Abstract
Objective
This study aimed to investigate the predictive value of inflammatory cells in peripheral blood on the prognosis of patients with acute coronary syndrome (ACS) undergoing percutaneous coronary intervention (PCI).
Methods
Patients (n=1558) were consecutively enrolled and the median follow-up was 1142 days. Patients were divided into the major adverse cardiac events (MACE) 1 group (n=63) (all-cause mortality [n=58] and rehospitalization for severe heart failure [n=5], no MACE1 group (n=1495), MACE2 group (n=38) (cardiac mortality [n=33] and rehospitalization for severe heart failure [n=5]), and no MACE2 group (n=1520). The neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR) were analyzed.
Results
The NLR, MLR, and PLR were higher in the MACE groups than in the no MACE groups. Different subsets of inflammatory cells had similar diagnostic values for MACE. Kaplan–Meier curves showed that the survival time gradually decreased with an increase in the degree of risk as determined by the NLR, MLR, and PLR. The risk of MACE was highest in the extremely high-risk group.
Conclusion
Peripheral blood inflammatory cell subsets can predict MACE in patients with ACS undergoing PCI. These cell subsets could be important laboratory markers for the prognosis and clinical treatment of these patients.
Keywords: Acute coronary syndrome, neutrophil-to-lymphocyte ratio, monocyte, platelet, prognosis, major adverse cardiac event
Introduction
Coronary artery disease (CAD) is the leading cause of death in middle-high income countries worldwide. 1 Standardized community diagnosis and treatment strategies have reduced mortality of CAD in developed countries. In the USA, the rate of CAD has decreased by 10% in the past 20 years under guidelines and the rapid development of percutaneous coronary intervention (PCI). 2 Acute coronary syndrome (ACS) is the most severe and common type of CAD. Evidence-based medicine has shown that PCI is an important treatment strategy for improving the prognosis of patients with ACS. 3
CAD is a chronic inflammatory disease and its pathological characteristics include changes in coronary plaques and vessel walls. Aggregation and release of platelets promote proliferation of smooth muscle cells after endothelial injury. Inflammation causes coronary plaque formation or rupture by adjusting the balance of anti- and pro-inflammatory adipocytokines.4,5 Therefore, inflammatory cells in peripheral blood may be related to the onset and progression of ACS. Inflammatory cells in peripheral blood mainly include neutrophils, monocytes, platelets, and lymphocytes. Previous studies have shown that neutrophils,6–8 monocytes,9–11 and platelets12,13 may promote development of atherosclerosis, whereas lymphocytes may inhibit atherosclerosis.14–17 The ratios of pro-atherosclerotic inflammatory cells to anti-atherosclerotic inflammatory cells, the neutrophil-to-lymphocyte ratio (NLR), monocyte-to-lymphocyte ratio (MLR), and platelet-to-lymphocyte ratio (PLR), may provide more information than a single inflammatory cell level. Recently, an association between the NLR and the prognosis of cardiovascular disease has been shown. 18 However, the associations of the MLR, PLR, and prognosis of ACS remain unclear. Therefore, the present study aimed to examine the diagnostic and prognostic value of peripheral blood inflammatory cell subsets in patients with ACS undergoing PCI.
Materials and methods
Participants
A total of 1773 inpatients with ACS who underwent PCI from January 2016 to December 2018 were consecutively enrolled in this study. The inclusion criteria were as follows: patients aged ≥40 years; those with ACS according to the global diagnostic criteria by the European Society of Cardiology; and those who underwent PCI for the first time and complete revascularization. The exclusion criteria were infectious diseases, malignant tumors, blood system diseases (e.g., anemia and leukopenia), serious heart diseases (e.g., aortic dissection and hypertrophic cardiomyopathy), and chronic kidney disease (stage ≥3). The patients were assigned to the following groups in relation to the prognosis: major adverse cardiac events (MACE) 1 group (n=63; all-cause mortality [n=58 of whom 33 had cardiac mortality] and rehospitalization for severe heart failure [n=5; heart function level IV based on the New York Heart Association [NYHA] classification), no MACE1 (NMACE1) group (no MACE occurred; n=1495), MACE2 group (n=38; cardiac mortality, n=33 and 5 were rehospitalized for severe heart failure [NYHA IV]), and no MACE2 (NMACE2) group (n=1520) (Figure 1). The study was approved by the Institutional Review Board of the Affiliated Hospital of Chengde Medical University (approval number: LL076). This study was carried out in accordance with the World Medical Association’s Code of Ethics (Helsinki Declaration). All patients provided written informed consent.
Figure 1.
Screening flowchart of all inpatients enrolled in the study
ACS, acute coronary syndrome; PCI, percutaneous coronary intervention; MACE, major adverse cardiac events.
Demographic, clinical, and follow-up data
Data of age, sex, and risk factors for CAD, including hypertension, diabetes, dyslipidemia, ischemic stroke, and smoking history, were carefully collected by students who were undergoing a Master's degree. Information on a previous history of heart failure, an echocardiogram, peripheral blood cell count, and blood biochemistry for subjects was recorded in a database. The NLR, MLR, and PLR were then calculated. Hypertension was defined as systolic blood pressure ≥140 mmHg (1 mmHg = 0.133 kPa) and/or diastolic blood pressure ≥90 mmHg at rest or a previous diagnosis of hypertension with antihypertensive therapy. 19 Diabetes was defined as having symptoms of diabetes (i.e., polydipsia, polyuria, and more food and weight loss) with random blood glucose levels ≥11.1 mmol/L, fasting plasma glucose levels ≥7.0 mmol/L, 2-hour oral glucose tolerance test blood glucose levels ≥11.1 mmol/L, or having no symptoms of diabetes, but with at least two blood glucose tests that met the above-mentioned criteria. 20 Hyperlipidemia was defined as serum total cholesterol levels ≥5.18 mmol/L, high-density lipoprotein cholesterol levels ≤1.04 mmol/L, low-density lipoprotein cholesterol levels ≥3.37 mmol/L, triglyceride levels ≥1.7 mmol/L, or a previous diagnosis of dyslipidemia with a prescribed medication. 21
Regular follow-up after PCI was carried out by cardiologists in the outpatient service at 1, 3, 6, and 12 months and once a year thereafter. The median duration of follow-up was 1142 days. The primary composite endpoint was MACE1, including all-cause mortality, cardiac mortality (death due to myocardial infarction, heart failure, fatal arrhythmia, and other heart-related causes), and rehospitalization for severe heart failure (NYHA IV). The secondary composite endpoint was MACE2, including cardiac mortality and rehospitalization for severe heart failure.
PCI
All patients received a loading dose of aspirin (300 mg) and clopidogrel (300 mg) or ticagrelor (180 mg) before PCI and an intravenous dose of heparin (70–100 U/kg) to maintain an activated clotting time (250–300 s) during the procedure. Prescription of glycoprotein IIb/IIIa inhibitors depended on the indications of the guidelines of ACS. Experienced cardiologists performed PCI with the Judkins technique using 6F right and left heart catheters. The successful standard of PCI was defined as a reduction in percentage diameter stenosis associated with thrombolysis in myocardial infarction grade 2 or 3 to <30%. Angiographic characteristics of all of the patients were determined and reported by the interventional cardiology teams.
Blood sample collection and neutrophil, monocyte, platelet, and lymphocyte counts
Fasting blood samples were collected at admission within the first 24 hours before PCI. Neutrophil, monocyte, platelet, and lymphocyte counts were performed by the Laboratory Department using an automatic hematology analyzer (Sysmex XE-2100; Sysmex, Kobe, Japan). A report was issued after being checked by a doctor.
Statistical analysis
Data processing and statistical analysis were performed using the IBM Statistical Package for Social Sciences, version 19.0 (IBM Corp, Armonk, NY, USA). The distribution of all continuous variables was skewed as shown by the Shapiro–Wilk test. Continuous variables are expressed as the median (interquartile range). The Mann–Whitney U test was used to compare continuous variables between two groups. Categorical variables are presented as number (%) and the chi-square test was used for comparison between the two groups. The diagnostic efficiency of the NLR, MLR, and PLR, the combination of two of the ratios, and the combination of three of the ratios were evaluated using diagnostic tests, and the optimal diagnostic value was determined. The Kaplan–Meier curve and log-rank test were used to compare the survival status of the different risk groups in relation to the NLR, MLR, and PLR (≥ the optimal diagnostic value). The Cox proportional hazards regression model was used to predict risk factors of MACE. All statistical analysis tests were two-sided and a P value <0.05 was considered statistically significant.
Results
Comparison of baseline characteristics
A total of 1558 patients were included in the study. The numbers of patients aged ≥65 years, and those with heart failure, cardiogenic shock, a NLR ≥2.67, a MLR ≥0.33, a PLR ≥225.49, elevated creatinine levels, and an ejection fraction <40% were significantly higher in the MACE1 group than in the NMACE1 group (all P<0.05) (Table 1). The numbers of patients aged ≥65 years, and those with heart failure, cardiogenic shock, a NLR ≥2.67, a MLR ≥0.43, a PLR ≥225.49, elevated creatinine levels, and an ejection fraction <40% were also significantly higher in the MACE2 group than in the NMACE2 group (all P<0.05) (Table 2).
Table 1.
Comparison of baseline clinical characteristics between the MACE1 group (all-cause mortality and rehospitalization for severe heart failure) and the NMACE1 group.
| Factors | MACE (n=63) | NMACE (n=1495) | χ2/t | P |
|---|---|---|---|---|
| Demographics and clinical data, n (%) | ||||
| Male sex | 46 (73.0) | 1120 (74.9) | 0.116 | 0.733 |
| Age ≥65 years | 32 (50.8) | 340 (22.7) | 26.171 | <0.001 |
| Hyperlipidemia | 36 (57.1) | 846 (56.6) | 0.008 | 0.931 |
| Hypertension | 35 (55.6) | 880 (58.9) | 0.273 | 0.601 |
| T2DM | 14 (22.2) | 380 (25.4) | 0.327 | 0.568 |
| History of stroke | 16 (25.4) | 206 (13.8) | 6.678 | 0.010 |
| History of TIA | 0 (0.0) | 5 (0.3) | 0.211 | 0.646 |
| History of HF | 20 (31.7) | 138 (9.2) | 33.629 | <0.001 |
| History of CGS | 8 (12.7) | 17 (1.1) | 51.178 | <0.001 |
| Current smoking | 29 (46.0) | 780 (52.2) | 0.914 | 0.339 |
| Family history of CAD | 5 (7.9) | 214 (14.3) | 2.036 | 0.154 |
| UA | 15 (23.8) | 601 (40.2) | 6.794 | 0.009 |
| STEMI | 34 (54.0) | 660 (44.1) | 2.360 | 0.124 |
| NSTEMI | 14 (22.2) | 234 (15.7) | 1.950 | 0.163 |
| Laboratory data, n (%) | ||||
| WBC↑ | 25 (40.3) | 485 (32.5) | 1.640 | 0.200 |
| PLT↑ | 4 (6.3) | 113 (7.6) | 0.127 | 0.721 |
| | NEU |↑ | 28 (45.9) | 519 (34.8) | 3.188 | 0.074 |
| | LYM |↓ | 16 (26.2) | 259 (17.3) | 3.174 | 0.075 |
| | MON |↑ | 16 (26.2) | 314 (21.0) | 0.947 | 0.331 |
| MPV↑ | 1 (1.7) | 5 (0.3) | 2.656 | 0.103 |
| PDW↑ | 1 (1.7) | 15 (1.0) | 0.264 | 0.607 |
| NLR↑ | 46 (75.4) | 780 (52.2) | 12.631 | <0.001 |
| MLR↑ | 31 (50.8) | 498 (94.1) | 7.961 | 0.005 |
| PLR↑ | 19 (31.1) | 192 (12.9) | 16.703 | <0.001 |
| ALB↓ | 7 (11.7) | 69 (4.6) | 6.077 | 0.014 |
| CK-MB↑ | 36 (65.5) | 688 (50.5) | 4.746 | 0.029 |
| Cr↑ | 6 (10.0) | 36 (2.4) | 12.638 | <0.001 |
| Echocardiography, n (%) | ||||
| LA↑ | 20 (35.1) | 338 (25.1) | 2.876 | 0.090 |
| LVEDD↑ | 18 (31.6) | 334 (24.7) | 1.363 | 0.243 |
| EF <40% | 8 (14.0) | 33 (2.4) | 25.971 | <0.001 |
| Coronary angiogram, n (%) | ||||
| One vascular lesion | 16 (25.4) | 472 (31.6) | 1.072 | 0.301 |
| Two vascular lesions | 22 (34.9) | 475 (31.8) | 0.276 | 0.599 |
| Three vascular lesions | 25 (39.7) | 548 (36.7) | 0.238 | 0.625 |
| Drugs, n (%) | ||||
| Aspirin | 49 (77.8) | 1483 (99.2) | 169.020 | <0.001 |
| Clopidogrel | 43 (68.3) | 1182 (79.1) | 4.203 | 0.040 |
| Ticagrelor | 5 (7.9) | 300 (20.1) | 5.650 | 0.017 |
| β-blockers | 32 (50.8) | 766 (51.2) | 0.005 | 0.945 |
| ACEIs/ARBs | 19 (30.2) | 679 (45.4) | 5.692 | 0.017 |
| Statins | 49 (77.8) | 1478 (98.9) | 137.816 | <0.001 |
| CCB | 7 (11.1) | 258 (17.3) | 1.618 | 0.203 |
| Diuretics | 11 (17.5) | 99 (6.6) | 10.822 | 0.001 |
MACE, major adverse cardiac events; NMACE, non-major adverse cardiac events; TIA, transient ischemic attack; T2DM, type 2 diabetes mellitus; HF, heart failure; CGS, cardiogenic shock; CAD, coronary artery disease; UA, unstable angina; STEMI: ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; WBC, white blood cells; PLT, platelets; | NEU |, absolute value of neutrophils; | LYM |, absolute value of lymphocytes; | MON |, absolute value of monocytes; MPV, mean platelet volume; PDW, platelet distribution width; NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALB, albumin; CK-MB, creatine phosphokinase isoenzyme; Cr, creatinine; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; EF, ejection fraction; ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; CCB, amlodipine besylate tablet; ↑, above the upper limit of normal values; ↓, below the lower limit of normal values.
Table 2.
Baseline clinical characteristics between the MACE2 group (cardiac mortality or rehospitalization for severe heart failure) and NMACE2 group.
| Factors | MACE2 (n=38) | NMACE2 (n=1520) | χ2/t | P |
|---|---|---|---|---|
| Demographics and clinical data, n (%) | ||||
| Male sex | 29 (76.3) | 1137 (74.8) | 0.045 | 0.832 |
| Age >65 years | 18 (47.4) | 354 (23.3) | 11.826 | 0.001 |
| Hyperlipidemia | 20 (52.6) | 862 (56.7) | 0.251 | 0.616 |
| Hypertension | 18 (47.4) | 897 (59.0) | 2.074 | 0.150 |
| History of stroke | 11 (28.9) | 211 (13.9) | 6.887 | 0.009 |
| History of TIA | 0 (0.0) | 5 (0.3) | 0.125 | 0.723 |
| T2DM | 8 (21.1) | 386 (25.4) | 0.370 | 0.543 |
| History of HF | 17 (44.7) | 141 (9.3) | 51.156 | <0.001 |
| History of CGS | 8 (21.1) | 17 (1.1) | 93.306 | <0.001 |
| Current smoking | 18 (47.4) | 791 (52.0) | 0.324 | 0.569 |
| Family history of | ||||
| CAD | 2 (5.3) | 217 (14.3) | 2.493 | 0.114 |
| UA | 7 (18.4) | 609 (40.1) | 7.266 | 0.007 |
| STEMI | 23 (60.5) | 671 (44.1) | 4.027 | 0.045 |
| NSTEMI | 8 (21.1) | 240 (15.8) | 0.767 | 0.381 |
| Laboratory data, n (%) | ||||
| WBC↑ | 16 (43.2) | 494 (32.6) | 1.860 | 0.173 |
| PLT↑ | 2 (5.3) | 115 (7.6) | 0.283 | 0.595 |
| | NEU |↑ | 16 (44.4) | 531 (35.0) | 1.381 | 0.240 |
| | LYM |↓ | 12 (33.3) | 263 (17.3) | 6.187 | 0.013 |
| | MON |↑ | 13 (36.1) | 317 (20.9) | 4.876 | 0.027 |
| MPV↑ | 1 (2.9) | 5 (0.3) | 5.675 | 0.017 |
| PDW↑ | 1 (2.9) | 15 (1.0) | 1.242 | 0.265 |
| NLR↑ | 28 (77.8) | 798 (52.6) | 8.975 | 0.003 |
| MLR↑ | 15 (41.7) | 282 (18.6) | 12.127 | <0.001 |
| PLR↑ | 14 (38.9) | 197 (13.0) | 20.121 | <0.001 |
| ALB↓ | 5 (14.3) | 71 (4.7) | 6.717 | 0.010 |
| CK-MB↑ | 22 (64.7) | 702 (50.7) | 2.597 | 0.107 |
| Cr↑ | 5 (14.3) | 37 (2.4) | 18.269 | <0.001 |
| Echocardiography, n (%) | ||||
| LA↑ | 13 (39.4) | 345 (25.2) | 3.435 | 0.064 |
| LVEDD↑ | 11 (33.3) | 341 (24.8) | 1.246 | 0.264 |
| EF <40% | 16 (48.5) | 323 (23.5) | 10.993 | 0.001 |
| Coronary angiogram, n (%) | ||||
| One vascular lesion | 8 (21.1) | 480 (31.6) | 1.910 | 0.167 |
| Two vascular lesions | 13 (34.2) | 484 (31.8) | 0.096 | 0.757 |
| Three vascular lesions | 17 (44.7) | 556 (36.6) | 1.061 | 0.303 |
| Drugs, n (%) | ||||
| Aspirin | 26 (68.4) | 1506 (99.1) | 212.348 | <0.001 |
| Clopidogrel | 20 (52.6) | 1205 (79.3) | 15.662 | <0.001 |
| Ticagrelor | 4 (7.4) | 301 (19.8) | 2.026 | 0.155 |
| β-blockers | 18 (47.4) | 780 (51.3) | 0.231 | 0.631 |
| ACEIs/ARBs | 8 (21.1) | 690 (45.4) | 8.883 | 0.003 |
| Statins | 26 (68.4) | 1501 (98.8) | 174.867 | <0.001 |
| CCB | 4 (10.5) | 261 (17.2) | 1.160 | 0.282 |
| Diuretics | 9 (23.7) | 101 (6.6) | 16.404 | <0.001 |
MACE, major adverse cardiac events; NMACE, non-major adverse cardiac events; TIA, transient ischemic attack; T2DM, type 2 diabetes mellitus; HF, heart failure; CGS, cardiogenic shock; CAD, coronary artery disease; UA, unstable angina; STEMI: ST-segment elevation myocardial infarction; NSTEMI, non-ST-segment elevation myocardial infarction; WBC, white blood cells; PLT, platelets; | NEU |, absolute value of neutrophils; | LYM |, absolute value of lymphocytes; | MON |, absolute value of monocytes; MPV, mean platelet volume; PDW, platelet distribution width; NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; ALB, albumin; CK-MB, creatine phosphokinase isoenzyme; Cr, creatinine; LA, left atrium; LVEDD, left ventricular end-diastolic diameter; EF, ejection fraction; ACEIs/ARBs, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers; CCB, amlodipine besylate tablet; ↑, above the upper limit of normal values; ↓, below the lower limit of normal values.
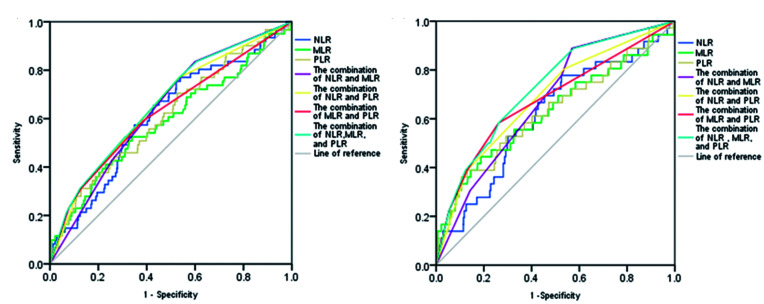
Receiver operating characteristic curve analysis of the NLR, MLR, and PLR for diagnosis of MACE
The area under the receiver operating characteristic curve of the NLR, MLR, PLR, combination of the NLR and MLR, combination of the NLR and PLR, combination of the MLR and PLR, and combination of these three ratios for the MACE1 group was 0.613 (95% confidence interval [CI] 0.541–0.686), 0.588 (95% CI 0.508–0.668), 0.610 (95% CI 0.535–0.685), 0.641 (95% CI 0.575–0.707), 0.652 (95% CI 0.581–0.723), 0.627 (95% CI 0.550–0.705), and 0.663 (95% CI 0.594–0.732), respectively. The optimal diagnostic value of the NLR was 2.67, and the sensitivity and specificity were 0.754 and 0.448, respectively. The optimal diagnostic value of the MLR was 0.33, and the sensitivity and specificity were 0.525 and 0.661, respectively. The best diagnostic threshold of the PLR was 225.49, and the sensitivity and specificity were 0.311 and 0.871, respectively (all P<0.05) (Figure 2a). The area under the receiver operating characteristic curve of the NLR, MLR, PLR, combination of the NLR and MLR, combination of the NLR and PLR, combination of the MLR and PLR, and combination of these three ratios for the MACE2 group were 0.615 (95% CI 0.521–0.710), 0.627 (95% CI 0.521–0.734), 0.629 (95% CI 0.526–0.732), 0.673 (95% CI 0.593–0.752), 0.687 (95% CI 0.596–0.777), 0.678 (95% CI 0.579–0.777), and 0.724 (95% CI 0.643–0.806), respectively. The optimal diagnostic value of the NLR was 2.67, and the sensitivity and specificity were 0.778 and 0.475, respectively. The optimal diagnostic value of the MLR was 0.43, and the sensitivity and specificity were 0.444 and 0.813, respectively. The best diagnostic value of the PLR was 225.49, and the sensitivity and specificity were 0.389 and 0.870, respectively (all P<0.05) (Figure 2b). The NLR and PLR showed similar optimal diagnostic value for the MACE1 and MACE2 groups, whereas the MLR had different optimal diagnostic values for the MACE1 and MACE2 groups.
Figure 2.
Receiver operating characteristic curve of the NLR, MLR, and PLR in predicting (a) MACE1 (all-cause mortality, cardiac mortality, and rehospitalization for severe heart failure) and (b) MACE2 (cardiac mortality and rehospitalization for severe heart failure).
NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MACE, major adverse cardiac events.
Kaplan–Meier survival curves
Patients with ACS who underwent PCI were classified in relation to their NLR, MLR, and PLR (≥ the optimal diagnostic value) as follows: low-risk group (the NLR, MLR, and PLR were all < the optimal diagnostic value), medium-risk group (one of the ratios was ≥ the optimal diagnostic value), high-risk group (two of the ratios were ≥ the optimal diagnostic value), and extremely high-risk group (the NLR, MLR, and PLR were ≥ the optimal diagnostic value). The median survival time of the low-, medium-, high-, and extremely high-risk groups for MACE1 was 1213.00 (903.75, 1449.25) days, 1137.50 (852.00, 1137.50) days, 985.00 (814.00, 1381.75) days, and 944.50 (763.00, 1276.00) days, respectively. The overall survival time was significantly different among the four groups (χ2=28.534, log-rank test, P<0.001) (Figure 3a). The median survival time of the low-, medium-, high-, and extremely high-risk groups for MACE2 was 1206.50 (901.25, 1445.00) days, 1088.00 (844.50, 1408.50) days, 974.50 (795.50, 1322.75) days, and 980.00 (762.00, 1391.00) days, respectively. The overall survival time was significantly different among the four groups (χ2=28.012, log-rank test, P<0.001) (Figure 3b).
Figure 3.
Kaplan–Meier survival curve of patients who underwent percutaneous coronary intervention in relation to the neutrophil-to-lymphocyte ratio, monocyte-to-lymphocyte ratio, and platelet-to-lymphocyte ratio for (a) MACE1 (all-cause mortality, cardiac mortality, and rehospitalization for severe heart failure) and (b) MACE2 (cardiac mortality and rehospitalization for severe heart failure).
MACE, major adverse cardiac events.
Forest graphs for predicting MACE in relation to the NLR, MLR, and PLR
Variables were screened for the Cox proportional hazards regression model to determine the risk factors for MACE1 and MACE2 in patients with ACS who underwent PCI. The forest graphs for predicting MACE1 in patients with ACS who underwent PCI in relation to the NLR, MLR, or PLR alone showed that the NLR and PLR were independent risk factors for predicting MACE1 (both P<0.05). The predictive value of the NLR was higher than that of PLR, with hazard ratios (HR) of 2.938 and 2.303, respectively (Figure 4). The forest graphs for predicting MACE2 in patients with ACS who underwent PCI in relation to the NLR, MLR, or PLR alone showed that the NLR, MLR, and PLR were independent risk factors for predicting MACE2 (all P<0.001). The predictive value of the NLR was the highest (13.268), followed by that of the PLR (11.551) and that of the MLR (6.064) (Figure 5).
Figure 4.
Forest graphs of the Cox proportional hazards regression model for predicting MACE1 (all-cause mortality, cardiac mortality, and rehospitalization for severe heart failure) in patients with acute coronary syndrome who underwent percutaneous coronary intervention in relation to (a) the NLR, (b) MLR, and (c) PLR alone.
HR, hazard ratio; CI, confidence interval; HF, heart failure; CGS, cardiogenic shock; Cr, creatinine; LVEF, left ventricular ejection fraction; NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MACE, major adverse cardiac events.
Figure 5.
Forest graphs of the Cox proportional hazards regression model for predicting MACE2 (cardiac mortality and rehospitalization for severe heart failure) in patients with acute coronary syndrome who underwent percutaneous coronary intervention in relation to (a) the NLR, (b) MLR, and (c) PLR alone.
HR, hazard ratio; CI, confidence interval; HF, heart failure; CGS, cardiogenic shock; Cr, creatinine; LVEF, left ventricular ejection fraction; NLR, neutrophil-to-lymphocyte ratio; MLR, monocyte-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; MACE, major adverse cardiac events.
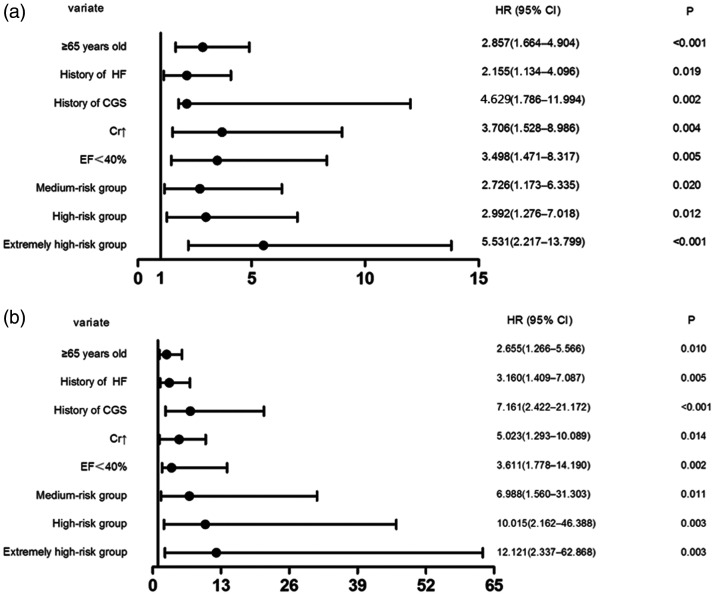
Age ≥65 years, a history of heart failure, a history of cardiogenic shock, elevated creatinine levels, and an ejection fraction <40% were significant independent risk factors for MACE1, with HRs of 2.857, 2.155, 4.629, 3.706, and 3.498, respectively (all P<0.05). An NLR ≥2.67, MLR ≥0.33, and PLR ≥225.49 were also significant independent risk factors for MACE1 (P=0.004). The HRs of the medium-risk, high-risk, and extremely high-risk groups were 2.726, 2.992, and 5.531, respectively. The HR of the medium-risk group was the lowest and close to that of age ≥65 years. The HR of the high-risk group was between that of age ≥65 years and an ejection fraction <40%, and higher than that of the medium-risk group (Figure 6a).
Figure 6.
Forest graphs of the Cox proportional hazards regression model to test the risk factors for (a) MACE1 (all-cause mortality, cardiac mortality, and rehospitalization for severe heart failure) and (b) MACE2 (cardiac mortality and rehospitalization for severe heart failure) in patients with acute coronary syndrome who underwent percutaneous coronary intervention.
HR, hazard ratio; CI, confidence interval; HF, heart failure; CGS, cardiogenic shock; Cr, creatinine; EF, ejection fraction; MACE, major adverse cardiac events.
Moreover, age ≥ 65 years, a history of heart failure, a history of cardiogenic shock, elevated preoperative creatinine levels, and an ejection fraction <40% were significant independent risk factors for MACE2, with HRs of 2.655, 3.160, 7.161, 5.023, and 3.611, respectively (all P<0.05). An NLR ≥2.67, MLR ≥0.43, and PLR ≥225.49 were also independent risk factors for MACE2 (P=0.018). The HRs of the medium-risk, high-risk, and extremely high-risk groups were 6.988, 10.015, and 12.121, respectively (Figure 6b). The NLR, MLR, PLR, and the combination of these three ratios were better for predicting MACE2 than for MACE1.
Discussion
In this study, we examined the diagnostic and predictive value of peripheral blood inflammatory cell subsets in patients with ACS who underwent PCI. We found that the NLR, MLR, and PLR had a similar diagnostic value for MACE in patients with ACS after PCI. Kaplan–Meier survival curves showed that the survival time gradually decreased as the degree of risk increased. The Cox proportional hazards regression model showed that the NLR, MLR, and PLR were independent risk factors for predicting cardiac mortality and rehospitalization for severe heart failure. The NLR had the highest predictive value and the MLR had the lowest predictive value. The risk of MACE in the medium-risk, high-risk, and extremely high-risk groups showed an upward trend compared with the low-risk group. The mechanism of this finding may be related to a more severe inflammatory response in the body resulting in a higher number of inflammatory cell subsets. The NLR, which was initially used for assessing prognosis of cancer, tuberculosis infection, and autoimmune diseases, is an inflammatory factor in peripheral blood that can be used to assess prognosis of patients with CAD.22–24 Previous studies have shown that the NLR is a predictor of short- and long-term poor prognosis in patients with ACS after PCI.25–30 The MLR has rarely been reported and is considered as a novel marker of inflammation. 31 Chen et al. 32 found that the MLR was correlated with the severity of CAD and had a higher predictive value for severity than NLR. These authors also found that the MLR was an independent predictor of MACE in patients with non-ST-segment elevation myocardial infarction.
At present, there are no relevant reports on the prediction of prognosis of ACS by the MLR in patients undergoing PCI. The PLR is a newly discovered marker of inflammation in peripheral blood. Previous studies have shown that an increased PLR is a manifestation of platelet activation and increased aggregation of microsomes. Platelets and monocytes activate the mechanism of coagulation and thrombosis, promote thrombosis and atherosclerosis, and intensify the inflammatory response, which may lead to a poor prognosis of cardiovascular disease.33,34 However, there is no clear conclusion on the relationship between the PLR and the prognosis of patients with CAD after PCI. Cho et al. and Zhou et al.35,36 found that the PLR was associated with the severity of coronary stenosis and major cardiovascular adverse events. However, Bressi et al. 37 reported that, in patients with stable CAD, an increased PLR was not associated with an increased risk of long-term clinical adverse events. The pathological basis of ACS is rupture of unstable coronary plaques and secondary thrombosis. Therefore, the different results of the above-mentioned studies compared with our study may be related to the stronger inflammatory response in ACS than in stable CAD. In our study, the PLR showed predictive value and was an independent risk factor for a poor prognosis in patients with ACS after PCI.
The present study has some limitations. First, the sample was relatively small and from a single hospital. Second, the design of this study was prospective and lacked a healthy control group. These findings need to be further validated in large-sample, multicenter, clinical studies.
In conclusion, the present study further confirms that inflammatory cell subsets have diagnostic and predictive value for MACE in patients with ACS undergoing PCI. As the risk stratification increases, a poor prognosis (all-cause mortality, cardiogenic mortality, and rehospitalization for severe heart failure) of patients with ACS undergoing PCI increases correspondingly with a gradual decrease in the median survival time.
Acknowledgements
The authors are grateful for the assistance of the cardiologists, laboratory staff, and nurses at the Affiliated Hospital of Chengde Medical University.
Footnotes
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This study was supported by grants from Hebei Province Government Science and Technology Agency (Grant no. 17277769D) to Dr. Lixian Sun and Technology Innovation Guidance Project-Science and Technology Work Conference from Hebei Provincial Department of Science and Technology (2020) to Dr. Lixian Sun.
ORCID iD: Lixian Sun https://orcid.org/0000-0001-9814-0965
References
- 1.WHO. The Top 10 causes of death, https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (2018, accessed 2 December 2020).
- 2.Yusuf S, Reddy S, Ounpuu S, et al. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition risk factors, and impact of urbanization. Circulation 2001; 104: 2746–2753. [DOI] [PubMed] [Google Scholar]
- 3.Hahn JY, Song YB, Oh JH, et al. 6-month versus 12-month or longer dual antiplatelet therapy after percutaneous coronary intervention in patients with acute coronary syndrome (SMART-DATE): a randomised, open-label, non-inferiority trial. Lancet 2018; 391: 1274–1284. [DOI] [PubMed] [Google Scholar]
- 4.Abolhasani S, Shahbazloo SV, Saadati HM, et al . Evaluation of serum levels of inflammation fibrinolysis and oxidative stress markers in coronary artery disease prediction: A cross-sectional study. Arq Bras Cardiol 2019; 113: 667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peikert A, Kaier K, Merz J, et al. Residual inflammatory risk in coronary heart disease: incidence of elevated high-sensitive CRP in a real-world cohort. Clin Res Cardiol 2020; 109: 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li C, Zhang F, Shen Y, et al. Impact of Neutrophil to Lymphocyte Ratio (NLR) Index and Its Periprocedural Change (NLRΔ) for Percutaneous Coronary Intervention in Patients With Chronic Total Occlusion. Angiology 2017; 68: 640–646. [DOI] [PubMed] [Google Scholar]
- 7.Park JJ, Jang HJ, Oh IY, et al. Prognostic value of neutrophil to lymphocyte ratio in patients presenting with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Am J Cardiol 2013; 111: 636–642. [DOI] [PubMed] [Google Scholar]
- 8.Pinheiro MG, Araujo GN, Carpes CK, et al. Elevated neutrophil-to-lymphocyte ratio can predict procedural adverse events in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron Artery Dis 2019; 30: 20–25. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto E, Sugiyama S, Hirata Y, et al. Prognostic significance of circulating leukocyte subtype counts in patients with coronary artery disease. Atherosclerosis 2016; 255: 210–216. [DOI] [PubMed] [Google Scholar]
- 10.Tabas I, Lichtman AH. Monocyte-macrophages and T cells in atherosclerosis. Immunity 2017; 47: 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman MS, Murphy AJ, Woollard KJ. Effects of dyslipidaemia on monocyte production and function in cardiovascular disease. Nat Rev Cardiol 2017; 14: 387–400. [DOI] [PubMed] [Google Scholar]
- 12.Nording H, Baron L, Langer HF. Platelets as therapeutic targets to prevent atherosclerosis. Atherosclerosis 2020; 307: 97–108. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim H, Kleiman NS. Platelet pathophysiology, pharmacology, and function in coronary artery disease. Coron Artery Dis 2017; 28: 614–623. [DOI] [PubMed] [Google Scholar]
- 14.Her AY, Cho KI, Singh GB, et al. Plaque characteristics and inflammatory markers for the prediction of major cardiovascular events in patients with ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging 2017; 33: 1445–1454. [DOI] [PubMed] [Google Scholar]
- 15.Wada H, Dohi T, Miyauchi K, et al. Pre-procedural neutrophil-to-lymphocyte ratio and long-term cardiac outcomes after percutaneous coronary intervention for stable coronary artery disease. Atherosclerosis 2017; 265: 35–40. [DOI] [PubMed] [Google Scholar]
- 16.Trakarnwijitr I, Li B, Adams H, et al. Age modulates the relationship between platelet-to-lymphocyte ratio and coronary artery disease. Int J Cardiol 2017; 248: 349–354. [DOI] [PubMed] [Google Scholar]
- 17.Ji H, Li Y, Fan Z, et al. Monocyte/lymphocyte ratio predicts the severity of coronary artery disease: a syntax score assessment. BMC Cardiovasc Disord 2017; 17: 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haybara H, Pezeshkib SMS and Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: An early indicator of prognosis? Exp Mol Pathol 2019; 110; 104267. [DOI] [PubMed] [Google Scholar]
- 19.Bakkum MJ, Danad I, Romijn MA, et al. The impact of obesity on the relationship between epicardial adipose tissue, left ventricular mass and coronary microvascular function. Eur J Nucl Med Mol Imaging 2015; 42: 1562–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chamberlain JJ, Rhinehart AS, Shaefer CF, et al. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann Intern Med 2016; 164: 542–552. [DOI] [PubMed] [Google Scholar]
- 21.Da Silva PM, Duarte JS, Von Hafe P, et al. Standardization of laboratory and lipid profile evaluation: A call for action with a special focus in 2016 ESC/EAS dyslipidaemia guidelines - Full report. Atheroscler Suppl 2018; 31: e1–e12. [DOI] [PubMed] [Google Scholar]
- 22.Stefaniuk P, Szymczyk A and Podhorecka M. The neutrophil to lymphocyte and lymphocyte to monocyte ratios as new prognostic factors in hematological malignancies -a narrative review. Cancer Manag Res 2020; 12: 2961–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rees CA, Pineros DB, Amour M, et al. The potential of CBC-derived ratios (monocyte-to-lymphocyte, neutrophil-to-lymphocyte, and platelet-to-lymphocyte) to predict or diagnose incident TB infection in Tanzanian adolescents. BMC Infect Dis 2020; 20: 609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mititelu RR, Pădureanu R, Băcănoiu M, et al. Inflammatory and oxidative stress markers-mirror tools in rheumatoid arthritis. Biomedicines 2020; 8: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akpek M, Kaya MG, Lam YY, et al. Relation of neutrophil/lymphocyte ratio to coronary flow to in-hospital major adverse cardiac events in patients with ST-elevated myocardial infarction undergoing primary coronary intervention. Am J Cardiol 2012; 110: 621–627. [DOI] [PubMed] [Google Scholar]
- 26.Kaya MG, Akpek M, Lam YY, et al. Prognostic value of neutrophil/lymphocyte ratio in patients with ST-elevated myocardial infarction undergoing primary coronary intervention: a prospective, multicenter study. Int J Cardiol 2013; 168: 1154–1159. [DOI] [PubMed] [Google Scholar]
- 27.Celik T, Balta S, Demir M, et al. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol J 2016; 23: 84–92. [DOI] [PubMed] [Google Scholar]
- 28.Balta S, Celik T, Ozturk C, et al. The Relation between Monocyte-HDL Ratio and No-reflow Phenomenon in with Acute ST Segment Elevation Myocardial Infarction. Am J Emerg Med 2016; 34: 1542–1547. [DOI] [PubMed] [Google Scholar]
- 29.Celik T, Balta S, Mikhailidis DP, et al. The Relation Between No-Reflow Phenomenon and Complete Blood Count Parameters. Angiology 2017; 68: 381–388. [DOI] [PubMed] [Google Scholar]
- 30.Adatia K, Farag MF, Gue YX, et al. Relationship of platelet reactivity and inflammatory markers to recurrent adverse events in patients with ST-elevation myocardial infarction. Thromb Haemost 2019; 119: 1785–1794. [DOI] [PubMed] [Google Scholar]
- 31.Fan Z, Li Y, Ji H, et al. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: a retrospective cohort study. BMJ Open 2018; 8: e023459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Li M, Liu L, et al. Monocyte/lymphocyte ratio is related to the severity of coronary artery disease and clinical outcome in patients with non-ST-elevation myocardial infarction. Medicine (Baltimore) 2019; 98: e16267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ugur M, Gul M, Bozbay M, et al. The relationship between platelet to lymphocyte ratio and the clinical outcomes in ST elevation myocardial infarction underwent primary coronary intervention. Blood Coagul Fibrinolysis 2014; 25: 806–811. [DOI] [PubMed] [Google Scholar]
- 34.Cetin EHO, Cetin MS, Aras D, et al. Platelet to Lymphocyte Ratio as a Prognostic Marker of In-Hospital and Long-Term Major Adverse Cardiovascular Events in ST-Segment Elevation Myocardial Infarction. Angiology 2016; 67: 336–345. [DOI] [PubMed] [Google Scholar]
- 35.Zhou D, Wang G, Fan Y, et al. Platelet to lymphocyte ratio is associated with the severity of coronary artery disease and clinical outcomes of percutaneous coronary intervention in the Chinese Han population. Exp Ther Med 2017; 13: 731–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cho KI, Ann SH, Singh GB, et al. Combined usefulness of the platelet-to-lymphocyte ratio and the neutrophil-to-lymphocyte ratio in predicting the long-term adverse events in patients who have undergone percutaneous coronary intervention with a drug-eluting stent. PLoS One 2015; 10: e0133934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bressi E, Mangiacapra F, Ricottini E, et al. Impact of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio on 5-year clinical outcomes of patients with stable coronary artery disease undergoing elective percutaneous coronary intervention. J Cardiovasc Transl Res 2018; 11: 517–523. [DOI] [PubMed] [Google Scholar]