 7A). However, berberine was a weaker inhibitor under our testing conditions with an IC50 of 19.64 μM, than crizotinib (13.75 nM) or PF-04217903 (11.72 nM). Given that MET is the receptor for HGF, through which HGF activates intracellular signaling including PI3K/Akt and MEK/ERK pathways (7), we further determined the effect of berberine on HGF-induced activation of MET signaling pathways. Treatment of both HCC827 and HCC827/AR cells with HGF increased the levels of p-MET, p-Akt and p-ERK1/2; the presence of berberine abrogated these effects caused by HGF (Fig. 7B), indicating that berberine effectively inhibits HGF-induced activation of MET signaling in these cell lines. Hence, we further demonstrated the MET-inhibitory activity of berberine in cells.
Fig. 7. Berberine inhibits MET kinase activity (A) and HGF-induced activation of MET and its downstream signaling (B).
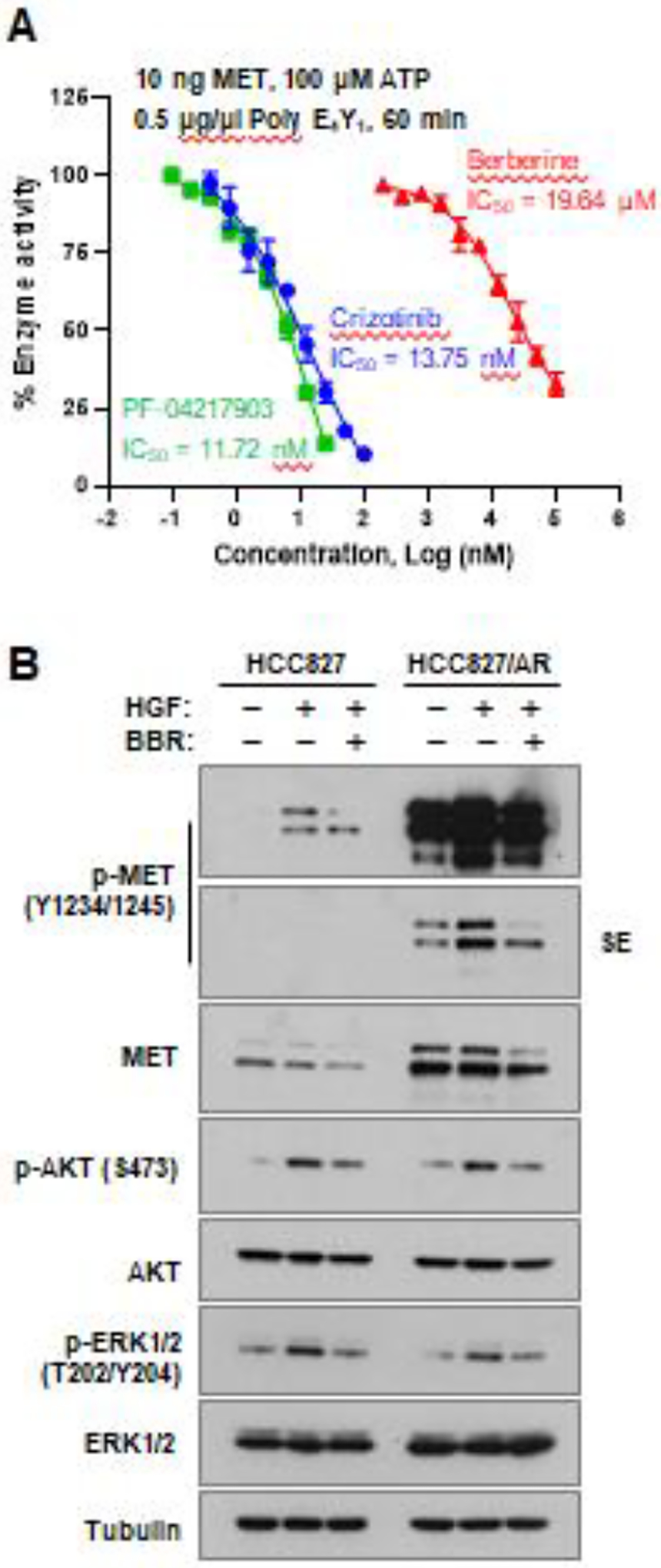
A, MET kinase assay was determined under the conditions indicated in the absence and presence of different concentrations of berberine and given MET inhibitors. B, HCC827 and HCC827/AR cell lines were pre-treated with 10 ng HGF for 2 h and then co-treated with DMSO or 100 μM berberine for another 8 h. Whole cell-protein lysates were prepared, and Western blot analysis was used to detect the indicated proteins.
4. Discussion
There are many studies demonstrating the potential effects of the natural product, berberine, in enhancing chemosensitivity and radiosensitivity (21–30), reversing chemoresistance (31–36) or augmenting efficacies of targeted therapies including overcoming acquired resistance (37–39) in different cancer types, although berberine was shown to positively regulate multidrug-resistant transporter (pgp-170) expression, leading to reduced response to chemotherapy (40, 41). The current study has demonstrated the previously undiscovered activity of berberine in selectively reversing acquired resistance of MET-amplified EGFRm NSCLC cells and tumors to osimertinib based on both in vitro and in vivo findings. Given that MET amplification is a well-characterized resistance mechanism to osimertinib and other third generation EGFR-TKIs and accounts for 5–30% of resistance cases (7), our findings are clearly of translational value or significance.
Although berberine has been widely studied either alone or in combination for its potential anticancer activity, the underlying molecular mechanisms, particularly its direct targets, are largely unclear albeit with many putative ones (11, 14, 15). Berberine was reported to directly bind to and target NEK7 protein to block NEK7-NLRP3 interaction, achieving anti-inflammatory efficacy in a NEK7-dependent manner (42). Another study using molecular docking modeling revealed that berberine inhibited B-Raf activity via directly binding to B-Raf protein (43). Beyond, berberine has been suggested to directly bind to and degrade BCR-ABL and BCR-ABL T315I mutant, leading to BCR-ABL inhibition in chronic myeloid leukemia cells (39). These targets may not be relevant to the biological activity of berberine in selectively sensitizing MET-amplified osimertinib-resistant EGFRm NSCLC cells to osimertinib.
Our findings clearly show that berberine shares some biological similarities with other known MET inhibitor such as crizotinib, PF-04217903, SGX523 and HQP8361 as we previously reported (8, 10) in effectively decreasing cell survival and inducing Bim-dependent apoptosis of MET-amplified osimertinib-resistant EGFRm NSCLC cell lines with modulation of Bim and Mcl-1 degradation, in inhibiting the growth of MET-amplified osimertinib-resistant EGFRm NSCLC xenografts in vivo, and in preferentially suppressing the MET/mTOR signaling in MET-amplified osimertinib-resistant cells, when combined with osimertinib. This led us to demonstrate another novel and important finding in this study revealing that berberine functions as a MET inhibitor through directly binding to and inhibiting MET activity. This finding can logically explain why the combination of berberine and osimertinib is effective only against osimertinib-resistant cell lines with MET amplification.
Though binding to the kinase domain of MET and inhibiting MET activity, berberine is a relatively weak MET inhibitor compared with other optimized MET inhibitors such as crizitonib and PF-04217903. Despite the well tolerability and safety, its therapeutic efficacy in potential treatment of cancer and other disease is significantly compromised due to its poor solubility in water, low absorption in intestines, rapid metabolism and low bioavailability. Efforts toward improving the pharmacologic and pharmaceutic properties of berberine have resulted in synthesis of various berberine derivatives with improved pharmacological and pharmacokinetic profiles (13, 15, 44). In line with this direction of effort, our finding on MET as a novel therapeutic target of berberine may be helpful for guiding future synthesis of the right berberine derivatives with optimized MET-inhibitory activity in addition to better pharmaceutic properties.
Patient selection is a key factor that determines the success of a given cancer therapy, particularly targeted therapy. Our study clearly showed that osimertinib-resistant EGFRm NSCLC cancer cell lines with MET amplification or high MET activity responded better than their parental cell line without MET amplification to berberine monotherapy and particularly to the combination of berberine and osimertinib. Our finding on MET as a target of berberine also implies that MET amplification or hyper-activation may be considered as a predictive biomarker to identify cancer patients such as those with MET-amplified EGFRm NSCLC who are likely to be responsive to therapeutic regimens based on berberine or its directives.
5. Conclusions
The current study has demonstrated berberine functions as a naturally-existing MET inhibitor which synergizes with osimertinib to preferentially decrease the survival of MET-amplified osimertinib-resistant EGFRm NSCLC cells with enhanced induction of Bim-dependent apoptosis and effectively inhibits the growth of MET-amplified osimertinib-resistant EGFRm NSCLC tumors in vivo. These novel findings warrant further investigation of the potential of berberine and its derivatives in overcoming acquired resistance to osimertinib and possibly other EGFR-TKIs.
Supplementary Material
Acknowledgement
This study was in part supported by the NIH/NCI R01 CA223220 (to SYS), R01 CA245386 (to SYS) and UG1 CA233259 (to SSR), Emory Winship Cancer Institute lung cancer research pilot funds (to SYS), and the John D. Stobo, M.D. Distinguished Chair Endowment Fund at UTMB (to JZ). SSR and SYS are Georgia Research Alliance Distinguished Cancer Scientists.
Abbreviations
- NSCLC
non-small cell lung cancer
- EGFR
epidermal growth factor receptor
- EGFR-TKIs
EGFR tyrosine kinase inhibitors
- EGFRm
EGFR-mutant
- AR
AZD9291-resistant
- CI
combination index
- CHX
cyclohexemide
- KO
knockout
- SRB
sulforhodamine B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
SSR is on consulting/advisory boards for AstraZeneca, BMS, Merck, Roche, Tesaro and Amgen. Other authors declare that they have no competing interests.
CRediT authorship contribution statement
Zhen Chen: Conceptualization, Investigation, Formal analysis, Data curation and Writing – Original Draft and Writing - Review & Editing. Karin A. Vallega: Investigation, Writing – Original Draft and Writing - Review & Editing. Haiying Chen: Investigation, Writing – Original Draft. Jia Zhou: Supervision, Writing – Original Draft. Suresh S. Ramalingam: Supervision, Writing – Original Draft and Funding acquisition. Shi-Yong Sun: Conceptualization, Supervision, Project administration, Writing – Original Draft, Writing - Review & Editing and Funding acquisition.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: a cancer journal for clinicians. 2021;71(1):7–33. [DOI] [PubMed] [Google Scholar]
- 2.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2021. [DOI] [PubMed] [Google Scholar]
- 3.Tartarone A, Lerose R. Clinical approaches to treat patients with non-small cell lung cancer and epidermal growth factor receptor tyrosine kinase inhibitor acquired resistance. Therapeutic advances in respiratory disease. 2015;9(5):242–50. [DOI] [PubMed] [Google Scholar]
- 4.Shah R, Lester JF. Tyrosine Kinase Inhibitors for the Treatment of EGFR Mutation-Positive Non-Small-Cell Lung Cancer: A Clash of the Generations. Clinical lung cancer. 2020;21(3):e216–e28. [DOI] [PubMed] [Google Scholar]
- 5.Kato Y, Hosomi Y, Watanabe K, Yomota M, Kawai S, Okuma Y, et al. Impact of clinical features on the efficacy of osimertinib therapy in patients with T790M-positive non-small cell lung cancer and acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Dis. 2019;11(6):2350–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piper-Vallillo AJ, Sequist LV, Piotrowska Z. Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review. J Clin Oncol. 2020:JCO1903123. [DOI] [PubMed] [Google Scholar]
- 7.Wang Q, Yang S, Wang K, Sun SY. MET inhibitors for targeted therapy of EGFR TKI-resistant lung cancer. J Hematol Oncol. 2019;12(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi P, Oh YT, Zhang G, Yao W, Yue P, Li Y, et al. Met gene amplification and protein hyperactivation is a mechanism of resistance to both first and third generation EGFR inhibitors in lung cancer treatment. Cancer Lett. 2016;380(2):494–504. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Li L, Han R, Jiao L, Zheng J, He Y. Clinical analysis by next-generation sequencing for NSCLC patients with MET amplification resistant to osimertinib. Lung Cancer. 2018;118:105–10. [DOI] [PubMed] [Google Scholar]
- 10.Yu D, Li Y, Sun KD, Gu J, Chen Z, Owonikoko TK, et al. The novel MET inhibitor, HQP8361, possesses single agent activity and enhances therapeutic efficacy of AZD9291 (osimertinib) against AZD9291-resistant NSCLC cells with activated MET. Am J Cancer Res. 2020;10(10):3316–27. [PMC free article] [PubMed] [Google Scholar]
- 11.Och A, Podgorski R, Nowak R. Biological Activity of Berberine-A Summary Update. Toxins (Basel). 2020;12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Yi H, Wu J, Kuang T, Zhang J, Li Q, et al. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed Pharmacother. 2021;133:110984. [DOI] [PubMed] [Google Scholar]
- 13.Gaba S, Saini A, Singh G, Monga V. An insight into the medicinal attributes of berberine derivatives: A review. Bioorg Med Chem. 2021;38:116143. [DOI] [PubMed] [Google Scholar]
- 14.Farooqi AA, Qureshi MZ, Khalid S, Attar R, Martinelli C, Sabitaliyevich UY, et al. Regulation of Cell Signaling Pathways by Berberine in Different Cancers: Searching for Missing Pieces of an Incomplete Jig-Saw Puzzle for an Effective Cancer Therapy. Cancers. 2019;11(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Liu Y, Du X, Ma H, Yao J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag Res. 2020;12:695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shi P, Oh YT, Deng L, Zhang G, Qian G, Zhang S, et al. Overcoming Acquired Resistance to AZD9291, A Third-Generation EGFR Inhibitor, through Modulation of MEK/ERK-Dependent Bim and Mcl-1 Degradation. Clin Cancer Res. 2017;23(21):6567–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun SY, Yue P, Dawson MI, Shroot B, Michel S, Lamph WW, et al. Differential effects of synthetic nuclear retinoid receptor-selective retinoids on the growth of human non-small cell lung carcinoma cells. Cancer Res. 1997;57(21):4931–9. [PubMed] [Google Scholar]
- 18.Liu X, Yue P, Zhou Z, Khuri FR, Sun SY. Death receptor regulation and celecoxib-induced apoptosis in human lung cancer cells. J Natl Cancer Inst. 2004;96(23):1769–80. [DOI] [PubMed] [Google Scholar]
- 19.Deng L, Vallega KA, Zhang S, Shi P, Sun SY. MET inhibition downregulates DR4 expression in MET-amplified lung cancer cells with acquired resistance to EGFR inhibitors through suppressing AP-1-mediated transcription. Neoplasia. 2021;23(8):766–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui JJ, McTigue M, Nambu M, Tran-Dube M, Pairish M, Shen H, et al. Discovery of a novel class of exquisitely selective mesenchymal-epithelial transition factor (c-MET) protein kinase inhibitors and identification of the clinical candidate 2-(4-(1-(quinolin-6-ylmethyl)-1H-[1,2,3]triazolo[4,5-b]pyrazin-6-yl)-1H-pyrazol-1 -yl)ethanol (PF-04217903) for the treatment of cancer. J Med Chem. 2012;55(18):8091–109. [DOI] [PubMed] [Google Scholar]
- 21.Liu S, Fang Y, Shen H, Xu W, Li H. Berberine sensitizes ovarian cancer cells to cisplatin through miR-21/PDCD4 axis. Acta Biochim Biophys Sin (Shanghai). 2013;45(9):756–62. [DOI] [PubMed] [Google Scholar]
- 22.Zhang C, Yang X, Zhang Q, Yang B, Xu L, Qin Q, et al. Berberine radiosensitizes human nasopharyngeal carcinoma by suppressing hypoxia-inducible factor-1alpha expression. Acta Otolaryngol. 2014;134(2):185–92. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Q, Zhang C, Yang X, Yang B, Wang J, Kang Y, et al. Berberine inhibits the expression of hypoxia induction factor-1alpha and increases the radiosensitivity of prostate cancer. Diagn Pathol. 2014;9:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Qin R, Fang Y, Li H. Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through miR-93/PTEN/Akt Signaling Pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2015;36(3):956–65. [DOI] [PubMed] [Google Scholar]
- 25.Pandey A, Vishnoi K, Mahata S, Tripathi SC, Misra SP, Misra V, et al. Berberine and Curcumin Target Survivin and STAT3 in Gastric Cancer Cells and Synergize Actions of Standard Chemotherapeutic 5-Fluorouracil. Nutr Cancer. 2015;67(8):1293–304. [DOI] [PubMed] [Google Scholar]
- 26.Gong J, Munoz AR, Pingali S, Payton-Stewart F, Chan DE, Freeman JW, et al. Downregulation of STAT3/NF-kappaB potentiates gemcitabine activity in pancreatic cancer cells. Molecular carcinogenesis. 2017;56(2):402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Y, Zhang F, Zhao Y, Shao D, Zheng X, Chen Y, et al. Berberine Enhances Chemosensitivity and Induces Apoptosis Through Dose-orchestrated AMPK Signaling in Breast Cancer. Journal of Cancer. 2017;8(9):1679–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Fan J, Ai G, Liu J, Luo N, Li C, et al. Berberine in combination with cisplatin induces necroptosis and apoptosis in ovarian cancer cells. Biol Res. 2019;52(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao X, Wang J, Li M, Wang J, Lv J, Zhang L, et al. Berberine attenuates XRCC1-mediated base excision repair and sensitizes breast cancer cells to the chemotherapeutic drugs. Journal of cellular and molecular medicine. 2019;23(10):6797–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kou Y, Tong B, Wu W, Liao X, Zhao M. Berberine Improves Chemo-Sensitivity to Cisplatin by Enhancing Cell Apoptosis and Repressing PI3K/AKT/mTOR Signaling Pathway in Gastric Cancer. Frontiers in pharmacology. 2020;11:616251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wen C, Wu L, Fu L, Zhang X, Zhou H. Berberine enhances the antitumor activity of tamoxifen in drugsensitive MCF7 and drugresistant MCF7/TAM cells. Molecular medicine reports. 2016;14(3):2250–6. [DOI] [PubMed] [Google Scholar]
- 32.Pan Y, Shao D, Zhao Y, Zhang F, Zheng X, Tan Y, et al. Berberine Reverses Hypoxia-induced Chemoresistance in Breast Cancer through the Inhibition of AMPK- HIF-1alpha. International journal of biological sciences. 2017;13(6):794–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang S, Zhou L, Zhang M, Wang Y, Wang M, Du J, et al. Berberine Maintains the Neutrophil N1 Phenotype to Reverse Cancer Cell Resistance to Doxorubicin. Frontiers in pharmacology. 2019;10:1658. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Wang Y, Liu Y, Du X, Ma H, Yao J. Berberine Reverses Doxorubicin Resistance by Inhibiting Autophagy Through the PTEN/Akt/mTOR Signaling Pathway in Breast Cancer. OncoTargets and therapy. 2020;13:1909–19. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Qu H, Song X, Song Z, Jiang X, Gao X, Bai L, et al. Berberine reduces temozolomide resistance by inducing autophagy via the ERK1/2 signaling pathway in glioblastoma. Cancer cell international. 2020;20(1):592. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36.Qian K, Tang CY, Chen LY, Zheng S, Zhao Y, Ma LS, et al. Berberine Reverses Breast Cancer Multidrug Resistance Based on Fluorescence Pharmacokinetics In Vitro and In Vivo. ACS Omega. 2021;6(16):10645–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang R, Qiao H, Chen S, Chen X, Dou K, Wei L, et al. Berberine reverses lapatinib resistance of HER2-positive breast cancer cells by increasing the level of ROS. Cancer Biol Ther. 2016;17(9):925–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang Y, Wang K, Gu C, Yu G, Zhao D, Mai W, et al. Berberine, a natural plant alkaloid, synergistically sensitizes human liver cancer cells to sorafenib. Oncol Rep. 2018;40(3):1525–32. [DOI] [PubMed] [Google Scholar]
- 39.Yin Z, Huang G, Gu C, Liu Y, Yang J, Fei J. Discovery of Berberine that Targetedly Induces Autophagic Degradation of both BCR-ABL and BCR-ABL T315I through Recruiting LRSAM1 for Overcoming Imatinib Resistance. Clin Cancer Res. 2020;26(15):4040–53. [DOI] [PubMed] [Google Scholar]
- 40.Lin HL, Liu TY, Lui WY, Chi CW. Up-regulation of multidrug resistance transporter expression by berberine in human and murine hepatoma cells. Cancer. 1999;85(9):1937–42. [PubMed] [Google Scholar]
- 41.Lin HL, Liu TY, Wu CW, Chi CW. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br J Cancer. 1999;81(3):416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zeng Q, Deng H, Li Y, Fan T, Liu Y, Tang S, et al. Berberine Directly Targets the NEK7 Protein to Block the NEK7-NLRP3 Interaction and Exert Anti-inflammatory Activity. J Med Chem. 2021;64(1):768–81. [DOI] [PubMed] [Google Scholar]
- 43.Jabbarzadeh Kaboli P, Ismail P, Ling KH. Molecular modeling, dynamics simulations, and binding efficiency of berberine derivatives: A new group of RAF inhibitors for cancer treatment. PLoS One. 2018;13(3):e0193941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang YX, Kong WJ, Li YH, Tang S, Li Z, Li YB, et al. Synthesis and structure-activity relationship of berberine analogues in LDLR up-regulation and AMPK activation. Bioorg Med Chem. 2012;20(22):6552–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


