Abstract
Purpose
Diagnosis of atrial tachycardia (AT) with 3D mapping system remains challenging due to fibrosis or previous ablation. This study aims to evaluate a new electroanatomical mapping annotation setting using a window of interest adjusted at the end of the P wave (WOIp wave) to identify the AT mechanism more accurately.
Methods
Twenty patients with successful ablation of left AT using navigation system CARTO3 were evaluated. Two maps for each patient were generated offline using either conventional settings of WOI (WOIconv.) or WOIp wave. Three investigators from two centres analysed the maps blindly.
Results
Mechanisms of AT were macroreentrant in 14/20 patients (70%) and focal in 6/20 (30%). WOIp wave resulted in a significant increase in the percentage of correct identification of the mechanism based on mapping alone (93.3 ± 13.7% vs 58.3 ± 33.9%; p = 0.0003) compared with WOIconv.. Diagnoses based on mapping were arrived at faster (27.8 ± 16.4 s vs 38.97 ± 13.64 s, respectively; p = 0.0231) and with a greater confidence in the diagnosis (confidence index 2.57 ± 0.45 vs 2.12 ± 0.45, respectively; p = 0.0024). With perimitral re-entry specifically “early meets late” was closer to the anatomical region of the mitral isthmus (15.9 ± 20.9 mm vs 48.77 ± 23.23 mm, respectively; p = 0.0028).
Conclusions
This study found that electroanatomical mapping acquisition with a window of interest set at the end of the P wave improves the ability to diagnose the arrhythmia mechanism based on the initial map. It is particularly beneficial in identifying area of interest for ablation in perimitral AT.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10840-021-00940-0.
Keywords: Atrial tachycardia, Window of interest, Electroanatomical mapping, Mitral isthmus
Introduction
Interpretation of activation mapping to determine the mechanism of atrial tachycardia (AT) may be challenging because of the presence of intrinsic scarring further complicated by previous ablation or fibrosis [1, 2]. With the CARTO3 navigation system, the windows of interest (WOI) is the main parameter used to create an activation map and is defined by the interval preceding and following a reference point that determines the colour of each point projected on the map. WOI onset positioned in mid-diastole has been shown to be effective by De Ponti et al. and is currently the conventional setting (WOIconv.) [3]. The accuracy of the activation map generated with this technique is crucial for determining the AT propagation. However, apparent circular activation alone may be related to a focus with abnormal conduction through scarred tissue while on the other hand apparent focal activation may be related to failure to appreciate a smaller circuit. Consequently, other settings for WOI have been suggested to better appreciate mechanism and identify a potential critical isthmus [4].
Of course, entrainment and other complementary are critically important diagnostically but are not always possible and AT induction after termination of an AT during entrainment may not be reproducible. In the present study, we aimed to assess whether setting the WOI set at the end of the P wave (WOIp wave) improves the ability to diagnose the arrhythmia mechanism based on the initial mapping. In typical atrial flutter (cavotricuspid isthmus-dependent), the plateau phase of slow conduction following the P wave corresponds to the region of a critical isthmus in macroreentrant AT [5]. Positioning the WOI at the end of the P wave prior to a relatively isoelectric component should be related to the entrance into the slow conduction and improve the ability to identify the critical slow conduction zone on the map. Focal AT is generally less problematic, but the P wave onset should reflect anatomical exit from the focus from which it spreads centrifugally [6]. This technique is feasible if there is an identifiable relatively isoelectric interval which there appears to be most of the time.
Methods
Study population
The study population included a series of 20 consecutive patients 15% female (mean age: 68.8 ± 9.16 years, range: 48–82 years) undergoing catheter ablation at our institution (Hôpital Privé Clairval, Ramsay Santé, France) for symptomatic sustained left AT. All patients gave their written informed consent before taking part in the study. The study was approved by the local institutional review board (COS-RGDS-2016-06-087-MECHULAN-A) and followed the principles of the Declaration of Helsinki.
AT ablation procedure
All procedures were performed under general anaesthesia. Using trans-septal access guided by transoesophageal echocardiography, two catheters were inserted into the left atrium via irrigated sheaths (SL0 sheath or Agilis St Jude Medical Inc., St Paul, MN, USA). The mapping catheter used was a duo decapolar electrode catheter (Pentaray®; Biosense Webster Inc., Diamond Bar, CA, USA) to create 3D-electroanatomical mapping of the left atrium (LA) and PVs using a navigation system (Version 4 of CARTO3™; Biosense Webster Inc., Diamond Bar, CA, USA). The second catheter was the contact force (CF) ablation catheter with advanced irrigated porous tip (ThermoCool SmarttouchSF™; Biosense Webster Inc., Diamond Bar CA, USA). 3D-electroanatomical mapping were performed after identification of P waves on the surface electrocardiogram (ECG). The lead V1 was used to identify P waves. When the P wave was not clearly observable, all 12 channels were analysed and ECG signal was amplified. The WOI was set at the end of the P wave and calculated as follows: WOI duration = tachycardia cycle length-10 ms. The WOI started after the P wave when the slope of the signal was equal to zero. Atrial signal on the coronary sinus electrograms was used as the reference signal. A steerable decapolar catheter (Xtrem Dynamic®, Microport CRM France, Clamart), 5 mm electrode spacing, was positioned in the coronary sinus. Points were collected using ConfiDense in wavefront annotation. The tissue proximity indicator (TPI) was activated with LAT filter on 4 ms, stability filter on 4 mm and density filter on 1 mm. The colour fill threshold was set at 5. Signals were colour coded according to their activation timing. After 3D reconstruction, ablations were performed depending on the mechanism identified using the CF catheter with power between 25 and 50 W. Algorithm CARTO3 VISITAG SURPOINT was used to guide the ablation (targeted values: 450 in anterior wall). Mitral line was performed from the superior aspect of the left inferior pulmonary vein to the lateral part of the mitral valve and roof line was done from the left superior pulmonary vein to the right superior pulmonary vein at the top of the posterior wall. The CF used was between 10 and 50 g of force according to the operator’s judgment. Final diagnosis of AT was established by activation map and entrainment and confirmed by the results of catheter ablation achieving AT termination.
Offline interpretation of map
Maps were generated offline to ensure that all the other parameters remained equal. An engineer used 20 consecutive maps realized in the operating room with the WOIp wave. The 20 maps were anonymized and duplicated with the WOIconv. All the points were analysed and re-annotated if necessary. The 40 maps were presented randomly to the three investigators from two centres to allow a blind interpretation (Clairval Private Hospital, Marseille and Pasteur University Hospital, Nice). The percentages of correct interpretation of the precise mechanism (defined as correct mechanism and location of AT), time for interpretation (the timer was engaged when the maps were presented to investigators for interpretation) and confidence index for the diagnoses were evaluated. The confidence index was defined as an indicator of the operators’ confidence in their diagnosis: 1, not confident; 2, moderately confident; 3, very confident.
Measurement of the distance from “early meets late” to the mitral isthmus
Colours on the activation maps were removed and a line was drawn between the inferior side of the left inferior PV and the lateral part of the mitral valve. The distance measured was the smallest distance between “early meets late” and the middle of the mitral isthmus (MI) line [7].
Statistical analysis
Descriptive variables are presented as means ± standard deviation (SD). Two-group comparisons of continuous variables were performed using Mann-Whitney test as data were not normally distributed according to Shapiro-Wilk tests. Two-tailed p values < 0.05 were considered to indicate statistical significance. Statistical analyses were performed using GraphPad Prism (GraphPad software 5.01).
Results
Patients and atrial tachycardia characteristics
The study population included a total of 40 maps in 20 patients who presented for first-time AT ablation (n = 2) or redo atrial arrhythmia ablation (n = 18) because of organized AT. The characteristics of the study population are shown in Table 1. Mean was 68.8 ± 9.16 years and 3 of 20 (15%) were females. Mean LA size was 157.2 ± 34.31 ml.
Table 1.
Clinical characteristics of the study population
| # | Age | Sex | TCL (ms) | LA size (mL) | Mechanism | AT Location | Circuit | Strategy of ablation for macroreentrant tachycardia | Number of previous procedures | Type of index procedure |
|---|---|---|---|---|---|---|---|---|---|---|
| 2 | 82 | F | 350 | 160 | Macroreentrant | Perimitral | clockwise | MI Line | 1 | parox AF |
| 3 | 63 | M | 280 | 230 | Macroreentrant | Perimitral | Clockwise | MI Line | Index procedure | – |
| 4 | 82 | M | 354 | 174 | Macroreentrant | Perimitral | Clockwise | Anterior Line | 1 | pers AF |
| 5 | 65 | M | 250 | 91 | Macroreentrant | Perimitral | Counterclockwise | MI Line | 1 | pers AF |
| 7 | 75 | M | 240 | 225 | Macroreentrant | Perimitral | Counterclockwise | MI Line | 1 | AF |
| 8 | 76 | M | 290 | 153 | Macroreentrant | Perimitral | Counterclockwise | MI Line | 1 | pers AF |
| 15 | 70 | M | 305 | 126 | Macroreentrant | Perimitral | Clockwise | MI Line | 1 | pers AF |
| 18 | 68 | M | 260 | 143 | Macroreentrant | Perimitral | Clockwise | MI Line | 1 | parox AF |
| 19 | 62 | M | 240 | 141 | Macroreentrant | Perimitral | Clockwise | MI Line | 1 | pers AF |
| 20 | 48 | M | 240 | 172 | Macroreentrant | Perimitral | Clockwise | MI Line | 1 | pers AF |
| 1 | 56 | M | 260 | 112 | Macroreentrant | LA Anterior wall | – | Line from LAA to RPV | 1 | pers AF |
| 12 | 78 | F | 480 | 150 | Macroreentrant | LA Anterior wall | – | Anterior Line | 1 | parox AF |
| 13 | 67 | M | 290 | 153 | Macroreentrant | LA Roof | – | Roof RPV to LPV | 1 | parox AF |
| 17 | 64 | F | 570 | 178 | Macroreentrant | Anterior LAA | – | LAA line | 1 | pers AF |
| 6 | 72 | M | 400 | 134 | Focal | LA Roof | – | – | 1 | parox AF |
| 9 | 77 | M | 270 | 175 | Focal | LA Anterior wall | – | – | 1 | left AT |
| 10 | 59 | M | 240 | 130 | Focal | LA Anterior wall | – | – | 1 | pers AF |
| 11 | 76 | M | 315 | 150 | Focal | LA Roof | – | – | Index procedure | – |
| 14 | 59 | M | 360 | 147 | Focal | Ridge | – | – | 1 | pers AF |
| 16 | 76 | M | 330 | 200 | Focal | LA Anterior wall | – | – | 1 | pers AF |
LA left atria, AF atrial fibrillation, MI mitral isthmus, LAA left atria appendage, RPV right pulmonary vein, LPV left pulmonary vein, TCL tachycardia cycle length (in ms)
Fourteen of the 20 patients (70%) displayed macroreentrant AT, with perimitral AT the most common arrhythmia occurring in 10 of 14 (71.4%). Six of the 20 patients (30%) displayed focal AT, in the anterior region of the LA (50%), localized in the roof (33.3%), or in the ridge between the LA appendage and left PV (16.7%).
3-dimensional electroanatomical mapping
P waves with an isoelectric component were clearly identified on all surface ECGs and WOIs were set. P wave identification was facilitated by clean tracings with patients under anaesthesia. The WOIconv. was set using the formula as described by De Ponti et al. [3] with onset of the WOI set in mid-diastole (Fig. 1a). The WOIp-wave was set at the end of the P wave (Fig. 1b). Maps contained an average of 2025.8 ± 978.4 points per map and covered 96.8 ± 1.3% of the AT cycle. Mean cycle length of the clinical ATs was 316.2 ± 86.4 ms (range: 240–570 ms).
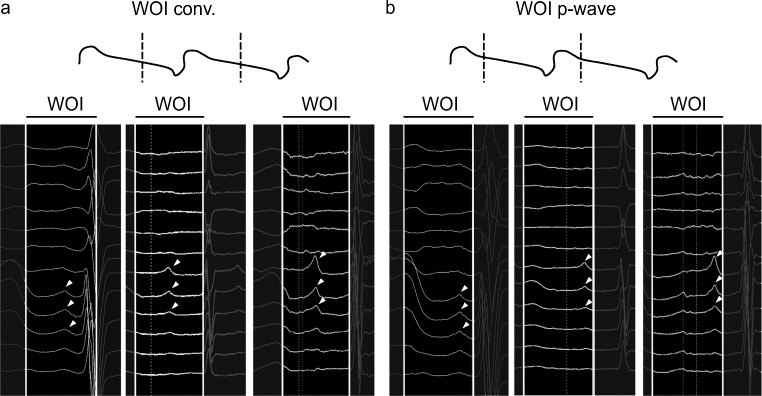
Fig. 1.
Annotation setting of window of interest on the electroanatomic mapping system interface. a Schematic representation of the position of the window of interest (WOI) using the conventional technique (WOIconv.) on the top. Examples of high magnification of ECG signal allowing clear identification of the P wave (white arrow) using the WOIconv. on the bottom. b Schematic representation of the position of the WOI set at the end of the P wave (WOIp-wave) on the top. Examples of high magnification of ECG signal allowing clear identification of the P wave (white arrow) using the WOIp-wave. WOI is the interval between the continuous vertical white line (clear area)
Generation of complete maps was achieved in all 20 ATs using the WOIconv. (Fig. 2a) or the WOIp-wave (Fig. 2b). The validated diagnosis of AT was defined by comprehensive mapping with clear propagation (Fig. 2c and Supplementary videos 1 and 2) and confirmed by the results of catheter ablation terminating AT. An example of one investigator’s diagnostic flowchart is shown in Fig. 3a. The percentage of correct identification of the mechanism was statistically higher with the WOIp-wave compared with the WOIconv. (93.3 ± 13.7% vs 58.3 ± 33.9%, respectively; p = 0.0003) (Fig. 3b). A superior degree of confidence in the diagnoses was obtained with the WOIp-wave (confidence index 2.57 ± 0.46 vs 2.12 ± 0.45, respectively; p = 0.0024) (Fig. 3c). Time needed for correct identification of the AT mechanism was lower with the WOIp-wave (27.8 ± 16.36 s vs 38.97 ± 13.65 s, respectively; p = 0.0231) (Fig. 3d). The inter-observer variability is shown in Table 2.
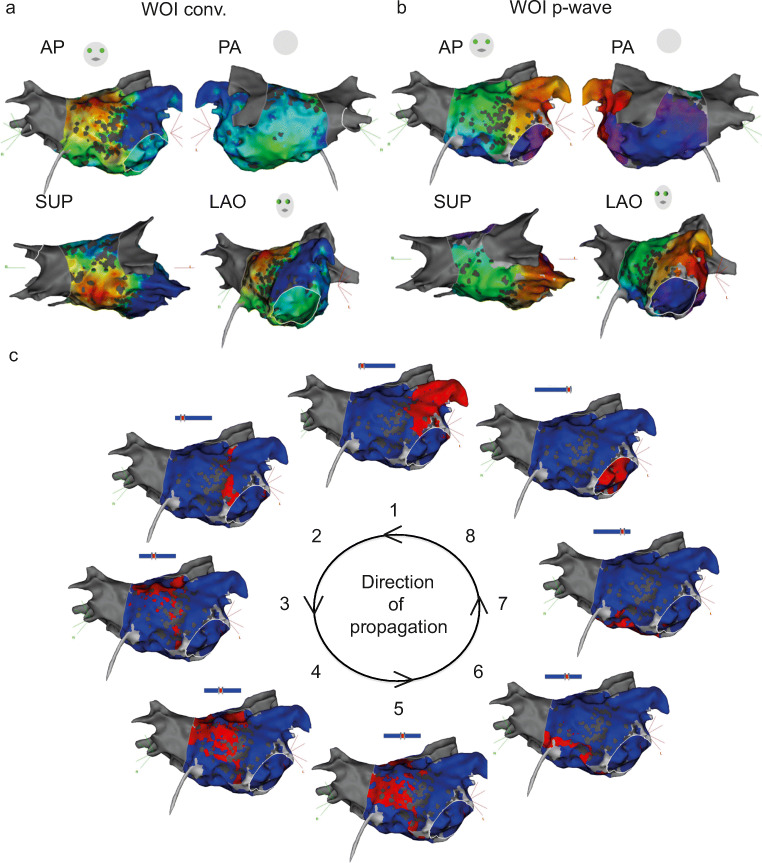
Fig. 2.
Left atrial 3-dimensional electroanatomical maps. a Electroanatomical maps of counterclockwise perimitral AT using the conventional annotation technique (WOIconv.). b Annotation set at the end of the P wave (WOIp-wave). c Propagation of the AT. AP anterior-posterior, LAO left anterior oblique, PA posterior-anterior, SUP superior view
Fig. 3.
Comparison of the percentage of correct identification of AT using the conventional method and for window of interest (WOI) set at the end of the P wave. a Example of diagnostic flowchart for one investigator. The vertical left dotted line represents the mechanism of AT suggested by the conventional WOI (WOIconv.), the middle one represents the investigational annotation (WOIp-wave) and the right one represents the validated diagnosis confirmed by ablation. Each horizontal line symbolizes one patient and the colours indicate the AT mechanism (blue for perimitral, green for focal and red for other macroreentrant ATs). Note that the green dotted line indicates an error of focal location. b Percentage of correct identification calculated for all operators; ***p = 0.0003. c Confidence in diagnosis; **p = 0.0024. D. Time for diagnosis; *p = 0.0231. In this and the following figure: error bars = SD
Table 2.
Inter-observer variability
| Operator 1 | Operator 2 | Operator 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WOI Conv. | WOI p-wave | WOI Conv. | WOI p-Wave | WOI Conv. | WOI p-wave | |||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Success (%) | 65 | 48.94 | 85 | 36.63 | 65.0 | 48.9 | 100 | 0.0 | 45 | 51.04 | 95 | 22.36 |
| Index | 2.25 | 0.72 | 2.55 | 0.76 | 1.9 | 0.8 | 2.5 | 0.8 | 2.25 | 0.55 | 2.65 | 0.59 |
| Time (s) | 40.9 | 26.82 | 22.45 | 17.37 | 52.6 | 38.07 | 44.4 | 39.41 | 23.4 | 12.78 | 16.6 | 10.83 |
Perimitral atrial tachycardias
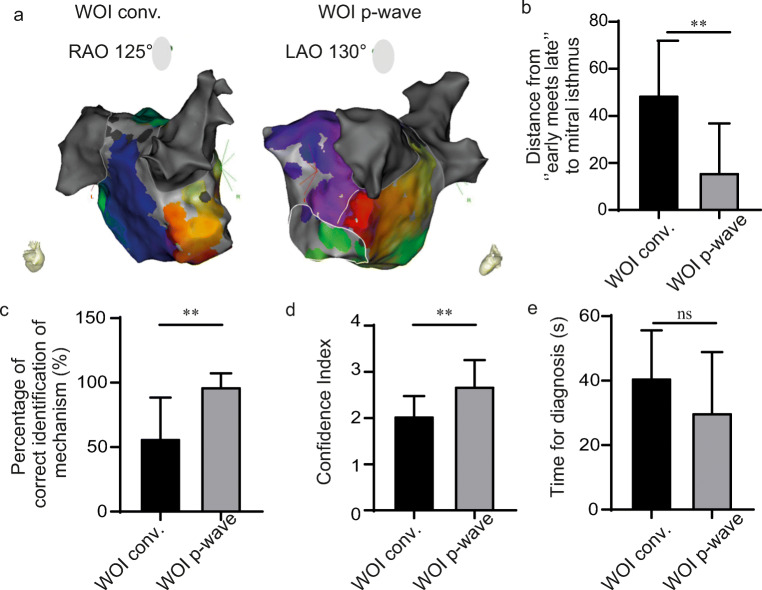
Using WOIp-wave, 90% of perimitral AT maps clearly showed an “early meets late” located close to the anatomical region of MI (Fig. 4a). This observation was quantified by blindly measuring the distance from “early meets late” to the anatomical MI in both settings. In maps obtained with the WOIconv., the distance of “early meets late” from the anatomical MI was higher compared with WOIp wave (48.8 ± 23.2 mm vs 15.9 ± 20.9 mm, respectively; p = 0.0028) (Fig. 4b). These results were associated with an increase in the percentage of successful identification of the mechanism with the WOIp wave (96.7 ± 10.5% vs 56.7 ± 31.6% with WOIconv.) (Fig. 4c) and with an increase in the confidence index (2.7 ± 0.55 vs 2.03 ± 0.43 with WOIconv.) (Fig. 4d). Time to obtain the diagnosis was not statistically different between the two configurations (30.0 ± 18.9 s vs 40.8 ± 14.9 s; p = 0.1903) (Fig. 4e).
Fig. 4.
“Early meets late” location for perimitral AT. a Electroanatomical map of perimitral AT using the conventional annotation technique (WOIconv.) (left) or window of interest set at the end of the P wave (WOIp-wave) (right). The white line indicates the theoretical location of the mitral isthmus. b Distance from “early meets late” to the mitral isthmus in both configurations; **p = 0.0028. c Percentage of correct identification calculated for all investigators; **p = 0.0031. d Confidence in diagnosis **p = 0.005. e Time for diagnosis; ns, p = 0.1903. LAO left anterior oblique, RAO right anterior oblique
Focal atrial tachycardias
Only 6 patients had focal AT and there was no difference between the WOIconv. and WOIp-wave. These results could be explained by the low number of cases. Generally, the p-wave onset should reflect anatomical exit from the focus. However it is not possible to distinguish micro-reentry from true focal activity with activation mapping. Interestingly, in one case, the origin of AT was colour coded in purple at the end of WOIconv. (Fig. 5a) and in red at the beginning of WOIp-wave (Fig. 5b). The origin of AT is separated by 10.4 mm between the two WOIs. This suggests that WOIp-wave increase the sensitivity to detect the first focal activities that could emerge from very slow conduction areas due to scar (Fig. 5 c–d).
Fig. 5.
Focal atrial tachycardias. a Electroanatomical map of focal AT using the conventional annotation technique (WOIconv.). b Electroanatomical map of focal AT using the investigational annotation technique (WOIp-wave). c Electroanatomical map showing ablation site indicated by red dots perfectly correlated with earliest point on WOIp-wave. d Voltage map showing low voltage in red explaining a slow conduction area. All maps are in superior view
Discussion
This study showed that WOIp-wave facilitated identifying the critical isthmus and the mechanism of AT with the initial maps with CARTO3 prior to further manoeuvres.
Wave-front propagation using activation mapping of scar-related AT can be difficult to interpret. A focus may exhibit apparent circular activation and early meets late even in re-entrant circuits is influenced by the WOI. It is desirable to get as much information from the mapping prior to manoeuvres since the tachycardia may be terminated or accelerated by the latter and be difficult to reinduce. The realization of a high definition activation map is the first essential step in the diagnosis. Other tools are also available to operators such as voltage maps and propagation maps to understand AT mechanism. Even if those tools are very important, they can be used only after completion of the map. On the contrary, the activation map built in real time may allow physician to focus on critical areas. The WOI setting is mandatory to create an activation map, and its modulation only induces a phase-shift of the activation pattern without affecting the sequence of activation [8, 9]. The conventional setting of WOI has been described by De Ponti et al. using a specific formula including the length of the tachycardia cycle, the duration of the P wave and the interval between the P wave and the reference signal. In this study, we evaluated a different approach for the setting of the WOI that allowed the WOI to reflect the transition into the critical slow conduction zone in macroreentrant circuits. In macroreentrant tachycardia, as in typical atrial flutter (cavotricuspid isthmus-dependent), the plateau phase following the P wave corresponds to the region of slow conduction where the cavotricuspid isthmus is located [5]. This proved to be applicable to other macrorentrant tachycardias in our study. WOIp-wave allowed accurate identification of the mechanism on the basis of the initial maps in 93% of cases compared with 58% with the WOIconv.. Diagnoses were more consistent between all blinded investigators explaining these results. These can be explained by a reduction in misinterpretation of perimitral macroreentrant AT as focal tachycardia. Investigators confused more perimitral macroreentrant ATs with the WOIconv. (43.3%). Perimitral were misinterpreted as being focal in 53.8% (n = 7) of maps, as macroreentry of the roof in 38.5% (n = 5) and as macroreentry of the anterior wall of the LA in 7.7% (n = 1). This observation is illustrated in the flowchart in Fig. 3a where 30% of perimitral ATs and 75% of other macroreentrant circuits were misinterpreted as focal ATs by one investigator.
Our study shows that the choice of projected colours plays an important role in the diagnostic accuracy. The colour interpolation feature of CARTO3 software and the physiology of the human eye regarding colour perception could be factors. First, scar due to previous linear lesions or ablation of fractionated signals induces complex patterns of propagation that can result in low-density mapping especially within and around scars. Limited point density in conduction block areas when it corresponds to “early meets late” location, allows over-interpolation within large areas. This could give the appearance of focal AT with centrifugal activation, masking a macroreentrant mechanism [10]. An example of this phenomenon is showed in Fig. 2; scars from the anterior region of the LA force the activation wave to propagate through a small channel. The insufficient number of points close to the scar will lead the system to interpolate colours that do not correspond to real measured points inducing investigators to misinterpret the tachycardia as focal. Biosense with an upgraded HD colouring module realized after our study has corrected this CARTO3 feature. Secondly, we observed that in 90% of maps, “early meets late” was located in the anatomical MI with the WOIp-wave (only one was not located close to the anatomical MI). This observation leads the software to colour wider areas in green. As the perception of eyes is most sensitive to green light [11] (located in the middle of the visible spectrum), eyes identify a large proportion of green in the isochronal colour scale. However, discrimination of fine contrast in shades of green appears to be more difficult and prevents operators from seeing details in those areas. Since the purple/red area was mainly located in the MI, this could explain why all investigators gave a correct interpretation of perimitral AT. Perimitral AT could be terminated with several methods; in our study the ablation line was performed in the MI. The WOIp-wave forces the colour distribution of the map in the region of the isthmus into the red and purple regions allowing better discrimination of the range of activation.
This study has limitations. Evaluation in a larger number of patients with a wider range of complex ATs and real-time assessment with upgraded version of CARTO software is required to further evaluate the clinical usefulness of our WOI. Finally, in the present study, no difference was made between micro-reentry and focal.
Conclusion
In conclusion, when compared retrospectively with conventional WOI settings, our novel WOI based on end of P wave performed better with respect to the investigator’s percentage of correct identification, confidence index and time of diagnosis. It has particular value for identifying perimitral AT without affecting the identification of other tachycardias.
Supplementary Information
AT propagation map shown in fig. 2 using the conventional window of interest. (MP4 6501 kb)
AT propagation map shown in fig. 2 using the window of interest set at the end of the P wave. (MP4 6794 kb)
Acknowledgements
The authors gratefully acknowledge the assistance of Julia Legrand, Céline Marra and Charlotte Butin. We particularly thank Georges Klein for his advices. We thank the physicians and nurses involved in the care of the patients who participated in this study.
Authors’ contributions
Alexis Mechulan: concept/design, data interpretation and collection, maps interpretation, drafting article, data analysis. Sok-Sithikun Bun: data interpretation, maps interpretation, drafting article. Alexandre Masse: concept/design, data interpretation and collection. Angélique Peret: data interpretation, drafting article, data analysis. Lauriane Leong-Feng: data collection. Frederic Pons: data interpretation. Ahmed Bouharaoua: data interpretation. Pierre Dieuzaide: data interpretation and collection. Sébastien Prévot: concept/design, data interpretation and collection, maps interpretation, drafting article, data analysis.
Funding
The work was supported by the Centre de Recherche en Rythmologie Interventionnelle de Clairval and by the Groupement de Coopération Sanitaire Ramsay Santé pour l’Enseignement et la Recherche.
Data availability
The data will not be deposited but are available on request.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The study was approved by the local institutional review board (Ethics approval number: COS-RGDS-2016-06-087-MECHULAN-A).
Consent to participate
Informed consent or non-opposition (MR004) was obtained from all individual participants included in the study.
Consent for publication
All participants consented to the publication.
Code availability
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kim Y-H, Chen S-A, Ernst S, Guzman CE, Han S, Kalarus Z, Labadet C, Lin YJ, Lo LW, Nogami A, Saad EB, Sapp J, Sticherling C, Tilz R, Tung R, Kim YG, Stiles MK. 2019 APHRS expert consensus statement on three-dimensional mapping systems for tachycardia developed in collaboration with HRS, EHRA, and LAHRS. J Arrhythmia. 2020;36(2):215–270. doi: 10.1002/joa3.12308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patel AM, d’Avila A, Neuzil P, Kim SJ, Mela T, Singh JP, et al. Atrial tachycardia after ablation of persistent atrial fibrillation: identification of the critical isthmus with a combination of multielectrode activation mapping and targeted entrainment mapping. Circ Arrhythm Electrophysiol. 2008;1(1):14–22. doi: 10.1161/CIRCEP.107.748160. [DOI] [PubMed] [Google Scholar]
- 3.De Ponti R, Verlato R, Bertaglia E, Del Greco M, Fusco A, Bottoni N, et al. Treatment of macro-re-entrant atrial tachycardia based on electroanatomic mapping: identification and ablation of the mid-diastolic isthmus. Europace. 2007;9(7):449–457. doi: 10.1093/europace/eum055. [DOI] [PubMed] [Google Scholar]
- 4.Rav-Acha M, Ng CY, Heist EK, Rozen G, Chalhoub F, Kostis WJ, et al. A novel annotation technique during mapping to facilitate the termination of atrial tachycardia following ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2016;27(11):1274–1281. doi: 10.1111/jce.13063. [DOI] [PubMed] [Google Scholar]
- 5.Bun SS, Latcu DG, Marchlinski F, Saoudi N. Atrial flutter: more than just one of a kind. Eur Heart J. 2015;36(35):2356–2363. doi: 10.1093/eurheartj/ehv118. [DOI] [PubMed] [Google Scholar]
- 6.Saoudi N, Cosío F, Waldo A, Chen S A, Iesaka Y, Lesh M, et al. Working Group of Arrhythmias of the European of Cardiology and the North American Society of Pacing and Electrophysiology. (2001). A classification of atrial flutter and regular atrial tachycardia according to electrophysiological mechanisms and anatomical bases; a Statement from a Joint Expert Group from The Working Group of Arrhythmias of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. European Heart Journal, 22(14), 1162–1182. [DOI] [PubMed]
- 7.Latcu DG, Squara F, Massaad Y, Bun SS, Saoudi N, Marchlinski FE. Electroanatomic characteristics of the mitral isthmus associated with successful mitral isthmus ablation. EP Europace. 2015;18(2):274–280. doi: 10.1093/europace/euv097. [DOI] [PubMed] [Google Scholar]
- 8.Del Carpio MF, Buescher TL, Asirvatham SJ. Three-dimensional mapping of cardiac arrhythmias: what do the colors really mean? Circulation. Arrhythmia Electrophysiol. 2010;3(6):e6–11. doi: 10.1161/CIRCEP.110.960161. [DOI] [PubMed] [Google Scholar]
- 9.Selvaraj RJ, Shankar B, Subramanian A, Nair K. Chasing red herrings: making sense of the colors while mapping. Circ Arrhythm Electrophysiol. 2014;7(3):553–556. doi: 10.1161/CIRCEP.113.001391. [DOI] [PubMed] [Google Scholar]
- 10.Namboodiri N. Electroanatomic contact mapping: how to use optimally to recognise the arrhythmia mechanism? Indian Pacing Electrophysiol J. 2010;10(1):1–7. doi: 10.1016/S0972-6292(16)30457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rolf GK. Variability in unique hue selection: a surprising phenomenon. Color Res Appl. 2004;29(2):158–162. doi: 10.1002/col.10237. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
AT propagation map shown in fig. 2 using the conventional window of interest. (MP4 6501 kb)
AT propagation map shown in fig. 2 using the window of interest set at the end of the P wave. (MP4 6794 kb)
Data Availability Statement
The data will not be deposited but are available on request.